Introduction
Osteopontin (OPN) is a secretory extracellular
matrix phos-phoglycoprotein that was originally identified in bone
tissue and is vital to control biomineralization, osteoclast
differentiation and bone resorption (1). They are expressed by cancer cells as
well, and participate in various metastasis-associated mechanisms
including proliferation, survival, adhesion, migration, invasion,
angiogenesis, malfunction of tumor-associated macrophages (TAMs)
and degradation of extracellular matrix (2–4).
Previous studies have shown that, in gastric cancer
tissues, OPN protein is diffusely located in the cytoplasm of tumor
cells as well as TAMs, which is in line with its implications in
the interactions between cancer cells and tumor stroma (5). Over the past decade, emerging
evidence has revealed a correlation of OPN overexpression and
clinicopathological features and prognosis in gastric cancers,
indicating its potential diagnostic and prognostic values in such
patients (6,7). Functional studies suggest that
targeting OPN and its related signaling network is likely to
provide an effective therapeutic approach for gastric cancers in
vitro and in vivo (8–10).
Due to the importance of OPN in gastric cancer, better
understanding of the implications of OPN in tumorigenesis might
facilitate development of therapeutic strategies in such
patients.
In this study, we investigated the biological
functions of OPN proteins in gastric cancer in vitro and
in vivo. The molecular events that define the role of OPN in
gastric cancer metastasis were investigated. We found that the OPN
status affected proliferation, apoptosis, invasion and migration of
gastric cancer in vitro, and OPN silencing decreased tumor
growth and metastasis in vivo. We also demonstrated that OPN
signaling, acting through the mitogen-activated protein kinase
(MAPK), phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) and
p65 nuclear factor κB (NF-κB) pathways, may contribute to cancer
processes by inducing matrix metalloproteinase 2 (MMP-2), MMP-9 and
urokinase-type plasminogen activator (uPA) activity, and inhibiting
caspase-3 cleavage.
Materials and methods
Cell lines
The human gastric cancer cell lines SGC7901, GES-1,
AGS, MKN45 and MKN28 were bought from the China Center for Type
Culture Collection (Wuhan, China). Cells were routinely maintained
in RPMI-1640 medium (Gibco, Grand Island, NY, USA) supplemented
with 10% fetal bovine serum (FBS) and 100 U/ml
penicillin/streptomycin at 37°C. The cells were cultured on tissue
culture dishes coated with 10 mg/ml fibronectin.
Animals
Four- to 6-week-old female/male athymic BALB/c nu/nu
mice were obtained from the Central Laboratory of Animal Science at
Wuhan University (Wuhan, China) and housed in laminar-flow cabinets
under specific pathogen-free conditions, with food and water ad
libitum. All experiments on mice were conducted in accordance
with the guidelines of National Institutes of Health (NIH;
Bethesda, MD, USA) for the care and use of laboratory animals. The
study protocol was also approved by Shanghai Medical Experimental
Animal Care Committee (China).
Lentivirus vector construction and
infection
Small interfering RNA (siRNA; GeneChem, Shanghai,
China) against OPN (GenBank accession no. NM_000582) mediated by
lentivirus and its mismatch mutants as negative control were
designed using an Internet application system (Invitrogen,
Carlsbad, CA, USA). These target sequences tagged with green
fluorescent protein (GFP) were annealed and inserted into pGC-LV
expression vector (Invitrogen), and then cotrans-fected into 293FT
cells with packaging vectors (pHelper 1.0 and pHelper 2.0;
Invitrogen) using Lipofectamine 2000 (Invitrogen). After a 48-h
incubation period post transfec-tion, viral supernatants were
collected, concentrated, and stored at −70°C. MKN45 and MKN28 cells
were infected with OPN-specific siRNA lentivirus or control
lentivirus with a multiplicity of infection (MOI) from 5 to 20 in
the presence of polybrene (10 mg/ml), respectively.
Growth curves
The cells were plated in 96-well plates and
maintained at 37°C in a humidified incubator. The cells were
harvested by trypsinization every day for 5 or 6 days and counted
with a hemocytometer.
Apoptosis assay
Cells were suspended at each chosen time-point.
Cells (2×106) were then centrifuged and washed twice
with ice-cold phosphate-buffered saline (PBS). Apoptotic cells were
detected by flow cytometric analysis using Annexin-V fluorescein
and propidium iodide (PI) (Molecular Probes, Invitrogen, Eugene,
OR, USA).
Invasion assay
Transwell invasion chambers (Millipore Corp.,
Bedford, MA, USA) consisting of a thin layer of Matrigel basement
membrane matrix were used. The upper surface of filters was 6.4 mm
in diameter with 8-μm pores. In the upper chambers,
1×105 cells in 200 μl of serum-free medium was
added. Then, 500 μl of RPMI-1640 medium containing 20% fetal
calf serum was added to the lower chambers as a chemoattractant.
After incubation for 24 h at 37°C in a humid atmosphere of 5%
CO2 and 95% air, the cells that had invaded through the
membrane and had reached the lower surface of the membrane were
fixed with methanol and stained with hematoxylin. Photographs were
taken and stained cells were counted under a microscope in five
randomly chosen fields.
Migration assay
Cells were cultured in fibronectin-coated 6-well
tissue culture plates as confluent monolayer. The near confluent
cell monolayer was then carefully scratched using 20 μl
sterile pipette tips. Cell debris was removed by washing with PBS.
The dishes were incubated in fresh regular culture medium
containing hydroxyurea (2 mM) for additional 24 h and photographs
were taken under a light microscope.
Western blotting
Proteins were extracted from the cultured cells and
then quantitated. Equal amounts of protein (30 /Ag) from different
cells were separated by a 10% SDS-polyacrylamide gel, transferred
onto nitrocellulose paper (Millipore, Billerica, MA, USA) and then
incubated overnight at 4°C with a 1 μg/ml of antibody
against target proteins. Protein bands were detected by the
enhanced chemiluminescence (ECL) kit (Pierce, Rockford, IL, USA)
and exposure to Biomax ML film (Eastman Kodak, Rochester, NY, USA).
The primary antibodies against mitogen-activated protein kinase
(MEK), phosphorylated MEK (p-MEK), extracellular signal-regulated
kinase 1/2 (ERK1/2), p-ERK1/2, Akt, p-Akt (Cell Signaling
Technology, Danvers, MA, USA), MMP-2, MM-9, uPA, p65 NF-κB,
Caspase-3, lamin B and actin (Santa Cruz Biotechnology, Santa Cruz,
CA, USA) were used.
Electrophoretic mobility shift assay
Electrophoretic mobility shift assay (EMSA) was
performed using an EMSA Gel-Shift kit (Pierce). Nuclear extracts
were prepared and incubated with NF-κB-32P-labeled
oligonucleotide (5′-AGCTATGTG GGTTTTCCCATGAGC-3′) for 30 min at
room temperature. Samples were electrophoresed at 100 V and 4°C,
transferred to Biodyne nylon membranes (Pierce), and then
cross-linked in an ultraviolet cross-linker (Stratagene Inc., La
Jolla, CA, USA). Gels were visualized using streptavidin-HRP
followed by chemiluminescence detection.
Gel zymography
The medium was collected to concentrate using
Centricon centrifugal filters devices with YM-30 membranes
(Millipore) and the protein concentration was detect using Bio-Rad
assay. Protein (10 μg) was loaded onto zymographic sodium
dodecyl sulfate gel containing gelatin (1 mg/ml). The gels were
washed twice in 2.5% (v/v) Triton X-100 at room temperature before
incubation in 50 mM Tris-HCl (pH 7.6), 10 mM CaCl2, 50
mM NaCl and 0.05% Brij35 overnight at 37°C. The gels were stained
with 0.5% Coomassie brilliant blue R-250 for ≥1 h and destained
with methanol/acetic acid to reveal zones of gelatinase activity.
The gels were analyzed using the Typhoon™ 9400 Scanner (GE
Healthcare). Image quantification was performed using ImageQuant ™
TL software (GE Healthcare).
In vivo tumor growth assay
Nude mice were divided into three groups, six mice
in each group. To evaluate in vivo tumor growth,
1×107 OPN silencing cells, mock virus-transfected cells
or untransfected cells (MKN28) were injected subcu-taneously into
the nude mice. Tumor growth was monitored with tumor volume, which
was calculated as follows: V (mm3) = width2
(mm2) × length (mm)/2. The mice were sacrificed 6 weeks
later, and tumors were harvested. The tumor weight was
recorded.
In vivo metastasis assays
Nude mice were injected subcutane-ously with
1×107 OPN silencing cells, mock virus-transfected cells
or untransfected cells (MKN28). After 15 days, the tumors were
extirpated. Cancer tissue was divided into small pieces, ∼1 mm in
diameter, for stomach surgical orthotopic implantation. During
surgical implantation, a small area of the gastric serosa was
removed, and 1 mm3 tumor fragment per mouse was
implanted. Tumor pieces were penetrated and sutured to the gastric
wall by a nylon surgical suture. Two months later, all the mice
were sacrificed, individual organs were excised, and metastases
were checked by hematoxylin-eosin (H&E) staining. The incidence
and classification of metastasis were calculated and evaluated
independently by two pathologists.
Statistical analysis
For statistical evaluation of cell culture
experiments, mean values and standard deviation of at least
triplicate experiments were calculated. Student’s t-test was used
to compare the difference of mean values. Difference in the
incidence of liver and lung metastasis was analyzed by Fischer’s
exact test. P-values <0.05 were defined as statistically
significant.
Results
Expression levels of OPN in gastric
cancer cell lines
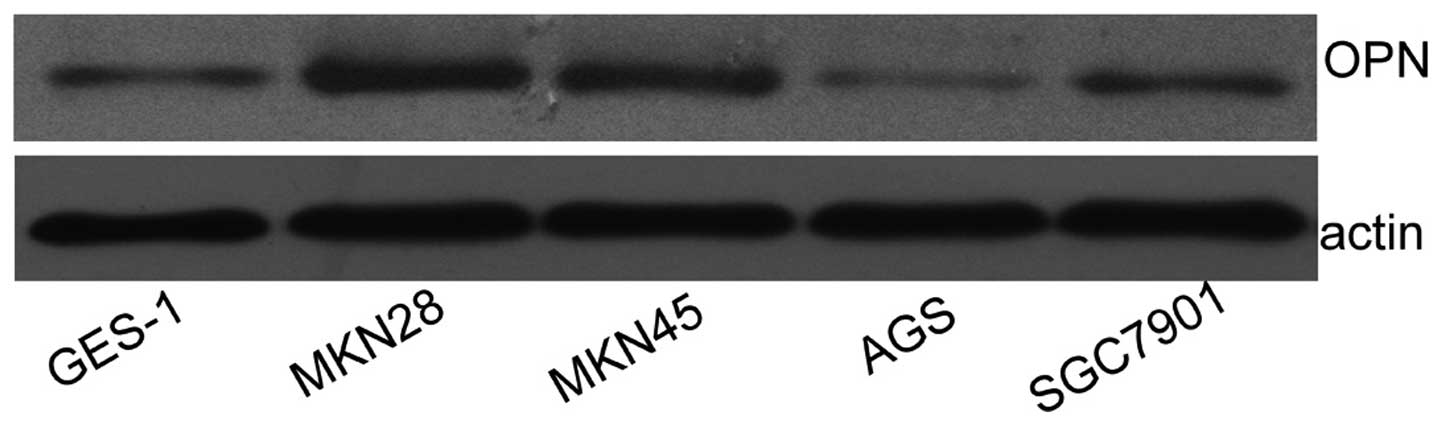
As shown in Fig. 1,
the expression levels of OPN in MKN45 and MKN28 cell lines were
much higher than that in SGC7901 and GES-1 cells, while the lowest
level of OPN among the 5 gastric cancer cells tested was in AGS
cells. Therefore, MKN45, MKN28 and AGS cells were selected and used
in the following experiments.
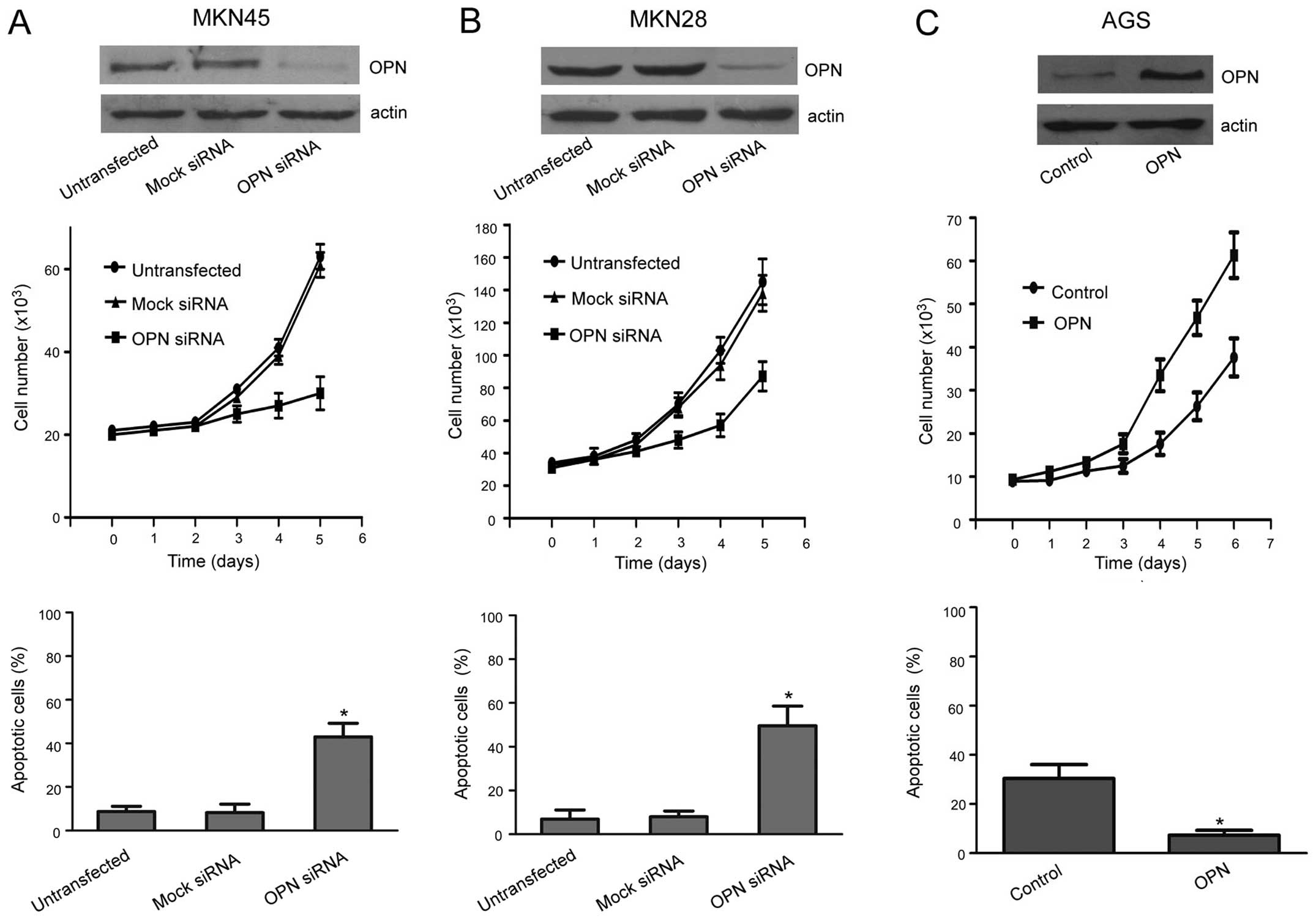
OPN status affects proliferation and
apoptosis of gastric cancer cells in vitro
OPN expression was inhibited in MKN45 and MKN28
cells which had higher expression levels of OPN, and OPN expression
was upregulated in AGS cells which slightly expressed OPN. First,
OPN expression was inhibited in MKN45 and MKN28 cells by
transfection with OPN-specific siRNA for 24 h. OPN silencing
significantly inhibited proliferation and promoted apoptosis in
both cell lines compared with the control (P<0.05, OPN silencing
relative to untransfected or mock control, Fig. 2A and B). Then, OPN expression was
upregulated in AGS cells by treatment with 10 μM exogenous
recombinant OPN for 24 h. OPN significantly promoted proliferation
and inhibited apoptosis in AGS cells (P<0.05, OPN upregulation
relative to control, Fig. 2C).
These data show that the OPN status strongly affects proliferation
and apoptosis in gastric cancer cells.
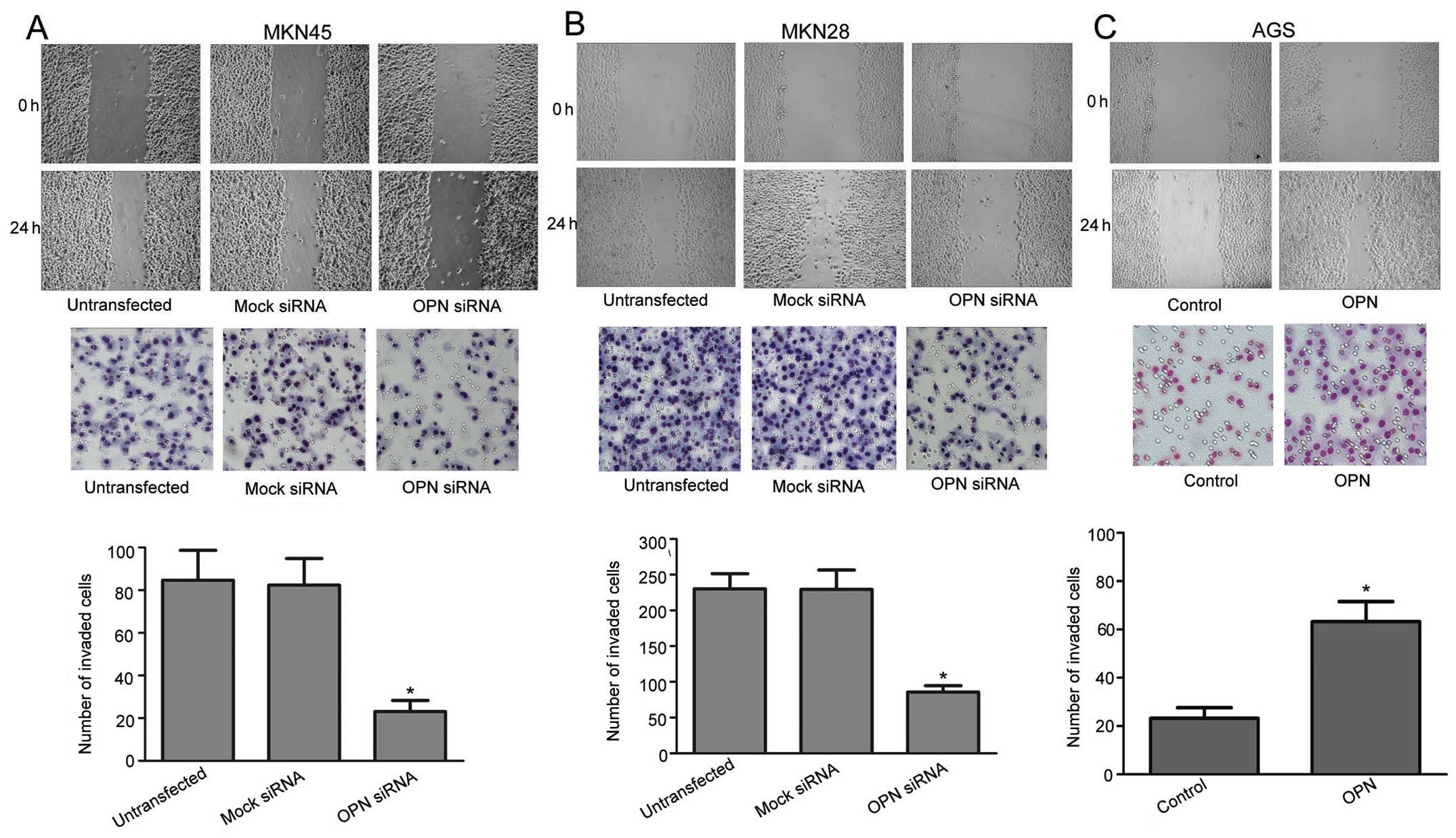
OPN status affects invasion and migration
of gastric cancer cells in vitro
Cell invasion and migration were analyzed as
described in Materials and methods. OPN silencing significantly
inhibited invasion and migration in MKN45 and MKN28 cells
(P<0.05, OPN silencing relative to untransfected or mock
control, Fig. 3A and B). OPN
expression significantly promoted invasion and migration in AGS
cells (P<0.05, OPN upregulation relative to control, Fig. 3C). Our findings suggest that the
OPN status significantly affects invasion and migration in gastric
cancer cells.
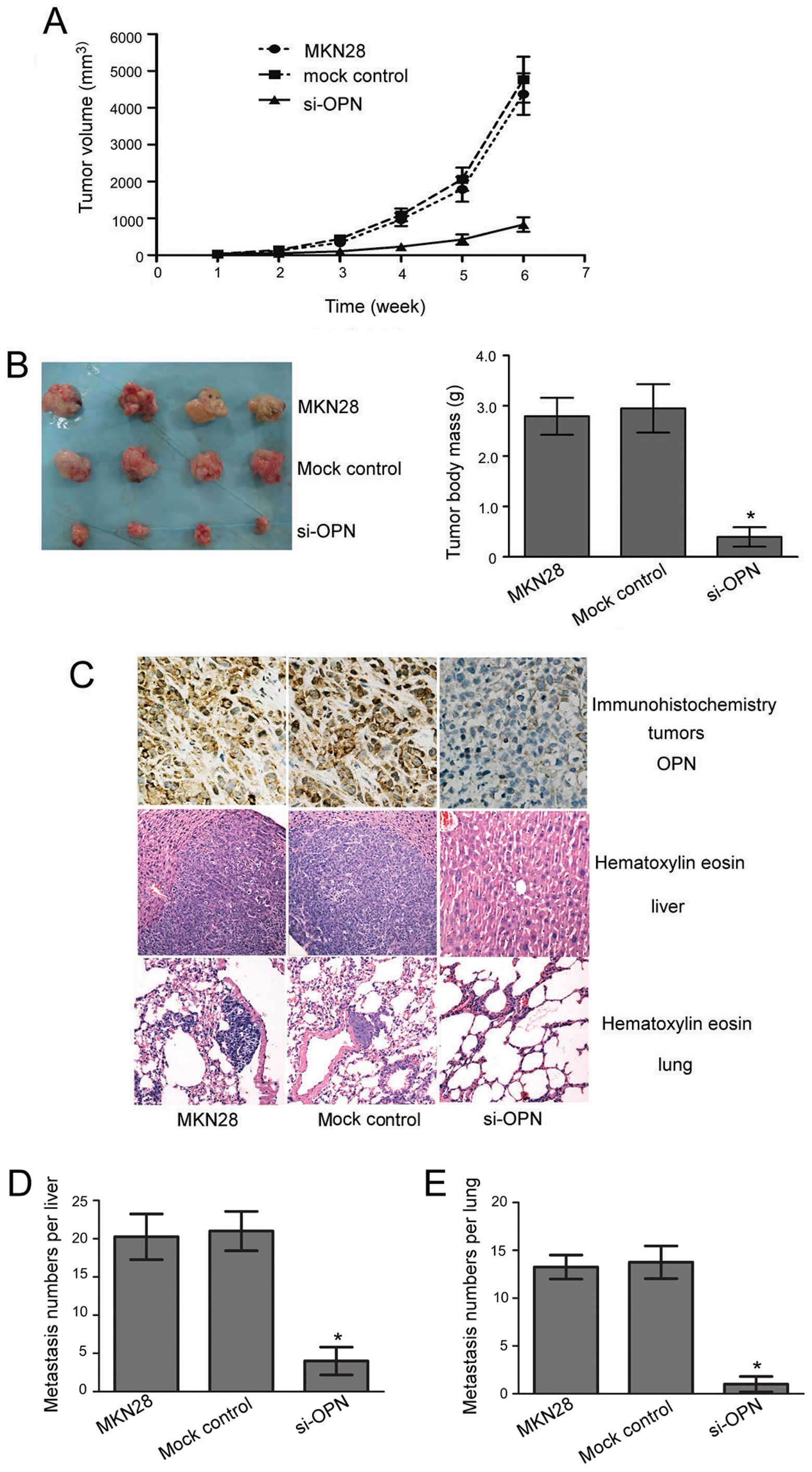
OPN siRNA inhibits gastric cancer
xenograft growth in vivo
MKN28 (parental control), control
lentivirus-infected cells (mock control) and OPN-specific siRNA
lentivirus-infected cells (si-OPN) were subcutaneously implanted
into nude mice, respectively. Tumor growth curves were plotted in
the course of the experiment (Fig.
4A), and the weight of tumor was evaluated at 6 weeks
post-inoculation (Fig. 4B).
Obvious tumor masses could be found in the two control groups of
mice inoculated with MKN28 cells (2.76±0.43 g) or mock control
cells (2.97±0.52 g), while much smaller tumors were detected in the
si-OPN groups (0.45±0.22 g) (P<0.05, si-OPN groups relative to
mock control or MKN28 groups). These data suggest that the
treatment of OPN siRNA blocked the growth of tumor compared to
control groups, the parental or the mock-transfected groups. In
addition, compared with the control groups, no significant body
weight change or other toxicity was observed in the treatment
groups (data not shown).
OPN siRNA inhibits gastric cancer
xenograft metastasis in vivo
The effect of OPN silencing on tumor metastasis was
further assayed in athymic mice. Autopsy was performed, and the
incidence of metastasis in the livers, lungs and other organs was
determined by macroscopic and histologic examinations. The
incidence of hepatic metastasis in mice of the parental and the
mock-transfected groups was 60% (6 of 10) and 70% (7 of 10),
respectively. In the si-OPN groups, only 30% (3 of 10) of animals
had hepatic metastasis (Fig. 4C).
Pulmonary metastases were found in 30% (3 of 10) of the parental
groups, 40% (4 of 10) of the mock-transfected groups and 10% (1 of
10) of the si-OPN groups (Fig.
4C). As shown in Fig. 4D, the
numbers of hepatic metastatic lesions in the si-OPN groups
(4.2±3.9) were greatly decreased compared with those in the
mock-transfected (20±3.4) and the parental groups (21.5±3.1). The
most lung metastases were observed in the mock-transfected
(13.8±2.1) and the parental mice (14.5±2.6). Only a few lung
metastases were observed in mice of si-OPN groups (2.5±1.6)
(Fig. 4E). Moreover, their
metastatic lesions were much smaller than those in the parental or
mock control. The difference between the si-OPN groups with the
parental or the mock transfected groups was significant
(P<0.05).
OPN mediates the MAPK and PI3K signaling
pathways in gastric cancer cells
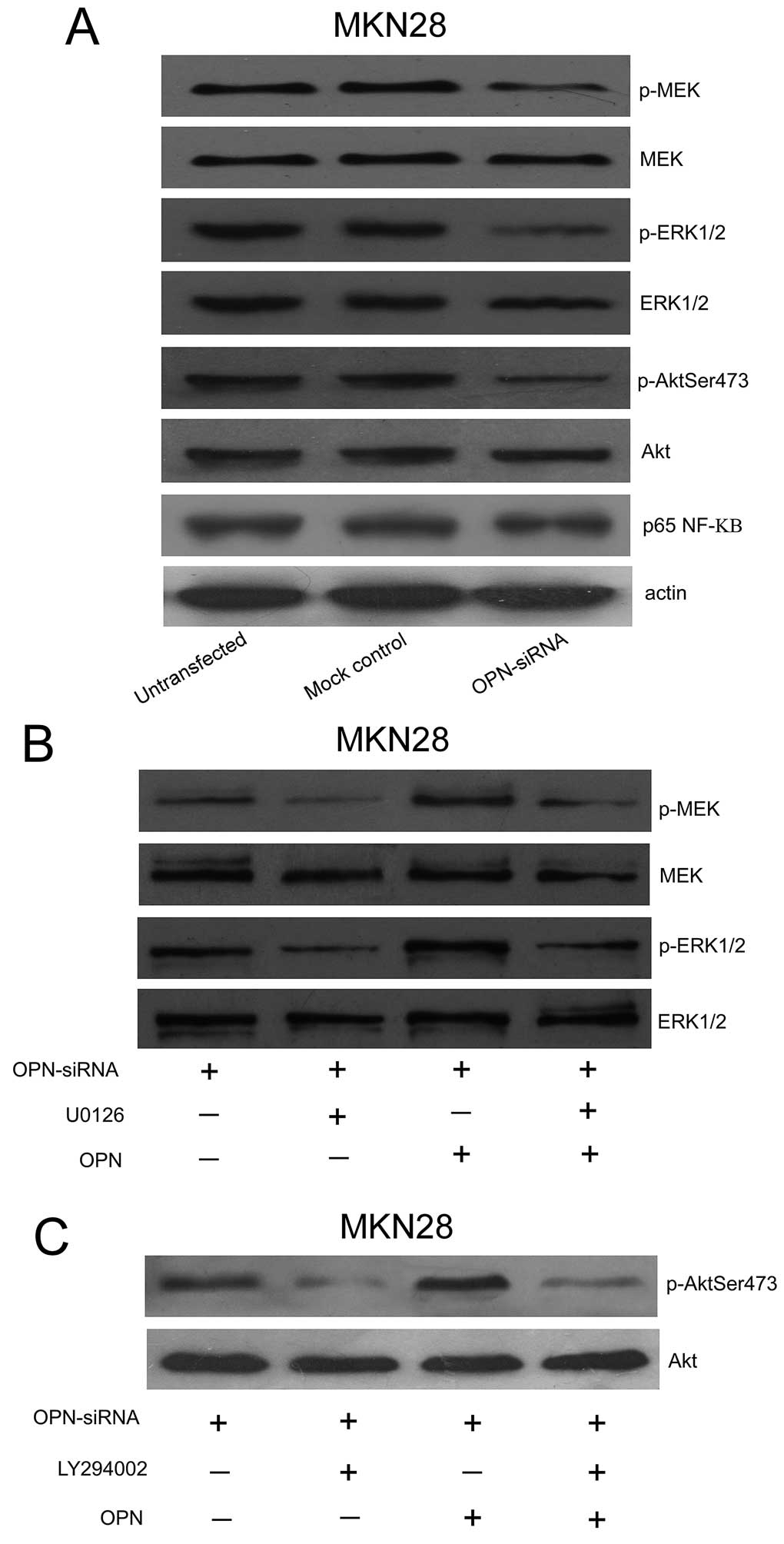
To explore the possible mechanisms of OPN in
progression of gastric cancer, the expression levels of NF-κB and
activation of Akt and MEK/ERK1/2 were investigated in gastric
cancer cells transfected with siRNA against OPN. Compared with
untransfected or mock control, OPN siRNA was demonstrated to induce
a significant decrease in the phosphorylation levels of Akt
(Ser473), MEK and ERK1/2; however, no significant alteration in p65
NF-κB was observed in the cells transfected with OPN-specific siRNA
(Fig. 5A).
To further evaluate the association of OPN with
activation of MEK and ERK1/2, MKN28 cells were transfected with OPN
siRNA, followed by incubation with OPN. Some of them were
pretreated with specific MEK inhibitor, 4-diamino-2, 3-dicyano-1,
4-bis[2-aminophenylthio] butadiene (U0126) (20 μM), and
those treated with OPN alone without pretreatment with U0126 were
used as control. OPN was demonstrated to significantly increase the
phosphorylation of MEK and ERK1/2, and U0126 could inhibit the
activation of MEK and ERK1/2 induced by OPN (Fig. 5B).
In Fig. 5C, the
si-OPN cells were treated with 10 μM exogenous recombinant
OPN. Some of them were pretreated with specific Akt inhibitor
2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) (25
μM), and those treated with OPN alone without pretreatment
with LY294002 were used as control. It was found that Akt could be
activated in OPN treated cells, and be inhibited in the cells
pretreated with LY294002.
The MAPK and PI3K signaling pathways are
involved in OPN-induced NF-κB activity in gastric cancer cells
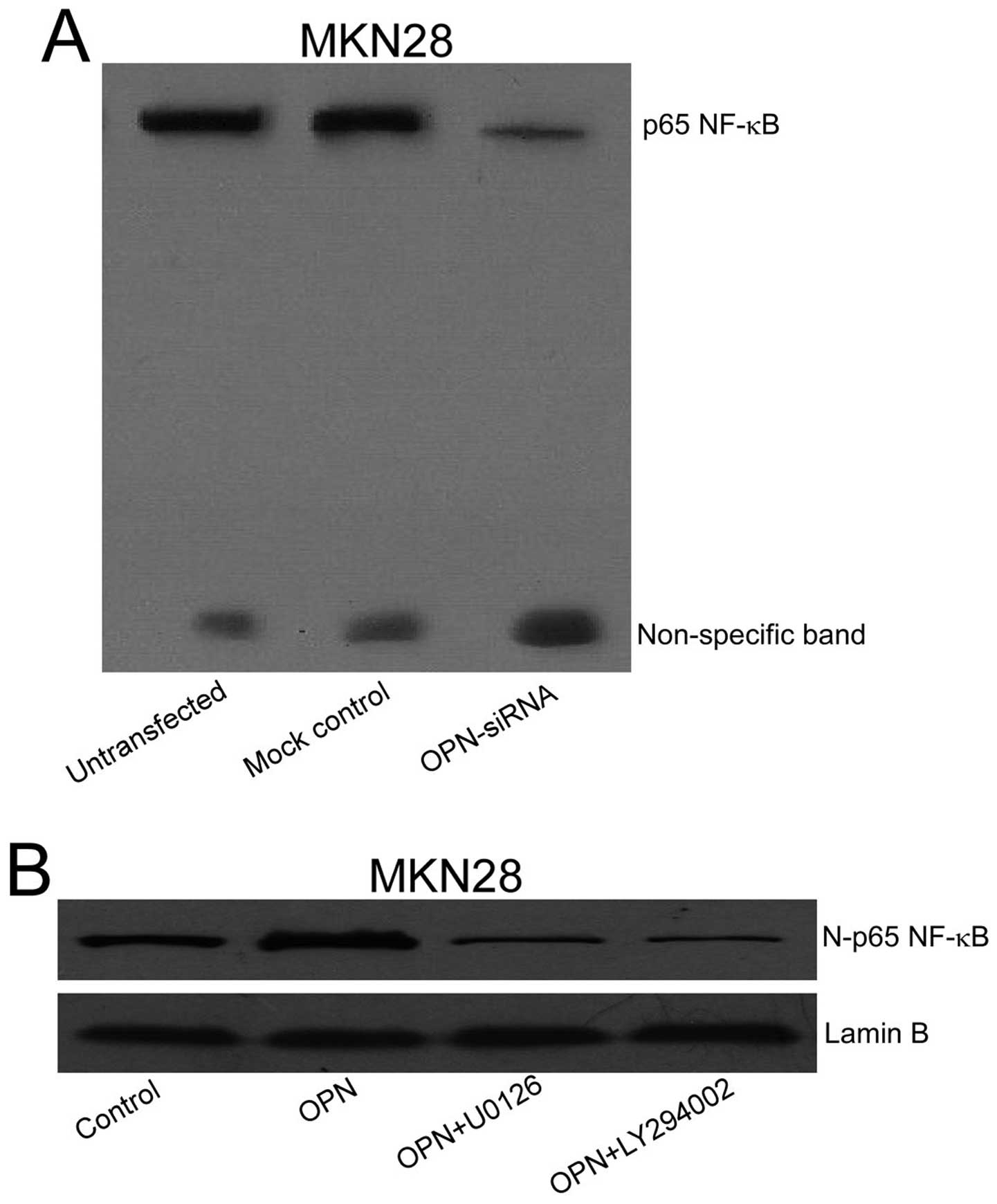
To investigate the effect of OPN siRNA on p65
subunit of NF-κB, EMSA of NF-κB was performed. OPN siRNA was
demonstrated to inhibit a translocation of p65 into nuclei in MKN28
cells. No significant alteration in NF-κB /DNA binding activity was
found in the cells transfected with mock control (Fig. 6A).
To further determine how OPN affects this
translocation, MKN28 cells were incubated with recombinant OPN for
90 min, some of the cells were preincubated with U0126 or LY294002
for 30 min. Cell lysates of nucleus were extracted and
immunoblotted with antibodies specific to p65. Western blot
analysis further confirmed the association of translocation of p65
with the OPN effects, and the effects of OPN on the p65
translocation in MKN28 cells were obviously inhibited by the MEK
and Akt inhibitor, respectively (Fig.
6B). Taken together, we suggest these results show that MEK and
Akt are partially mediated, OPN-induced the translocation of p65
from cytoplasm to the nuclei in MKN28 cells.
NF-κB signaling pathway mediates
OPN-induced MMP-2, MMP-9 and uPA activity, and caspase-3 inhibition
in gastric cancer cells
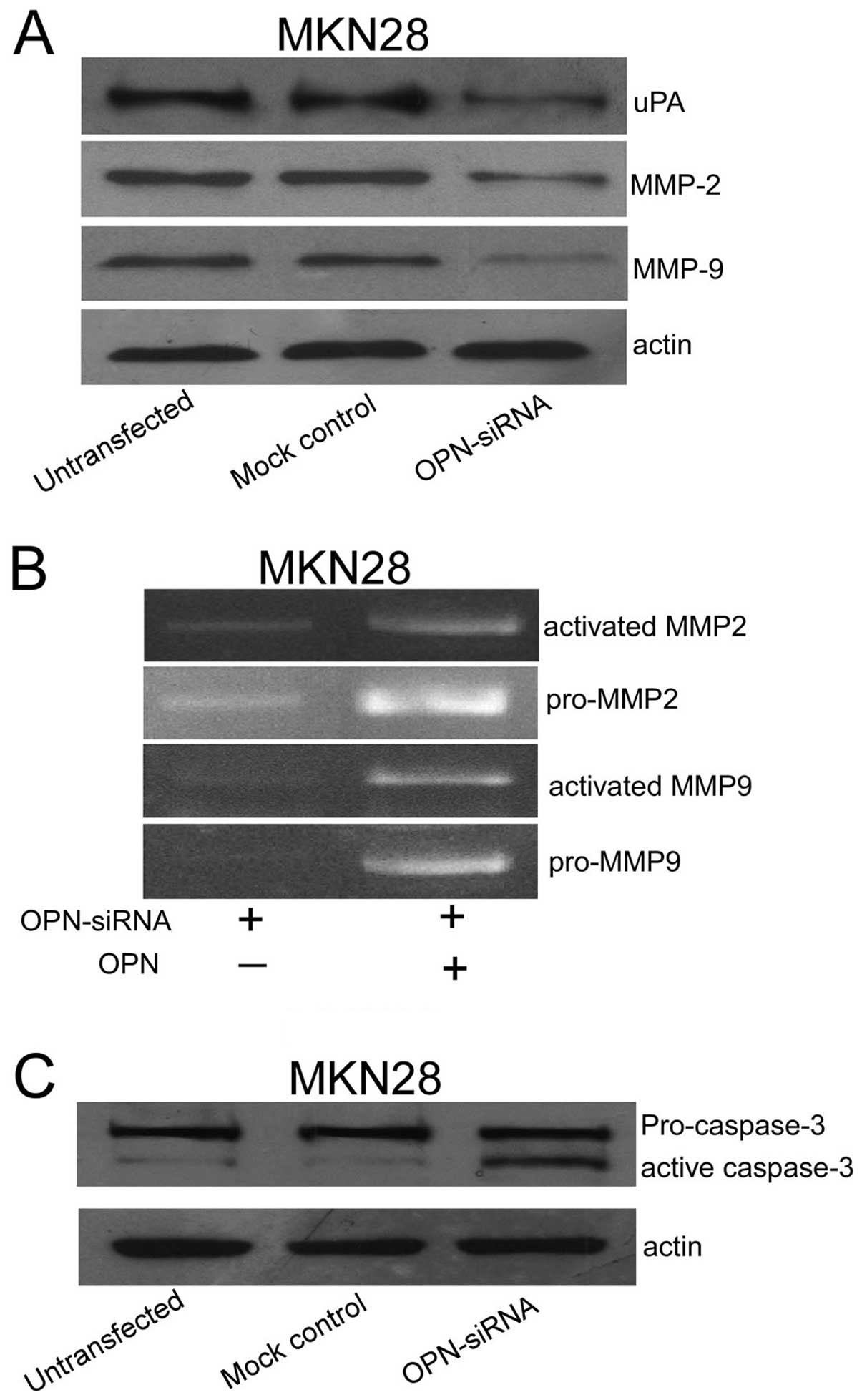
Western blotting was performed to investigate the
expression levels of MMP-2, MMP-9 and uPA. The expression levels of
MMP-2, MMP-9 and uPA were decreased in MKN28 cells treated with OPN
siRNA (Fig. 7A). Then, gelatin
zymography was used to investigate the activity of MMP-2 and MMP-9.
OPN siRNA-transfected cells were treated with exogenous recombinant
OPN (10 μM), cells without OPN treatment were used as
control. The levels of both the pro-and active forms of MMP-2 and
MMP-9 in OPN-treated cells were significantly enhanced compared
with those control cells without OPN treatment (Fig. 7B). OPN siRNA was also demonstrated
to induce a significant increase in the cleavage of caspase-3.
However, it did not induce a specific alteration in the cleavage
levels of caspase-3 in the cells transfected with mock control
(Fig. 7C).
Pyrrolidine dithiocarbamate (PDTC), an NF-κB
inhibitor, was used to explore the possible mechanisms of OPN in
regulating MMP-2, MMP-9, uPA and caspase-3. MKN28 cells were
preincubated with PDTC for 30 min followed by incubation with OPN
for 90 min. A translocation of p65 into the nuclei and a
significant increase in MMP-2, MMP-9 and uPA level were observed in
the cells incubated with OPN, even though a significantly decreased
expression of MMP-2, MMP-9 and uPA and an inhibited translocation
of p65 were detected in cells treated with PDTC. Caspase-3 was
found to be inhibited in OPN treated cells, and was activated in
the cells pretreated with PDTC (Fig.
8). Together, these results suggest that OPN promotes MMP-2,
MMP-9 and uPA expression and caspase-3 inhibition by activation of
the NF-κB signaling.
Discussion
Abundant clinical evidence and experimental studies
indicate that OPN signaling is involved in gastric cancer
progression (4,5,11–13).
In the present study, the function role of OPN signaling in gastric
cancer progression was investigated using multiple experiments,
both in vitro and in vivo. We showed that OPN
functions as an inducer of cell proliferation, invasion and
migration and an inhibitor of apoptosis, resulting tumor growth and
metastasis of gastric cancer. These data suggest that the effects
of OPN on malignancy likely occur at multiple levels.
OPN can utilize a wide variety of cell-surface
receptors including several integrin pairs and CD44, and can result
in activation of several downstream signaling cascades (14). A previous study showed that OPN
activates several downstream signaling cascades including the MAPK,
PI3K and NF-κB pathways in a cell-specific manner (3). Furthermore, NF-κB is also best
understood in the context of a major cascade stimulating cell
proliferation, invasion and migration, and inhibiting apoptosis in
various human cancers. In our study, OPN activated both the MAPK
and PI3K pathways as evidenced by phosphorylation of ERK and Akt,
respectively following OPN inhibition treatment. Furthermore,
according to our finding, OPN-mediated activation of ERK and Akt is
important for the enhancement of NF-κB/DNA binding activity.
Previous studies also demonstrated that NF-κB-inducing kinase
(NIK), Ras and Src are involved in the regulation of OPN-mediated
NF-κB activity (15–17). Although the role of MAPK and PI3K
pathways in regulating the OPN signaling varies between cell types,
OPN-induced nuclear translocation of NF-k B through MAPK and PI3K
pathways might be critical for OPN-mediated progression in gastric
cancer. However, the precise mechanisms controlling OPN-induced
NF-κB activity in gastric cancer need to be elucidated in detail.
In addition, the accurate mechanism of MAPK and PI3K signaling to
OPN-induced progression in gastric cancer is also to be
defined.
OPN can regulate the process of tumorigenesis and
metastasis by the degradation of the basement membrane and
extracellular matrix (ECM), which is critically depended on the
proteolytic activity generated by MMPs and the plasminogen
activator (PA)-plasmin system. The impact of OPN regulating MMPs
and the PA-plasmin system is cell type-dependent and through a
variety of overlapping intracellular signaling pathway. Previous
research has shown that OPN-induction of uPA and MMP-2 secretion is
NF-κB/IκBα/IKK mediated and depends on PI3K activity (18). In other systems, OPN stimulates
NIK-dependent NF-κB-mediated uPA secretion and MMP-9 activation
through both IKKα/β and MAPK-mediated pathways (16,19,20).
OPN can also upregulate uPA and MMP-2 activity through
integrin-linked kinase (ILK) and AP-1 signaling during tumor cell
invasion (21). Here, our findings
indicated that OPN, via a mechanism involving the NF-κB pathway,
promotes the expression and/or activation of MMP-2, MMP-9 and uPA,
which is in good agreement with earlier findings. Although NF-κB
activity is important for OPN-induced metastatic function,
activation may not be sufficient to promote gastric cancer
metastasis. Therefore, AP-1 might also play an important role in
the regulation of OPN-mediated metastatic potential of gastric
cancer cells as its activation leads to the induction of c-Fos,
which associates with c-Jun to form an AP-1 heterodimeric complex
(22).
Prevention of apoptosis is an essential trait during
tumor progression. OPN can impact cellular apoptosis through
intracellular signaling network. OPN has been shown to prevent
apoptosis through activation of the PI3K and NF-κB pathway
(23). Recently, more research has
demonstrated that the downstream target of the MAPK pathway, ERK
has cytoplasmic targets including the p90 ribosomal S6 kinase (RSK)
that target the proapoptotic protein Bad, leading to its
inactivation and reduced apoptosis (24). In our study, OPN-mediated
activation of NF-κB pathway was also important for inhibition of
caspase-3 cleavage in gastric cancer cells. Future studies will
need to focus on delineating the precise role of the MAPK, PI3K and
NF-κB pathways on OPN-mediated apoptosis inhibition.
In our study, the interesting findings were that OPN
was also expressed in normal gastric epithelial GES-1 cells, and
OPN expression was not absolutely correlated with the metastasis
potential of four gastric cancer cell lines (data not shown).
Although OPN is known to be vulnerable to metastasis potential of
gastric cancer cells in this study, the accurate role of OPN in
gastric cancer cells is largely unknown. Previous studies have
shown the functional diversity of OPN. One of the mechanisms
underlying the functional diversity of OPN is the existence of
splice variants (a, b and c), which exert different clinical and
biological features by different signal pathways (25–27).
A recent study showed that OPN-a was the major one in normal
gastric GES-1 cells, and OPN-b was the dominant isoform in gastric
cancer cell lines. OPN-c was also upregulated in most of gastric
cancer cell lines but barely detected in GES-1. OPN-b most strongly
promoted gastric cancer cell survival, while OPN-c most effectively
stimulated gastric cancer cell metastatic activity (28). These data may partly explain our
unexpected findings. Further investigations on the alternative
splicing and post-translational modification such as
phosphorylation, glycosylation, sulfation, enzymatic cleavage and
protein crosslinking, need to perform to help elucidate the exact
malignant potential of OPN in gastric cancer.
Here, we provided potentially beneficial insight
into the role of OPN in regulating various pathophysiological
processes and the molecular events of tumor progression, and we
believe that OPN might be useful in developing novel molecular
diagnostics and targeted therapy for the treatment of gastric
cancers.
Acknowledgements
The authors thank Dr Honggang Yu
(Department of Gastroenterology, Renmin Hospital of Wuhan
University, Wuhan, China) for his valuable discussion and
suggestions.
References
|
1.
|
Prince CW, Oosawa T, Butler WT, et al:
Isolation, characterization, and biosynthesis of a phosphorylated
glycoprotein from rat bone. J Biol Chem. 262:2900–2907.
1987.PubMed/NCBI
|
|
2.
|
Ramaiah SK and Rittling S:
Pathophysiological role of osteo-pontin in hepatic inflammation,
toxicity, and cancer. Toxicol Sci. 103:4–13. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Rangaswami H, Bulbule A and Kundu GC:
Osteopontin: role in cell signaling and cancer progression. Trends
Cell Biol. 16:79–87. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Higashiyama M, Ito T, Tanaka E and Shimada
Y: Prognostic significance of osteopontin expression in human
gastric carcinoma. Ann Surg Oncol. 14:3419–3427. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Imano M, Satou T, Itoh T, et al:
Immunohistochemical expression of osteopontin in gastric cancer. J
Gastrointest Surg. 13:1577–1582. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Junnila S, Kokkola A, Mizuguchi T, et al:
Gene expression analysis identifies over-expression of CXCL1,
SPARC, SPP1, and SULF1 in gastric cancer. Genes Chromosomes Cancer.
49:28–39. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Wu CY, Wu MS, Chiang EP, et al: Elevated
plasma osteopontin associated with gastric cancer development,
invasion and survival. Gut. 56:782–789. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Tang H, Wang J, Bai F, et al: Inhibition
of osteopontin would suppress angiogenesis in gastric cancer.
Biochem Cell Biol. 85:103–110. 2007.PubMed/NCBI
|
|
9.
|
Gong M, Lu Z, Fang G, Bi J and Xue X: A
small interfering RNA targeting osteopontin as gastric cancer
therapeutics. Cancer Lett. 272:148–159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Wang ZM, Cui YH, Li W, Chen SY and Liu TS:
Lentiviral-mediated siRNA targeted against osteopontin suppresses
the growth and metastasis of gastric cancer cells. Oncol Rep.
25:997–1003. 2011.PubMed/NCBI
|
|
11.
|
Ue T, Yokozaki H, Kitadai Y, et al:
Co-expression of osteopontin and CD44v9 in gastric cancer. Int J
Cancer. 79:127–132. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Dai N, Bao Q, Lu A and Li J: Protein
expression of osteopontin in tumor tissues is an independent
prognostic indicator in gastric cancer. Oncology. 72:89–96. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Zhang X, Tsukamoto T, Mizoshita T, et al:
Expression of osteopontin and CDX2: indications of phenotypes and
prognosis in advanced gastric cancer. Oncol Rep. 21:609–613.
2009.PubMed/NCBI
|
|
14.
|
Bellahcene A, Castronovo V, Ogbureke KU,
Fisher LW and Fedarko NS: Small integrin-binding ligand N-linked
glycoproteins (SIBLINGs): multifunctional proteins in cancer. Nat
Rev Cancer. 8:212–226. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Scatena M, Almeida M, Chaisson ML, Fausto
N, Nicosia RF and Giachelli CM: NF-kappaB mediates alphavbeta3
integrin-induced endothelial cell survival. J Cell Biol.
141:1083–1093. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Rangaswami H, Bulbule A and Kundu GC:
Nuclear factor-inducing kinase plays a crucial role in
osteopontin-induced MAPK/IkappaBalpha kinase-dependent nuclear
factor kappaB-mediated promatrix metalloproteinase-9 activation. J
Biol Chem. 279:38921–38935. 2004. View Article : Google Scholar
|
|
17.
|
Rittling SR and Chambers AF: Role of
osteopontin in tumour progression. Br J Cancer. 90:1877–1881. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Philip S and Kundu GC: Osteopontin induces
nuclear factor kappa B-mediated promatrix metalloproteinase-2
activation through I kappa B alpha/IKK signaling pathways, and
curcumin (diferulolylmethane) down-regulates these pathways. J Biol
Chem. 278:14487–14497. 2003. View Article : Google Scholar
|
|
19.
|
Das R, Mahabeleshwar GH and Kundu GC:
Osteopontin stimulates cell motility and nuclear factor
kappaB-mediated secretion of urokinase type plasminogen activator
through phosphati-dylinositol 3-kinase/Akt signaling pathways in
breast cancer cells. J Biol Chem. 278:28593–28606. 2003. View Article : Google Scholar
|
|
20.
|
Jain S, Chakraborty G and Kundu GC: The
crucial role of cyclooxygenase-2 in osteopontin-induced protein
kinase C alpha/c-Src/IkappaB kinase alpha/beta-dependent prostate
tumor progression and angiogenesis. Cancer Res. 66:6638–6648. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Mi Z, Guo H, Wai PY, Gao C and Kuo PC:
Integrin-linked kinase regulates osteopontin-dependent MMP-2 and
uPA expression to convey metastatic function in murine mammary
epithelial cancer cells. Carcinogenesis. 27:1134–1145. 2006.
View Article : Google Scholar
|
|
22.
|
Shaulian E and Karin M: AP-1 as a
regulator of cell life and death. Nat Cell Biol. 4:E131–E136. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Lin YH and Yang-Yen HF: The
osteopontin-CD44 survival signal involves activation of the
phosphatidylinositol 3-kinase/Akt signaling pathway. J Biol Chem.
276:46024–46030. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Lee KW, Kim SG, Kim HP, et al:
Enzastaurin, a protein kinase C beta inhibitor, suppresses
signaling through the ribosomal S6 kinase and bad pathways and
induces apoptosis in human gastric cancer cells. Cancer Res.
68:1916–1926. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Chae S, Jun HO, Lee EG, et al: Osteopontin
splice variants differentially modulate the migratory activity of
hepatocellular carcinoma cell lines. Int J Oncol. 35:1409–1416.
2009.PubMed/NCBI
|
|
26.
|
He B, Mirza M and Weber GF: An osteopontin
splice variant induces anchorage independence in human breast
cancer cells. Oncogene. 25:2192–2202. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Zhao B, Sun T, Meng F, et al: Osteopontin
as a potential biomarker of proliferation and invasiveness for lung
cancer. J Cancer Res Clin Oncol. 137:1061–1070. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Tang X, Li J, Yu B, et al: Osteopontin
splice variants differentially exert clinicopathological features
and biological functions in gastric cancer. Int J Biol Sci.
9:55–66. 2013. View Article : Google Scholar : PubMed/NCBI
|