Introduction
Osteosarcoma (OS) is the most common malignant
primary bone tumor in childhood (1), with an age-standardized incidence of
∼5 per million per year in America (2). It has a high propensity to
metastasize to the lungs, and the prognosis for localized extremity
OS treated with surgery alone is poor (<20% 2-year survival)
(3). Despite improvements in
multidisciplinary treatment, 30% of patients with localized disease
and 80% with metastatic disease at diagnosis will relapse (4,5),
even if they have received large dose of adjuvant/neoadjuvant
chemotherapy. Failure of standard multimodal therapy for the OS is
associated with a very poor prognosis. Surgical resection has been
shown to prolong survival among patients with pulmonary metastases
(6,7), including soft tissue sarcoma
(7,8), and similar results are obtained for
lung metastases from OS (9–16).
China is a large country consisting of various people with
different cultural backgrounds, and some patients are unwilling to
undergo operation because of their traditional cultural custom,
which limits the use of surgical resection. Besides, the efficacy
of second-line chemotherapy still remains controversial while many
investigators (13,14) have reported that outcome of
patients suffered from relapsed OS treated only by surgery or by
surgery combined with second-line chemotherapy was almost the same.
Thus, development of novel techniques for OS patients with
pulmonary metastasis is highly desired.
Stereotactic radiosurgery (SRS), such as gamma
knife, is defined as the delivery of external beam radiation
treatment technique by the use of several precisely aimed, highly
focused beams targeted to ablate a specific lesion with a fixed, or
virtual, stereotactic frame. Given its excellent rates of local
disease control with limited toxicity to surrounding tissues, it
has increasingly been accepted in the management of intracranial
neoplasms, and interest has developed in noncranial applications.
Obvious opportunities included tumors of the spine, lung, pancreas
and prostate (17–20). Many cases of primary or secondary
pulmonary tumors (17, 21–24),
include pulmonary metastasis from soft tissue sarcoma (24,25),
SRS offers a very effective treatment option without significant
complications, especially in medically-impaired patients who are
not surgical candidates or refuse surgery.
SRS-induced lung injury, or radiation pneumonitis,
is usually related with tumor size and tumor location (17). Tumors >50 ml in size and those
associated with the central airways also are associated with
greater frequencies of radiation pneumonitis (26). Some investigators find that the
incidence of severe radiation toxicity (including a decrease in
pulmonary function, pneumonia and pleural and pericardial
effusions) is 60% greater for central tumors compared with
peripheral ones (27). The unique
metastatic characteristics of OS, including affinity for the lung,
usually small lesions and often location at peripheral parts of the
lung, may make SRS as a potential therapy for this disease. Until
now, there are few data in the literature on the role of SRS in
pulmonary metastases from OS.
The stereotactic gamma-ray body therapeutic system,
or body gamma knife, is developed as a new technology by the OUR
International Technology & Science Co. Ltd. (Shenzhen, China).
The body gamma knife uses a stereotactic body frame and 30
Co60 sources scattered throughout the cavity of the
primary chambers. Combination of rotating multiple beam angles with
different sources (collimator) and stereotactic body frames can
result in sharp dose gradients, high-precision localization, and a
high dose per fraction in extracranial locations. This approach
delivers a very high biological effective dose (BED) to the center
of the target while delivering a microscopic dose to the clinical
target volume and a minimized dose to normal tissues. Previous
literature from China reported in early stage non-small cell lung
cancer, body gamma knife could achieve promising local control and
survival with minimal toxicity (28,29).
Thus, we reviewed the clinical data of OS patients
suffered from pulmonary metastases treated with body gamma knife in
our hospital (Shanghai), and compare the efficacy with that of
surgical resection to determine if SRS could achieve acceptable
local control and survival in this subset of OS patients.
Materials and methods
From January 2005 to December 2012, patients with
nonmetastatic OS of the extremity were diagnosed and treated at our
institution in Shanghai, according to four different neoadjuvant
protocols of chemotherapy reported in detail in previous studies
(30–34). Diagnosis of OS, established by
clinical and radiological findings, was always confirmed on
histologic slides of tumor tissue obtained from an open or trocar
biopsy and from the resected specimens.
A complete medical history was obtained for all
patients, who also underwent a thorough physical examination and
several chemical laboratory tests. The primary tumor was evaluated
on standard radiographs, and in the most recent cases, also by MRI.
The absence or presence of bone metastases was ascertained by total
body scans, whereas chest CT scans of the chest were used to
exclude lung metastases. Surgery consisted of amputation or limb
salvage. During postoperative chemotherapy, besides the clinical
evaluation, patients were checked every two months with CT scans of
the chest and treated limb. After completion of adjuvant
chemotherapy, patients were followed in the outpatient clinic with
CT every two months for two years, every three months in the 3rd
year and then every six months.
Patient selection after relapse to enter
this review
All the patients who relapsed with only pulmonary
metastasis during the period of postoperative chemotherapy or
follow-up were eligible for this study since it was designed to
precisely evaluate the efficiency of SRS on pulmonary metastasis.
After relapse with pulmonary metastasis, all patients were treated
at our institution. In chest CT scan, the number of pulmonary
metastasis was defined from consensus among at least 3 radiological
oncologists.
Treatment for pulmonary metastasis:
surgical resection
For patients who received surgical resection for
pulmonary metastasis. Criteria for resectability of lung metastases
were: i) no pleural or pericardial effusions; ii) no metastases in
other organs besides lungs; and iii) complete resectability leaving
adequate residual pulmonary functions (by removing all evident
metastatic lesions with no tumor tissue at the resection margins).
They underwent a monolateral or bilateral thoracotomy at the same
time. If found to be necessary during surgery, wedge resections
were performed manually using vascular clamps and resorbable
sutures. A lobectomy or a pneumonectomy was performed when
necessary depending on the extension, number and site of the
pulmonary lesions. Dissection of hilar lymph nodes was performed
when necessary. After resection, all the specimens were checked by
histological examination.
Radiotherapy equipment
The body gamma knife uses rotary conical surface
focusing to focalize 30 Co60 sources with total activity
of 8500 Ci, the focal dose rate at the initial source setting was 3
Gy/min. The body gamma knife consists of a radiation source,
collimator and treatment bed. The head of radiation source is an
iron ball rind with 30 Co60 sources scattered throughout
the cavity of the primary collimator. The source body rotates
horizontally around the central axis with the 30 bundles of gamma
ray directed toward a focal target. In the present study, three
chamber groups of with collimator aperture diameters of 3, 12 and
18 mm, respectively, were used; the full width at half-height of
the dose-field range at the target was 10, 30 and 50 mm,
respectively. As the aperture diameter of the collimator decreased,
the density of the distributed dose increased, and the periphery
dose decreased. Three groups of terminal collimators with different
apertures direct the focusing of the radials. Target volume of 1–10
cm in diameter could be treated using a combination of collimators
with different aperture diameters. The treatment bed can move in X,
Y and Z directions and can automatically adjust the target to the
focal point of the radials.
SRS planning and delivery
Supine or prone position was selected according to
CT scan. Patients were immobilized using a vacuum bag covering them
from the head to the pelvis. Each patient underwent slow CT
simulation at 10 sec/slide with a CT-slide thickness of 5 mm and
CT-slide interval of 5 mm to take into consideration tumor motion.
Selected patients with significant tumor motion (>1 cm) were
evaluated fluoroscopically. Additional margins for tumor motion
were added based on the results of the fluoroscopic analysis. After
the scan was finished, the positional parameters were recorded in
order to repeat the position when the patient was irradiated. The
images of CT simulation were then imported into the treatment
planning system (OUR WB-GR TPS99). Reconstructions were performed
on a three-dimensional conformal radiotherapy planning
algorithm.
The gross target volume (GTV) was delineated in the
lung window, and a minimum margin of 1 cm was used to form the
planning tumor volume (PTV) from the GTV. Either a single focus or
multiple foci of radiation beams were used based on the size of the
PTV. For example, if treatment of a round <3 cm PTV was
required, a single focus of radiation beams using collimators with
an aperture diameter of 12 mm was used. However, if treatment of a
>3–10 cm PTV was required, a combination of multiple foci of
radiation beams using different collimators with different aperture
diameters depending on the shape of the PTV was applied to ensure
conformal radiotherapy.
Until now, there are few data on the proper
radiation dosage of SRS in treating pulmonary metastases from OS.
Therefore, we used the radiation dosage reported by Xia et
al (28) as a reference,
considering the similarity of the location of pulmonary lesions and
size (most were periphery and <3 cm). A radiation dose of 50 Gy
was prescribed to the 50% isodose line covering at least 95% of the
PTV (5 Gy/fraction). In addition, delivery of a total dose of 70 Gy
(70% isodose line) covering at least 90% of the GTV (7 Gy/fraction)
was required. Three-dimensional imaging of isodose coverage of GTV
and PTV was used to select aperture diameter, and number and
location of target foci depending on the size and shape of the
target volume. Radiotherapy was delivered over 2 weeks in 5
fractions per week. In general, the volume of the lung receiving at
least 20 Gy was required to be <20%. Moreover, the dose
delivered to critical structures such as the main bronchi,
esophagus, trachea, heart and major blood vessels was required to
be <50 Gy (5 Gy/fraction), and the dose delivered to the spinal
cord was required to be <30 Gy (3 Gy/fraction) (28).
Data collection and assessments
Patients were evaluated through physical examination
and routine laboratory analyses (blood count, renal and liver
functions) every 2 weeks. Radiologic investigations were performed
at every 2 month until progress (assessed by RECIST 1.1).
The aim of this study was to compare efficiency of
SRS on pulmonary metastasis with that of surgical resection. The
primary endpoint of the study was post-relapse progress-free
survival (PRPFS), which was calculated from the date of pulmonary
metastasis until progress or last follow-up (assessed by RECIST
1.1). The secondary endpoint was post-relapse overall survival
(PROS), which was calculated from the date of pulmonary metastasis
until death or last follow-up. Toxicity was assessed according to
the National Cancer Institute Common Toxicity Criteria (V3.0). The
radiation reaction was classified as early or late side effects
according to Radiation Therapy Oncology Group toxicity
criteria.
Ethics approval for the study was provided by the
independent ethics committee, Sixth People’s Hospital, Shanghai
JiaoTong University. Informed and written consents were obtained
from all patients or their advisers according to ethics committee
guidelines.
Statistics
The evaluation of PRPFS and PROS was performed using
the Kaplan-Meier method for calculating survival curves.
Differences among the SRS and surgical-resection groups were
compared by means of the χ2 test and t-test.
Multivariate analyses of survival including the variables that
correlated with PRPFS and PROS (i.e., interval from to pulmonary
metastasis, number of lesions, monolateral or bilateral lungs) were
carried out using the Cox proportional hazards model. The
significance was defined at a 2-sided, P-value of <0.05.
Statistical analysis was performed using the SPSS software, version
13.0 (SPSS Inc., Chicago, IL, USA).
Results
Characteristics of patient and pulmonary
metastasis at relapse
Fifty-eight patients were eligible for this
retrospective review. At initial diagnosis, the median age of all
58 patients was 21 years (range, 8–59 years), among them, there
were 41 males (70.7%) and 17 females (29.3%), 26 received
amputation (44.8%) while the other 32 (55.2%) received limb
salvage, the initial tumor sites were 33 at femur (56.8%), 19 at
tibia (32.7%), 5 at humerus (8.6%) and 1 at fibula (1.7%).
The median interval from the start of treatment to
pulmonary metastasis was 12.4 months (range, 2–70.7). Among the 58
patients with pulmonary metastasis, 13 relapsed after 24 months
(22.4%), 17 relapsed within 12–24 months (29.3%), 28 relapsed
within 12 months (48.3%). Twenty-nine patients relapsed with
monolateral lesions (50%) while the other half with bilateral
lesions. Twenty-seven (46.5%) patients relapsed with only one
nodule while 31 (53.4%) patients relapsed with two nodules or more.
The number of nodules are defined by CT scans of the chest before
onset of treatment for relapse.
The number of pulmonary lesions in 58 patients range
from 1 to 9, median 2 lesions. From CT scan, number of pulmonary
lesions in the SRS group (range from 1 to 9 lesions, mean
2.19±1.79) was parallel to (P>0.05) that in the surgical group
(range from 1 to 5 lesions, mean 2.59±1.44).
Treatment of relapse
Of the patients 31 underwent surgical resection
(surgical group, consist of 14 wedge resections and 17 lobectomies)
and 27 underwent gamma knife SRS (SRS group). For patients in
surgical group, radiologically metastatic nodules were all proven
to be metastasis from OS histologically after resection. The
characteristics of the patients in two groups are listed in
Table I. Factors such as age,
gender, initial tumor site, histotype of tumor, method of surgery
for initial tumor, time to relapse were well balanced between the
groups. No difference was observed in aspects of pulmonary lesion
site and pulmonary lesion number between surgical group and SRS
group.
 | Table I.Characteristics of patients wth
occurrence of pulmonary metastasis. |
Table I.
Characteristics of patients wth
occurrence of pulmonary metastasis.
| Demographic data | Resection | SRS | P-value |
|---|
| No. of subjects | 31 | 27 | |
| Gender | | | 0.96 |
| Male | 22 | 19 | |
| Female | 9 | 8 | |
| Age (years) | | | |
| >18 | 22 | 17 | 0.51 |
| ≤18 | 9 | 10 | |
| Site | | | |
| Femur | 18 | 15 | 0.84 |
| Tibia | 10 | 9 | |
| Humerus | 2 | 3 | |
| Fibula | 1 | 0 | |
| Surgery | | | |
| Amputation | 15 | 11 | 0.56 |
| Limb salvage | 16 | 16 | |
| Histotype | | | |
| Classic | 29 | 24 | 0.87 |
| Others | 2 | 3 | |
| Site of pulmonary
lesions | | | |
| Monolateral | 19 | 10 | 0.11 |
| Bilateral | 12 | 17 | |
| No. of pulmonary
lesions | | | |
| Solitary | 17 | 10 | 0.17 |
| Multiple lesions
(≥2) | 14 | 17 | |
| Time to relapse
(years) | | | |
| ≤2 | 22 | 23 | 0.20 |
| >2 | 9 | 4 | |
Outcome
Until last follow-up in October 2013, the follow-up
time after the treatment for relapse in surgical and SRS group was
5–96 months (median, 22 months) and 8–50 months (median, 18
months), respectively. In patients without progression or still
alive after treatment for relapse, their minimum follow-up time had
reached 2 years.
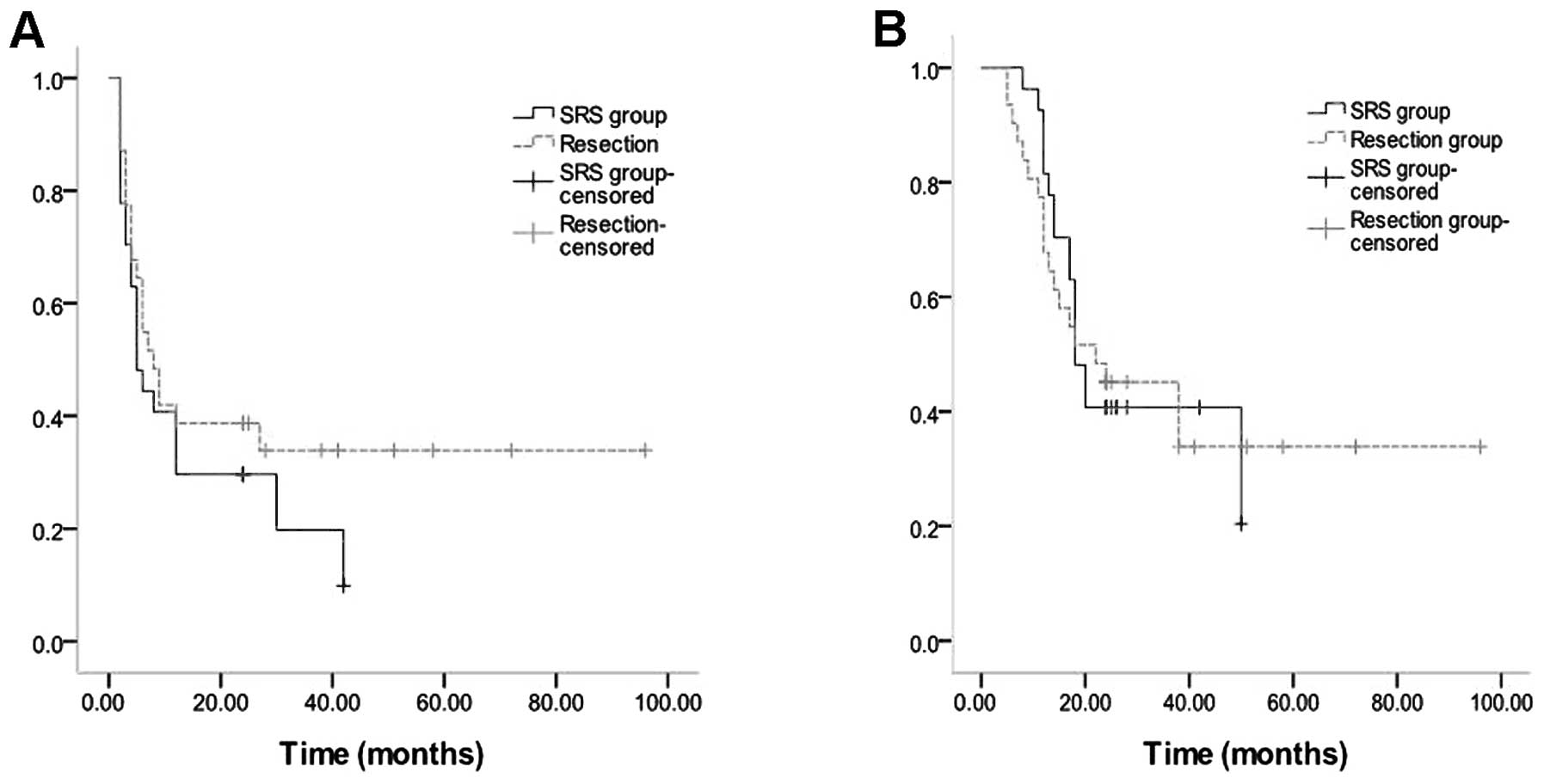
Of 58 patients, the median time of PRPFS and PROS
was 6 months (range, 2–96 months) and 18 months (range, 5–96
months), respectively (Fig. 1A and
B). Until last follow-up in October 2013, there were 15
relapses with pulmonary metastasis, 3 with bone metastasis, 1 with
pulmonary and bone metastasis in surgical group. In the SRS group,
there were 4 relapses with local pulmonary recurrences, 13 with
pulmonary metastasis, 2 with bone metastasis, 1 with pulmonary and
bone metastasis, and 1 with local recurrence at initial tumor
site.
Four months after completion of SRS, the CR rate was
59.2% (16/27), the PR rate was 11.1% (3/27), the disease control
rate was 70.3%. In the surgical group, 4 months after operation,
the CR rate was 70.9% (22/31), the disease control rate was also
70.9% (No PR in surgical resection). The two-year progression-free
survival rate was 38.7% (12 CRs, 9/31) in the surgical group and
33.3% (8 CRs and 1 PR, 9/27) in the SRS group. The two-year
survival rate was 48.3% (15/31) in the surgical group and 40.7%
(11/27) in the SRS group. The median time of PRPFS in the surgical
and SRS group was 8 and 5 months, respectively. The median time of
PROS in the surgical and SRS group was 22 and 18 months,
respectively (Fig. 2A and B).
Differences in median time of PRPFS, median time of PROS, two-year
progression-free survival rate and two-year survival rate between
two groups were not significant (P>0.05).
Cox proportional hazards model indicated that time
to relapse and number of pulmonary lesions are associated
significantly with PRPFS and PROS. Moreover, these two factors were
well balanced between the groups (Table I).
Adverse events for SRS and surgical
resection
Acute toxicity was defined as events occurring in
the first 3 months after SRS treatment, and late toxicity was
defined as events occurring >3 months after SRS treatment.
Generally speaking, the addition of SRS for patients was well
tolerated. There have been no cases of grade 3–5 toxicity or
possible treatment-related death observed. The most common acute
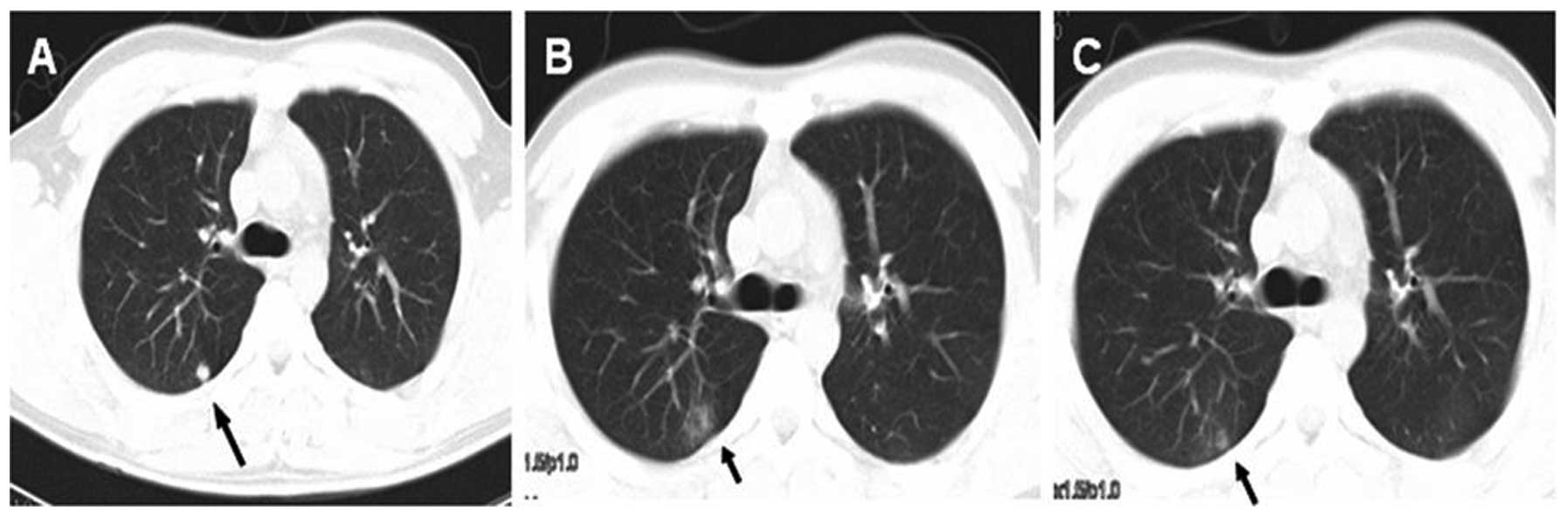
toxicity from SRS was grade 1–2 pneumonitis in 9 patients (7
patients with grade 1 while 2 patients with grade 2), account for
33.3%. All cases of radiation pneumonitis occurred 1–2 months after
treatment and resolved within 1–2 months (Fig. 3). We did not observe other
complications include the development of pleural effusions,
hemoptysis, tracheoesophageal fistula, pericardial effusions, or
pneumothoraces. In terms of late toxicity, until the last follow-up
at October 2013, there was no incidence of radiation pulmonary
fibrosis or grade 3–5 radiation pneumonitis.
For the 31 patients who underwent surgical
resection, there were no surgery-related deaths. The most common
complication is persistent pneumothorax in 3 patients. These
pneumothorax resolved by keeping the drainage in situ for a
longer period of time (10–15 days) and by placing a unidirectional
valve removed once the pneumothorax resolved. Other complications
were respiratory insufficiency for 1 patient, superficial wound
infections for 2, prolonged air leak for 2, pneumonia for 1,
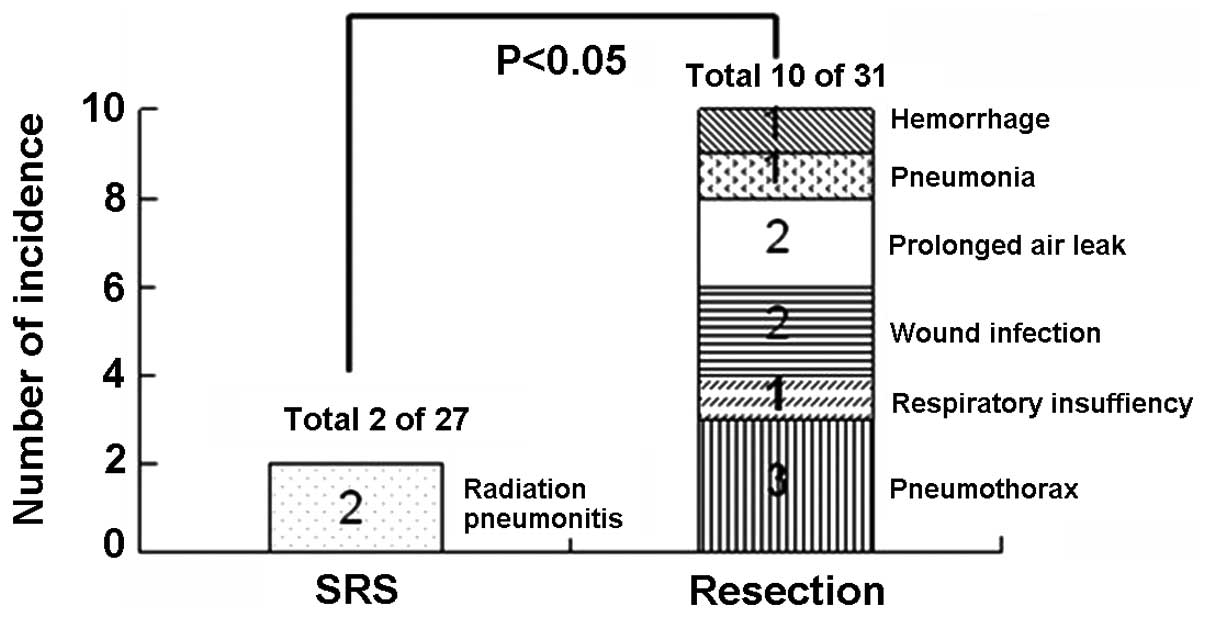
hemorrhage for 1 (Fig. 4). With
aggressive medical intervention, all the 10 patients recovered
within 2 months. More patients with complications needing medical
intervention (10 out of 31) was recorded in the surgical group than
that (2 out of 27) in the SRS group (P<0.05, Fig. 5).
Discussion
In the past, 80–90% of patients with osteosarcoma
(OS) of the extremities treated by surgery alone, mostly
amputation, relapsed in the first 12 months (3). In the last 25 years, adjuvant and
neoadjuvant chemotherapy have greatly improved prognosis of these
patients. However, still 30–40% will relapse. Lung is the most
frequent metastatic site. Of 570 patients with OS of the
extremities treated at the Istituto Ortopedico Rizzoli from
1983–1995, 206 (36%) relapsed only in the lung (35). In addition, nearly 40% of patients
who had undergone complete remission of lung metastases relapsed in
the lung (11). Although advances
in chemotherapeutic strategy and surgical approach have
significantly improved the prognosis of these patients (9–16),
optimal treatment strategy is still undefined.
The advances of technology in radiation therapy have
enabled stereotactic radiosurgery (SRS) as a novel technique for
lung cancer. SRS allows the delivery of a higher dose of radiation
to the tumor while reducing the irradiation of the surrounding
normal tissue. Previous studies have shown that SRS was feasible
and efficient in patients with primary and secondary pulmonary
tumors, including pulmonary metastasis from soft tissue sarcoma
(24). Moreover, body gamma knife,
developed as a new technology in China, has showed its comparable
efficacy with surgical resection in early stage NSCLC (28,29).
In addition, some patients in China are unwilling to undergo
surgery because of traditional cultural customs, and they are more
willing to choose SRS, which further makes body gamma knife a
promising treatment.
In our study, for the first time, we found that the
delivery of SRS treatment by body gamma knife on OS patients with
pulmonary metastasis could yield an outcome which was equivalent to
that from surgical resection. Although there was a 3 months
difference in PRPRS and a 4 months difference in PROS with better
results for the surgical group, the difference is not significant.
We believed that it may be contributed to a higher proportion of
patients with one pulmonary metastatic lesion in the surgical group
(17 out of 31, while 10 out of 27 in the SRS group), as Cox
proportional hazards model indicated that number of pulmonary
lesions was a key factor which could affect PRPFS and PROS. It is
reasonable for us to believe that SRS treatment might have an
opportunity to play a role in pulmonary metastasis from OS as it
has played in intracranial neoplasms.
A major limitation of the body gamma knife is that
the isodose distribution is mainly circuital or elliptical. If the
tumor shape is anomalous, obtaining satisfactory conformity of the
isodose line is difficult. In OS, the unique distribution pattern
of pulmonary metastatic lesion (small, peripheral and round
lesions) makes this limitation unobvious. Besides, consideration of
tumor motion is critical in SRS in a patient whose tumor moves
significantly during radiotherapy. A recent study used
four-dimensional CT to investigate patients with lung cancer and
found that the tumor moved >1 cm during breathing in 13% of the
patients, particularly those with small lower lobe tumors close to
the diaphragm (36), suggesting an
individualized tumor-motion margin should be considered for such
patients. In our study, slow CT simulation and fluoroscopy were
used to compensate for tumor motion during breathing. We found 8
patients in SRS group had significant tumor motion (>1 cm).
Although 4 patients with local pulmonary recurrences were well
balanced in patients with significant tumor motion (2 out of 8) or
not (2 out of 19), we could not exclude the influence by small
number of local pulmonary recurrences which might limit statistical
power to show the difference. In addition, we believe better
evaluation and targeting of tumor motion using improved on-board
imaging may allow further dose escalation and reduce the risk of
local recurrence.
In general, the most common acute toxicity from SRS
are pneumonitis and pneumothoraces, other side effects include
pleural effusions, hemoptysis, tracheoesophageal fistula and
pericardial effusions. Some of these side effects might be
life-threatening. The most common later toxicity from SRS are
fibrosis and grade 3 to 5 radiation pneumonitis. Encouragingly, the
toxicity of body gamma-knife radiosurgery in our study was well
tolerated. The 27 patients who underwent SRS experienced no
treatment-related death or serious side effects, and more
inspiring, the SRS group needed less medical intervention to
resolve side effects than the resection group, due to only 9
patients with grades 1–2 pneumonitis. The safety of SRS on
pulmonary metastasis from OS may be contributed to not only the
technical improvements but also the unique metastatic
characteristics of OS, which are usually small lesions and often
located at peripheral parts of the lung. For SRS, smaller lesions
at peripheral site of the lung without association with central
airways means less possibility of lung injury (22,23),
which implies that pulmonary metastasis from OS is a potentially
good candidate for SRS.
There are several shortcomings in our study. First,
our study was limited to its retrospective nature with possible
patient selection bias which could affect the prognosis. In China,
patients have to pay for SRS treatment as it is not covered by
medical insurance. This might make some patients eligible for SRS
treatment to choose surgery. Secondly, due to the limited number of
patients, we believed the efficiency of SRS treatment on OS
patients with pulmonary metastasis, compared with that of
resection, is still far from being defined. Thirdly, because our
study is the first to investigate the efficacy of body gamma knife
in treatment of pulmonary metastasis from OS, we took the
prescribed radiation dosage reported by Xia et al (28), which investigated NSCLC, as a
reference. The radiation dosage might not be optimal.
SRS is a potentially alternative treatment for
pulmonary metastasis of OS after the failure of adjuvant
chemotherapy, especially for those patients who were medically
unfit for a resection (on the basis of age, comorbidities), or who
refused surgery. Further studies to confirm the results, especially
prospective clinical trials, focusing on the efficiency of SRS
treatments for OS patients with pulmonary metastasis compared with
surgical resection, should be strongly considered.
Acknowledgements
Editorial assistance in the
preparation of this study was provided by Dr Daliu Min from
Department of Oncology, Affiliated Sixth People’s Hospital,
Shanghai Jiaotong University.
References
|
1.
|
Arndt CA and Crist WM: Common
musculoskeletal tumors of childhood and adolescence. N Engl J Med.
341:342–352. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar
|
|
3.
|
Friedman MA and Carter SK: The therapy of
osteogenic sarcoma: current status and thoughts for the future. J
Surg Oncol. 4:482–510. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Ozaki T, Flege S, Kevric M, et al:
Osteosarcoma of the pelvis: experience of the Cooperative
Osteosarcoma Study Group. J Clin Oncol. 21:334–341. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Tabone MD, Kalifa C, Rodary C, Raquin M,
Valteau-Couanet D and Lemerle J: Osteosarcoma recurrences in
pediatric patients previously treated with intensive chemotherapy.
J Clin Oncol. 12:2614–2620. 1994.PubMed/NCBI
|
|
6.
|
Sternberg DI and Sonett JR: Surgical
therapy of lung metastases. Semin Oncol. 34:186–196. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Casiraghi M, De Pas T, Maisonneuve P, et
al: 10-year single-center experience on 708 lung metastasectomies:
the evidence of the ‘international registry of lung metastases’. J
Thorac Oncol. 6:1373–1378. 2011.PubMed/NCBI
|
|
8.
|
Sardenberg RA, Figueiredo LP, Haddad FJ,
Gross JL and Younes RN: Pulmonary metastasectomy from soft tissue
sarcomas. Clinics. 65:871–876. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Kempf-Bielack B, Bielack SS, Jürgens H, et
al: Osteosarcoma relapse after combined modality therapy: an
analysis of unselected patients in the Cooperative Osteosarcoma
Study Group (COSS). J Clin Oncol. 23:559–568. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Saltzman DA, Snyder CL, Ferrell KL,
Thompson RC and Leonard AS: Aggressive metastasectomy for pulmonic
sarcomatous metastases: a follow-up study. Am J Surg. 166:543–547.
1993.PubMed/NCBI
|
|
11.
|
Biccoli A, Rocca M and Salone M: Resection
of recurrent pulmonary metastases in patients with osteosarcoma.
Cancer. 104:1721–1725. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Bielack SS, Kempf-Bielack B, Branscheid D,
et al: Second and subsequent recurrences of osteosarcoma:
presentation, treatment, and outcomes of 249 consecutive
cooperative osteosarcoma study group patients. J Clin Oncol.
27:557–565. 2009. View Article : Google Scholar
|
|
13.
|
Briccoli A, Rocca M, Salone M, Guzzardella
GA, Balladelli A and Bacci G: High grade osteosarcoma of the
extremities metastatic to the lung: long-term results in 323
patients treated combining surgery and chemotherapy, 1985–2005.
Surg Oncol. 19:193–199. 2010.PubMed/NCBI
|
|
14.
|
Bacci G, Briccoli A, Longhi A, et al:
Treatment and outcome of recurrent osteosarcoma: experience at
Rizzoli in 235 patients initially treated with neoadjuvant
chemotherapy. Acta Oncol. 44:748–755. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Temeck BK, Wexler LH, Steinberg SM,
McClure LL, Horowitz M and Pass HI: Metastasectomy for sarcomatous
pediatric histologies: results and prognostic factors. Ann Thorac
Surg. 59:1385–1389. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Chen F, Miyahara R, Bando T, et al: Repeat
resection of pulmonary metastasis is beneficial for patients with
osteosarcoma of the extremities. Interact Cardiovasc Thorac Surg.
9:649–653. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Whyte RI: Stereotactic radiosurgery for
lung tumors. Semin Thorac Cardiovasc Surg. 22:59–66. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Shin JH, Chao ST and Angelov L:
Stereotactic radiosurgery for spinal metastases: update on
treatment strategies. J Neurosurg Sci. 55:197–209. 2011.PubMed/NCBI
|
|
19.
|
Katz AJ: CyberKnife radiosurgery for
prostate cancer. Technol Cancer Res Treat. 9:463–472. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Murphy MJ, Martin D, Whyte R, Hai J,
Ozhasoglu C and Le QT: The effectiveness of breath-holding to
stabilize lung and pancreas tumors during radiosurgery. Int J
Radiat Oncol Biol Phys. 53:475–482. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Christie NA, Pennathur A, Burton SA and
Luketich JD: Stereotactic radiosurgery for early stage non-small
cell lung cancer: rationale, patient selection, results, and
complications. Semin Thorac Cardiovasc Surg. 20:290–297. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Banki F, Luketich JD and Chen H:
Stereotactic radiosurgery for lung cancer. Minerva Chir.
64:589–598. 2009.PubMed/NCBI
|
|
23.
|
Nuyttens JJ and van de Pol M: The
CyberKnife radiosurgery system for lung cancer. Expert Rev Med
Devices. 9:465–475. 2012. View Article : Google Scholar
|
|
24.
|
Zhang Y, Xiao JP, Zhang HZ, et al:
Stereotactic body radiation therapy favors long-term overall
survival in patients with lung metastases: five-year experience of
a single-institution. Chin Med J. 124:4132–4137. 2011.PubMed/NCBI
|
|
25.
|
Dhakal S, Corbin KS, Milano MT, et al:
Stereotactic body radiotherapy for pulmonary metastases from
soft-tissue sarcomas: excellent local lesion control and improved
patient survival. Int J Radiat Oncol Biol Phys. 82:940–945. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Le QT, Loo BW, Ho A, et al: Results of a
phase I dose-escalation study using single-fraction stereotactic
radiotherapy for lung tumors. J Thorac Oncol. 1:802–809. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Timmerman R, McGarry R, Yiannoutsos C, et
al: Excessive toxicity when treating central tumors in a phase II
study of stereotactic body radiation therapy for medically
inoperable early-stage lung cancer. J Clin Oncol. 24:4833–4839.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Xia T, Li H, Sun Q, et al: Promising
clinical outcome of stereotactic body radiation therapy for
patients with inoperable Stage I/II non-small-cell lung cancer. Int
J Radiat Oncol Biol Phys. 66:117–125. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Wu D, Zhu H, Tang H, Li C and Xu F:
Clinical analysis of stereo-tactic body radiation therapy using
extracranial gamma knife for patients with mainly bulky inoperable
early stage non-small cell lung carcinoma. Radiat Oncol. 6:842011.
View Article : Google Scholar
|
|
30.
|
Bacci G, Picci P, Ruggieri P, et al:
Primary chemotherapy and delayed surgery (neoadjuvant chemotherapy)
for osteosarcoma of the extremities. The Istituto Rizzoli
experience in 127 patients treated peroperatively with intravenous
methotrexate (high versus moderate doses) and intraarterial
cisplatin. Cancer. 65:2539–2553. 1990.
|
|
31.
|
Bacci G, Picci P, Ferrari S, et al:
Primary chemotherapy and delayed surgery for nonmetastatic
osteosarcoma of the extremities. Results in 164 patients
preoperatively treated with high doses of methotrexate followed by
cisplatin and doxorubicin. Cancer. 72:3227–3238. 1993. View Article : Google Scholar
|
|
32.
|
Ferrari S, Mercuri M, Picci P, et al:
Nonmetastatic osteosarcoma of the extremity: results of a
neoadjuvant chemotherapy protocol (IOR/OS-3) with high-dose
methotrexate, intraarterial or intravenous cisplatin, doxorubicin,
and salvage chemotherapy based on histologic response. Tumori.
85:458–464. 1999.
|
|
33.
|
Bacci G, Briccoli A, Ferrari S, et al:
Neoadjuvant chemotherapy for osteosarcoma of the extremity:
long-term results of the Rizzoli’s 4th protocol. Eur J Cancer.
37:2030–2039. 2001.
|
|
34.
|
Bacci G, Ferrari S, Longhi A, et al:
High-dose ifosfamide in combination with high-dose methotrexate,
doxorubicin and cisplatin in the neoadjuvant treatment of extremity
osteosarcoma: preliminary results of an Italian Sarcoma
Group/Scandinavian Sarcoma Group pilot study. J Chemother.
14:198–206. 2002. View Article : Google Scholar
|
|
35.
|
Bacci G, Ruggieri P, Picci P, et al:
Pattern of relapse in patients with osteosarcoma of the extremities
treated with neoadjuvant chemotherapy. Eur J Cancer. 37:32–38.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Liu HH, Balter P, Tutt T, et al: Assessing
respiration-induced tumor motion and internal target volume using
four-dimensional computed tomography for radiotherapy of lung
cancer. Int J Radiat Oncol Biol Phys. 68:531–540. 2007.PubMed/NCBI
|