Introduction
CXCR4 is a seven-span transmembrane G-protein
coupled receptor for SDF-1 (stromal derived factor-1). The
interaction between SDF-1 and its receptor CXCR4 activates multiple
biological processes e.g. organogenesis and hematopoiesis,
migration, proliferation, adhesion, inflammation and plays a
critical role during tumor growth and metastasis (1–4).
SDF-1 -CXCR4 axis has been shown to direct tumor cells to organs
that highly express SDF-1 e.g., lymph nodes, lungs, liver or bones
(5–7). It was also shown that cells
expressing CXCR4 are clonally selected during their growth in the
mammary fat pad of nude mice. A further increase in CXCR4
expression, and SDF-1-mediated migration was observed in cancer
cells that metastasized to the lungs (8). Furthermore, blockade of CXCR4
inhibits tumor growth in a mouse model (9).
CXCR4 receptor is involved in a multistage process
of metastasis (10,11). Scientific reports clearly indicate
a significant role of CXCR4 receptor in breast, prostate, ovarian
or melanoma cancer progression (12,13).
In addition, CXCR4 tumor positive cells are characterized by high
self-renewal abilities, tumor initiation and resistance to
treatment (11,14). The ability of cancer cells to
spread throughout the body is dependent on the interactions of
their cell surface molecules with the microenvironment and the
presence of cancer stem cells (CSCs) (11,15).
CSCs initiate primary tumor growth, have the ability to self-renew
and give rise to more differentiated cell types. They participate
in cancer cell migration to distant tissue where they are able to
form secondary tumor from a single cell (11,16).
CSCs are very difficult to identify but recognition of them seems
to be necessary for efficient cancer treatment. Their resistance to
chemo- and radiotherapy is an additional exertion to elaborate
relevant anticancer targeted therapy. Many of CSC markers are still
unrecognized. Moreover, their expression profile may be addicted to
the origin and type of the tumor. As previously described, CSCs
demonstrate high expression of the CXCR4 receptor (17).
Cervical carcinoma (CC) is highly associated with
human papillomavirus (HPV) infection that is one of the major risk
factor for CC development. At early stages, CC cells occupy the
surrounding tissue whereas in advanced stages they migrate and form
metastases in regional lymph nodes, bone marrow, lungs, spleen and
liver (18–20). However, despite the development of
vaccine against HPV infection, cervical carcinoma is one of the
most frequently diagnosed tumor among women with no available
effective therapy at advanced stages.
Downregulation of gene expression is the appropriate
method to evaluate the role of genes of interest (21,22).
In our study we efficiently blocked CXCR4 gene expression with
lentivirus shRNA construct directed against the CXCR4 gene and
investigated the role of CXCR4 receptor in CC development and
metastasis.
Materials and methods
Cell culture
HTB-35 cell line was purchased from ATCC (Rockville,
MD, USA) and maintained in culture medium MEM (minimal essential
medium, PAA Laboratories GmbH) supplemented with 10%
heat-inactivated FBS (Fetal Bovine Serum, PAA) and 0.05 mg/ml
gentamycin (PAA). Cells were cultured in a humidified atmosphere at
5% CO2 and 37°C. They were split usually twice a
week.
Lentiviral transduction
HTB-35 cells were transduced with Fusin shRNA
Lentiviral Particles (Santa Cruz Biotechnology, Santa Cruz, CA,
USA) in 6 μg/ml polybrene (Sigma, St. Louis, MO, USA)
according to the manufacturer’s protocol. Transduced cells were
selected with 2.5 μg/ml puromycin (InvivoGen, San Diego, CA,
USA) for 16 days. Next, the cell line was analyzed to confirm the
reduction of CXCR4 gene expression at the mRNA and protein level
and subjected to further characterization. For control cell line,
HTB-35 cells were transduced with Control shRNA Lentiviral
Particles (Santa Cruz Biotechnology) as described above.
Flow cytometry
Extracellular staining of CXCR4 receptor was tested
by flow cytometry. Cells were split with nonenzymatic Cell
Dissociation Solution (Sigma). Cells (1×105) in 90
μl staining buffer (PBS supplemented with 2% FBS) were added
to a test tube containing the appropriate amount of monoclonal
PE-conjugated mouse anti-human CD184 antibody (clone 12G5, BD
Bioscience-Pharmingen, San Jose, CA, USA). Isotype-matched mouse
PE-conjugated immunoglobulin (IgG2aκ) served as control (clone
G155-178, BD Pharmingen, San Jose, CA, USA). After 30 min of
incubation on ice in the dark, cells were washed with staining
buffer twice and collected using a FACSCanto cytometer
(Becton-Dickinson, San Jose, CA, USA). Data were analyzed with FACS
Diva (Becton-Dickinson), WIN MDI 2.9 (free program available) and
Cyflogic v.1.2.1. software (free program available).
Quantitative real-time PCR (qRT-PCR)
analysis
The total RNA isolation was performed using RNeasy
Mini kit (Qiagen, Hilden, Germany). followed by DNAse treatment
(Promega, Madison, WI, USA). RNA (1 μg) was used for the
reverse transcriptase reaction that was carried out using M-MLV
reverse transcriptase (Promega) according to the manufacturer’s
protocol. qRT-PCR analysis was performed on ABI PRISM 7300 Sequence
Detection System (Applied Biosystems, Inc., Foster City, CA, USA)
using TaqMan Gene Expression Master MiX (Applied Biosystems, Inc.).
Probes used in this study were as follows: human GAPDH
(Hs99999905_m1), MMP9 (Hs00234579_m1), TIMP1 (Hs00171558_m1), TIMP2
(Hs00234278_m1), HIF-1α (Hs00153153_m1), VEGF (Hs00900055_m1) and
mouse GAPDH (Mm99999915_g1). The mRNA expression level was
normalized to the housekeeping gene GAPDH. The experiments were
performed three times in duplicate. Data are presented as % of
control cells (wild-type).
MTS assay
To examine mitochondrial activity of tumor cells MTS
test was done. Cells (2×103) were seeded on 96-well
plates. Analysis was performed in two different conditions: culture
medium supplemented with FBS (MEM 10% FBS) and culture medium
supplemented with bovine serum albumin (MEM 0.5% BSA). After 24,
48, 72 and 96 h CellTiter 96® AQueous One Solution assay
(Promega) was added according to the manufacturer’s protocol. After
2 h the level of absorbance was read at a wavelength of 490 nm
using the EL×800 Universal Microplate Reader (BioTek Instruments,
Highland Park, USA) and analyzed with KC4 v3.0 with PowerReports
software. The results are presented as mean absorbance value in an
appropriate time. The experiment was carried out three times in
triplicates.
Colony-forming assay
To determine different colony morphologies,
1×103 cells were plated as a single cell on a 6-well
plate in culture medium. As a colony, we recognized a cluster
consisting of at least 6 cells. After 4 days different colony
morphologies were observed. Paraclone-, holoclone- and
meroclone-like colonies were identified. Colonies were fixed,
stained using Wright’s reagent (Merck, Darmstadt, Germany) and
counted in 10 fields at ×100 magnification under a light Olympus
BX-51 microscope. Two independent experiments were performed. The
percentage of paraclone- and holoclone-like colonies recognized as
a result of cell culture in low density ± D are presented.
Suspension growth assay
Cells were seeded at a density of 3×104
on a non-adherent 24-well plate (Thermo Scientific, Rockford, IL,
USA) in culture medium for 48 h at 37°C and 5% CO2.
After this time cells were collected and seeded at 1×103
on a 6-well plate in order to observe the clonal growth potential
and in the amount of 2×103 on a 96-well plate to
determine the mitochondrial activity potential (MTS assay).
Experiments were performed two times according to the schemes
described above.
Cell stimulation
Cells (2.5×105) were seeded on 6-well
plates in culture medium. The next day, culture medium was changed
to MEM 0.5% BSA to make cells quiescent. Stimulation was performed
with SDF-1β (100 ng/ml) (PeproTech, Rocky Hill, NJ, USA) for 2, 5,
10 and 30 min. Medium containing 10% FBS and 0.5% BSA were positive
and negative control, respectively.
Western blot analysis
Cells were lysed (for 10 min) on ice in M-Per lysing
buffer (Pierce) containing protease and phosphatase inhibitors
(Sigma). Protein concentration was determined by Bradford protein
assay. Protein samples [containing 20 μg of protein, LDS (NuPage
LDS sample buffer; Invitrogen Life Technologies, Carlsbad, CA,
USA), Bond Breaker (Thermo Scientific) and M-Per buffer] were
separated on a 12% sodium dodecyl sulfate-PAGE gel and transferred
into a PVDF membrane (Bio-Rad Laboratories, Hercules, CA, USA). The
phosphorylation of AKT and MAPK was assessed using primary rabbit
anti-phospho-AKT (Ser 473, Cell Signaling, Danvers, MA, USA) and
primary mouse anti-phospho-MAPK (Thr202/Tyr204, Cell Signaling)
antibodies and subsequently detected with horseradish peroxidase
(HRP)-conjugated goat anti-rabbit IgG secondary antibody (sc-2054;
Santa Cruz Biotechnology) and (HRP)-conjugated goat anti-mouse IgG
secondary antibody (sc-2055; Santa Cruz Biotechnology). The
membranes were developed with an enhanced chemiluminescence reagent
(ECL, Amersham Life Sciences, Buckinghamshire, UK) dried and
subsequently exposed to the HyperFilm (Amersham Life Sciences) or
imaged by Gel Logic Imaging System 1500 (Kodak; Molecular Imaging
System, New Haven, CT, USA). An equal loading in the lanes was
evaluated by probing with anti-rabbit monoclonal anti-GAPDH
antibody (14C10; Cell Signaling). The experiment was performed two
times with similar results. Representative data are presented.
Chemotaxis assay
To check the tumor cells the migration ability
towards SDF-1β gradient, modified Boyden’s chamber with 8-μm
pore polycarbonate membrane inserts (Costar Transwell;
Costar-Corning, Lowell, MA, USA) were used. Cells were harvested
with non-enzymatic Cell Dissociation Solution (Sigma). Cell
suspension at the density of 3×104 in 100 μl MEM
0.5% BSA was placed in the upper chamber of the insert. In the
lower chamber 650 μl medium contained SDF-1β [100 ng/ml] was
placed. After 24-h incubation in a humidified atmosphere at 5%
CO2 and 37°C the transmigrated cells were fixed, stained
with Wright solution (Merck) and counted in five fields of view at
×100 magnification using an inverted light microscope (Olympus
IX70). Medium containing 10% FBS, or 0.5% BSA was the positive and
negative control, respectively. Experiment was performed two times
in duplicates.
In vivo tumor models (animal
experiments)
The 6- to 8-week old male NOD-SCID mice (non-obese
diabetes severe combined immunodeficiency mice) were used to
evaluate the in vivo meta-static behavior of tumor
cells.
Subcutaneous injection: mice were injected with
5×106 tumor cells/mouse. Twice a week the volume of
primary tumors was quantified using the formula
[(x2y)/2] for an ellipsoid. After 30 days mice were
subjected to anesthesia, tumors were weighed and fixed in 10%
formalin. Immunohistochemical evaluation was prepared using
hematoxylin and eosin staining and primary mouse monoclonal
antibodies anti-Ki-67 (clon MIB-1; 1:75, Biocompare,
DakoCytomation, Glostrup, Denmark).
Intravenous injection: mice were injected through
the eyeball with 1×106 tumor cells per mouse for 24 h
and 30 days. After this time, mice were sacrificed. Isolation of
bone marrow was performed. Organ tissues such us lungs and spleen
was isolated and homogenized using Cell Strainer (BD Bioscience)
with a 40-micron pore size. To assess the potential sites of
metastasis, RNA isolation, reverse transcription and qPCR (as
previously described) was performed to define human to mouse GAPDH
proportion.
Animal experiments were approved by the Local Ethics
Committee for Experiments on Animals acting at the Jagiellonian
University in Krakow (Resolution No. 56/2011). Two independent
experiments were carried out with 10 NOD-SCID mice/group.
Statistical analysis
Statistical analysis was performed using Statistica
v10 software by one-way ANOVA and the Tukey test. The results with
P-values <0.05 were considered as statistically significant, and
labeled by an asterisk in the figures.
Results
Downregulation of CXCR4 gene
expression
In order to efficiently knock down the CXCR4 gene
expression, HTB-35 cell line was transduced with Fusin shRNA
lentiviral particles, and shRNA lentiviral particles were used as a
control. After transduction and antibiotic selection, we obtained
80% and 90% reduction of CXCR4 gene expression at mRNA and protein
level, respectively, compared to control cells: wild-type (WT) and
shCONTROL (Fig. 1A and B).
Next, we examined the effectiveness of the CXCR4
gene knockdown. Western blot analysis showed strong phosphorylation
of AKT and MAPK kinases after 5 min stimulation in control cells.
The shCXCR4 cells also responded to the chemokine but at a lower
level. The weak stimulation might be caused by CXCR7 receptor
activity, the second SDF-1 receptor (Fig. 1C). Moreover, downregulation of
CXCR4 receptor led to almost 7-fold decrease in the chemotactic
activity toward SDF-1β gradient compared to control cells (Fig. 1D).
CXCR4 receptor maintains the diversity of
clonal morphology
The epithelial origin of HTB-35 cell line is
associated with the capacity to form colony-like structures as a
result of culture beginning at low density. In order to analyze
whether the CXCR4 receptor is involved in the diversity of clonal
morphology, colony-forming assay was used. After 6-day culture at
low density, ‘holoclone’-, ‘meroclone’- and ‘paraclone’-like
colonies were identified (Fig.
2A). Our results suggest that CXCR4 receptor downregulation
increases the number of ‘paraclone’-like colonies in comparison to
control cells (Fig. 2B). Cell
culture under the suspension condition for 48 h has no influence on
the colony formation but changes the percentage participation of
different types of colonies. Control cell lines had lost cells
which are able to form ‘paraclone’-like colonies. Similar effect
was observed in shCXCR4 cells where the percentage of
‘paraclone’-like colonies decreased about 50% compared to control
condition (Fig. 2C). Furthermore,
growth in suspension has no influence on mitochondrial activity of
examined cells (Fig. 2D).
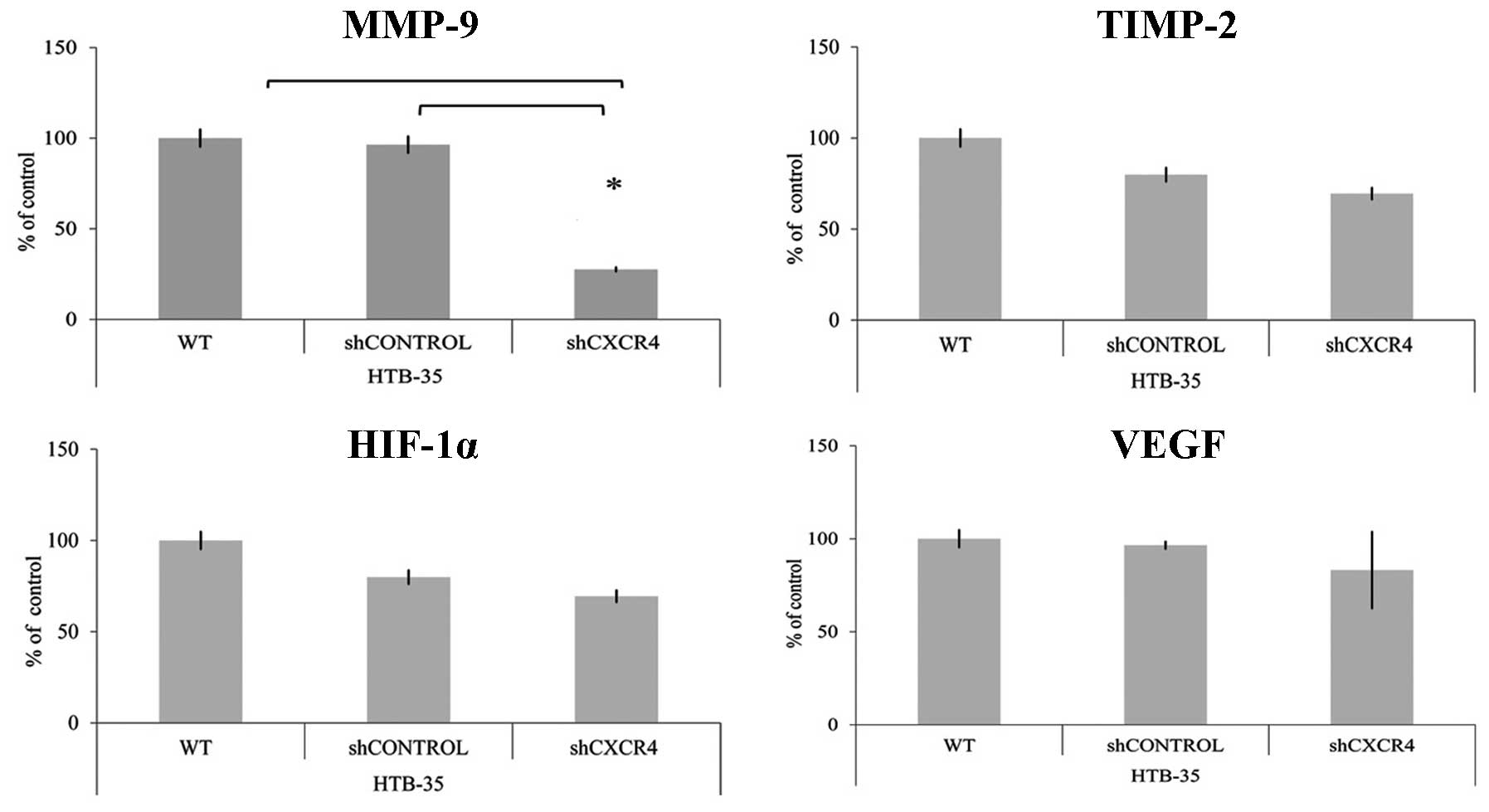
CXCR4 receptor modulates the expression
of MMP-9
To evaluate if CXCR4 receptor mediates the
expression of genes related to angiogenesis and metastasis,
quantitative real-time RT-PCR was performed. We observed
significant positive correlation between CXCR4 and matrix
metalloproteinase (MMP-9) level. CXCR4 downregulation resulted in
the reduction of MMP-9 and had no influence on tissue matrix
metalloproteinase inhibitor-2 (TIMP-2) expression. In HTB-35 cell
line, the interaction at mRNA level between CXCR4 and
hypoxia-inducible factor 1-α (HIF-1α) or vascular endothelial
growth factor (VEGF) was not observed (Fig. 3).
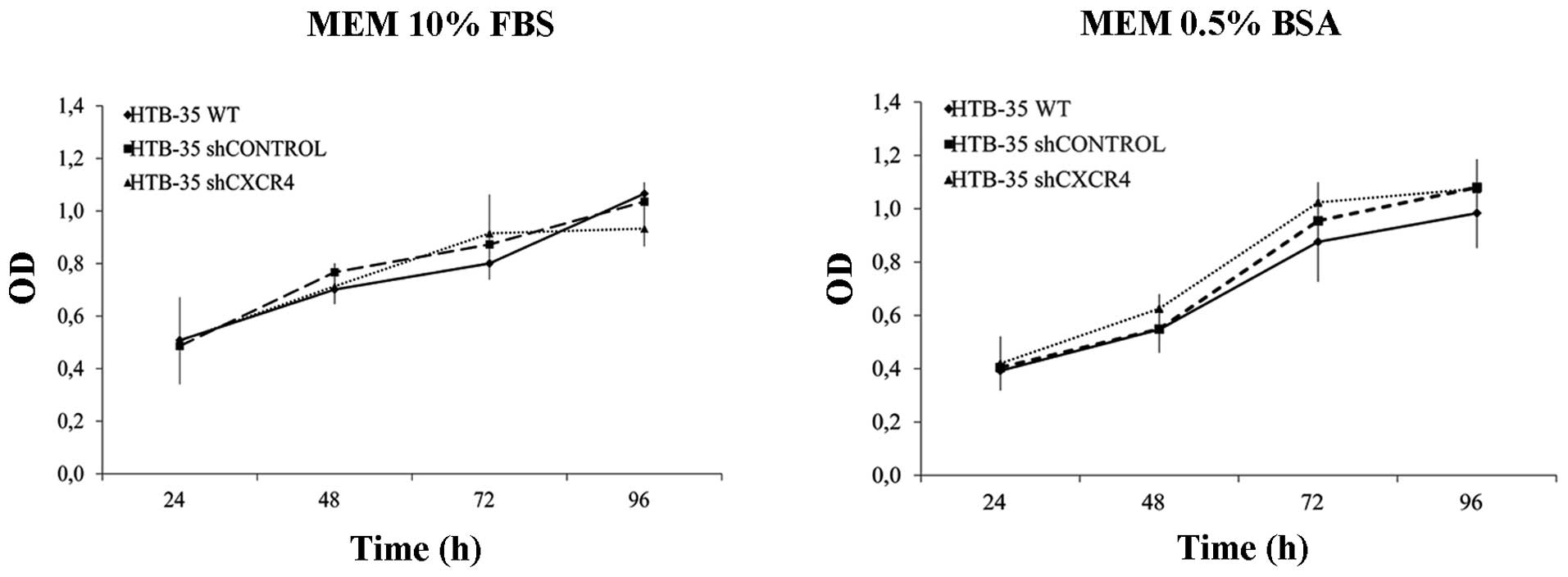
CXCR4 downregulation does not influence
the cell proliferation rate in vitro but efficiently reduces tumor
growth and metastasis in a murine model in vivo
The effect of CXCR4 downregulation on HTB-35 tumor
cell proliferation rate was measured by MTS assay. Cells were
cultured for 96 h in medium supplemented with 0.5% BSA or 10% FBS.
We observed no differences in the proliferation rate between
shCXCR4 cells and control cells either under starvation or control
conditions (Fig. 4).
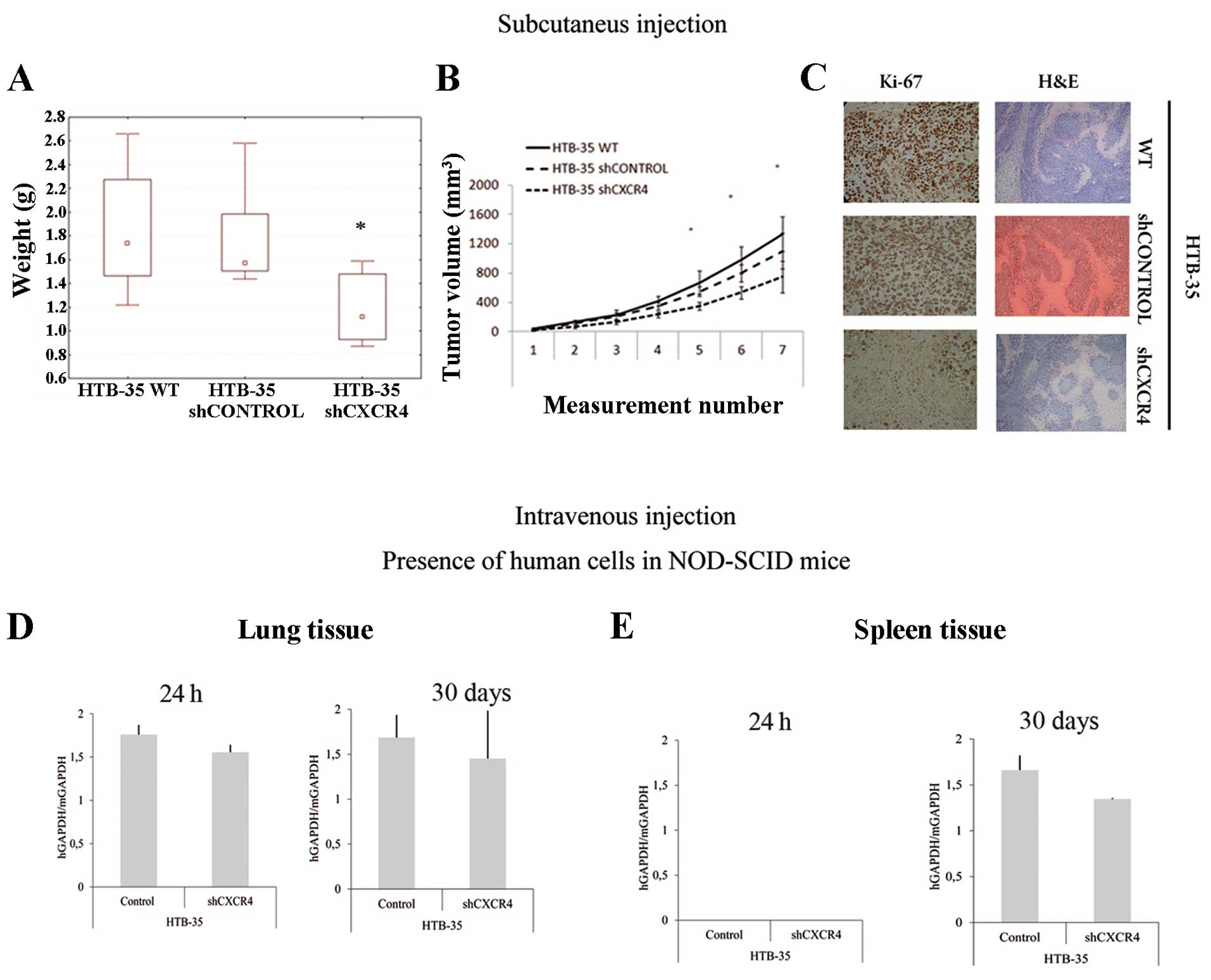
Interestingly, the results from in vitro
tests were different from in vivo results. Our studies
showed that CXCR4 down-regulation reduces HTB-35 cell growth and
metastasis. Subcutaneous injection with shCXCR4 cells in NOD-SCID
mice was associated with a significant decrease (about 30%) in
tumor growth potential (Fig. 5A and
B) compared to control cells. In addition, Ki-67 staining
showed decreased proliferation activity in tumors formed by shCXCR4
cells (Fig. 5C). However, H&E
staining revealed no differences between morphology of the tumors
formed by shCXCR4 cells and control cells (Fig. 5C).
Intravenous injection demonstrated the participation
of SDF-1/CXCR4 axis in CC cell migration to the lungs and spleen.
High expression of human GAPDH in lungs tissue in both control
groups of NOD-SCID mice was observed. In contrast, CXCR4 receptor
downregulation decreased the metastatic ability of HTB-35 cells. We
observed 12% and 20% decrease in metastasis in short- and long-term
murine models, respectively (Fig.
5D). We also showed that the period of 24 h was not sufficient
for tumor cells to extravasate into spleen tissue. After 30 days of
injection, we observed 30% reduction in metastasis for shCXCR4 in
comparison to control group (Fig.
5E). We detected no human cells in the bone marrow after
injection with HTB-35 cells (data not shown).
Discussion
Growth disorders usually manifest by excessive
proliferation which cause e.g., rapid primary tumor growth and
subsequently its invasion and metastasis (23). The role of the chemokine receptor
CXCR4 in the regulation of tumor growth has been recognized as an
important issue. In HTB-35 cell line, CXCR4 downregulation did not
change the mitochondrial activity and growth potential in
vitro. Interestingly, we observed a significant decrease in
primary tumor growth and weight in vivo. It is possible that
the CXCR4 receptor is required for the initiation of cell
proliferation and/or promotes the survival of cervical cancer
cells, similarly to breast cancer cells (24). The observed effect of limiting
growth in vivo might be explained by low expression of CXCR4
which is associated with induction of apoptosis and/or a decrease
level of proliferation potential (24). In addition, Ki-67 staining was
performed to check the proliferation activity of tumor cells in
vivo. Ki-67 antigen is expressed from late G1 to the M phase.
It has a prognostic value and its high level is associated with
poor prognosis and short patient survival (25). In this study, we observed that a
decrease of proliferation activity in primary tumors formed by
shCXCR4 cells, was associated with the reduction of Ki-67 level.
Similar data were reported for breast and lung cancer, where
increased growth was directly related to Ki-67 upregulation and
provides enhanced risk of metastasis (26,27).
Our data define the CXCR4 receptor as a very
important factor in the metastatic process of cervical cancer.
Morphological diversity is a common feature of cancer cells and
might be associated with the ability to generate colonies and
self-renewal population from single cell (11,28–30).
The study of CSCs and the mechanisms that regulate their biology is
challenging. CSCs constitute a small percentage of the tumor
population. However, we are still unable to define precisely
markers of these cells for many types of tumors. One possible
pre-selection of epithelial cells as stem cells is to assess their
clonogenic potential (11,28). Such cells are capable of forming
specific clones of different morphology which is an indicator of
their properties (29). There are:
i) holoclones, the structure typical for epithelial cells, composed
of small, closely adjacent cells with increased capacity for
self-renewal and differentiation properties, ii) paraclones, the
structure formed by the cells with irregular shape, loosely
arranged in the clone with a high degree of differentiation
capacity and iii) meroclones, intermediate forms of clones
(28,30). Among holoclones, there is the
greatest probability of stem cell presence [e.g. keratinocytes
(31)] or CSCs [e.g. prostate
cancer (29)]. Only cells derived
from holoclones are able to initiate tumor development after
transplantation into mice. Furthermore, they express surface
markers characteristic of CSCs (such as CXCR4 and CD44).
Interestingly, meroclone-derived cells are not able to form tumor
tissue. Furthermore, paraclone-derived cells die during the in
vitro culture (29) which was
also observed in our study in suspension culture. Our results
indicate that CXCR4/SDF-1 axis play a very important role in
maintaining the metastatic potential. Morphological diversity
showed that CXCR4 downregulation caused the reduction of
‘holoclone’-like structures possibly indicating a decreased number
of CSCs. However, further analysis and characterization of colonies
formed by HTB-35 cells are required, but these results reflect a
decreased metastatic potential to the lung and spleen tissue in the
shCXCR4 cell line. Our results are consistent with a study of human
breast cancer that also demonstrated the importance of CXCR4/SDF-1
signaling at the primary tumor microenvironment (32). It is possible that SDF-1 in the
tumor microenvironment promotes breast cancer proliferation,
migration and invasion. The impairment of CXCR4 and/or SDF-1
activity could interrupt this paracrine signaling pathway reducing
growth potential of primary tumors (24,32).
As previously described, CXCR4 receptor is also related to bone
marrow metastasis in e.g. rhabdomyosarcoma (33), and breast cancer (34). Our observation indicates the
attenuation of AKT and MAPK pathways and inhibition of chemotaxis
to the SDF-1 gradient in shCXCR4 cells. It may suggest a similar
mechanism of CXCR4/SDF-1 axis in the cervical cancer cells as in
the breast cancer and rhabdomyosarcoma (9,24,33).
High level of MMP expression is directly associated
with tumor invasiveness and poor prognosis (35–37).
We observed positive relationship between CXCR4 and MMP-9 level in
HTB-35 cell line. Significant decrease of MMP-9 mRNA expression
level in the shCXCR4 cells might be associated with reduced
capacity to form lung and spleen metastasis. Surprisingly, the
level of HIF-1α and VEGF was not changed. The obtained results are
different from studies of breast cancer angiogenesis. It has been
showed that HIF-1α is a potent inducer of VEGF but the expression
of VEGF can be unregulated via HIF-1α independent mechanisms as
well (38). It is related to the
activation of PI3K/AKT pathway, which can be stimulated, for
example, by CXCR4/SDF-1 interaction. The inhibition of this axis
significantly decreases the VEGF expression and angiogenesis in
MDA-MB-231 cells (39).
Inhibition of CXCR4 expression and function
significantly impairs the growth potential of the HTB-35 cervical
carcinoma cell line in vivo and efficiently decreases the
lung and spleen metastasis in an animal NOD-SCID model. Thus, our
data suggest CXCR4 as a novel target for prevention of cervical
carcinoma growth and metastasis.
Acknowledgements
This study was supported by research
grants to K.M. (N N401 010036, N N401 142339) and M.M. (N N 401
615840) from the Polish Ministry of Science and Higher
Education.
References
|
1.
|
Zlotnik A and Yoshie O: Chemokines: a new
classification system and their role in immunity. Immunity.
12:121–127. 2000. View Article : Google Scholar
|
|
2.
|
Zlotnik A: Chemokines and cancer. Int J
Cancer. 119:2026–2029. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Braunersreuther V, Mach F and Steffens S:
The specific role of chemokines in atherosclerosis. Thromb Haemost.
97:714–721. 2007.PubMed/NCBI
|
|
4.
|
Ara T, Tokoyoda K, Sugiyama T, Kawabata K,
et al: Long-term hematopoietic stem cells require stromal
cell-derived factor-1 for colonizing bone marrow during ontogeny.
Immunity. 19:257–267. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Taichman RS, Cooper C, Keller ET, et al:
Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate
cancer metastasis to bone. Cancer Res. 62:1832–1837.
2002.PubMed/NCBI
|
|
6.
|
Kato M, Kitayama J, Kazama S and Nagawa H:
Expression pattern of CXC chemokine receptor-4 is correlated with
lymph node metastasis in human invasive ductal carcinoma. Breast
Cancer Res. 5:144–150. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Kucia M, Jankowski K, Reca R, et al:
CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol
Histol. 35:233–245. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Helbig G, Christopherson KW,
Bhat-Nakshatri P, et al: NF-kappaB promotes breast cancer cell
migration and metastasis by inducing the expression of the
chemokine receptor CXCR4. J Biol Chem. 278:21631–21638. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Liang Z, Yoon Y, Votaw J, et al: Silencing
of CXCR4 blocks breast cancer metastasis. Cancer Res. 65:967–971.
2005.PubMed/NCBI
|
|
10.
|
Kucia M, Reca R, Miekus K, et al:
Trafficking of normal stem cells and metastasis of cancer stem
cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4
axis. Stem Cells. 23:879–894. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Shiozawa Y, Nie B, Pienta KJ, et al:
Cancer stem cells and their role in metastasis. Pharmacol Ther.
138:285–293. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Raman D, Baugher PJ, Thu YM and Richmond
A: Role of chemokines in tumor growth. Cancer Lett. 256:137–165.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Teicher BA and Fricker SP: CXCL12
(SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 16:2927–2931.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Orimo A, Gupta PB, Sgroi DC, et al:
Stromal fibroblasts present in invasive human breast carcinomas
promote tumor growth and angiogenesis through elevated SDF-1/CXCL12
secretion. Cell. 121:335–348. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Pecorino L: Molecular Biology of Cancer.
Oxford University Press; 2012
|
|
16.
|
LaBarge MA: The difficulty of targeting
cancer stem cell niches. Clin Cancer Res. 16:3121–3129. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Majka M, Drukala J, Lesko E, et al: SDF-1
alone and in co-operation with HGF regulates biology of human
cervical carcinoma cells. Folia Histochem Cytobiol. 44:155–164.
2006.PubMed/NCBI
|
|
18.
|
Schiffman M, Wentzensen N, Wacholder S, et
al: Human papillomavirus testing in the prevention of cervical
cancer. J Natl Cancer Inst. 103:368–383. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Kodama J, Hasengaowa, Kusumoto T, et al:
Association of CXCR4 and CCR7 chemokine receptor expression and
lymph node metastasis in human cervical cancer. Ann Oncol.
18:70–76. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
González Martín A: Molecular biology of
cervical cancer. Clin Transl Oncol. 9:347–354. 2007.
|
|
21.
|
Sharp FA: RNA interference - 2001. Genes
Dev. 15:485–490. 2001. View Article : Google Scholar
|
|
22.
|
Felekkis K and Deltas C: RNA
intereference: a powerful laboratory tool and its therapeutic
implications. Hippokratia. 10:112–115. 2006.PubMed/NCBI
|
|
23.
|
Wu J, Chen C and Zhao KN:
Phosphatidylinositol 3-kinase signaling as a therapeutic target for
cervical cancer. Curr Cancer Drug Targets. 13:143–156. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Smith M, Luker KE, Garbow JR, et al: CXCR4
regulates growth of both primary and metastatic breast cancer.
Cancer Res. 64:8604–8612. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Kruse AJ, Baak JPA, de Bruin PC, et al:
Ki-67 immunoquantitation in cervical intraepithelial neoplasia
(CIN): a sensitive marker for grading. J Pathol. 193:48–54. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Fasching PA, Heusinger K, Haeberle L, et
al: Ki-67, chemo-therapy response, and prognosis in breast cancer
patients receiving neoadjuvant treatment. BMC Cancer. 11:486–499.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Martin B, Paesmans M, Mascaux C, et al:
Ki-67 expression and patients survival in lung cancer: systematic
review of the literature with meta-analysis. Br J Cancer.
91:2018–2025. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Locke M, Heywood M, Fawell S and Mackenzie
IC: Retention of intrinsic stem cell hierarchies in
carcinoma-derived cell lines. Cancer Res. 65:8944–8950. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Li H, Chen X, Calhoun-Davis T, et al: PC3
human prostate carcinoma cell holoclones contain self-renewing
tumor-initiating cells. Cancer Res. 68:1820–1825. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Papini S, Grivel JC, Cecchetti D, et al:
Isolation and clonal analysis of human epidermal keratinocyte stem
cells in long-term culture. Stem Cells. 21:481–494. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Barrandon Y and Green H: Three clonal
types of keratinocyte with different capacities for multiplication.
Proc Natl Acad Sci USA. 84:2302–2306. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Allinen M, Beroukhim R, Cai L, et al:
Molecular characterization of the tumor microenvironment in breast
cancer. Cancer Cell. 6:17–32. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Miekus K, Lukasiewicz E, Jarocha D, et al:
The decreased meta-static potential of rhabdomyosarcoma cells
obtained through MET receptor downregulation and the induction of
differentiation. Cell Death Dis. 17:e4592013. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Muller A, Homey B, Soto H, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Davidson B, Goldberg I, Kopolovic J, et
al: MMP-2 and TIMP-2 expression correlates with poor prognosis in
cervical carcinoma - a clinicopathologic study using
immunohisto-chemistry and mRNA in situ hybridization. Gynecol
Oncol. 73:372–382. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Zhai Y, Hotary KB, Nan B, et al:
Expression of membrane type 1 matrix metalloproteinase is
associated with cervical carcinoma progression and invasion. Cancer
Res. 65:6543–6550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Sheu BC, Lien HC, Ho HN, et al: Increased
expression and activation of gelatinolytic matrix
metalloproteinases is associated with the progression and
recurrence of human cervical cancer. Cancer Res. 63:6537–6542.
2003.PubMed/NCBI
|
|
38.
|
Arsham AM, Plas DR, Thompson CB and Simon
MC: Akt and hypoxia-inducible factor-1 independently enhance tumor
growth and angiogenesis. Cancer Res. 64:3500–3507. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Lianga Z, Brooksa J, Willarda M, et al:
CXCR4/CXCL12 axis promotes VEGF-mediated tumor angiogenesis through
Akt signaling pathway. Biochem Biophys Res Commun. 359:716–722.
2007. View Article : Google Scholar : PubMed/NCBI
|