Introduction
All tissues are dependent on angiogenesis for their
sustained growth. In the case of pathological tissue growth, i.e.,
in malignant tumors and endometriosis, angiogenesis is also
required for the formation of metastases and ectopic endometriotic
lesions, respectively (1,2). Angiogenesis is considered to be the
result of a net balance between the actions of pro- and
anti-angiogenic factors (3–5).
Pro-angiogenic factors include growth factors such as vascular
endothelial growth factor (VEGF) (6,7),
proteases (plasminogen activators, matrix metalloproteinases or
MMPs; (8,9), integrin adhesion molecules
(αvβ3, αvβ5) (10,11),
extracellular matrix components (collagens, laminins,
proteoglycans) and many others. The discovery of angiostatin, a
proteolytic fragment of plasminogen (12), and endostatin (13), a proteolytic fragment of collagen
XVIII, have prompted studies on anti-angiogenic factors. Both
angiostatin and endostatin have been shown to possess potent
anti-angiogenic and, as a consequence, antitumor activity in
several animal tumor models (14,15).
It has been shown that a combination of a
plasminogen activator (PA) such as urokinase- and tissue type PA
(uPA, tPA), and a free sulfhydryl donor (FSD), can adequately
generate angiostatin in vitro. The plasminogen activator is
required for the conversion of plasminogen to the active protease
plasmin. Subsequently plasmin, in the presence of an FSD, excises
the angiostatin fragment from other plasmin molecules (16). We found in a panel of 75 different
cell lines derived from 8 different tumor types, that plasminogen
activator expression and the ability to generate angiostatin in
vitro were clearly linked (17). Other reports have shown that
several species of MMPs (18,19),
PSA (20), as well as cathepsin V
(21) also play a role in
angiostatin generation. The fact that several (combinations of)
proteases, that each cleave the plasminogen/plasmin molecule at a
different location, are able to generate angiostatin, explains the
formation of several different angiostatin species. The angiostatin
species described in the original paper by O’Reilly et al
(12) consisted of the first 3
kringle domains (out of 5) of plasminogen (K1-3), but also K1-4,
K1-4.5 and K5 species have been reported (22). Although each angiostatin species
has anti-angiogenic properties (probably by inducing apoptosis in
vascular cells), the systemic and/or site-specific occurrence of
these different species, as well as their (relative) contributions
to the (anti-)angiogenic balance is unclear. In addition, little is
known about the mechanisms involved in the in vivo
generation of angiostatin. Rotenberg et al (23) determined the levels of angiostatin,
uPA and MMPs in ascitic and pleural effusions of 21 cancer
patients, and found no correlation between the presence of
angiostatin and either enzyme.
To address this question in a different patient
group, we collected cyst fluids from benign and malignant ovarian
tumors, and from functional cysts. In these fluids, we determined
the presence of plasminogen and angiostatin semiquantitatively by
western blot analysis, and the corresponding levels of uPA and tPA
by specific ELISAs. The absence or presence of angiostatin in the
cyst fluids was linked to the levels of plasminogen, uPA and
tPA.
Materials and methods
Patients
Patients with a cystic ovarian process scheduled for
surgical treatment were included in the cyst fluid collection
procedure (n=124). Informed consent was obtained from all the
patients. We included samples from ovarian carcinoma (n=31),
borderline tumors (n=17), benign mucinous ovarian tumors (n=27),
benign serous ovarian tumors (n=28), dermoid cysts (n=11) and
endometriotic lesions (n=10). After surgical removal, the tissue
was immediately transported to the pathology laboratory where
aseptic fine needle aspiration was performed to collect cyst fluid
samples. Subsequently, cooled fluid samples were centrifuged at
3,000 × g for 10 min. The supernatant was collected and stored at
−35°C until further analyses. Histopathological evaluation was
performed by an experienced gynecologic pathologist, and
clinicopathologic characteristics were retrieved from the medical
records of the patients. For comparison we used follicle fluid
collected routinely from functional cysts during an IVF procedure
from 24 women.
ELISAs for plasminogen activator
components
Determination of uPA and tPA concentration was
performed by specific double determinant ELISAs as described
previously (24,25).
Purification of plasminogen and
angiostatin
Lysine-sepharose slurry (50 μl) prepared
according to the manufacturer’s instructions (Pharmacia, Uppsala,
Sweden) was added to 200 μl of cyst fluid, and incubated
overnight on a roller bank at 4°C. Lysine-sepharose was washed
twice with phosphate buffered saline, and bound material was eluted
with 100 μl 0.2 M ε-amino-capronic acid.
Western blot analyses for plasminogen and
angiostatin
SDS-PAGE and western blot analyses were performed
essentially as described previously (17). Briefly, samples of 15 μl
purified plasminogen/angiostatin plus 15 μl sample buffer
were run on a 10% polyacrylamide gel. After electrophoresis,
samples were electroblotted onto nitrocellulose membranes
(Schleicher & Schuell, Dassel, Germany). Blots were blocked for
60 min in blocking solution [PBS/0.05% Tween-20/2% block (Roche,
Basel, Switzerland)], followed by overnight incubation with
polyclonal rabbit anti-human antibody, affinity purified against
plasminogen kringle domains 1–3, diluted in blocking solution.
After washing, blots were incubated for two hours with
peroxidase-conjugated swine anti-rabbit secondary antibody (Dako,
Glostrup, Denmark). After washing, blots were developed by
chemiluminescence according to the manufacturer’s protocol (Roche).
Plasminogen and angiostatin content were assessed
semiquantitatively by visually scoring the intensity of the
resulting bands as absent (0), low (1), moderate (2) or high (3).
In addition, the conversion level of plasminogen to angiostatin
(irrespective of the accompanying plasminogen and angiostatin
levels) was scored in 5 different categories. Category 1 refers to
cyst fluids in which no plasminogen was converted at all, and
category 5 to cases of complete conversion of plasminogen to
angiostatin (in other words no remaining plasminogen present). A
further three categories of partial conversion were defined as: 2,
<50% of plasminogen converted to angiostatin (plasminogen band
more intense); 3, ∼50% of plasminogen converted (bands comparable
in intensity); and 4, >50% of plasminogen converted (angiostatin
band more intense).
Statistical analysis
In samples with no conversion of plasminogen to
angiostatin, the 75% percentile of plasminogen activator levels was
7.0 and 18.5 ng/ml for uPA and tPA, respectively. We used these
values to define an increased level of activator components in the
analyses. Univariable logistic regression was used to study the
occurrence of the conversion of plasminogen to angiostatin, to the
probability of increased plasminogen activator component. The
dependent variable was the probability of increased uPA, increased
tPA or an increase in both, respectively. The independent variable
was conversion of plasminogen to angiostatin, in five categories as
described above. The odds ratios with 95% confidence intervals are
presented.
Results
Plasminogen and angiostatin levels in
ovarian tumor cyst fluid and functional cyst fluid samples
Cyst fluid plasminogen and angiostatin levels were
assessed semiquantitatively by western blot analysis. Two examples
from each source of cyst fluid are shown in Fig. 1. Whereas the molecular weight (MW)
of the plasminogen we found in the different samples appeared to be
constant, variations were observed in the molecular weights of the
different angiostatin species. In most samples at least two
different angiostatin bands were observed. This is particularly
clear in sample 1 in Fig. 1,
showing two bands of ∼37 and 42 kDa. In functional cyst samples,
two angiostatin bands in the 50 kDa range could be observed. Next
to these rather large differences in molecular weight, some more
subtle differences were observed as well. The angiostatin bands in
the two mucinous benign cyst fluids, for example (samples 5 and 6
in Fig. 1) are within the 37–40
kDa 3 kringle range but have clearly somewhat different molecular
weights.
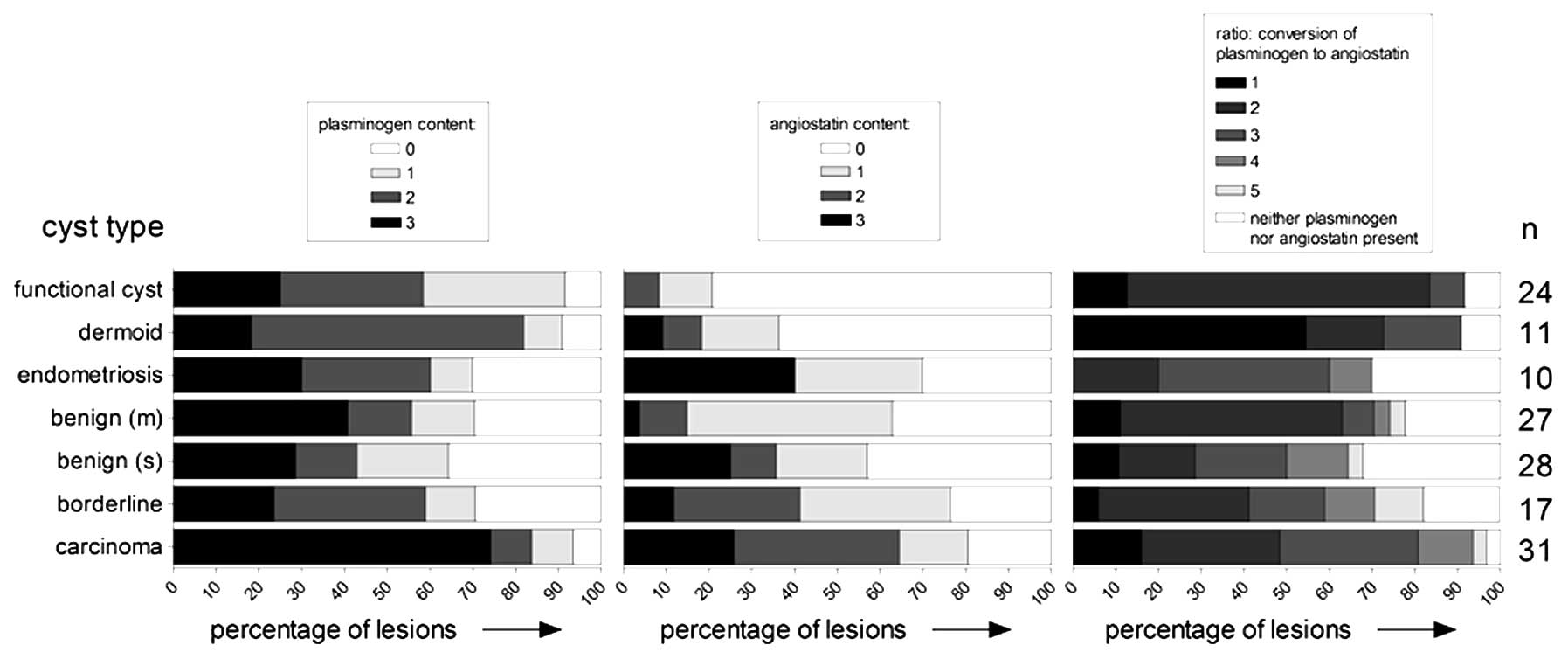
A summary of the semiquantitative assessment of the
levels of plasminogen and angiostatin in each cyst fluid sample is
shown in Fig. 2 (left panel,
plasminogen; middle panel, angiostatin). High levels of plasminogen
were notably found in the majority of carcinoma derived samples. In
the fluid samples derived from other pathological cysts, a large
variation in the levels of these proteins was observed. In these
types of cyst fluids, samples with very large amounts of
plasminogen were found, next to samples with no plasminogen at all.
Compared to plasminogen, the observed level of angiostatin was
generally lower and there were more samples with no angiostatin
protein at all. The highest percentages of samples with moderate or
high amounts of angiostatin were derived from carcinoma,
borderline, and, remarkably, endometriosis cysts.
In contrast to the samples derived from pathological
cysts, the functional cyst samples all presented with a rather
consistent pattern of moderate amounts of plasminogen and only
small amounts (if any) angiostatin. With the exception of the
instances mentioned above, no further obvious correlations between
source of the cyst fluid, and the presence and/or quantity of
either plasminogen or angiostatin were observed.
In the right panel of Fig. 2, the degree of plasminogen to
angiostatin conversion is shown, divided in different categories.
Categories were based on the ratio between plasminogen and
angiostatin content. Category 1 contained those samples where
sufficient amounts of plasminogen were present with no conversion
to angiostatin whatsoever. Category 5 contained those samples where
plasminogen was completely absent due to conversion to angiostatin.
These two extremes where rather uncommon, as in many samples both
plasminogen as angiostatin was observed, indicating a partial
conversion of plasminogen to angiostatin. Category 3 contained
those samples where the levels of plasminogen and angiostatin were
approximately equal, while in category 2 the conversion to
angiostatin was low and in category 4 this was high. The last
category contained samples where both plasminogen and angiostatin
were not present.
Plasminogen activators in tumor cyst
fluid and functional cyst fluid samples
As plasminogen activators are in vitro potent
mediators of plasminogen to angiostatin conversion, we assessed uPA
and tPA levels in the cyst fluid samples to study whether the same
mechanism is involved in in vivo angiostatin generation. In
Fig. 3, uPA (left panel) and tPA
levels (right panel) in the different types of cyst fluid are
shown. All cyst fluid groups with the exception of endometriosis
(see below) contained a number of samples with no detectable uPA.
Median uPA levels tended to be lower in benign lesions. A notable
exception was the endometriosis samples, that all contained uPA,
some to a very high level. In fact, the mean uPA level in this
group was by far the highest and significantly different from all
other groups (one-way ANOVA, p<0.001). This pattern was not
observed for tPA, as this enzyme showed highest median levels in
serous benign tumors cyst fluid, followed by borderline tumors and
endometriosis. Both uPA and tPA were low in dermoid tumors and
functional cysts. The tPA levels in the endometriosis group were
not strikingly different from the other cyst fluid groups.
Plasminogen activator levels are
associated with the conversion of plasminogen to angiostatin
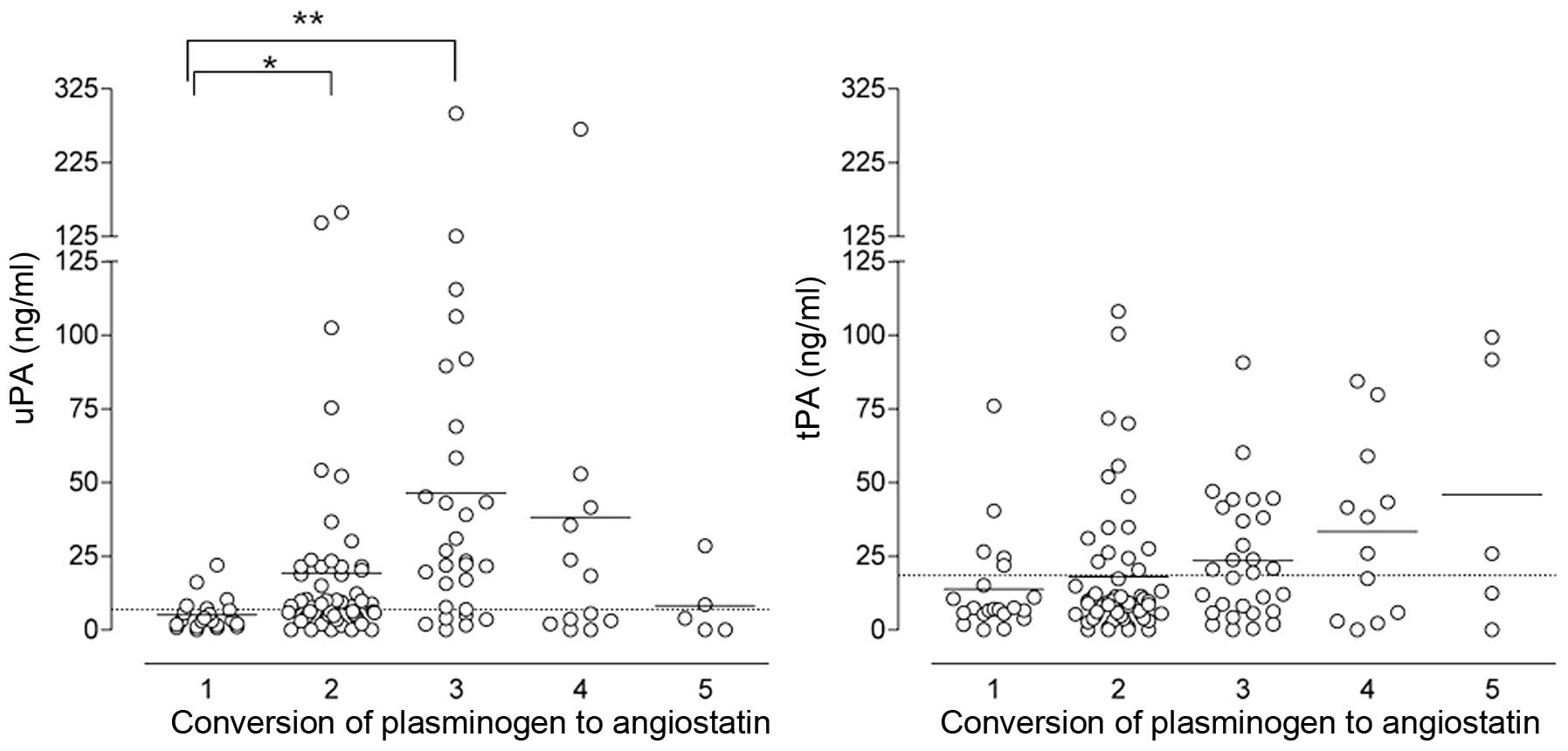
Fig. 4 shows the
levels of uPA (left panel) and tPA (right panel) in relation to the
conversion level of plasminogen to angiostatin. Samples that
contained neither plasminogen nor angiostatin were excluded from
this plot, as these are not informative.
Mean uPA levels were clearly lowest in the samples
in which no conversion of plasminogen to angiostatin occurred
(category 1). In samples with a low to moderate levels of
conversion (categories 2 and 3), uPA levels were significantly
higher (t-test, p=0.046 and 0.003, respectively). With increasing
levels of plasminogen to angiostatin conversion (category 4), the
mean uPA declined again. Remarkably, in the small group (n=5) of
samples with a complete conversion of plasminogen to angiostatin,
uPA levels were comparable to those in the group of samples with no
conversion at all.
The differences in tPA content between the
categories with different levels of plasminogen to angiostatin
conversion were less obvious and not significantly different,
although the median tPA level increased with increasing conversion
levels.
As it is possible that both uPA and tPA are involved
in angiostatin generation, and that insufficient levels of one
enzyme are compensated for by a sufficient level of the other, we
also plotted uPA against tPA concentration for each group of
plasminogen to angiostatin conversion (Fig. 5). As in Fig. 4, samples that contained neither
plasminogen nor angiostatin were left out of the plot. In 12 of the
21 fluid samples in which no conversion occurred (category 1;
Fig. 5 left panel), uPA as well as
tPA was <75% percentile in this group. In 4 samples, only the
tPA concentration (≤78 ng/ml) was increased, while in another 4
only the uPA concentration was increased (≤16.2 ng/ml). In one
sample, both tPA and uPA plasminogen activators (40 and 22 ng/ml,
respectively) were increased. Apparently, the presence of
plasminogen and uPA and/or tPA is not always sufficient to convert
plasminogen to angiostatin.
In categories 2–5 samples, where at least some
degree of plasminogen to angiostatin conversion was observed
(Fig. 5 right panel), ∼40% of
samples with a low conversion level (category 2) also showed low
uPA and tPA levels (below dotted lines; lower left quadrant of
Fig. 5 right panel). Twenty-three
percent of category 2 samples, however, presented with high uPA and
tPA levels (upper right quadrant), again indicating that the
combination of plasminogen and a plasminogen activator is not
always sufficient for angiostatin generation. Samples displaying an
intermediate, high or complete conversion of plasminogen to
angiostatin (categories 3–5), were found predominantly in the upper
quadrants, indicating high uPA and/or tPA levels. The probability
of increased uPA and/or tPA levels given a certain conversion level
is given in Table I. A
considerable amount of samples was found in the upper left quadrant
(only high tPA levels, 22%) or lower right quadrant (only high uPA
levels, 33%). Interestingly, a small minority (five samples,
including one with a complete conversion of plasminogen to
angiostatin) was positioned in the lower left quadrant, suggesting
that in these samples neither uPA nor tPA was involved in
angiostatin generation. When analyzing our data for each source of
cyst fluid separately, we did not find any tumor-specific
angiostatin generating pathways (data not shown).
 | Table I.The odds ratio with 95% confidence
interval of enhanced conversion of plasminogen to angiostatin for
the probability of increased uPA, increased tPA and increased
either uPA or tPA, respectively, using univariable logistic
regression. |
Table I.
The odds ratio with 95% confidence
interval of enhanced conversion of plasminogen to angiostatin for
the probability of increased uPA, increased tPA and increased
either uPA or tPA, respectively, using univariable logistic
regression.
| | uPA >7.0
| tPA >18.5
| (uPA >7.0) or (tPA
>18.5)
|
|---|
| Conversion level | n | OR | (95% CI) | p-value | OR | (95% CI) | p-value | OR | (95% CI) | p-value |
|---|
| 1 | 21 | 1.0 | (ref) | | 1.0 | (ref) | | 1.0 | (ref) | |
| 2 | 56 | 4.0 | (1.3–12.3) | 0.017 | 1.2 | (0.4–3.8) | 0.791 | 1.9 | (0.7–5.3) | 0.093 |
| 3 | 29 | 10.1 | (2.7–37.5) | 0.001 | 3.4 | (1.0–11.9) | 0.052 | 11.6 | (2.6–50.5) | 0.022 |
| 4 | 12 | 3.2 | (0.7–14.5) | 0.132 | 4.5 | (1.0–20.6) | 0.054 | 6.7 | (1.2–38.25) | 0.121 |
| 5 | 5 | 2.1 | (0.3–16.6) | 0.469 | 4.8 | (0.6–37.4) | 0.134 | 5.3 | (0.5–56.2) | 0.289 |
Discussion
Angiogenesis is a prerequisite for (pathological)
tissue growth and the formation of metastatic or ectopic tissue.
Angiogenesis is the net result of the balance between of pro- and
anti-angiogenic molecules. Angiostatin, a proteolytic fragment of
plasminogen with potent anti-angiogenic properties, has been
extensively studied with regard to the molecules involved in
excising the different angiostatin fragments from its parent
molecule. Circulating angiostatin has been demonstrated in
tumor-bearing as well as control mice, and also in human cancer
patients and healthy subjects.
In our angiostatin containing samples we usually
observed at least two angiostatin bands, that differed ∼ 4 kDa in
molecular weight. These tandem bands were either found in the 37–40
kDa range, or in the 48–52 kDa range. As the molecular weight of
one plasminogen kringle domain is ∼12 kDa, the two sets of tandem
bands may represent angiostatin species containing 3 or 4 kringle
domains, respectively. As reported previously, both the 3 kringle
and 4 kringle angiostatin species possess anti-angiogenic
properties. We also observed angiostatin species in the 37–40 kDa
range that were only slightly (1–2 kDa, or even less) different
from each other with regard to their molecular weight. Whether
these bands represent different glycosylation forms of the same
proteolytic fragment, or proteolytic fragments of different sizes,
is unknown. Interestingly, the angiostatin species with a molecular
weight between 48 and 52 kDa were observed exclusively in the
functional cyst samples, whereas the 37–40 kDa species were only
found in the pathological samples. This finding suggests that
angiostatin generation is different under physiological conditions
compared to pathological conditions. We could, however, not detect
any differences between the levels of uPA or tPA on one hand, and
the molecular weight form of the angiostatin that was generated on
the other.
Thus far, the mechanisms involved in physiological
as well as pathological angiostatin generation in vivo, have
not been elucidated. Rotenberg et al (23) have studied angiostatin levels in
blood, and in ascitic and pleural effusions from 21 patients with
malignant disease. No link between angiostatin levels, and
concentrations of either MMPs or plasminogen activators could be
established. Murthi et al (26) analyzed plasminogen levels and uPA
activity in tissue extracts and in the urine of patients with
normal, benign, borderline, and invasive serous tumors and
suggested that proteolytic activity of the plasminogen activation
cascade increases in serous epithelial ovarian carcinoma in
combination with a decrease in plasminogen and angiostatin levels.
Drenberg et al (27)
assayed angiostatin levels in plasma and urine by ELISA in normal
samples, benign gynecological disease, primary peritoneal cancer
and epithelial ovarian cancer. Angiostatin was elevated in urine of
ovarian cancer patients regardless of tumor grade, stage, size,
histological subtype, creatinine levels, menopausal status, or
patient age.
In our study, we show a clear correlation between
the concentration of uPA, and angiostatin content in cyst fluids
derived from malignant and benign ovarian disease, endometriotic
lesions, and functional cysts. We also identified, however, a
number of samples containing angiostatin but no uPA or tPA, and, on
the other hand, plasminogen containing samples with substantial
levels of uPA, tPA or both, but no angiostatin. In the first group
of samples other proteases, for instance members from the MMP
family may have been involved in excising the angiostatin moiety
from the plasminogen parent molecule. In the second group of
samples, molecules such as plasminogen activator inhibitor-1
(PAI-1) may have modulated the enzymatic activity of the
plasminogen activator, thus preventing angiostatin generation.
Our data are in accordance with a previous report in
which we analyzed the angiostatin generating capacity of a large
number of tumor cell lines (17).
In this study we found a strong correlation between uPA and tPA
production and angiostatin generation, but we also observed
angiostatin generation by a number of uPA/tPA negative cell
lines.
Our results may explain the dual role that
plasminogen activators appear to play in tumor progression. These
proteases have been described to be positively- as well as
negatively-correlated with the degree of malignancy of several
tumor types, e.g., breast cancer (28–32).
The role of plasminogen activators in in vivo angiostatin
generation may lead to a shift in the angiogenic balance towards
the inhibitory side, resulting in a less malignant tumor and,
consequently, in a negative correlation between plasminogen
activator expression and tumor malignancy and/or progression.
Using angiostatin as a (potential) treatment, is
hampered by problems producing sufficient amounts of active
angiostatin, and subsequent effective administration of the
compound. In parallel studies (33,34)
we have attempted to bypass these problems by trying to stimulate
the endogenous in vivo angiostatin production. In a human
melanoma xenograft model, administration of a combination of tPA
and a free sulfhydryl donor to tumor bearing mice resulted in
increased levels of circulating angiostatin, and in an inhibition
of tumor growth. Further elucidation of the mechanism(s) involved
in in vivo angiostatin production, may eventually lead to
the development of alternative anti-angiogenic therapeutic
approaches aimed at increasing the circulating levels of
angiostatin.
In conclusion, our data show that plasminogen
activators are clearly involved in angiostatin formation in
vivo in ovarian cysts. Most likely, however, other proteases,
as well as inhibitors of plasminogen activators, are involved as
well.
References
|
1.
|
Folkman J: Seminars in Medicine of the
Beth Israel Hospital, Boston. Clinical applications of research on
angiogenesis. N Engl J Med. 333:1757–1763. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Folkman J: Angiogenesis in cancer,
vascular, rheumatoid and other disease. Nat Med. 1:27–31. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Carmeliet P and Collen D: Molecular
analysis of blood vessel formation and disease. Am J Physiol.
273:H2091–H2104. 1997.PubMed/NCBI
|
|
4.
|
Iruela-Arispe ML and Dvorak HF:
Angiogenesis: a dynamic balance of stimulators and inhibitors.
Thromb Haemost. 78:672–677. 1997.PubMed/NCBI
|
|
5.
|
Talks KL and Harris AL: Current status of
antiangiogenic factors. Br J Haematol. 109:477–489. 2000.
View Article : Google Scholar
|
|
6.
|
Bicknell R and Harris AL: Mechanisms and
therapeutic implications of angiogenesis. Curr Opin Oncol. 8:60–65.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Liekens S, De Clercq E and Neyts J:
Angiogenesis: regulators and clinical applications. Biochem
Pharmacol. 61:253–270. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Hofmann UB, Westphal JR, Van Muijen GN and
Ruiter DJ: Matrix metalloproteinases in human melanoma. J Invest
Dermatol. 115:337–344. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Pozzi A, Moberg PE, Miles LA, Wagner S,
Soloway P and Gardner HA: Elevated matrix metalloprotease and
angiostatin levels in integrin alpha 1 knockout mice cause reduced
tumor vascularization. Proc Natl Acad Sci USA. 97:2202–2207. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Friedlander M, Brooks PC, Shaffer RW,
Kincaid CM, Varner JA and Cheresh DA: Definition of two angiogenic
pathways by distinct alpha v integrins. Science. 270:1500–1502.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Brooks PC, Clark RA and Cheresh DA:
Requirement of vascular integrin alpha v beta 3 for angiogenesis.
Science. 264:569–571. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
O’Reilly MS, Holmgren L, Shing Y, et al:
Angiostatin: a novel angiogenesis inhibitor that mediates the
suppression of metastases by a Lewis lung carcinoma. Cell.
79:315–328. 1994.
|
|
13.
|
O’Reilly MS, Boehm T, Shing Y, et al:
Endostatin: an endogenous inhibitor of angiogenesis and tumor
growth. Cell. 88:277–285. 1997.
|
|
14.
|
Holmgren L, O’Reilly MS and Folkman J:
Dormancy of micro-metastases: balanced proliferation and apoptosis
in the presence of angiogenesis suppression. Nat Med. 1:149–153.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
O’Reilly MS, Holmgren L, Chen C and
Folkman J: Angiostatin induces and sustains dormancy of human
primary tumors in mice. Nat Med. 2:689–692. 1996.PubMed/NCBI
|
|
16.
|
Gately S, Twardowski P, Stack MS, et al:
The mechanism of cancer-mediated conversion of plasminogen to the
angiogenesis inhibitor angiostatin. Proc Natl Acad Sci USA.
94:10868–10872. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Westphal JR, Van’t Hullenaar R,
Geurts-Moespot A, et al: Angiostatin generation by human tumor cell
lines: involvement of plasminogen activators. Int J Cancer.
86:760–767. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Cornelius LA, Nehring LC, Harding E, et
al: Matrix metalloproteinases generate angiostatin: effects on
neovascularization. J Immunol. 161:6845–6852. 1998.PubMed/NCBI
|
|
19.
|
Dong Z, Kumar R, Yang X and Fidler IJ:
Macrophage-derived metalloelastase is responsible for the
generation of angiostatin in Lewis lung carcinoma. Cell.
88:801–810. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Heidtmann HH, Nettelbeck DM, Mingels A,
Jager R, Welker HG and Kontermann RE: Generation of
angiostatin-like fragments from plasminogen by prostate-specific
antigen. Br J Cancer. 81:1269–1273. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Puzer L, Barros NM, Paschoalin T, et al:
Cathepsin V, but not cathepsins L, B and K, may release
angiostatin-like fragments from plasminogen. Biol Chem.
389:195–200. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Soff GA: Angiostatin and
angiostatin-related proteins. Cancer Metastasis Rev. 19:97–107.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Rotenberg RG, Rozas NS, Guerri L, et al:
Elevated levels of angiostatin in effusions from patients with
malignant disease. Oncol Rep. 11:523–528. 2004.PubMed/NCBI
|
|
24.
|
Grebenschikov N, Geurts-Moespot A, De
Witte H, et al: A sensitive and robust assay for urokinase and
tissue-type plasminogen activators (uPA and tPA) and their
inhibitor type I (PAI-1) in breast tumor cytosols. Int J Biol
Markers. 12:6–14. 1997.PubMed/NCBI
|
|
25.
|
Span PN, Grebenchtchikov N, Geurts-Moespot
J, Westphal JR, Lucassen AM and Sweep CG: EORTC Receptor and
Biomarker Study Group Report: a sandwich enzyme-linked
immunosorbent assay for vascular endothelial growth factor in blood
and tumor tissue extracts. Int J Biol Markers. 15:184–191.
2000.
|
|
26.
|
Murthi P, Barker G, Nowell CJ, et al:
Plasminogen fragmentation and increased production of extracellular
matrix-degrading proteinases are associated with serous epithelial
ovarian cancer progression. Gynecol Oncol. 92:80–88. 2004.
View Article : Google Scholar
|
|
27.
|
Drenberg CD, Saunders BO, Wilbanks GD, et
al: Urinary angiostatin levels are elevated in patients with
epithelial ovarian cancer. Gynecol Oncol. 117:117–124. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
de Witte JH, Sweep CG, Klijn JG, et al:
Prognostic value of tissue-type plasminogen activator (tPA) and its
complex with the type-1 inhibitor (PAI-1) in breast cancer. Br J
Cancer. 80:286–294. 1999.PubMed/NCBI
|
|
29.
|
de Witte JH, Sweep CG, Klijn JG, et al:
Prognostic impact of urokinase-type plasminogen activator (uPA) and
its inhibitor (PAI-1) in cytosols and pellet extracts derived from
892 breast cancer patients. Br J Cancer. 79:1190–1198.
1999.PubMed/NCBI
|
|
30.
|
Look MP, van Putten WL, Duffy MJ, et al:
Pooled analysis of prognostic impact of urokinase-type plasminogen
activator and its inhibitor PAI-1 in 8377 breast cancer patients. J
Natl Cancer Inst. 94:116–128. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Manders P, Tjan-Heijnen VC, Span PN, et
al: Predictive impact of urokinase-type plasminogen activator:
plasminogen activator inhibitor type-1 complex on the efficacy of
adjuvant systemic therapy in primary breast cancer. Cancer Res.
64:659–664. 2004. View Article : Google Scholar
|
|
32.
|
Manders P, Tjan-Heijnen VC, Span PN, et
al: The complex between urokinase-type plasminogen activator (uPA)
and its type-1 inhibitor (PAI-I) independently predicts response to
first-line endocrine therapy in advanced breast cancer. Thromb
Haemost. 91:514–521. 2004.
|
|
33.
|
de Groot-Besseling RR, Ruers TJ,
Lamers-Elemans IL, Maass CN, de Waal RM and Westphal JR:
Angiostatin generating capacity and anti-tumour effects of
D-penicillamine and plasminogen activators. BMC Cancer.
6:1492006.PubMed/NCBI
|
|
34.
|
de Groot-Besseling RR, Ruers TJ, van
Kraats AA, et al: Anti-tumor activity of a combination of
plasminogen activator and captopril in a human melanoma xenograft
model. Int J Cancer. 112:329–334. 2004.
|