Introduction
MicroRNAs (miRNAs) are short, non-coding RNA
molecules that post-transcriptionally regulate the expression of
target genes, and play a role in diverse cellular, physiological
and pathophysiological processes (1–3).
MicroRNA-126 (miR-126) is located within intron 7 of epidermal
growth factor-like domain 7 (EGFL7) and is highly expressed in
vascular endothelial cells (4,5). To
date, it has been reported that the miR-126 expression differs
between normal tissues and derived tumors (6–8).
miR-126 is also strongly downregulated in pancreatic cancer, with
an associated elevation in K-Ras (9), and lower expression of miR-126 is
significantly correlated with short survival in non-small cell lung
carcinoma (NSCLC) and renal cell carcinoma (10). Recent studies have shown that some
miRNAs are present in the systemic circulation and are associated
with exosomes and microparticles (11,12).
The levels of some circulating miRNAs have been reported to be
differentially expressed in the presence of a variety of cancers
(13,14).
However, to our knowledge, no previous study exists
showing the relationship between miR-126 and laryngeal squamous
cell carcinoma (LSCC). In this study, we assessed the levels of
circulating miR-126 in serum of the patients with LSCC. In
addition, we focused on a target protein of miR-126, Camsap1. We
also investigated the function and mechanism of miR-126 and Camsap1
in the LSCC cells. This study provides new insights into the
potential mechanisms of LSCC oncogenesis and metastasis.
Materials and methods
Sample collection
After obtaining Our University Ethics Committee
approval and informed consent from all study participants, tissue
samples and blood samples were drawn at the Department of
Otorhinolaryngology, Shengjing Hospital, China Medical University
in 2010 and 2011. Up to 8 ml of whole blood were collected from
each participant in an ethylene diamine tetracetic acid tube. Blood
samples were centrifuged at 1,200 × g for 10 min at 4°C to separate
the blood cells, and the supernatant was transferred into
microcentrifuge tubes and then centrifuged a second time at 12,000
× g for 10 min at 4°C to completely remove the cellular components.
Plasma was aliquoted and stored at −80°C until use. Blood samples
were processed and plasma was frozen within 4 h of collection.
Real-time PCR
Total RNA (2 μg) was reverse-transcribed
using Transcript First-strand cDNA Synthesis SuperMix (TransGen
Biotech, Beijing, China) according to the manufacturer’s protocol.
In short, the 50 μl reactions were incubated for 60 min at
42°C, 10 min at 70°C, and then stored at 4°C. qRT-PCR analyses were
performed using the Bulge-Loop™ miRNA qRT-PCR Detection kit
(Ribobio Co., Guangzhou, China) and TransStart™ Green qPCR SuperMix
(TransGen Biotech) according to with the manufacturer’s protocol
with the Rotor-Gene 6000 system (Qiagen, Hilden, Germany). Briefly,
the reactions were incubated at 95°C for 30 sec, followed by 40
cycles of 95°C for 30 sec, 60°C for 20 sec, 70°C for 1 sec. The
relative expression level for miRNA-126 was computed using the
comparative CT method. It is important to note that to control for
possible diversity in the amount of starting RNA, miRNA expression
was normalized to small nucleolar RNA U6.
Isolation and enumeration of CTCs
The CellSearch system (Veridex, Warren, NJ, USA) is
the only test sanctioned by the United States Food and Drug
Administration for enumeration of CTCs in clinical practice. Blood
samples (5 ml) from patients were drawn into CellSave tubes, which
were maintained at room temperature and processed within 72 h of
collection. CTCs were defined as nucleated epithelial cell adhesion
molecule (EpCAM)-positive cells, lacking cluster of differentiation
(CD)45 but expressing cytoplasmic cytokeratins 8, 18 and 19. All
CTC evaluations were performed by qualified and trained
personnel.
Western blot analysis
Specimens were lysed using lysis buffer [50 mM
Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 0.1% sodium dodecyl
sulfate (SDS), 1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM
Na3VO4, 1 mM NaF, protease inhibitor
cocktail]. The extracts were incubated on ice for 20 min,
centrifuged at 12,000 × g for 20 min at 4°C and supernatants
collected. Protein concentrations were determined using Bradford
assay (Bio-Rad, Hercules, CA, USA), and proteins were resolved by
10% Bis-Tris gel electrophoresis, transferred to a nitrocellulose
membrane and western blot analysis performed. Anti-Camsap1 and
anti-β-actin were purchased from Santa Cruz Biotechnology (Santa
Cruz, CA, USA).
Cell line and culture
The human laryngeal cancer cell line Hep-2 was
purchased from Shanghai Institutes for Biological Sciences, Chinese
Academy of Sciences. The cells were cultured in PRMI-1640
supplemented with 10% heat-inactivated fetal bovine serum (FBS) in
a humidified cell incubator with an atmosphere of 5% CO2
at 37°C. Exponentially growing cells were used for experiments.
Transfection
Hep-2 cells were transfected with Precursor
Molecules mimicking miR-126 (Pre-miR-126) (Applied Biosystems,
Foster, CA, USA) or Camsap1 siRNA (sc-92757, Santa Cruz) by using
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s instructions.
Xenograft assays
All experiments with animals were performed
according to the guidelines of the China Medical University Ethics
Committee. NU/NU Nude mice (Crl: NU-Foxn1nu) aged 6-8-weeks were
purchased from Charles River (Wilmington, MA, USA). Hep-2 cells
(3×107 in 200 μl) were subcutaneously injected
into the axilla of each mouse. After the tumor diameters reached
3–5 mm, the mice were divided randomly into three groups
(untreated, miR-126 mimic, Camsap1 knockdown) and received a 100
μl intratumoral injection of PBS, miR-126 mimic, Camsap1
siRNA. Three injections were administered at 9 a.m., 3 p.m. and 9
p.m. every three days. Tumor growth was then monitored for 30 days.
Every five days until the end of the experiment, one mouse from
each group was randomly selected to be anesthetized, photographed
and sacrificed. For each tumor, measurements were made using
calipers, and tumor volumes were calculated as follows: length ×
width2 × 0.52. Tumors were subsequently fixed in 4%
paraformaldehyde for 24 h, then embedded in paraffin.
Survival curves
Additional mice (n=60) were used to establish
xenografts to obtain survival curves. Mice with xenografted tumors
(as described above) that reached 3–5 mm in diameter were divided
into three treatment groups (n=20 for each). Survival was monitored
until the experiments were terminated due to heavy tumor
burden.
Quantification of intratumoral
microvessels
For immunohistochemical staining of CD31, endogenous
peroxidase activity was blocked in 4-μm tumor sections with
3% hydrogen peroxide for 30 min. Antigen retrieval was performed in
citrate buffer (10 mM, pH 6.0) for 30 min at 95°C in a pressure
cooker. CD31 antibodies (Sigma-Aldrich, Carlsbad, CA, USA) were
incubated with sections at 1:500 overnight at 4°C. Sections were
then incubated with a biotinylated secondary antibody for 1 h at
RT, followed by incubation with a streptavidin horse-radish
peroxidase (HRP) complex (Beyotime, Beijing, China) for 60 min at
room temperature. Bound antibody was visualized with
3,3’-diaminobenzidine tetrahydrochloride (DAB, Beyotime). Sections
were also counterstained with hematoxylin (Beyotime). Microvessel
density was detected using the method of Ivkovic-Kapic et al
(15). Regions of highest vessel
density were located at low magnification (×40), then the number of
vessels present were counted at ×200 magnification. Three high
magnification fields were counted for each tumor section and the
mean microvessel density value was recorded for each. Any
individual endothelial cells, or endothelial cell cluster, that was
clearly separated from adjacent microvessels was counted as a
single microvessel.
miRNA target prediction
The miRNA targets predicted by TargetScan
(http://www.targetscan.org/) are based on
the presence of conserved 8 mer, 7 mer and 6 mer sites that match
the seed region of each miRNA (16).
Negative staining electron
microscopy
Tubulin was absorbed for 1 min onto glow-discharged
formvar- and carbon-coated grids. The samples were stained in 1.5%
uranyl acetate for 25 sec. Images were recorded on a FEI Morgagni
268D transmission electron microscope. Tubulin oligomers were
imaged at ×120,000 magnification resulting in a pixel size of 0.53
nm.
Immunofluorescence
Transfected cells were washed with PBS, fixed in 4%
paraformaldehyde, permeabilized in 1% Triton X-100 for 5 min, and
blocked with 5% bovine serum albumin in PBS containing 0.5% Triton
X-100 for 1 h. Camsap1 expression and tubulin expression were
detected using anti-Camsap1 (Santa Cruz) antibody and anti-tubulin
(Santa Cruz) antibody, respectively. Cells were washed with PBS and
incubated with appropriate secondary fluorophore-conjugated
antibody for 1 h at room temperature. Secondary antibody used for
detection of Camsap1 was Alexa Fluor® 488 donkey
anti-goat IgG (H+L) (Invitrogen) and tubulin was Alexa
Fluor® 594 rat anti-mouse IgG (H+L) (Invitrogen).
Phylogenetic analysis
Thirty-four separate protein sequences of Camsap1
from a wide range of organisms were extracted from NCBI. An
alignment of these sequences was made using Mega 5.0. To determine
the phylogenetic relationships of these sequences, we used maximum
likelihood (ML), neighbor-joining (NJ), and Bayesian Markov chain
Monte Carlo (MCMC) approaches to infer three individual trees.
Statistical analysis
All statistical analyses were carried out using SPSS
version 17.0 (Statistical Package for the Social Sciences). The
experiments were conducted in triplicates. All numerical data are
expressed as the means ± SD. Differences among the mean values were
evaluated using Student’s t-test. P-values <0.05 were considered
statistically significant.
Results
miR-126 levels in plasma and Camsap1
levels in tissue from the patients with LSCC
Using qRT-PCR assays, we measured the circulating
levels of miR-126 in the patients. Based on this result, we
analyzed the potential relationship between the circulating levels
of miR-126 and the clinicopathological characteristics of the
sampled patients. Results are summarized in Table I. No correlation was found with
sex, age, lymph node metastasis, T classification and clinical
stage (P>0.05). However, miR-126 expression was significantly
associated with differentiation of LSCC (P<0.05). Western blot
analysis and real-time PCR analysis were performed in order to
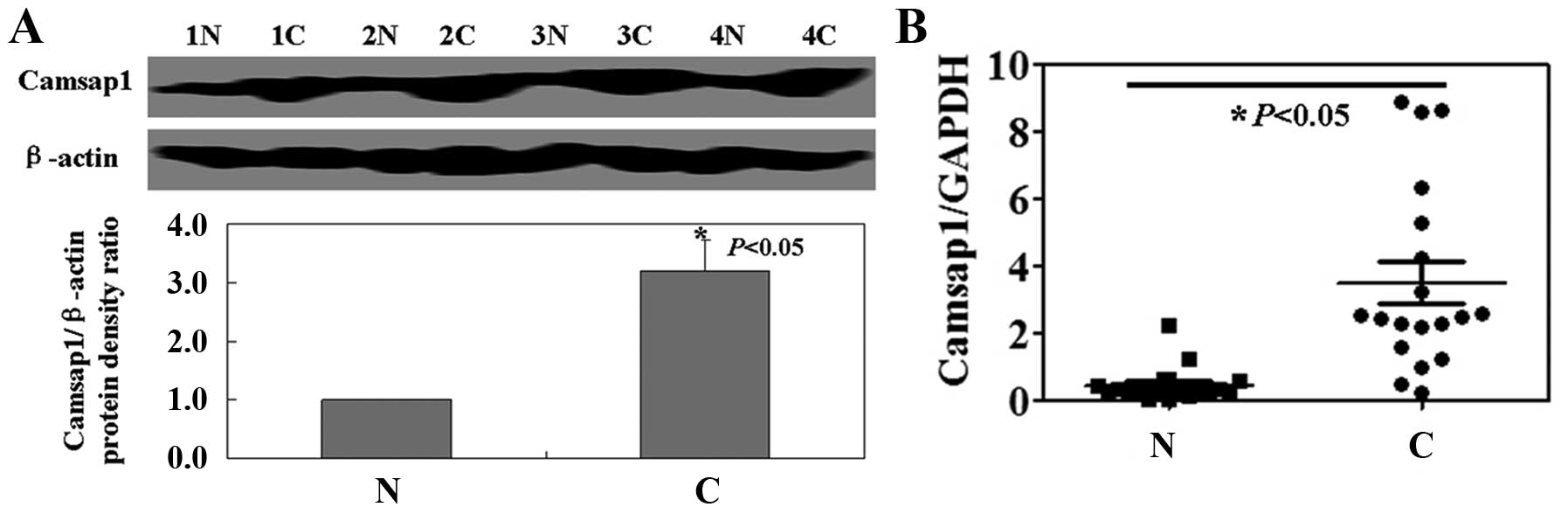
determine the levels of Camsap1 protein and mRNA in LSCC specimens.
Both Camsap1 protein and mRNA in cancer tissue was significantly
higher than that in matched normal tissue (P<0.05, Fig. 1).
 | Table I.Relationship between miR-126 levels
and clinical pathology in LSCC patients. |
Table I.
Relationship between miR-126 levels
and clinical pathology in LSCC patients.
| Clinicopathological
features | n | mir-126 high | mir-126 low | χ2 | P-value |
|---|
| Sex | | | | 0.07 | 0.79 |
| Female | 12 | 4 | 8 | | |
| Male | 26 | 6 | 20 | | |
| Age (years) | | | | 0.82 | 0.37 |
| <55 | 14 | 2 | 12 | | |
| ≥55 | 24 | 8 | 16 | | |
| Differentiation | | | | 4.68 | 0.03 |
| Well | 8 | 5 | 3 | | |
|
Moderately/poorly | 30 | 5 | 25 | | |
| Lymph node
metastasis | | | | 0.28 | 0.60 |
| − | 16 | 3 | 13 | | |
| + | 22 | 7 | 15 | | |
| T classification | | | | 1.21 | 0.27 |
| T1–2 | 12 | 2 | 10 | | |
| T3–4 | 26 | 8 | 18 | | |
| Clinical stage | | | | 1.01 | 0.31 |
| I–II | 15 | 6 | 9 | | |
| III–IV | 23 | 4 | 19 | | |
Correlation of miR-126, Camsap1 and
CTCs
In the present study, we isolated and enumerated the
CTCs in patients using the CellSearch® system (Fig. 2A). The patients with <10 CTCs
showed a higher survival rate when compared with the patients with
>10 CTCs (P<0.05, Fig. 2B).
miR-126 expression was closely correlated with the favorable
prognosis of the patients with LSCC, whereas Camsap1 expression was
correlated with a poor prognosis (P<0.05, Fig. 2B). The plasma level of miR-126 and
the number of CTCs were significantly negatively correlated
(r=−0.848, P<0.01; Fig. 2C).
The plasma level of miR-126 was also negatively related with
Camsap1 expression (r=−0.937, P<0.05; Fig. 2C). However, Camsap1 expression was
positively related with the number of CTCs (r=−0.776, P<0.01;
Fig. 2C).
The roles of miR-126 and Camsap1 in LSCC
mouse model
The antitumor properties of miR-126 and Camsap1 were
further evaluated using LSCC mouse models. As shown in Fig. 3A, compared to untreated mice, both
miR-126 mimics and Camsap1 knockdown mice had a significant lower
tumor volume (P<0.05). Correspondingly, the tumor weights of
miR-126 mimics or Camsap1 knockdown mice also were lower than that
of untreated ones (P<0.05, Fig.
3B). In addition, the survival rate of mice with miR-126 mimics
and Camsap1 knockdown was significantly improved (P<0.05;
Fig. 3C). Furthermore, the
anti-angiogenic effects were shown in vivo by anti-CD31
immunohistochemistry following injection of miR-126 mimics or
Camsap1 knockdown into tumors (P<0.05; Fig. 3D).
The antitumor mechanism of miR-126 mimics
or Camsap1 knockdown in LSCC cells
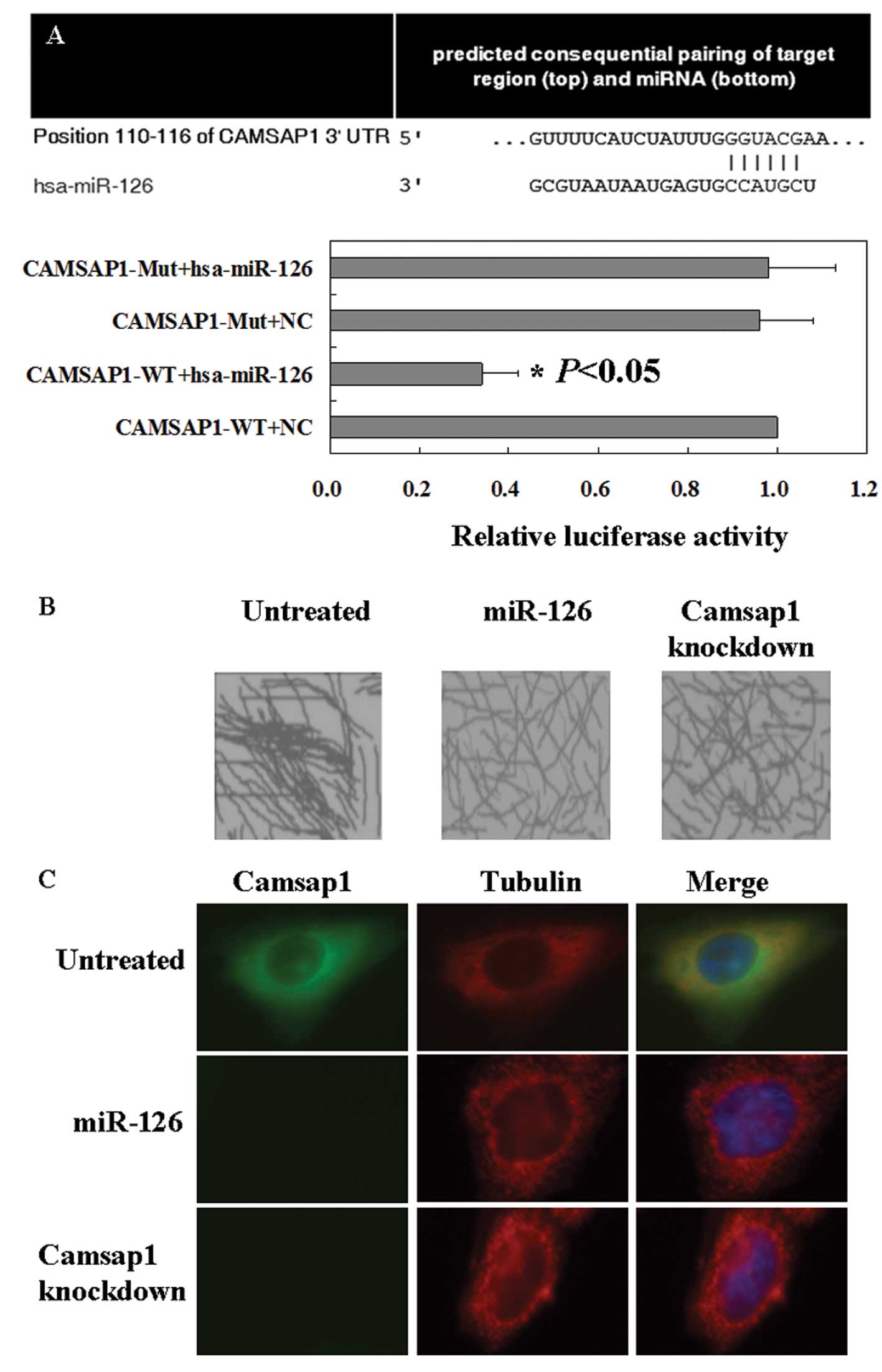
To clarify the mechanism of miR-126 or Camsap1 in
LSCC cells, TargetScan were used to determine whether Camsap1 is a
target gene of miR-126 or not. The prediction results are shown in
Fig. 4A. Furthermore, microtubules
formed bundles and aggregated together in untreated cells by using
negative stain electron microscopy (Fig. 4B). After knockdown treatment of
miR-126 mimics or Camsap1, microtubules did not form such
aggregates in LSCC cells. These results indicated Camsap1 induced
the formation of bundles by directly interacting with microtubules
(Fig. 4B). Interestingly, we
demonstrated that Camsap1 and tubulin colocalized in the cytoplasm
by using immunofluorescence (IF) (Fig.
4C).
Phylogeny of the CAMSAP1 family
To further study the functions of CAMSAP1 protein,
we made use of it in a phylogenetic analysis of the family.
Fig. 5 shows a tree generated by
maximum likelihood analysis of a codon-based alignment. It appears
that a CAMSAP1 gene arose in the ancestors of simple animals.
During the evolution of the vertebrates, this gene was multiplied
so that extant vertebrate genomes encode three classes of CAMSAP1
genes. These results indicated that we could get more information
on CAMSAP1 gene from adjacent species, such as Gorilla.
Discussion
Loss of miR-126 has been observed in many cancers,
such as breast cancer (8), lung
cancer (10) and prostate cancer
(17). The decreased expression of
miR-126 was associated with poor metastasis-free survival of breast
cancer patients (18). In this
study, we detected the level of miR-126 in plasma of the patients
with LSCC. We also found loss of miR-126 was related with poor
prognosis of the patients with LSCC. Similarly, the relationship
between low miR-126 expression and worse disease prognosis has been
reported in glioblastoma (19),
and gastric cancer patients (20).
Contrary to our results, Donnem et al (10) demonstrated that high miR-126
expression in tumor samples correlates with a shorter survival of
NSCLC patients. Recent studies indicated that circulating miRNA may
become valuable biomarkers for different diseases. Long et
al (21) found that
circulating miR-126 might be useful biomarkers for ischemic stroke
in humans. In this study, we confirmed that the plasma miR-126
could predict the survival rate of the patients with LSCC.
Furthermore, we found the plasma level of miR-126 was also
negatively related with Camsap1 expression. Based on this result,
we hypothesized Camsap1 may be a target gene of miR-126.
CAMSAP1 is a protein expressed in the nervous system
of mammals in neurons and astrocytes (22). CAMSAP1 contains a C-terminal CKK
domain which binds microtubules, and was overexpressed in the model
cell line PC12 (23). In this
study, we also found that microtubules did not form aggregates in
LSCC cells after miR-126 mimics or Camsap1 knockdown treatment.
Interestingly, we confirmed Camsap1 and tubulin colocalized in the
cytoplasm by using immunofluorescence. We constructed the
evolutionary tree of Camsap1 by using phylogenetic analysis. In our
study, we confirmed Camsap1 expression is higher in cancer tissues
than normal tissues and its expression is related with the
prognosis of the patients with LSCC. However, previous studies on
Camsap1 are very scarce. We are not able yet to provide the exact
mechanism of Camsap1 in LSCC, thus, further study is required.
In conclusion, we found miR-126 was able to inhibit
LSCC partly by suppressing Camsap1 expression. Camsap1 expression
induced microtubule formation and aggregation. The reported
mechanism possibly explains why loss of miR-126 is frequently
associated with tumor metastasis.
Acknowledgements
This study was supported by the Grants
from National Natural Science Foundation of China (no.
82072196).
References
|
1.
|
Du T and Zamore PD: microPrimer: the
biogenesis and function of microRNA. Development. 132:4645–4652.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Chang TC and Mendell JT: microRNAs in
vertebrate physiology and human disease. Annu Rev Genomics Hum
Genet. 8:215–239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Latronico MV, Catalucci D and Condorelli
G: Emerging role of microRNAs in cardiovascular biology. Circ Res.
101:1225–1236. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Fish JE, Santoro MM, Morton SU, et al:
miR-126 regulates angiogenic signaling and vascular integrity. Dev
Cell. 15:272–284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Wang S, Aurora AB, Johnson BA, et al: The
endothelial-specific microRNA miR-126 governs vascular integrity
and angiogenesis. Dev Cell. 15:261–271. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Feng R, Chen X, Yu Y, et al: miR-126
functions as a tumour suppressor in human gastric cancer. Cancer
Lett. 298:50–63. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Liu B, Peng XC, Zheng XL, Wang J and Qin
YW: MiR-126 restoration down-regulate VEGF and inhibit the growth
of lung cancer cell lines in vitro and in vivo. Lung Cancer.
66:169–175. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Zhu N, Zhang D, Xie H, et al:
Endothelial-specific intron-derived miR-126 is down-regulated in
human breast cancer and targets both VEGFA and PIK3R2. Mol Cell
Biochem. 351:157–164. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Slaby O, Redova M, Poprach A, et al:
Identification of MicroRNAs associated with early relapse after
nephrectomy in renal cell carcinoma patients. Genes Chromosomes
Cancer. 51:707–716. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Donnem T, Fenton CG, Lonvik K, et al:
MicroRNA signatures in tumor tissue related to angiogenesis in
non-small cell lung cancer. PLos One. 7:e296712012. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
D’Alessandra Y, Devanna P, Limana F, et
al: Circulating microRNAs are new and sensitive biomarkers of
myocardial infarction. Eur Heart J. 31:2765–2773. 2010.PubMed/NCBI
|
|
12.
|
Su X, Chakravarti D, Cho MS, et al: TAp63
suppresses metastasis through coordinate regulation of Dicer and
miRNAs. Nature. 467:986–990. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Watahiki A, Macfarlane RJ, Gleave ME, et
al: Plasma miRNAs as biomarkers to identify patients with
castration-resistant meta-static prostate cancer. Int J Mol Sci.
14:7757–7770. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Jung EJ, Santarpia L, Kim J, et al: Plasma
microRNA-210 levels correlate with sensitivity to trastuzumab and
tumor presence in breast cancer patients. Cancer. 118:2603–2614.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Ivkovic-Kapic T, Knelevic-Usaj S,
Panjkovic M and Mastilovic K: Immunohistochemical analysis of
angiogenesis in invasive ductal breast carcinoma with correlations
to clinicopathological factor. Vojnosanit Pregl. 63:635–642. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Sun X, Liu Z, Yang Z, et al: Association
of microRNA-126 expression with clinicopathological features and
the risk of biochemical recurrence in prostate cancer patients
undergoing radical prostatectomy. Diagn Pathol. 8:2082013.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Zhang Y, Yang P, Sun T, et al: miR-126 and
miR-126* repress recruitment of mesenchymal stem cells
and inflammatory monocytes to inhibit breast cancer metastasis. Nat
Cell Biol. 15:284–294. 2013.
|
|
19.
|
Feng J, Kim ST, Liu W, et al: An
integrated analysis of germline and somatic, genetic and epigenetic
alterations at 9p21.3 in glioblastoma. Cancer. 118:232–240. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Li X, Zhang Y, Zhang Y, et al: Survival
prediction of gastric cancer by a seven-microRNA signature. Gut.
59:579–585. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Long G, Wang F, Li H, et al: Circulating
miR-30a, miR-126 and let-7b as biomarker for ischemic stroke in
humans. BMC Neurol. 13:1782013. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Yamamoto M, Yoshimura K, Kitada M, et al:
A new monoclonal antibody, A3B10, specific for astrocyte-lineage
cells recognizes calmodulin-regulated spectrin-associated protein 1
(Camsap1). J Neurosci Res. 87:5035132009. View Article : Google Scholar
|
|
23.
|
Baines AJ, Bignone PA, King MD, et al: The
CKK domain (DUF1781) binds microtubules and defines the CAMSAP/ssp4
family of animal proteins. Mol Biol Evol. 26:2005–2014. 2009.
View Article : Google Scholar : PubMed/NCBI
|