| 202952_s_at | ADAM12 | Proteolysis, cell
adhesion, epidermal growth factor receptor signaling pathway,
myoblast fusion |
| 222862_s_at | AK5 |
Nucleobase-containing compound metabolic
process, nucleoside diphosphate phosphorylation, ADP biosynthetic
process, dADP biosynthetic process, signal transduction, nucleoside
triphosphate biosynthetic process, pyrimidine ribonucleotide
biosynthetic process, nucleobase-containing small molecule
interconversion, phosphorylation, small molecule metabolic process,
ATP metabolic process, nucleobase-containing small molecule
metabolic process |
| 228367_at | ALPK2 | Protein
phosphorylation, phosphorylation |
| 235548_at | APCDD1L | - |
| 210121_at | B3GALT2 | Protein
glycosylation, oligosaccharide biosynthetic process |
| 217452_s_at | | |
| 239367_at | BDNF | Ureteric bud
development, behavioral fear response, response to hypoxia, chronic
inflammatory response, mitochondrial electron transport, NADH to
ubiquinone, nervous system development, negative regulation of
neuroblast proliferation, axon guidance, axon target recognition,
behavior, learning or memory, feeding behavior, neuron recognition,
response to hormone stimulus, glutamate secretion, response to
fluoxetine, dendrite development, regulation of metabolic process,
nerve development, response to nutrient levels, response to vitamin
A, mechanoreceptor differentiation, response to drug, fear
response, negative regulation of apoptotic process, regulation of
neuron apoptotic process, negative regulation of neuron apoptotic
process, positive regulation of neuron differentiation, negative
regulation of striated muscle tissue development, regulation of
retinal cell programmed cell death, regulation of synaptic
plasticity, regulation of long-term neuronal synaptic plasticity,
positive regulation of long-term neuronal synaptic plasticity,
regulation of short-term neuronal synaptic plasticity, inner ear
development, cognition, positive regulation of synapse assembly,
response to hyperoxia, regulation of excitatory postsynaptic
membrane potential, response to anesthetic |
| 206382_s_at | | |
| 1552487_a_at | BNC1 | Transcription,
DNA-dependent, regulation of transcription, DNA-dependent,
regulation of transcription from RNA polymerase I promoter,
regulation of transcription from RNA polymerase II promoter,
positive regulation of cell proliferation, epidermis development,
wound healing, positive regulation of epithelial cell
proliferation, chromosome organization |
| 236532_at | C11orf87 | - |
| 1557180_at | | |
| 1557181_s_at | | |
| 229641_at | CCBE1 | Angiogenesis,
lymphangiogenesis, sprouting angiogenesis, multicellular organismal
development, venous blood vessel morphogenesis |
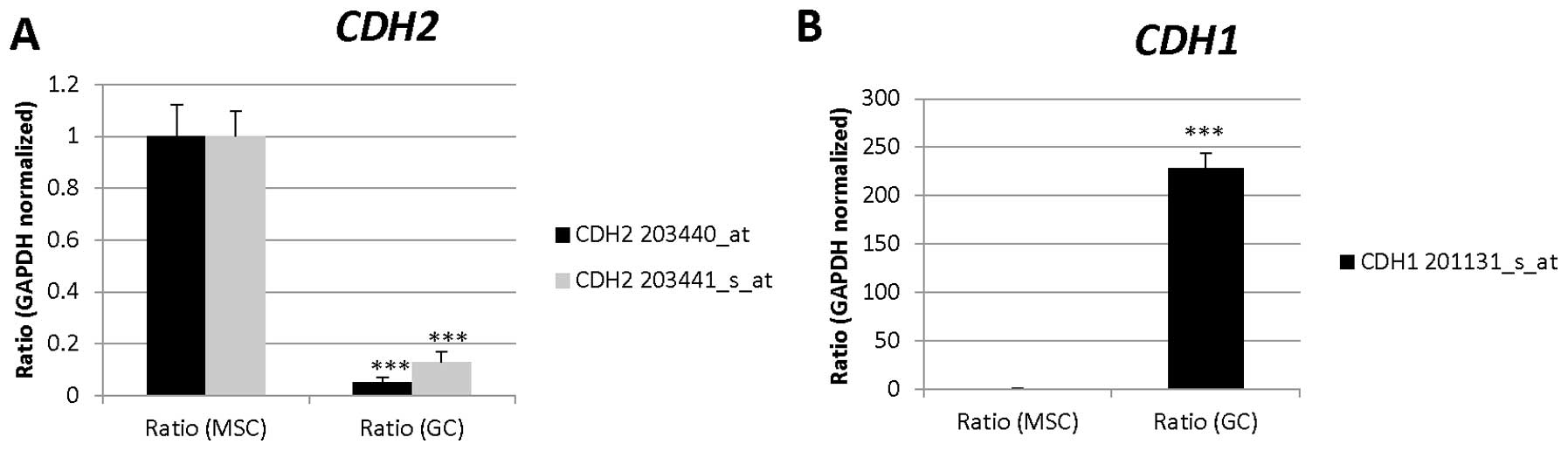
| 203440_at | CDH2 | Cell adhesion,
homophilic cell adhesion, heterophilic cell-cell adhesion, synapse
assembly, cell- cell adhesion, calcium-dependent cell-cell
adhesion, cell migration, regulation of myelination, regulation of
protein localization, cell junction assembly, adherens junction
organization, regulation of Rho protein signal transduction, muscle
cell differentiation, positive regulation of MAPK cascade,
cell-cell junction organization, blood vessel morphogenesis,
regulation of axonogenesis, striated muscle cell differentiation,
positive regulation of muscle cell differentiation, negative
regulation of canonical Wnt receptor signaling pathway |
| 204602_at | DKK1 | Negative regulation
of transcription from RNA polymerase II promoter, cell
morphogenesis involved in differentiation, endoderm formation,
mesoderm formation, hair follicle development, regulation of
receptor internalization, multicellular organismal development,
endoderm development, Wnt receptor signaling pathway, regulation of
Wnt receptor signaling pathway, negative regulation of Wnt receptor
signaling pathway, embryonic limb morphogenesis, negative
regulation of BMP signaling pathway, forebrain development,
negative regulation of protein complex assembly, response to
retinoic acid, negative regulation of peptidyl-serine
phosphorylation, negative regulation of mesodermal cell fate
specification, regulation of endodermal cell fate specification,
negative regulation of skeletal muscle tissue development, head
morphogenesis, face morphogenesis, negative regulation of
pathway-restricted SMAD protein phosphorylation, positive
regulation of heart induction by negative regulation of canonical
Wnt receptor signaling pathway, negative regulation of canonical
Wnt receptor signaling pathway, Wnt receptor signaling pathway
involved in somitogenesis, extracellular negative regulation of
signal transduction, negative regulation of canonical Wnt receptor
signaling pathway involved in cardiac muscle cell fate commitment,
negative regulation of cardiac muscle cell differentiation |
| 213707_s_at | DLX5 | Skeletal system
development, ossification, osteoblast differentiation, endochondral
ossification, transcription, DNA-dependent, regulation of
transcription, DNA-dependent, multicellular organismal development,
nervous system development, axonogenesis, axon guidance, cell
proliferation, embryonic limb morphogenesis, BMP signaling pathway,
epithelial cell differentiation, inner ear morphogenesis, ear
development, positive regulation of osteoblast differentiation,
positive regulation of transcription, DNA-dependent, positive
regulation of transcription from RNA polymerase II promoter,
anatomical structure formation involved in morphogenesis, positive
regulation of epithelial cell proliferation, palate development,
olfactory pit development, head development, face morphogenesis,
bone morphogenesis, cellular response to BMP stimulus, positive
regulation of canonical Wnt receptor signaling pathway, positive
regulation of transcription from RNA polymerase II promoter
involved in cellular response to chemical stimulus |
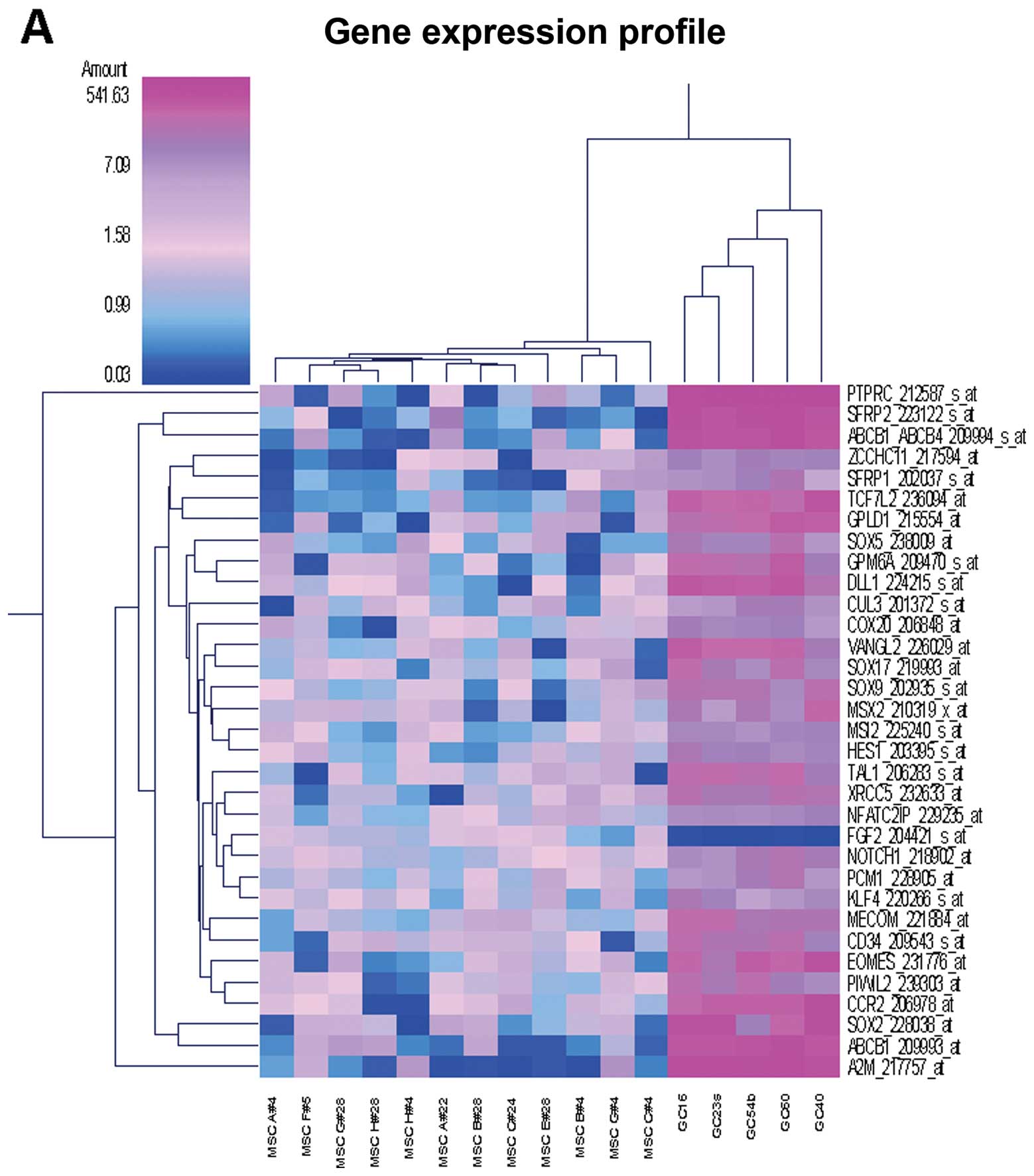
| 204421_s_at | FGF2 | Activation of MAPKK
activity, activation of MAPK activity, MAPK import into nucleus,
angiogenesis, branching involved in ureteric bud morphogenesis,
organ induction, positive regulation of protein phosphorylation,
positive regulation of endothelial cell proliferation, cell
migration involved in sprouting angiogenesis, regulation of
transcription, DNA-dependent, phosphatidyl-inositol biosynthetic
process, C21-steroid hormone biosynthetic process, apoptotic
process, chemotaxis, signal transduction, epidermal growth factor
receptor signaling pathway, intracellular protein kinase cascade,
Ras protein signal transduction, synaptic transmission,
multicellular organismal development, nervous system development,
positive regulation of cell proliferation, negative regulation of
cell proliferation, insulin receptor signaling pathway, fibroblast
growth factor receptor signaling pathway, fibroblast growth factor
receptor signaling pathway, embryo development, organ
morphogenesis, glial cell differentiation, positive regulation of
endothelial cell migration, positive regulation of gene expression,
negative regulation of fibroblast migration, positive regulation of
phospholipase C activity, regulation of calcium ion-dependent
exocytosis, substantia nigra development, positive regulation of
cerebellar granule cell precursor proliferation, cell
differentiation, extracellular matrix organization, hyaluronan
catabolic process, negative regulation of cell growth, lung
development, inositol phosphate biosynthetic process, Fc-epsilon
receptor signaling pathway, wound healing, positive regulation of
cell fate specification, positive regulation of blood vessel
endothelial cell migration, negative regulation of blood vessel
endothelial cell migration, positive regulation of
phosphatidylinositol 3-kinase activity, innate immune response,
positive regulation of cell differentiation, positive regulation of
osteoblast differentiation, regulation of angiogenesis, positive
regulation of angiogenesis, negative regulation of transcription,
DNA-dependent, positive regulation of transcription, DNA-dependent,
positive regulation of transcription from RNA polymerase II
promoter, regulation of retinal cell programmed cell death,
neurotrophin TRK receptor signaling pathway,
phosphatidylinositol-mediated signaling, embryonic morphogenesis,
response to axon injury, stem cell development, positive regulation
of epithelial cell proliferation, positive chemotaxis, release of
sequestered calcium ion into cytosol, regulation of cell cycle,
positive regulation of cell division, positive regulation of
cardiac muscle cell proliferation, corticotropin hormone secreting
cell differentiation, thyroid-stimulating hormone-secreting cell
differentiation, negative regulation of cell death, chondroblast
differentiation, mammary gland epithelial cell differentiation,
negative regulation of wound healing, positive regulation of ERK1
and ERK2 cascade |
| 223618_at | FMN2 | Transport,
apoptotic process, response to stress, response to DNA damage
stimulus, meiotic meta-phase I, multicellular organismal
development, protein transport, cellular component organization,
vesicle-mediated transport, meiotic chromosome movement towards
spindle pole, actin cytoskeleton organization, intracellular signal
transduction, polar body extrusion after meiotic divisions,
negative regulation of protein catabolic process, negative
regulation of apoptotic process, actin nucleation, intracellular
transport, oogenesis, establishment of meiotic spindle
localization, homologous chromosome movement towards spindle pole
involved in homologous chromosome segregation, formin-nucleated
actin cable assembly, cellular response to hypoxia |
| 1555471_a_at | | |
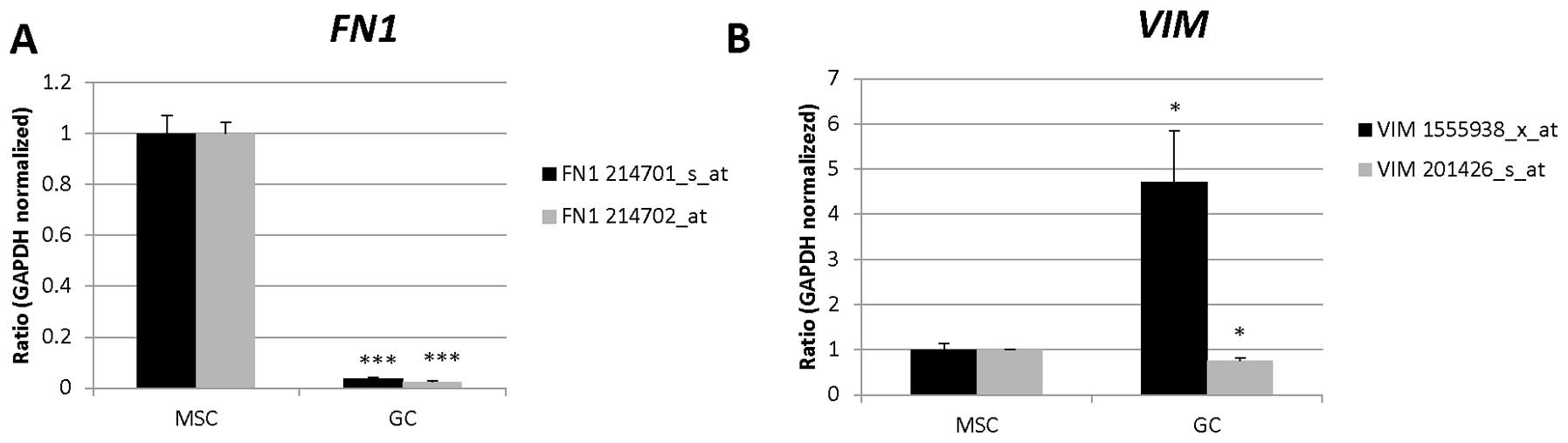
| 214701_s_at | FN1 | Angiogenesis,
platelet degranulation, acute-phase response, cell-substrate
junction assembly, celladhesion, cell-matrix adhesion,
calcium-independent cell-matrix adhesion, blood coagulation,
regulation of cell shape, response to wounding, positive regulation
of peptidase activity, cell migration, peptide cross-linking,
platelet activation, extracellular matrix organization, substrate
adhesion-dependent cell spreading, wound healing, leukocyte
migration |
| 214702_at | | |
| 206307_s_at | FOXD1 | Neural crest cell
migration, transcription, DNA-dependent, regulation of
transcription, DNA-dependent, pattern specification process,
peripheral nervous system development, embryo development, positive
regulation of gene expression, melanocyte differentiation, positive
regulation of BMP signaling pathway, negative regulation of
transcription, DNA-dependent, positive regulation of transcription
from RNA polymerase II promoter, enteric nervous system
development, sympathetic nervous system development, axon extension
involved in axon guidance, lateral line nerve glial cell
development, iridophore differentiation, regulation of
sequence-specific DNA binding transcription factor activity,
cartilage development, dichotomous subdivision of terminal units
involved in ureteric bud branching, metanephric capsule
development, metanephric capsule specification, positive regulation
of kidney development |
| 204948_s_at | FST | Negative regulation
of transcription from RNA polymerase II promoter, hematopoietic
progenitor cell differentiation, gamete generation, pattern
specification process, female gonad development, BMP signaling
pathway, hair follicle morphogenesis, negative regulation of
activin receptor signaling pathway, odontogenesis of
dentin-containing tooth, keratinocyte proliferation, negative
regulation of cell differentiation, negative regulation of
follicle-stimulating hormone secretion, positive regulation of hair
follicle development |
| 207345_at | | |
| 226847_at | | |
| 209905_at | HOXA10-HOXA9,
HOXA9, MIR196B | Transcription,
DNA-dependent, regulation of transcription, DNA-dependent,
multicellular organismal development, anterior/posterior pattern
specification, proximal/distal pattern formation, mammary gland
development, embryonic forelimb morphogenesis, endothelial cell
activation, negative regulation of myeloid cell differentiation,
embryonic skeletal system development, definitive hemopoiesis |
| 203851_at | IGFBP6 | Regulation of cell
growth, signal transduction, negative regulation of cell
proliferation, cellular protein metabolic process |
| 210261_at | KCNK2 | Transport, ion
transport, potassium ion transport, G-protein coupled receptor
signaling pathway, synaptic transmission, regulation of ion
transmembrane transport, potassium ion transmembrane transport |
| 244623_at | KCNQ5 | Protein complex
assembly, transport, ion transport, potassium ion transport,
synaptic transmission, regulation of ion transmembrane transport,
transmembrane transport, potassium ion transmembrane transport |
| 233533_at | KRTAP1-5 | - |
| 243813_at | LINC00968 | - |
| 204298_s_at | LOX | Blood vessel
development, cellular protein modification process, response to
hormone stimulus, extracellular matrix organization, collagen
fibril organization, lung development, wound healing, response to
drug, elastic fiber assembly, response to steroid hormone stimulus,
oxidation-reduction process |
| 213640_s_at | | |
| 215446_s_at | | |
| 230112_at | MARCH4 | Protein
ubiquitination |
| 219054_at | NPR3 | Skeletal system
development, osteoclast proliferation, adenylate cyclase-inhibiting
G-protein coupled receptor signaling pathway, negative regulation
of adenylate cyclase activity, phospholipase C-activating G-protein
coupled receptor signaling pathway, regulation of blood pressure,
regulation of osteoblast proliferation, positive regulation of
urine volume, positive regulation of nitric-oxide synthase
activity |
| 219789_at | | |
| 219790_s_at | | |
| 213791_at | PENK | Behavioral fear
response, signal transduction, neuropeptide signaling pathway,
behavior, sensory perception of pain |
| 207558_s_at | PITX2 | Negative regulation
of transcription from RNA polymerase II promoter, patterning of
blood vessels, vasculogenesis, in utero embryonic development,
neuron migration, extraocular skeletal muscle development,
atrioventricular valve development, cardiac neural crest cell
migration involved in outflow tract morphogenesis, pulmonary
myocardium development, regulation of transcription, DNA-dependent,
regulation of transcription from RNA polymerase II promoter,
transcription from RNA polymerase II promoter, multicellular
organismal development, determination of left/right symmetry, brain
development, heart development, skeletal muscle tissue development,
myoblast fusion, male gonad development, female gonad development,
anatomical structure morphogenesis, response to hormone stimulus,
organ morphogenesis, Wnt receptor signaling pathway, subthalamic
nucleus development, hypothalamus cell migration, pituitary gland
development, neuron differentiation, lung development, regulation
of cell migration, embryonic camera-type eye development, response
to vitamin A, embryonic hindlimb morphogenesis, hair cell
differentiation, vascular smooth muscle cell differentiation,
deltoid tuberosity development, regulation of cell proliferation,
odontogenesis of dentin-containing tooth, odontogenesis,
camera-type eye development, positive regulation of DNA binding,
positive regulation of transcription, DNA-dependent, positive
regulation of transcription from RNA polymerase II promoter, spleen
development, embryonic digestive tract morphogenesis, cardiac
muscle tissue development, cardiac muscle cell differentiation,
atrial cardiac muscle tissue morphogenesis, ventricular cardiac
muscle cell development, digestive system development, somatotropin
secreting cell differentiation, prolactin secreting cell
differentiation, ventricular septum morphogenesis, left lung
morphogenesis, pulmonary vein morphogenesis, superior vena cava
morphogenesis, endodermal digestive tract morphogenesis, iris
morphogenesis, cell proliferation involved in outflow tract
morphogenesis, left/right axis specification, positive regulation
of myoblast proliferation |
| 219729_at | PRRX2 | Positive regulation
of mesenchymal cell proliferation, regulation of transcription,
DNA-dependent, multicellular organismal development, embryonic limb
morphogenesis, inner ear morphogenesis, middle ear morphogenesis,
positive regulation of smoothened signaling pathway, embryonic
cranial skeleton morphogenesis, embryonic skeletal system
morphogenesis, artery morphogenesis, cartilage development |
| 210367_s_at | PTGES | Prostaglandin
biosynthetic process, acute inflammatory response, chronic
inflammatory response, lipid metabolic process, fatty acid
metabolic process, fatty acid biosynthetic process, prostaglandin
metabolic process, signal transduction, negative regulation of cell
proliferation, response to organic cyclic compound, arachidonic
acid metabolic process, cyclooxygenase pathway, response to
lipopolysaccharide, response to retinoic acid, response to cytokine
stimulus, small molecule metabolic process, response to calcium
ion |
| 206157_at | PTX3 | Response to yeast,
inflammatory response, opsonization, positive regulation of nitric
oxide biosynthetic process, positive regulation of
phagocytosis |
| 239202_at | RAB3B | GTP catabolic
process, transport, intracellular protein transport,
nucleocytoplasmic transport, signal transduction, small GTPase
mediated signal transduction, protein transport, regulation of
exocytosis, peptidyl-cysteine methylation |
| 235417_at | SPOCD1 | Transcription,
DNA-dependent, negative regulation of phosphatase activity |
| 203438_at | STC2 | Cellular calcium
ion homeostasis, response to oxidative stress, cell surface
receptor signaling pathway, cell-cell signaling, embryo
implantation, response to nutrient, endoplasmic reticulum unfolded
protein response, response to vitamin D, response to endoplasmic
reticulum stress, negative regulation of multicellular organism
growth, response to peptide hormone stimulus, decidualization,
calcium ion homeostasis, cellular response to hypoxia, regulation
of store-operated calcium entry |
| 201107_s_at | THBS1 | Activation of MAPK
activity, response to hypoxia, negative regulation of endothelial
cell proliferation, negative regulation of cell-matrix adhesion,
sprouting angiogenesis, chronic inflammatory response, platelet
degranulation, negative regulation of antigen processing and
presentation of peptide or polysaccharide antigen via MHC class II,
negative regulation of dendritic cell antigen processing and
presentation, outflow tract morphogenesis, endocardial cushion
development, growth plate cartilage development, induction of
apoptosis, inflammatory response, immune response, cell cycle
arrest, cell adhesion, blood coagulation, response to glucose
stimulus, positive regulation of endothelial cell migration,
negative regulation of endothelial cell migration, negative
regulation of plasma membrane long-chain fatty acid transport,
negative regulation of nitric oxide mediated signal transduction,
negative regulation of cGMP-mediated signaling, negative regulation
of plasminogen activation, positive regulation of macrophage
chemotaxis, positive regulation of fibroblast migration, positive
regulation of cell-substrate adhesion, cell migration, negative
regulation of angiogenesis, peptide cross-linking, platelet
activation, positive regulation of blood coagulation, extracellular
matrix organization, positive regulation of cell migration,
positive regulation of transforming growth factor beta receptor
signaling pathway, response to magnesium ion, response to
progesterone stimulus, negative regulation of interleukin-12
production, positive regulation of transforming growth factor beta1
production, cellular response to heat, response to endoplasmic
reticulum stress, negative regulation of fibroblast growth factor
receptor signaling pathway, positive regulation of phosphorylation,
response to drug, positive regulation of tumor necrosis factor
biosynthetic process, positive regulation of macrophage activation,
negative regulation of apoptotic process, negative regulation of
cysteine-type endopeptidase activity involved in apoptotic process,
positive regulation of blood vessel endothelial cell migration,
negative regulation of blood vessel endothelial cell migration,
engulfment of apoptotic cell, positive regulation of translation,
positive regulation of angiogenesis, behavioral response to pain,
blood vessel morphogenesis, positive regulation of chemotaxis,
response to calcium ion, negative regulation of focal adhesion
assembly, positive regulation of protein kinase B signaling
cascade, negative regulation of fibrinolysis, positive regulation
of execution phase of apoptosis, positive regulation of extrinsic
apoptotic signaling pathway via death domain receptors, positive
regulation of endothelial cell apoptotic process, positive
regulation of reactive oxygen species metabolic process, negative
regulation of extrinsic apoptotic signaling pathway |
| 201387_s_at | UCHL1 | Proteolysis,
ubiquitin-dependent protein catabolic process, response to stress,
axonogenesis, axon target recognition, adult walking behavior, cell
death, cell proliferation, protein deubiquitination, sensory
perception of pain, axon transport of mitochondrion, eating
behavior, negative regulation of MAP kinase activity, muscle fiber
development, neuromuscular process |
| 209946_at | VEGFC | Angiogenesis,
positive regulation of neuroblast proliferation, platelet
degranulation, substrate-dependent cell migration, signal
transduction, multicellular organismal development, blood
coagulation, positive regulation of cell proliferation, organ
morphogenesis, morphogenesis of embryonic epithelium, cell
differentiation, platelet activation, regulation of vascular
endothelial growth factor receptor signaling pathway, positive
regulation of protein autophosphorylation, response to drug,
positive regulation of blood vessel endothelial cell migration,
negative regulation of blood pressure, vascular endothelial growth
factor receptor signaling pathway, positive regulation of
epithelial cell proliferation, positive regulation of protein
secretion, positive chemotaxis, induction of positive chemotaxis,
positive regulation of cell division, positive regulation of mast
cell chemotaxis, positive regulation of lymphangiogenesis |
| 232122_s_at | VEPH1 | - |