Introduction
Prostate cancer (PCa) represents a major health
concern in Western countries. In the USA alone, this tumor accounts
for 29% of all newly diagnosed cancers (1). This high incidence is due to both the
progressive aging of the male population and opportunistic
screening for prostate-specific antigen (PSA). The PSA test has two
major limitations: i) the lack of specificity because PSA is
frequently elevated in benign prostate hyperplasia and prostatitis,
and ii) the inability to discriminate between a less aggressive
disease, characterized by an indolent behavior, and a more
aggressive one with a very poor outcome (2). Several parameters, such as the tumor
volume, pathological grade and the Gleason score, have been
associated with the malignant potential of PCa (3). However, these parameters have proven
to be inadequate, both in the selection of patients who require
immediate local treatment and in the discrimination of high-risk
patients who might require systemic therapy in the context of a
multimodal approach (4–6). Therefore, new diagnostic and
prognostic biomarkers are greatly needed for clinicians to improve
the risk stratification of patients with PCa.
Recently, studies carried out in our and other
laboratories, have shown that heterogeneous nuclear
ribonucleoprotein K (hnRNP K) may play a key role in the
carcinogenesis process of PCa (7,8).
HnRNP K is a protein with pleiotropic functions present primarily
in the nucleus (9) and active at
the chromatin level, where it is localized at a higher density near
transcribed genes compared with silent ones (10). Many human tumors manifest an
overexpression of hnRNP K and, in several cases, an aberrant
cytoplasmic localization as well. Furthermore, a correlation
between the protein expression and the patient’s prognosis has been
frequently observed (11–18). In 2004, Nagano et al
(19) demonstrated that hnRNP K
was strongly overexpressed in a primary culture of human PCa with
respect to normal cell lines derived from the same patient. In
addition, Wang et al (20)
have shown that a novel transcriptional repressor complex
containing Purα and hnRNP K binds to the androgen receptor (AR)
gene both in cell lines and in primary human prostate tissues. More
recently, evidence has been provided for a role played by hnRNP K
in the regulation of AR expression via a post-transcriptional
mechanism (7). Using a proteomic
approach, we have previously reported that one phosphorylated
isoform of hnRNP K present in the nuclear matrix, if co-expressed
with another nuclear matrix protein (NM-8), is strongly associated
with the clinical outcome of patients following radical
prostatectomy (21,22). Moreover, in prostate cancer cell
lines, we have demonstrated by immunoprecipitation and confocal
microscopy techniques that hnRNP K and AR colocalize in the
nucleoplasm in a complex and both proteins are synchronously
modulated by treatment with bicalutamide, the anti-androgen widely
used in PCa therapy (23).
These results support the hypothesis that hnRNP K is
implicated in the network of mechanisms that control AR expression
and that this protein could be a good biomarker for disease
diagnosis, prognosis and progression in PCa. Based on these
observations, in the present study we analyzed, by
immunohistochemical analysis, hnRNP K and AR expression both in PCa
and benign peritumoral tissues obtained from radical prostatectomy
specimens, we have evaluated the diagnostic and prognostic
potential of these proteins and whether a relationship between
their expression levels also exists. The in vivo results
were compared with a PCa in vitro model.
Materials and methods
Patient characteristics and assessment of
clinical outcome
From 1995 to 2007, 105 patients who had undergone
radical prostatectomy for biopsy-proven PCa were selected for the
present study that was approved by the Ethics Committee of the
National Cancer Research Institute of Genoa (OMB06.004). This
cohort included all the patients who consented that their tissue
specimens could be utilized for this research project and who were
subsequently referred to our Unit for treatment or follow up. The
patient characteristics are summarized in Table I. All specimens were subjected to a
uniform histopathology protocol and clinical stage was reviewed and
assigned using the 2011 TNM staging system. Due to the relatively
small size of patient population, patients presenting Gleason score
7, 8 and 9 were arbitrarily grouped.
 | Table I.Patient demographics and tumor
characteristics. |
Table I.
Patient demographics and tumor
characteristics.
|
Characteristics | N=105 | (%) |
|---|
| Median preoperative
age, years (range) | 64 (48–77) | |
| Median PSA level at
surgery, ng/ml (range) | 11
(1.70–167.4) | |
| PSA ≤10 ng/ml | 45 | 42.9 |
| Tumor stage | | |
| pT2a | 2 | 1.9 |
| pT2b | 5 | 4.8 |
| pT2c | 46 | 43.8 |
| pT3a | 24 | 22.9 |
| pT3b | 27 | 25.7 |
| pT3c | 1 | 0.9 |
| Pelvic nodes
involved | 23 | 21.9 |
| Surgical margins
involved | 41 | 39.0 |
| Seminal vesicles
involved | 30 | 28.6 |
| Gleason score | | |
| <7 | 34 | 32.4 |
| =7 | 37 | 35.2 |
| >7 | 34 | 32.4 |
PSA failure-free and overall survivals were the main
endpoints of the present analysis. Patients were followed at
regular intervals and PSA was determined at each clinical
examination. Any PSA level of at least 0.4 ng/ml following
prostatectomy, which was confirmed by another assay four weeks
later, was considered a biochemical failure. Therefore, PSA
failure-free survival was defined by the time elapsed from the date
the patient was submitted to prostatectomy to the date PSA
progression was documented, and overall survival was the time
elapsed from the date of prostatectomy to the date of death,
independent of the cause. After a median follow-up time of 10.7
years [95% Confidence interval (CI) 9.7–11.6], 54 (51%) patients
were found to have experienced biochemical progression and 21 (20%)
had died.
Cell culture
The immortalized but non-transformed human prostate
epithelial cell line BPH-1 was kindly provided by Dr Pfeffer of our
Institute; the human prostate cancer cell line LNCaP was obtained
from ATCC (CRL-1740; Rockville, MD, USA). The cells were maintained
in phenol red-positive RPMI-1640 containing heat-inactivated 10%
fetal bovine serum, 1% penicillin, 1% streptomycin, 1% glutamine,
10 mM HEPES, 1 mM sodium pyruvate and 4.5 mg/ml glucose (Celbio).
Two different LNCaP cell populations were utilized: LNCaP cells
with a low passage number (less than 28 and designated as LNCaPlp)
and with a high passage number (higher than 54 and designed as
LNCaPhp). These two populations recapitulate the progression of
human PCa towards a more aggressive disease (24).
Immunohistochemical analysis
Immunohistochemistry was carried out on
formalin-fixed, paraffin-embedded whole sections using mouse
monoclonal antibodies anti-hnRNP K (sc-28380, Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA, diluted 1:800) and anti-AR
(AR441, 200M-14, Cell Marque, CA, USA, diluted 1:50). Anti-hnRNP K
was raised against amino acids 1–300 of the protein of human origin
whereas anti-AR antibody recognizes both full-length and truncated
AR proteins (25). For each
antibody, two different sections (3 μm) of the same sample
were treated independently. The more representative tumor sections
were deparaffinised and in the case of anti-AR antibody,
heat-induced antigen retrieval was carried out using Cell
Conditioning 1 solution (CC1, Ventana Medical Systems, S.A.
Strasbourg, France) for 30 min.
Immunostaining was carried out using a BenchMark XT
automated stainer (Ventana Medical Systems). Sections were
incubated for 16 min at 37°C with the anti-hnRNP K antibody or for
2 h at room temperature with the anti-AR antibody, and then the
antigen-antibody complex was detected using the Ventana Medical
System/ultraView diaminobenzidine (DAB) detection kit. The sections
were counterstained with modified Gill’s haematoxylin and mounted
in Eukitt (Bio-Optica, Milan, Italy). An appropriate positive
tissue control was used for each staining run; the negative control
consisted of performing the entire immunohistochemistry procedure
on adjacent sections in the absence of primary antibody. For each
patient, both PCa and NT adjacent tissues were analyzed. The
sections were observed with an Olympus BX41 light microscope
(Olympus, Tokyo, Japan). Two observers independently examined the
immunostaining of both proteins and the extent of immunochemical
reactivity was evaluated exclusively in benign or malignant
epithelial cells; stromal cells were not considered.
The expression of proteins was graded according to
the number of immunoreactive cells and the staining intensity using
the scoring system described by Carpenter et al (12), which we have already applied
successfully to prostate tissues analyses (8). In this system, the extent of
positively labeled nuclei or cytoplasm was ranked as follows: 0,
0%; 1, 1–25%; 2, 26–50%; 3, 51–75%; and 4, 76–100%. Staining
intensity was graded into four steps with 0, negative; 1, low; 2,
intermediate; and 3, high staining. The results are presented as
the sum of the two assessments, thus ranging from 0 to 7 for each
cellular compartment. The percentage of AR-positive nuclei was
estimated by averaging the values, obtained by Qwin Standard image
analysis software (Leica, Cambridge, UK), of 10 fields chosen at
random. To study the correlation between AR expression and patient
follow-up, the intensity and the percentage of the positive areas
were analyzed separately.
Western blot analysis
Cultured cells were mechanically harvested with a
sterile plastic disposable cell scraper and recovered by
centrifugation at 3000 x g for 15 min. The pellet was washed twice
in PBS and resuspend in RIPA lysis buffer containing protease
inhibitor and 1 mM dithiothreitol. Cell lysates were prepared as
already reported (26). Protein
concentration was determined using Bio-Rad (Munich, Germany)
protein microassay with bovine serum albumin as a standard. Equal
amounts of samples were resolved by SDS-PAGE, transferred to PVDF
and probed at 4°C overnight with the following antibodies: mouse
anti-hnRNP K (sc-28380, Santa Cruz Biotechnology Inc., diluted
1:8000); rabbit polyclonal anti-phospho-AKT-1 (pAKT, Ser473, Cell
Signaling Technology, Beverly, MA, USA diluted 1:2000) and
anti-phospho-ERK 1/2 (pERK 1/2, Thr202/Tyr204, Cell Signaling
Technology, diluted 1:2000). After washing, the membranes were
incubated for 1 h at room temperature with horseradish
peroxidase-conjugated secondary antibodies (GE Healthcare, Milan,
Italy) and immunoreactive bands were revealed by enhanced
chemiluminescence (Immobilon, Millipore, MA, USA). HPR-conjugated
rabbit anti-β-Actin antibody (Cell Signaling Technology, diluted
1:10000) was used as a loading control.
Immunocytochemistry
The cells were grown on chamber slides coated with
poly-L-lysine. Cells were washed three times in PBS containing 2%
sucrose, fixed for 15 min in 3.7% formaldehyde and treated for 15
min with PBS containing 2% sucrose and 0.2% Triton X-100. Cells
were then incubated 5 min with peroxidase-blocking solution (Dako,
Milan, Italy) and washed three times with PBS. After 30 min at room
temperature in PBS containing 2% BSA, cells were incubated with
mouse anti-hnRNP K antiserum (sc-28380, Santa Cruz Biotechnology
Inc., diluted 1:1000) for 60 min. Cells were then washed three
times with PBS before a 30-min incubation with
peroxidase-conjugated anti-mouse IgG (Santa Cruz Biotechnology
Inc.). The reaction was visualized using DAB Substrate Chromogen
(Dako). The cells were observed by a light microscope (Leica,
DM-LB2), photographed in a standardized manner under ×40
magnification and stored as TIFF without compression, 24-bit (RGB)
with 2040×1536 pixels. The original RGB color images were converted
to a grayscale and adjusted homogeneously for contrast.
Quantitative evaluation of hnRNP K compartmentalization was
manually carried out using ImageJ 1.45 software (http://imagej.nih.gov/ij). The mean gray value,
measured in approximately 300 cells, was converted in optical
density according to Kim et al (27).
Statistical analysis
The t-test was applied to compare mean values of
immunoreactive score in PCa with those in non-tumor (NT) tissues.
Associations among the principal variables under study, i.e., hnRNP
K, AR, preoperative PSA, Gleason score, extracapsular extension,
regional lymph nodes, surgical margins status and seminal vesicle
involvement, were investigated with the Pearson’s correlation
coefficient (r) (28). To this
aim, variables were categorized as follows: Gleason score (<7
vs. ≥7), extracapsular extension (no vs. yes), surgical margins
status (negative vs. positive), seminal vesicles (not involved vs.
involved) and regional lymph nodes (not involved vs. involved). PSA
was considered as a continuous variable. PSA failure-free and
overall survival curves were constructed by means of the
Kaplan-Meier method and compared by the log-rank test (29).
To evaluate the possible interactions among all of
the variables under study, multi-parametric models were constructed
according to the Cox proportional hazard technique (30) by including within the models, all
of the covariates that also predicted for either PSA failure-free
or overall survival after univariate analysis. The following
covariates were included in all models: pre-surgery PSA levels (≤10
ng/ml, >10 ng/ml); extracapsular extension (No, Yes); surgical
margins status (negative, positive); involvement of seminal
vesicles (No, Yes); Gleason score (<7, ≥7); involvement of
regional lymph nodes (pN0, pN1); cytoplasmic hnRNP K (<6, ≥6);
AR expression (>75%, ≤75%); AR and cytoplasmic hnRNP K
(>75%+hK<6 vs. ≤75% and >75%+hK≥6). The cut-off values
used for hnRNP K and AR were those that better discriminated the
patient-cohort according to the clinical endpoints under study. A
stepwise procedure was used with a significance level of p=0.05 to
retain variables in the model (31). Hazard ratio (HR) estimates and
their 95% CIs were also calculated. All p-values were two-tailed.
The IBM software Statistical Package for Social Sciences (SPSS)
version 19.0 for Windows (SPSS Inc. Chicago, IL, USA) was used for
data analysis.
Results
HnRNP K and AR expression in PCa and
paired NT tissues
HnRNP K was expressed in all of the specimens
examined but with a different compartmentalization. In PCa, the
protein displayed a more frequent and strong immunoreactivity, both
cytoplasmic and nuclear, compared with NT tissues, where it was
primarily localized in the nucleus with very little cytoplasmic
staining observed (Fig. 1A). More
than 85% of patients had tumor cells with a high nuclear score
(≥6), and only 7% had negative cytoplasm. In contrast, in
approximately 60% of the NT samples examined, hnRNP K was expressed
with an intermediate score (4 or 5) in the nucleus, while in 35% of
cases, it was not present in the cytoplasm (Fig. 1B). The difference in expression
between PCa and paired NT tissues was highly significant
(p<0.00001) in both cellular compartments (Fig. 1C), confirming the potential
diagnostic value of the hnRNP K protein level.
 | Figure 1.The analysis of hnRNP K and AR
expression in prostate tissues. Consecutive PCa sections were
stained using anti-hnRNP K (A) or anti-AR antibodies (D and G) and
were evaluated by immunohistochemistry. Representative images of
PCa specimens are reported; higher magnifications of the areas
enclosed in boxes are shown in the inset. (D) An example of
positive and (G) of negative staining with the anti-AR antibody.
The bars correspond to 100 μm in (A), (D) and (G) and 50
μm in the insets. PCa and NT mark tumor and non-tumor area,
respectively. (B) The distribution of cytoplasmic (Cyt) and nuclear
(Nu) hnRNP K scores in the different patients analyzed. The scores
were banded as follows: 0, negative; 1, 2, 3, low; 4, 5,
intermediate; 6, 7, high. (C) Comparison of the scores of hnRNP K
between NT and PCa tissues. The ordinate represents the mean score
± SE. HnRNP K expression was significantly higher (p<0.0001) in
PCa compared with NT in both cellular compartments. (E)
Distribution of the percentage of area positive for AR expression
in NT and PCa in all patients analyzed. (F) Comparison of AR scores
between NT and PCa. AR expression was significantly higher
(p=0.004) in PCa. Only patients with a score >0 were considered.
In the same patients, regression analysis (H) demonstrated a
significant direct correlation between nuclear hnRNP K and AR
expression (r=0.363, p=0.0009). |
Specific immunostaining of the AR was exclusively
visible in the nuclei (Fig. 1D).
The staining intensity was almost homogeneous both in NT and PCa
tissues where more than 70% of patients had a low or intermediate
intensity. In contrast, the distribution of the percentage of the
area that reacted positively to the antibody was more flattened
(kurtosis −1.27) in tumor than in NT specimens (kurtosis −0.39),
suggesting a great heterogeneity of the malignant tissues among
different patients (Fig. 1E). The
tissue sections from 25 PCas (24%) showed negative staining
(Fig. 1G), whereas in the
remaining 80 samples, the percentage of AR-positive cells, as well
as the total score, was statistically higher in malignant than
non-malignant epithelial cells (p=0.002 and p=0.004, respectively)
(Fig. 1F).
Since we found that hnRNP K and AR colocalize in the
nucleoplasm of prostate cancer cell lines (LNCaP and TRAMP) giving
rise to a complex (23), we
analyzed whether a correlation between the expression levels of the
two proteins also exists in human tissue. As shown in Fig. 1H, in AR-positive PCas, protein
expression was directly correlated with the level of nuclear hnRNP
K (r=0.363, p=0.0009), and the correlation was also maintained when
all PCas were considered (r=0.256, p=0.009). No relationship was
demonstrated between cytoplasmic expression of hnRNP K and AR. This
result suggests that the interaction between the two proteins found
in vitro is a more widespread behavior, which is also
present in vivo.
Patient characteristics and hnRNP K
expression
Statistical analyses revealed no specific
correlations between cytoplasmic or nuclear expression levels of
hnRNP K and clinicopathological characteristics of patients
(Table II).
 | Table II.Relationship between the expression
levels of hnRNP K and AR and clinicopathological characteristics of
patients. |
Table II.
Relationship between the expression
levels of hnRNP K and AR and clinicopathological characteristics of
patients.
|
Characteristics | Cytoplasmic hnRNP K
| Nuclear hnRNP K
| AR
|
|---|
| r | p-value | r | p-value | r | p-value |
|---|
| PSA | 0.145 | ≤0.1 | 0.126 | ≤0.2 | −0.249 | 0.01 |
| Extra-prostatic
extension | 0.029 | ≤0.7 | 0.191 | ≤0.051 | 0.024 | 0.8 |
| Pelvic nodes
involved | 0.022 | ≤0.8 | 0.142 | ≤0.1 | −0.049 | 0.6 |
| Surgical margins
involved | −0.038 | ≤0.7 | 0.007 | ≤0.9 | −0.021 | 0.8 |
| Seminal vesicles
involved | 0.073 | ≤0.4 | 0.165 | ≤0.1 | 0.044 | 0.6 |
| Gleason score
<7 | −0.063 | ≤0.5 | 0.027 | ≤0.8 | −0.110 | 0.3 |
| Gleason score
≥7 | −0.055 | ≤0.6 | 0.043 | ≤0.7 | −0.238 | 0.01 |
To evaluate whether the expression of hnRNP K was
correlated with PSA failure-free and/or overall survival, a
Kaplan-Meier analysis was performed. We stratified the patients
into three groups based on the hnRNP K expression level within both
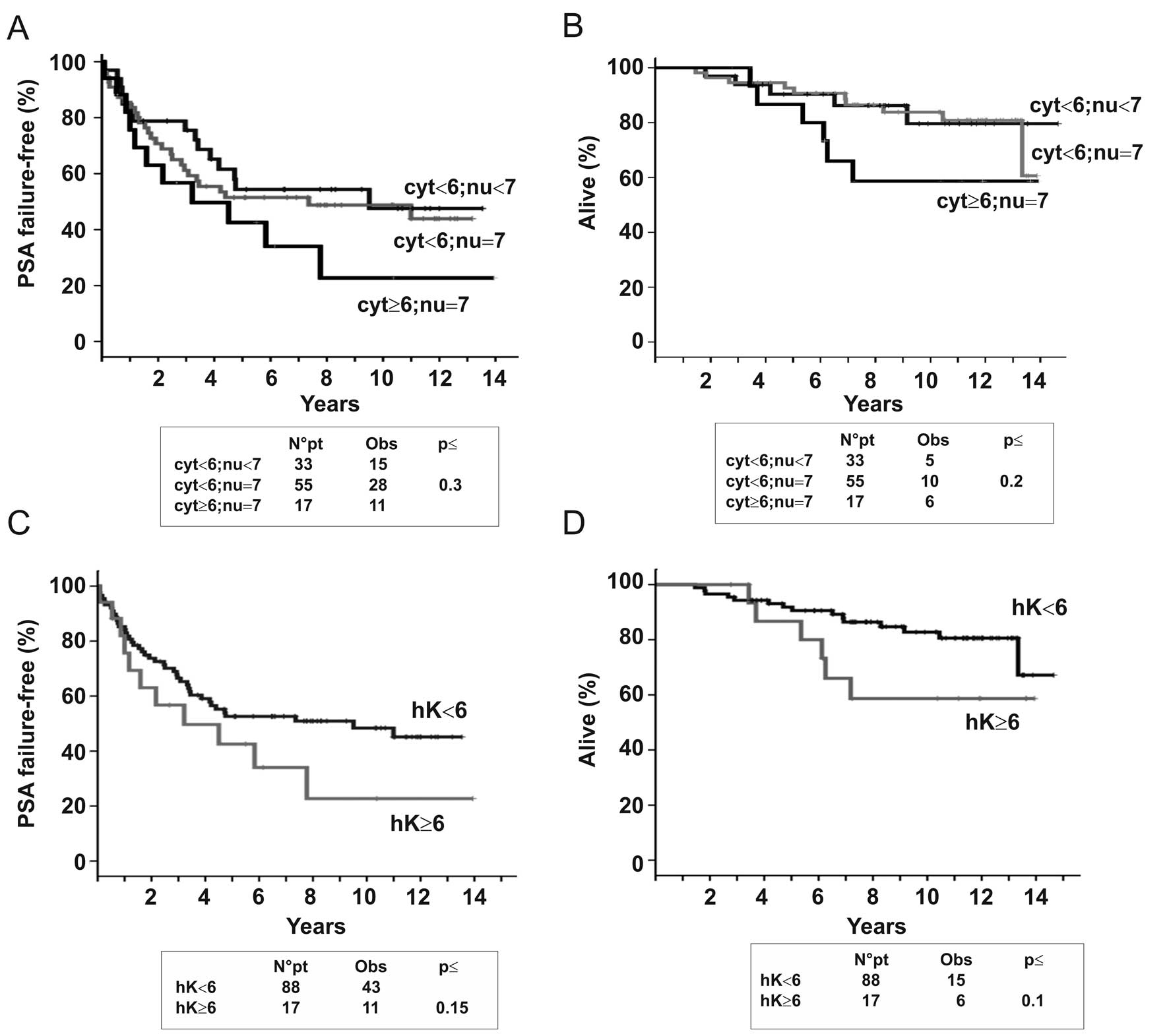
of the cellular compartments. Group 1, which included 17 patients
whose tumors presented high cytoplasmic (≥6) and nuclear expression
(=7), showed the worst outcome, whereas in other groups (group 2:
cytoplasmic <6 and nuclear <7 and group 3: cytoplasmic <6
and nuclear =7), the clinical outcome was independent of nuclear
expression and the curves for both PSA failure-free and overall
survival were almost superimposable (Fig. 2A and B). Therefore, for the
following analysis, we considered only the cytoplasmic expression
of hnRNP K and divided patients into two groups, those with low
(<6, 88 patients) and those with high (≥6, 17 patients)
expression. As illustrated by Kaplan-Meier curves shown in Fig. 2C and D, patients with high
cytoplasmic hnRNP K levels showed a trend for a highest risk of
biochemical failure (HR=1.63, 0.84–3.16 CI 95%, p=0.15) and death
(HR=2.23, 0.86–5.81 CI 95%, p=0.1) confirming that the aberrant
cytoplasmic accumulation of the protein has an important role in
the aggressiveness of PCa, as already reported for several human
tumors (12–18).
Patient characteristics and AR
expression
A significant inverse correlation of AR expression
with the Gleason score ≥7 (r=−0.238; p≤0.010) and with PSA
(r=−0.249; p≤0.010) was found (Table
II). The first observation is in agreement with previous
results showing a direct relationship between a higher degree of
AR-positive cells and lower Gleason score (32). Whereas, the inverse correlation
between the AR score and the level of PSA could depend on a
limitation of immunohistochemical methods that only requires the
presence of an immunoreactive epitope and does not distinguish
between a functional vs. non-functional AR.
Since there has been no consensus for measuring the
AR expression by immunohistochemistry in clinically localized PCa
(33), we performed a Kaplan-Meier
analysis considering the intensity and the percentage of
AR-positive cells separately. No correlation was observed between
the staining intensity in tumor cells and PSA failure-free survival
(data not shown), whereas when we considered the percentage of
AR-positive cells a strong association with a worse prognosis was
evident in PCa exhibiting low percentage of AR-positive cells
(<75%) (HR=3.38, 1.44–7.89 CI 95%, p=0.005) (Fig. 3A). This variable was the most
significant (p=0.001) in a multi-parametric model build up by
including all variables significantly correlated with the risk of
PSA progression at univariate analysis (Table III). No statistical correlation was
observed between the percentage of area positive for AR staining
and overall survival (data not shown).
 | Table III.PSA failure-free according to
principal clinicophatological variables and AR or AR in combination
with cytoplasmic hnRNP K expression. |
Table III.
PSA failure-free according to
principal clinicophatological variables and AR or AR in combination
with cytoplasmic hnRNP K expression.
| Variables | Univariate analysis
| Multivariate
analysis
| Multivariate
analysis
|
|---|
| HR | (95% CI) | p-value | HR | (95% CI) | p-value | HR | (95% CI) | p-value |
|---|
| PSA | | | | | | | | | |
| ≤10 ng/ml | 1.0 | | | 1.0 | | | 1.0 | | |
| >10 ng/ml | 1.88 | (1.06–3.35) | ≤0.03 | 1.21 | (0.64–2.28) | ≤0.5 | 1.18 | (0.63–2.23) | ≤0.6 |
| Extra-prostatic
penetration | | | | | | | | | |
| No | 1.0 | | | 1.0 | | | 1.0 | | |
| Yes | 2.98 | (1.67–5.32) | ≤0.000 | 3.2 | (1.41–7.26) | ≤0.005 | 3.18 | (1.39–7.24) | ≤0.006 |
| Pelvic nodes
involved | | | | | | | | | |
| pN0 | 1.0 | | | 1.0 | | | 1.0 | | |
| pN1 | 2.92 | (1.66–5.11) | ≤0.000 | 1.29 | (0.63–2.61) | ≤0.5 | 1.29 | (0.63–2.65) | ≤0.5 |
| Surgical margins
status | | | | | | | | | |
| Negative | 1.0 | | | 1.0 | | | 1.0 | | |
| Positive | 2.45 | (1.43–4.19) | ≤0.001 | 1.69 | (0.88–3.24) | ≤0.1 | 1.6 | (0.84–3.07) | ≤0.2 |
| Seminal vesicles
involved | | | | | | | | | |
| No | 1.0 | | | 1.0 | | | 1.0 | | |
| Yes | 1.71 | (0.98–2.99) | ≤0.06 | 0.47 | (0.23–0.95) | ≤0.04 | 0.47 | (0.23–0.96) | ≤0.04 |
| Gleason score | | | | | | | | | |
| <7 | 1.0 | | | 1.0 | | | 1.0 | | |
| ≥7 | 2.71 | (1.36–5.39) | ≤0.004 | 1.41 | (0.63–3.09) | ≤0.4 | 1.63 | (0.75–3.55) | ≤0.2 |
| AR | | | | | | | | | |
| >75% | 1.0 | | | 1.0 | | | | | |
| ≤75% | 3.38 | (1.44–7.89) | ≤0.005 | 4.25 | (1.76–10.26) | ≤0.001 | | | |
| AR and cytoplasmic
hK | | | | | | | | | |
| >75% + hK
<6 | 1.0 | | | | | | 1.0 | | |
| ≤75% and >75%
+ hK ≥6 | 4.59 | (1.66–12.72) | ≤0.003 | | | | 5.71 | (2.01–16.23) | ≤0.001 |
Effect of the association of hnRNP K and
AR expression on clinical outcome
We hypothesized that patient prognosis is most
likely dependent on an interplay between AR and cytoplasmic hnRNP
K. Therefore, we performed an analysis combining these two
variables and stratifying patients into four distinct groups,
depending on the level of expression of each protein: AR>75% and
hnRNP K<6; AR≤75% and hnRNP K<6; AR>75% and hnRNP K≥6;
AR≤75% and hnRNP K≥6. The combination of higher AR (>75%) and
lower hnRNP K (<6) expression was strongly associated with a
good prognosis (Fig. 3B) and
demonstrated even better than considering AR alone (Fig. 3A). Moreover, this association
emerged as the most significant independent prognostic marker for
PSA failure-free survival in a multivariate analysis (Table III).
HnRNP K expression in an in vitro model
of human PCa
It is possible to mimic the natural history of PCa
utilizing cell lines reflecting the various steps of the tumor
development (24). Therefore, to
understand the role of hnRNP K in PCa progression we studied hnRNP
K expression in BHP-1, LNCaPlp and LNCaPhp human prostate cells.
Light microscopy and western blot analysis (Fig. 4) showed that hnRNP K was weakly
expressed in BPH-1 cells, where it was localized mainly in the
nucleus whereas the cytoplasm was faintly stained. In LNCaP cancer
cells a significant increase of protein expression was detected and
it was localized both in the nucleus and in the cytoplasm. More
interestingly, hnRNP K expression was higher in LNCaPhp cells with
respect to less aggressive LNCaPlp (Fig. 4A and B). These data are in
agreement with in vivo observations reported above. Since it
is known that ERK phosphorylation drives cytoplasmic hnRNP K
accumulation (34) and in PCa
progression upregulated ERK activity is often correlated with AKT
activation (35), we studied the
expression of p-AKT and p-ERK in this in vitro model. pAKT
expression was absent in BPH-1 cells and present in both LNCaP cell
populations where no difference in the protein level was observed.
pERK 1/2 expression was weakly detectable in BPH-1 and was elevated
in cancer cells. In LNCaPhp the protein expression was
approximately 2.5 times higher than in LNCaPlp (Fig. 4B). This result is consistent with
the more elevated cytoplasmic localization of hnRNP K in this cell
line. Of note, higher hnRNP K and pERK expressions were associated
with higher PSA level (Fig. 4B),
suggesting a relationship between hnRNP K phosphorylation and
AR-regulated genes.
Discussion
Despite extensive research efforts, Gleason grade,
tumor stage and PSA are still the only parameters utilized for the
stratification of patients in prognostic groups (36). The pathogenesis of PCa is complex
and heterogeneous, and an understanding of the mechanisms that
regulate this process and its principal actors is fundamental to
the identification of new prognostic markers and new therapeutic
strategies.
In this study, we have demonstrated that AR and
hnRNP K are more highly expressed in cancer than normal prostate
tissues. Moreover, we found that a high percentage of AR-positive
cells (>75%) was strongly associated with a favorable prognosis
and this prognostic value increased when it was associated with low
cytoplasmic expression of hnRNP K.
While there is a widespread consensus that the
ligand-stabilized AR is nuclear and that AR expression is more
variable in PCa with respect to NT, the prognostic value of AR
expression in the epithelia of PCa and its clinical relevance is
still debated (33). In this
study, heterogeneous expression of AR, due to the presence of both
negative and positive cells in PCa (AR cell positive ≤75%), was
significantly associated with shorter PSA-free survival in
agreement with well-established results demonstrating that a high
variability of AR protein content in PCa cells correlates
significantly with a worse prognosis (37,38).
The interaction between AR and hnRNP K could have a
pivotal role in the development and progression of PCa. The modular
structure of hnRNP K causes a functional versatility that allows it
to interact with both nucleic acids and proteins, thus acting as a
‘docking platform’ to co-ordinate nucleic acid metabolism and to
facilitate cross-talk between signaling pathways (39). It has been demonstrated that hnRNP
K is an inhibitor of AR mRNA translation (7) and Shi et al (40) have reported that in the aging rat
liver and in oxidatively stressed hepatoma cells, the
transcriptional downregulation of the AR involves hnRNP K, which
participates in the activation of the complex that governs AR
stimulation; in this model, silencing hnRNP K decreased AR
expression. Moreover, we have shown in two cell lines (human LNCaP
and mouse TRAMPC2 cells) that the AR and hnRNP K colocalize in the
nucleoplasm forming a complex ligand-dependent (23). Notably, in a loss-of-function
screening, it has been found that hnRNP K is a potential target for
metastasis and that the cytoplasmic accumulation of this protein
was essential for its role in promoting metastasis (41). Additionally, the knockdown of hnRNP
K expression gives rise to a loss of the angiogenic and migratory
phenotype of prostate carcinoma cells (42). Therefore, hnRNP K could have
different roles in PCa as a function of its cellular
compartmentalization.
It is known that the cytoplasmic localization of
hnRNP K is phosphorylation-dependent and that the
translation-regulatory function of the protein depends on its
cytoplasmic localization. The increase of the phosphorylation grade
of hnRNP K that occurs during PCa progression (8,21,22)
could be responsible for its cytoplasmic accumulation. This
accumulation is not peculiar to PCa but seems to be a general
characteristic, indeed, it has been observed in several human
tumors, and it is often associated with a worse prognosis (12–18).
Moreover, utilizing an in vitro model, in this study we have
demonstrated that the aggressiveness of cancer cell lines
correlates with an increase in cytoplasmic expression of hnRNP K
and increased levels of pERK 1/2.
Somatic mutations, gene amplification, increased
protein stability and an altered level or function of coregulators
are the principal molecular events that have been proposed to act
in determining the level of AR in PCa. HnRNP K is one of the dozens
of AR-interacting coregulators (43) and post-translational modifications
of AR coregulators play a critical role in the formation of the
transcriptional complex and growth factor-induced enhancement of AR
transcription (44). We have
recently demonstrated in vitro that the bond between AR and
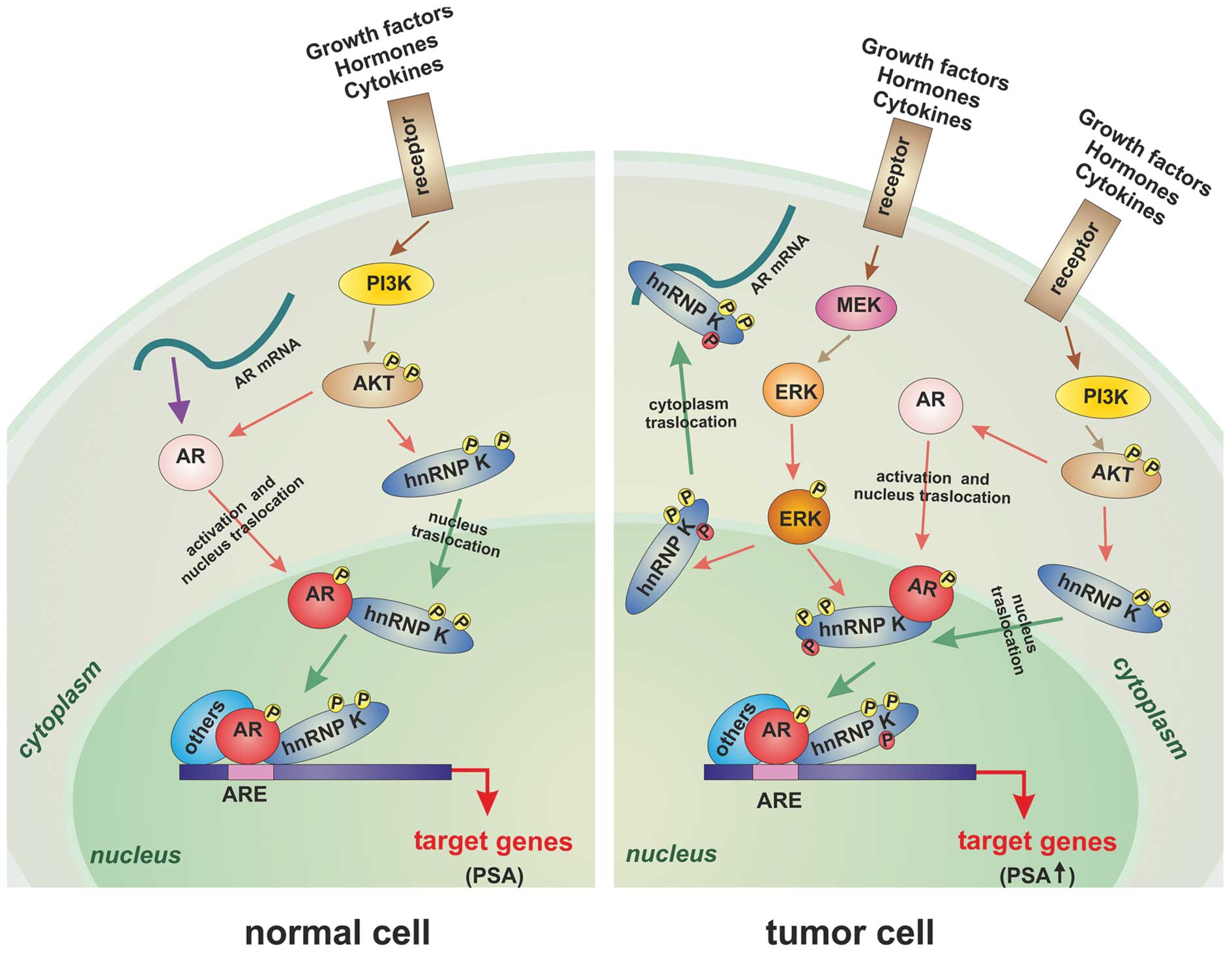
hnRNP K depends on the phosphorylation of the latter (45). Therefore, we propose a model where
alterations in the expression level and phosphorylation status of
hnRNP K could modify the nuclear interaction between AR and hnRNP
K, cause an increase of the PSA level and promote the migration of
the hnRNP K into the cytoplasm, where it accumulates and is
incapable of correctly regulating AR mRNA translation, as shown
schematically in Fig. 5. This
hypothesis is supported by several experimental observations.
Habelhah et al (34) have
demonstrated that ERK efficiently phosphorylates hnRNP K primarily
in the nucleus, after which phosphorylated protein translocates to
the cytoplasm and inhibits mRNA translation. Interestingly,
increased expression of the Ras/Raf/MEK/ERK pathway has been
associated with PCa progression (46). Furthermore, altered levels of pAKT
in the AKT/hnRNP K/AR/β-catenin pathway are critical for
neuroendocrine differentiation (47). Finally, more recently Gao et
al (48) have demonstrated
that hnRNP K overexpressing cells show enhanced malignant and
metastatic properties by regulation of extracellular matrix
components through the ERK signaling pathway.
Our results indicate that the association of AR and
hnRNP K expression has a potential prognostic value in PCa. The
possibility of detecting the expression of these two proteins with
immunohistochemistry, an easy-to-handle technique, could extend the
study of PCa progression to material obtained through core biopsies
and could significantly improve upon the sensitivity and
specificity with which PCa is diagnosed. Determining the expression
levels of AR and hnRNP K could allow for the stratification of
patients into different prognostic subgroups and could provide a
rationale for developing new chemotherapeutic agents directed
against phosphorylated hnRNP K.
Acknowledgements
This study was partially supported by
grants from Compagnia di San Paolo (Grant no. 2009.127); Liguria
Region, Department of Health (Grant no. 563/2009) and Ministero
dell’Universita’ e Ricerca Scientifica (MIUR) project PRIN (Grant
no. 2005065404_003, Italy).
References
|
1.
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2.
|
Stamey TA: Preoperative serum
prostate-specific antigen (PSA) below 10 μg/l predicts
neither the presence of prostate cancer nor the rate of
postoperative PSA failure. Clin Chem. 47:631–634. 2001.PubMed/NCBI
|
|
3.
|
Bostwick DG, Grignon DJ, Hammond ME, Amin
MB, Cohen M, Crawford D, Gospadarowicz M, Kaplan RS, Miller DS,
Montironi R, Pajak TF, Pollack A, Srigley JR and Yarbro JW:
Prognostic factors in prostate cancer. College of American
Pathologists Consensus Statement 1999. Arch Pathol Lab Med.
124:995–1000. 2000.PubMed/NCBI
|
|
4.
|
Humphrey PA and Vollmer RT: Percentage
carcinoma as a measure of prostatic tumor size in radical
prostatectomy tissues. Mod Pathol. 10:326–333. 1997.PubMed/NCBI
|
|
5.
|
Tefilli MV, Gheiler EL, Tiguert R, Sakr W,
Grignon DJ, Banerjee M, Pontes JE and Wood DP Jr: Should Gleason
score 7 prostate cancer be considered a unique grade category?
Urology. 53:372–377. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Montironi R, Mazzuccheli R, Scarpelli M,
Lopez-Beltran A, Fellegara G and Algaba F: Gleason grading of
prostate cancer in needle biopsies or radical prostatectomy
specimens: contemporary approach, current clinical significance and
sources of pathology discrepancies. BJU Int. 95:1146–1152.
2005.
|
|
7.
|
Mukhopadhyay NK, Kim J, Cinar B,
Ramachandran A, Hager MH, Di Vizio D, Adam RM, Rubin MA,
Raychaudhuri P, De Benedetti A and Freeman MR: Heterogeneous
nuclear ribonucleoprotein K is a novel regulator of androgen
receptor translation. Cancer Res. 69:2210–2218. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Barboro P, Repaci E, Rubagotti A, Salvi S,
Boccardo S, Spina B, Truini M, Introini C, Puppo P, Ferrari N,
Carmignani G, Boccardo F and Balbi C: Heterogeneous nuclear
ribonucleo-protein K: altered pattern of expression associated with
diagnosis and prognosis of prostate cancer. Br J Cancer.
100:1608–1616. 2009. View Article : Google Scholar
|
|
9.
|
Bomsztyk K, Denisenko O and Ostrowski J:
hnRNP K: one protein multiple processes. Bioessays. 26:629–638.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Ostrowski J and Bomsztyk K: Nuclear shift
of hnRNP K protein in neoplasms and other states of enhanced cell
proliferation. Br J Cancer. 89:1493–1501. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Carpenter B, MacKay C, Alnabulsi A, MacKay
M, Telfer C, Melvin WT and Murray GI: The roles of heterogeneous
nuclear ribonucleoproteins in tumour development and progression.
Biochim Biophys Acta. 1765:85–100. 2006.PubMed/NCBI
|
|
12.
|
Carpenter B, McKay M, Dundas SR, Lawrie
LC, Telfer C and Murray GI: Heterogeneous nuclear ribonucleoprotein
K is over expressed, aberrantly localized and is associated with
poor prognosis in colorectal cancer. Br J Cancer. 95:921–927. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Chen LC, Hsueh C, Tsang NM, Liang Y, Chang
KP, Hao SP, Yu JS and Chang YS: Heterogeneous nuclear
ribonucleoprotein K and thymidine phosphorylase are independent
prognostic and therapeutic markers for nasopharyngeal carcinoma.
Clin Cancer Res. 14:3807–3813. 2008. View Article : Google Scholar
|
|
14.
|
Matta A, Tripathi SC, DeSouza LV, Grigull
J, Kaur J, Chauhan SS, Srivastava A, Thakar A, Shukla NK, Duggal R,
DattaGupta S, Ralhan R and Siu KWM: Heterogeneous ribonucleoprotein
K is a marker of oral leukoplakia and correlates with poor
prognosis of squamous cell carcinoma. Int J Cancer. 125:1398–1406.
2009. View Article : Google Scholar
|
|
15.
|
Chen LC, Chung IC, Hsueh C, Tsang NM, Chi
LM, Liang Y, Chen CC, Wang LJ and Chang YS: The antiapoptotic
protein FLIP, is regulated by heterogeneous nuclear
ribonucleoprotein K and correlates with poor overall survival of
nasopharyngeal carcinoma patients. Cell Death Differ. 17:1463–1473.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Wen F, Shen A, Shanas R, Bhattacharyya A,
Lian F, Hostetter G and Shi J: Higher expression of the
heterogeneous nuclear ribonucleoprotein K in melanoma. Ann Surg
Oncol. 17:2619–2627. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Hope NR and Murray GI: The expression
profile of RNA-binding proteins in primary and metastatic
colorectal cancer: relationship of heterogeneous nuclear
ribonucleoproteins with prognosis. Human Pathol. 42:392–402.
2011.
|
|
18.
|
Wang F, Zhang P, Shi C, Yang Y and Qin H:
Immunohisto-chemical detection of HSP27 and hnRNP K as prognostic
and predictive biomarkers for colorectal cancer. Med Oncol.
29:1780–1788. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Nagano K, Masters JR, Akpan A, Yang A,
Corless S, Wood C, Hastie C, Zvelebil M, Cramer R and Naaby-Hansen
S: Differential protein synthesis and expression levels in normal
and neoplastic human prostate cells and their regulation by type I
and II interferons. Oncogene. 23:1693–1703. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Wang LG, Johnson EM, Kinoshita Y, Babb JS,
Buckley MT, Liebes LF, Melamed J, Liu XM, Kurek R, Ossowski L and
Ferrari AC: Androgen receptor overexpression in prostate cancer
linked to Purα loss from a novel repressor complex. Cancer Res.
68:2678–2688. 2008.
|
|
21.
|
Boccardo F, Rubagotti A, Carmignani G,
Romagnoli A, Nicolò G, Barboro P, Parodi S, Patrone E and Balbi C:
Nuclear matrix proteins changes in cancerous prostate tissues and
their prognostic value in clinically localized prostate cancer.
Prostate. 55:259–264. 2003. View Article : Google Scholar
|
|
22.
|
Ricci F, Rubagotti A, Zinoli L, Mangerini
R, Nuzzo PV, Carmignani G, Simonato A, Barboro P, Balbi C and
Boccardo F: Prognostic value of nuclear matrix protein expression
in localized prostate cancer. J Cancer Res Clin Oncol.
138:1379–1384. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Barboro P, Repaci E, Ferrari N, Rubagotti
A, Boccardo F and Balbi C: Androgen receptor and heterogeneous
nuclear ribonucleoprotein K colocalize in the nucleoplasm and are
modulated by bicalutamide and 4-hydroxy-tamoxifen in prostatic
cancer cell lines. Prostate. 71:1466–1479. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Igawa T, Lin FF, Lee MS, Karan D, Batra SK
and Lin MF: Establishment and characterization of
androgen-independent human prostate cancer LNCaP cell model.
Prostate. 50:222–235. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Tepper CG, Boucher DL, Ryan PE, Ma AH, Xia
L, Lee LF, Pretlow TG and Kung HJ: Characterization of a novel
androgen receptor mutation in a relapsed CWR22 prostate cancer
xenograft and cell line. Cancer Res. 62:6606–6614. 2002.PubMed/NCBI
|
|
26.
|
Barboro P, Rubagotti A, Orecchia P, Spina
B, Truini M, Repaci E, Carmignani G, Romagnoli A, Introini C,
Boccardo F, Carnemolla B and Balbi C: Differential proteomic
analysis of nuclear matrix in muscle-invasive bladder cancer:
potential to improve diagnosis and prognosis. Cell Oncol. 30:13–26.
2008.
|
|
27.
|
Kim D, Gregory CW, Smith GJ and Mohler JL:
Immunohistochemical quantitation of androgen receptor expression
using color video image analysis. Cytometry. 35:2–10. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Fleiss JL: Statistical Methods for Rates
and Proportions. 2nd edition. John Wiley & Sons, Inc; New York:
1981
|
|
29.
|
Kaplan EL and Meier P: Nonparametric
estimation from incomplete observations. J Am Stat Assoc.
53:457–481. 1958. View Article : Google Scholar
|
|
30.
|
Cox DR: Regression models and life tables.
JR Stat Soc B. 34:187–220. 1972.
|
|
31.
|
Armitage P, Berry G and Matthews JNS:
Statistical methods in Medical Research. 4th edition. Blackwell
Publishing; Oxford: 2000
|
|
32.
|
Heinlein CA and Chang C: Androgen receptor
in prostate cancer. Endocr Rev. 25:276–308. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Mohler JM: A role for the
androgen-receptor in clinically localized and advanced prostate
cancer. Best Pract Res Clin Endocrinol Metab. 22:357–372. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Habelhah H, Shah K, Huang L,
Ostareck-Lederer A, Burlingame AL, Shokat KM, Hentze MW and Ronai
Z: ERK phosphorylation drives cytoplasmic accumulation of hnRNP-K
and inhibition of mRNA translation. Nat Cell Biol. 3:325–330. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Hong SK, Jeong JH, Chan AM and Park JI:
AKT upregulates B-Raf Ser445 phosphorylation and ERK1/2 activation
in prostate cancer cells in response to androgen depletion. Exp
Cell Res. 319:1732–1743. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Fiorentino M, Capizzi E and Loda M: Blood
and tissue biomarkers in prostate cancer: State of the art. Urol
Clin North Am. 37:131–141. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Sadi M and Barrack ER: Image analysis of
androgen receptor immunostaining in metastatic prostate cancer.
Heterogeneity as a predictor of response to hormonal therapy.
Cancer. 71:2574–2580. 1993. View Article : Google Scholar
|
|
38.
|
Magi-Galluzzi C, Xu X, Hlatky L, Hahnfeldt
P, Kaplan I, Hsiao P, Chang C and Loda M: Heterogeneity of androgen
receptor content in advanced prostate cancer. Mod Pathol.
10:8398–8445. 1997.
|
|
39.
|
Mikula M and Ostrowski J: HNRNP K
(heterogeneous nuclear ribonucleoprotein K). Atlas Genet Cytogenet
Oncol Haematol. 14:127–129. 2010.
|
|
40.
|
Shi L, Ko S, Kim S, Echchgadda I, Oh TS,
Song CS and Chatterjee B: Loss of androgen receptor in aging and
oxidative stress through Myb protooncoprotein-regulated reciprocal
chromatin dynamics of p53 and poly(ADP-ribose) polymerase PARP-1. J
Biol Chem. 283:36474–36485. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Inoue A, Sawata SY, Taira K and Wadhwa R:
Loss-of-function screening by randomized intracellular antibodies:
identification of hnRNP-K as a potential target for metastasis.
Proc Natl Acad Sci USA. 104:8983–8988. 2007. View Article : Google Scholar
|
|
42.
|
Benelli R, Monteghirfo S, Balbi C, Barboro
P and Ferrari N: Novel antivascular efficacy of metronomic
docetaxel therapy in prostate cancer: hnRNP K as a player. Int J
Cancer. 124:2989–2996. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Paliouras M, Zaman N, Lumbroso R,
Kapogeorgakis L, Beitel LK, Wang E and Trifiro M: Dynamic rewiring
of the androgen receptor protein interaction network correlates
with prostate cancer clinical outcomes. Integr Biol. 3:1020–1032.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Chmelar R, Buchanan G, Need EF, Tilley W
and Greenberg NM: Androgen receptor coregulators and their
involvement in the development and progression of prostate cancer.
Int J Cancer. 120:719–733. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Barboro P, Borzì L, Repaci E, Ferrari N
and Balbi C: Androgen Receptor activity is affected by both nuclear
matrix localization and the phosphorylation status of the
heterogeneous nuclear ribonucleoprotein K in anti-androgen-treated
LNCaP cells. PLoS One. 8:e792122013. View Article : Google Scholar
|
|
46.
|
McCubrey JA, Steelman LS, Chappell WH,
Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M,
Tafuri A, Stivala F, Libra M, Basecke J, Evangelisti C, Martelli AM
and Franklin RA: Roles of the Raf/MEK/ERK pathway in cell growth,
malignant transformation and drug resistance. Biochim Biophys Acta.
1773:1263–1284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Ciarlo M, Benelli R, Barbieri O, Minghelli
S, Barboro P, Balbi C and Ferrari N: Regulation of neuroendocrine
differentiation by AKT/hnRNPK/AR/β-catenin signaling in prostate
cancer cells. Int J Cancer. 131:582–590. 2012.PubMed/NCBI
|
|
48.
|
Gao R, Yu Y, Inoue A, Widodo N, Kaul SC
and Wadhwa R: Heterogeneous nuclear ribonucleoprotein K (hnRNP-K)
promotes tumor metastasis by induction of genes involved in
extracellular matrix, cell movement, and angiogenesis. J Biol Chem.
288:15046–15056. 2013. View Article : Google Scholar : PubMed/NCBI
|