Introduction
Cancer stem cells (CSCs) have been identified in
numerous human cancers including both hematological malignancies
and solid tumors. These pluripotent cells with unlimited
proliferative potential, which are characterized by the ability to
self-renew and differentiate, constitute a subset of cells within a
tumor capable to initiate and propagate tumor growth (1). Therefore, to cure patients with
malignant diseases, CSCs need to be eradicated. Unfortunately, CSCs
are resistant to chemotherapy, radiotherapy, and targeted molecular
therapy due to slow proliferation and production of membrane
transporter proteins and detoxifying enzymes. As CSCs can express
high level of MHC class I molecules and tumor antigens (2), immunization against CSC markers might
be an important method of CSC elimination. To identify CSCs, cell
surface markers are usually utilized, but unique CSC-specific
markers are still missing.
Gene expression profiling of human stem cells has
identified different expression programs for adult and embryonic
stem cells (ESC) (3,4). It has also shown a shared
transcriptional program of ESCs and CSCs (3). Moreover, ESC-like expression has been
found in aggressive human tumors (5). These observations suggest that the
regulatory network controlling the function of ESCs may also be
active in CSCs. The key regulators of ESC identity including
self-renewal are the transcription factors OCT4, SOX2, and NANOG
that co-operate in the regulation of transcription of large sets of
genes (6). Production of these
transcription factors has also been shown in various human tumors.
For instance, SOX2 overexpression was proved in small cell lung
cancer (7), monoclonal gammopathy,
multiple myeloma (8), glioblastoma
(2,9), Merkel cell carcinoma, melanoma
(10), gastric carcinoma (11), and breast carcinoma (12). Amplification of the SOX2 gene was
found in a proportion of squamous cell carcinomas (SCC) of various
organ locations including the esophagus, lung (1315), oral cavity
(16), uterine cervix, skin and
penis (17). In SCC of the lung,
SOX2 was identified as a lineage survival oncogene (13,15)
and its overexpression was associated with favorable prognosis
(18) while in most other cancers,
high SOX2 expression was predictive of poor prognosis.
SOX2 is a high mobility group (HMG) domain protein
that contains two nuclear localization signals (NLS), a bipartite
NLS motif and a basic cluster NLS motif, that are conserved in the
HMG domain of transcription factors (19). This protein is highly immunogenic,
both humoral and cell-mediated immune reactions were demonstrated
in patients with premalignant and malignant diseases (7,8).
Moreover, the activation of SOX2-specific CD8+ T
lymphocytes was shown after immunization with dendritic cells
primed with apoptotic CSCs (2).
SOX2 plays a pivotal role in the maintenance of stemness and
tumorigenicity of CSCs and is also involved in cell proliferation,
growth, migration, and chemoresistance (reviewed in ref. 20). Consequently, the SOX2 protein may
be an appropriate target for tumor therapy (9,21).
In this study, we modified the Sox2 gene and
constructed DNA vaccines against the Sox2 protein, determined the
immunogenicity of these vaccines, and utilized the depletion of
regulatory T lymphocytes (Treg) for the enhancement of vaccine
efficiency. We also characterized Sox2 expression in the mouse
oncogenic TC-1 cell line and its clones and demonstrated the
antitumor effect against TC-1/B7 cells that was not augmented by
Treg depletion.
Materials and methods
Plasmids
The wild-type (wt) Sox2 gene was isolated from the
F9 mouse testicular teratoma cell line (obtained from CLC,
Eppelheim, Germany). Total RNA was extracted from these cells by
the RNeasy kit (Qiagen, Hilden, Germany) and after the treatment
with DNase (Promega, Madison, WI, USA), a reverse transcription was
performed with the M-MLV reverse transcriptase (Promega). The Sox2
cDNA was amplified by PCR using the Phusion High-Fidelity DNA
Polymerase (Finnzymes, Espoo, Finland) and the Sox2-derived primers
5′-GTCTCGA GCCGCCATGTATAACATGATGGAGA-3′ (forward) and
5′-GTCTCGAGTCACATGTGCGACAGGGGCA-3′ (reverse) with the Kozak
sequence and XhoI restriction sites added. Subsequently, the
PCR product was cloned into the XhoI site of the pBSC
plasmid (22). The Sox2opt gene
with codons optimized for expression in human cells and the Kozak
sequence added was designed and synthesized by GeneArt (Regensburg,
Germany) and cloned into the EcoRI site of the pBSC plasmid.
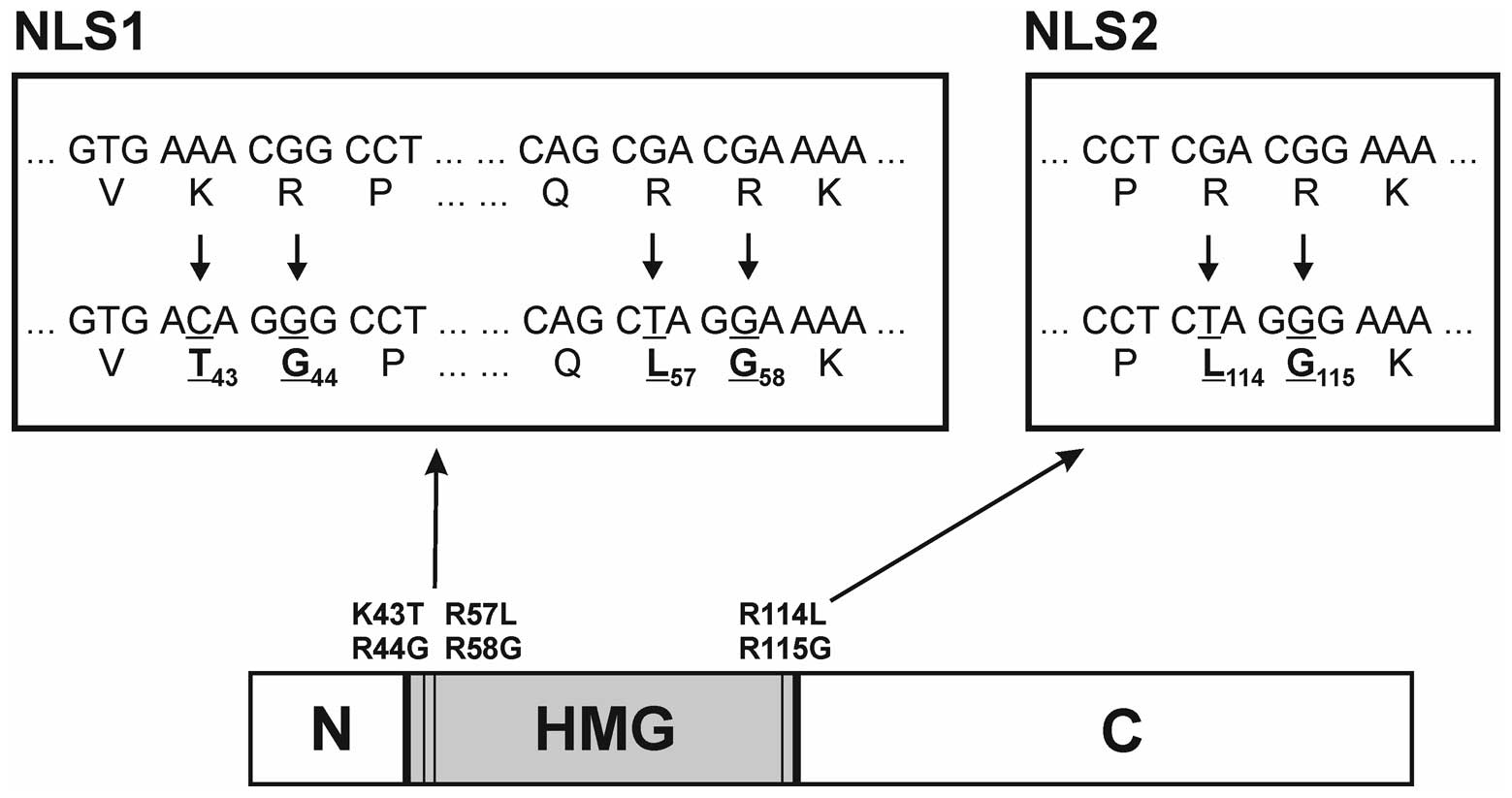
Then, the Sox2opt gene was mutated with the GeneEditor in
vitro Site-Directed Mutagenesis system (Promega). To abolish
the two NLSs present in Sox2 (19), altogether six nucleotides were
changed in one reaction using three mutagenic oligonucleotides
(Fig. 1). This mutagenesis
resulted in six substitutions in the amino acid sequence. The
substitutions K43T together with R44G and R57L together with R58G
in the first NLS (NLS1) were created using the oligonucleotides
5′-ATCGGGTGACAGGGCCTATG-3′ and
5′-AGGCCAGCTAGGAAAAATGG-3′ (the mutated
nucleotides are underlined), respectively, and the substitutions
R114L and R115G in the second NLS (NLS2) were introduced using the
5′-ACCGACCTCTAGGGAAAACC-3′ mutagenic
oligo-nucleotide. The resultant gene Sox2opt-cyt was amplified with
primers 5′-TCGTCTCGAGTACAATATGATGGAAACCG-3′ (forward) and
5′-TCGTCTCGAGTTAGCTAGCCATGTGAG ACAGAGGCAG-3′ (reverse) and cloned
into the XhoI site of the pBSC/Kozak+ATG plasmid that
contains the Kozak sequence, NsiI site, XhoI site,
and STOP codon inserted in the EcoRI site of pBSC. Into the
NsiI site, the Pan DR epitope (PADRE) (23) was cloned using annealed
oligonucleotides 5′-TGCCAAGTTCGT GGCTGCCTGGACCCTGAAGGCTG
CCGCTATGCA-3′ and 5′-TAGCGGCAGCCTTCAGGGTCC
AGGCAGCCACGAACTTGGCATGCA-3′, thus generating the PADRE.Sox2opt-cyt
gene.
The sequence of all constructed genes was verified
by DNA sequencing. Plasmids were propagated in E. coli
XL1-blue strain cultured in Luria Broth medium with 100
μg/ml of ampicillin added and purified with the Qiagen
Plasmid Maxi kit (Qiagen).
Peptides
The PADRE peptide (AKFVAAWTLKAAA; >90% pure) was
custom synthesized by GenScript (Piscataway, NJ, USA). The PepMix
Human SOX2 (JPT Peptide Technologies, Berlin, Germany) is a mixture
of 77 peptides covering the SOX2 protein (>70% pure; 15-mers
with an overlap of 11 amino acids).
Cell lines
NIH/3T3 fibroblasts established from mouse embryo
culture (24) were obtained from
the German National Resource Centre for Biological Material. TC-1
cells (provided by T.-C. Wu, Johns Hopkins University, Baltimore,
MD, USA) were prepared by transformation of primary C57BL/6 mouse
lung cells with the activated H-ras and human papillomavirus type
16 (HPV16) E6/E7 oncogenes (25).
TC-1/B7 cell line was derived from a TC-1-induced tumor formed in a
mouse immunized against the E7 oncoprotein (26). All cells were grown in Dulbecco’s
modified Eagle’s medium (DMEM; PAA Laboratories, Linz, Austria)
supplemented with 10% fetal calf serum, 2 mM L-glutamine, 100 U/ml
penicillin, and 100 μg/ml streptomycin (PAA
Laboratories).
Mice
Seven- to eight-week-old female C57BL/6 mice
(Charles River, Sulzfeld, Germany) were used in immunization
experiments. Animals were maintained under standard conditions and
in accordance with the guidelines for the proper treatment of
laboratory animals at the Center for Experimental Biomodels,
Charles University, Prague. Animal protocols were approved by the
First Faculty of Medicine Animal Use Committee.
Immunoblot staining
To verify the expression of the prepared genes,
NIH/3T3 cells seeded in 4-cm dishes were transfected with 2.5
μg of plasmid DNA using the Lipofectamine 2000 Transfection
Reagent (Invitrogen, Carlsbad, CA, USA). Cotransfection with 0.5
μg of the β-galactosidase encoding plasmid that served as an
internal control of the transfection efficacy was conducted. After
two days, the cells were collected and lysed in a modified Laemmli
lysis buffer (4% sodium dodecyl sulfate, 20% glycerol, 10%
mercaptoethanol, 2 mM EDTA, 100 mM Tris, pH 8.0) (27). Proteins from cell lysates were
separated by 10% sodium dodecyl sulfate/polyacryl-amide gel
electrophoresis (SDS-PAGE) and electroblotted onto a polyvinylidene
difluoride (PVDF) membrane (GE Healthcare, Little Chalfont, UK).
The membrane was blocked with 10% non-fat milk in PBS and incubated
with Sox2 (clone L1D6A2; Cell Signaling Technology, Danvers, MA,
USA) and β-galactosidase (Promega) mouse monoclonal antibodies.
Subsequently, the membrane was stained with
horseradishperoxidase-conjugated anti-mouse IgG secondary antibody
(GE Healthcare). The blots were stained using the ECL Prime Western
Blotting Detection reagent (GE Healthcare) and scanned by the UVP
EC3 Imaging System (UVP, Upland, CA, USA).
For the determination of the Sox2 production in cell
lines, proteins corresponding to 2×105 cells were
analyzed by SDS-PAGE and stained as described above. β-tubulin was
also stained with rabbit antibodies (Sigma-Aldrich, St. Louis, MO,
USA) for comparison of protein load.
Immunofluorescence staining
The NIH/3T3 cells grown on 8-well Lab-Tek II Chamber
Slides (Thermo Fisher Scientific, Waltham, MA, USA) were
transfected with 0.4 μg of plasmids using Lipofectamine 2000
(Invitrogen). Two days after transfection, cells were fixed and
permeabilized with methanol at −20°C for 10 min. The Sox2 protein
was stained with mouse monoclonal antibody (clone L1D6A2) followed
by Alexa Fluor 488-conjugated goat anti-mouse IgG antibodies
(Invitrogen). The nuclei of the cells were labeled with DAPI. The
slides were examined by an Eclipse E600 microscope (Nikon, Tokyo,
Japan).
Preparation of cartridges for the gene
gun
Plasmid DNA was coated onto 1-μm gold
particles (Bio-Rad, Hercules, CA, USA) by the procedure recommended
by the manufacturer. Each cartridge contained 1 μg DNA
coated onto 0.5 mg of gold particles.
Immunization experiments
Mice were immunized with three 2-μg doses of
plasmid DNA at 1-week intervals by the gene gun (Bio-Rad). Vaccines
were delivered into the shaven skin of the abdomen at a discharge
pressure of 400 psi. One week after the last vaccination, pools of
mononuclear cells were isolated from splenocytes (3 mice per group)
using Ficoll-Paque (GE Healthcare) and analyzed by an ELISPOT assay
or mice were s.c. inoculated with 3×104 TC-1/B7 cells
into the back under anesthesia with intraperitoneal (i.p.)
etomidate (0.5 mg/mouse; Janssen Pharmaceutica, Beerse, Belgium).
The tumor growth in the challenged animals was monitored twice a
week and the tumor size was calculated from three perpendicular
measurements using the formula (π/6)(a × b × c). In some
experiments, 200 μg of anti-CD25 antibody (clone PC61; Bio X
Cell, West Lebanon, NH, USA) was i.p. inoculated four days before
the first immunization.
ELISPOT assay
For detection of IFN-γ, multiScreen 96-well
filtration plates with a PVDF membrane (Millipore, Billerica, MA,
USA) were coated with 10 μg/ml of rat anti-mouse IFN-γ
antibody (BD Biosciences Pharmingen, San Diego, CA, USA) in 50
μl of PBS and incubated overnight at 4°C. Mononuclear cells
resuspended in serum-free CTL-Test medium (Cellular Technology
Ltd., Shaker Heights, OH, USA) were added to the plate
(8×105/well) and incubated at 37°C in 5% CO2
for 20 h either with or without 1 μg/ml of SOX2-derived pool
of peptides (PepMix Human SOX2) or 1 μg/ml of the PADRE
peptide. The cells were removed by three washes with PBS and three
washes with PBS-0.05% Tween-20. Then, 4 μg/ml of
biotinylated rat anti-mouse IFN-γ antibody (BD Biosciences
Pharmingen) in 50 μl PBS were added per well and cultured at
4°C overnight. The wells were washed four times with PBS-0.05%
Tween-20 and incubated for 30 min with 50 μl of 1:100
dilution of streptavidin-horseradish peroxidase (BD Biosciences
Pharmingen) in PBS at room temperature. After washing three times
with PBS-0.05% Tween-20, followed by three washing steps with PBS
alone, the spots were developed using the AEC Substrate Set (BD
Biosciences Pharmingen). The induced spots were counted by a
CTL-ImmunoSpot S5 UV Analyzer (Cellular Technology Ltd.).
Statistical analysis
The specific activation of mononuclear cells in
ELISPOT was determined by Student’s t-test. Tumor growth after DNA
immunization was evaluated by the two-way analysis of variance and
Bonferroni post-tests. A difference between groups was considered
significant if P<0.05. Calculations were performed using the
Prism software, version 5.02 (Graph-Pad Software, San Diego, CA,
USA).
Results
Construction of DNA vaccines
To increase potentially the production of the Sox2
protein, we ordered a codon-optimized Sox2 gene (Sox2opt) from the
GeneArt Co. and cloned it into the pBSC plasmid. The expression of
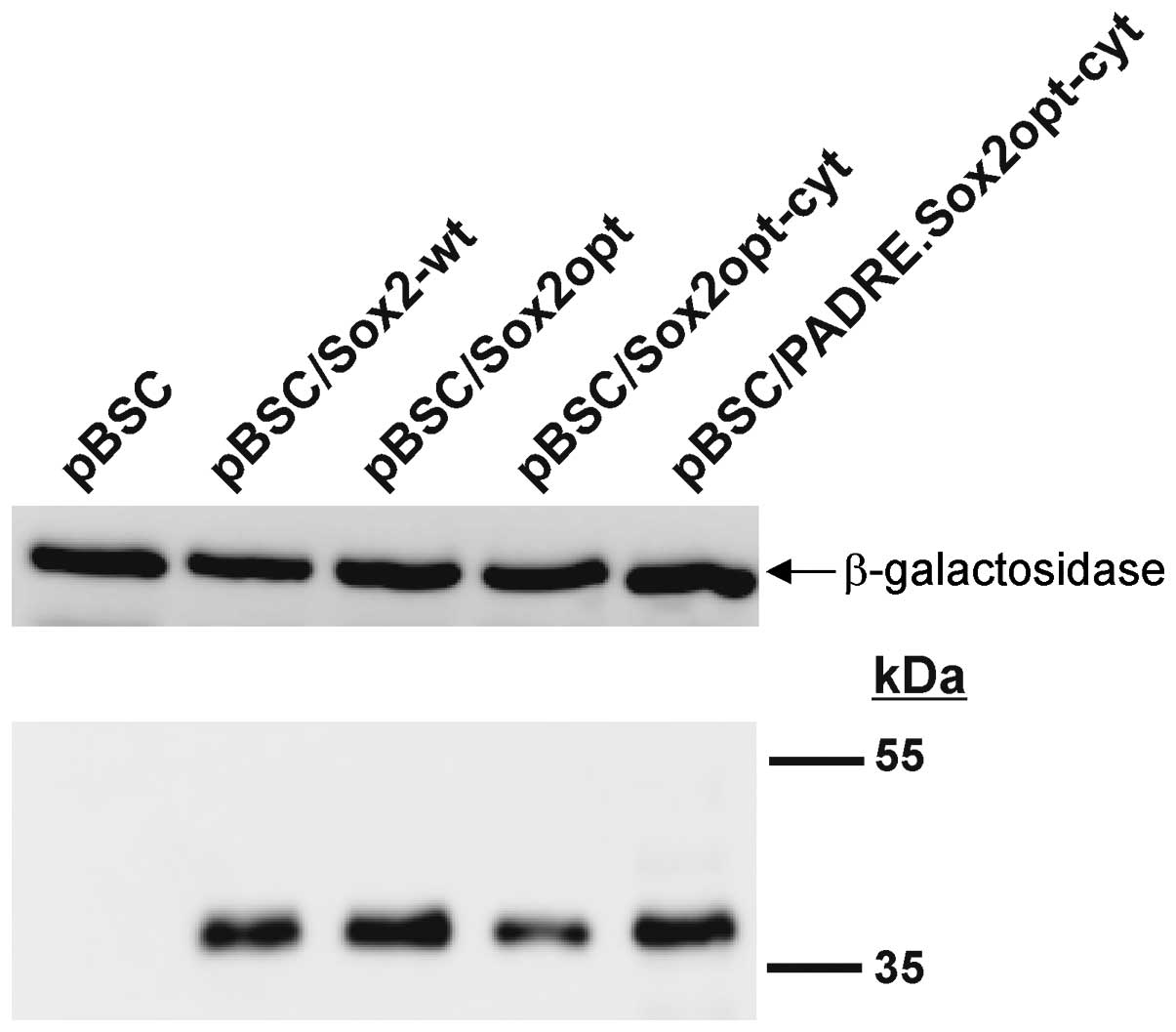
the optimized gene was evaluated by immunoblotting after
transfection of NIH/3T3 cells. The steady-state level of the Sox2
protein from the codon-optimized gene was comparable with that of
the wt Sox2 gene (Fig. 2).
As another step in the modification of the Sox2
gene, we attempted to prevent the transcriptional activity of the
Sox2 protein by the abolition of its two NLSs. This alteration
should increase the safety of a DNA vaccine against Sox2. In
previous studies, only mutational inactivation of both NLS motifs
in the HMG domain led to complete cytoplasmic localization of the
SRY and SOX9 proteins (28) and
analogous mutations in the Sox2 transcription factor substantially
reduced its nuclear localization (19). The site-directed mutagenesis of the
Sox2opt gene substituting six nucleotides in the sequences
corresponding to the two NLSs of the Sox2 protein resulted in the
Sox2opt-cyt gene (Fig. 1).
The expression of the Sox2opt-cyt gene was verified
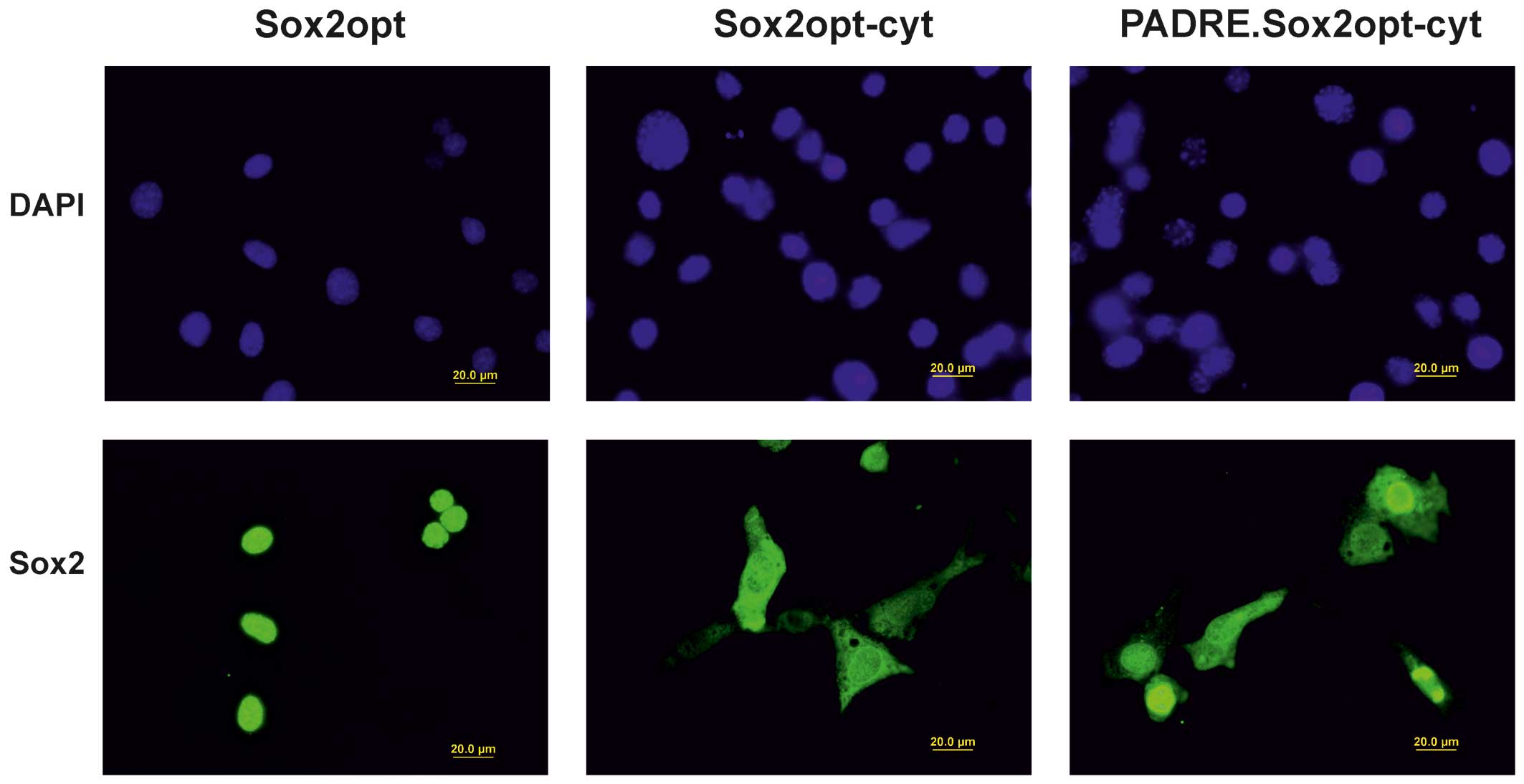
after transfection of NIH/3T3 cells by immunoblot (Fig. 2) and immunofluorescence analyses
(Fig. 3). Immunofluorescence
staining of transfected cells showed that the mutations in the NLS
motifs reduced the ability of the Sox2 protein to translocate to
the nucleus, but besides localization in the cytoplasm, the Sox2
protein was still present in the nuclei of most cells.
Finally, to support the immunogenicity of the DNA
vaccine by the activation of T helper (Th) cells, we fused the
sequence encoding the strong PADRE helper epitope with the 5’
terminus of the Sox2opt-cyt gene. The production (Fig. 2) of the fusion protein was
comparable with that of the parental Sox2opt-cyt protein. The
addition of PADRE did not change the mobility of the protein in
SDS-PAGE, but the induction of the PADRE-specific response (see
below) proves that the fusion protein was really synthesized. We
also observed this phenomenon after the fusion of PADRE with the
modified E7 oncoprotein (E7GGG). After immunofluorescent staining
of the transfected cells, we found nuclei with highly variable
intensity of labeling. The cytoplasm of the positive cells was
uniformly stained (Fig. 3).
The PADRE epitope enhanced the
immunogenicity of the DNA vaccine
The immunogenicity of the modified Sox2 genes was
examined after gene gun immunization of C57BL/6 mice with plasmid
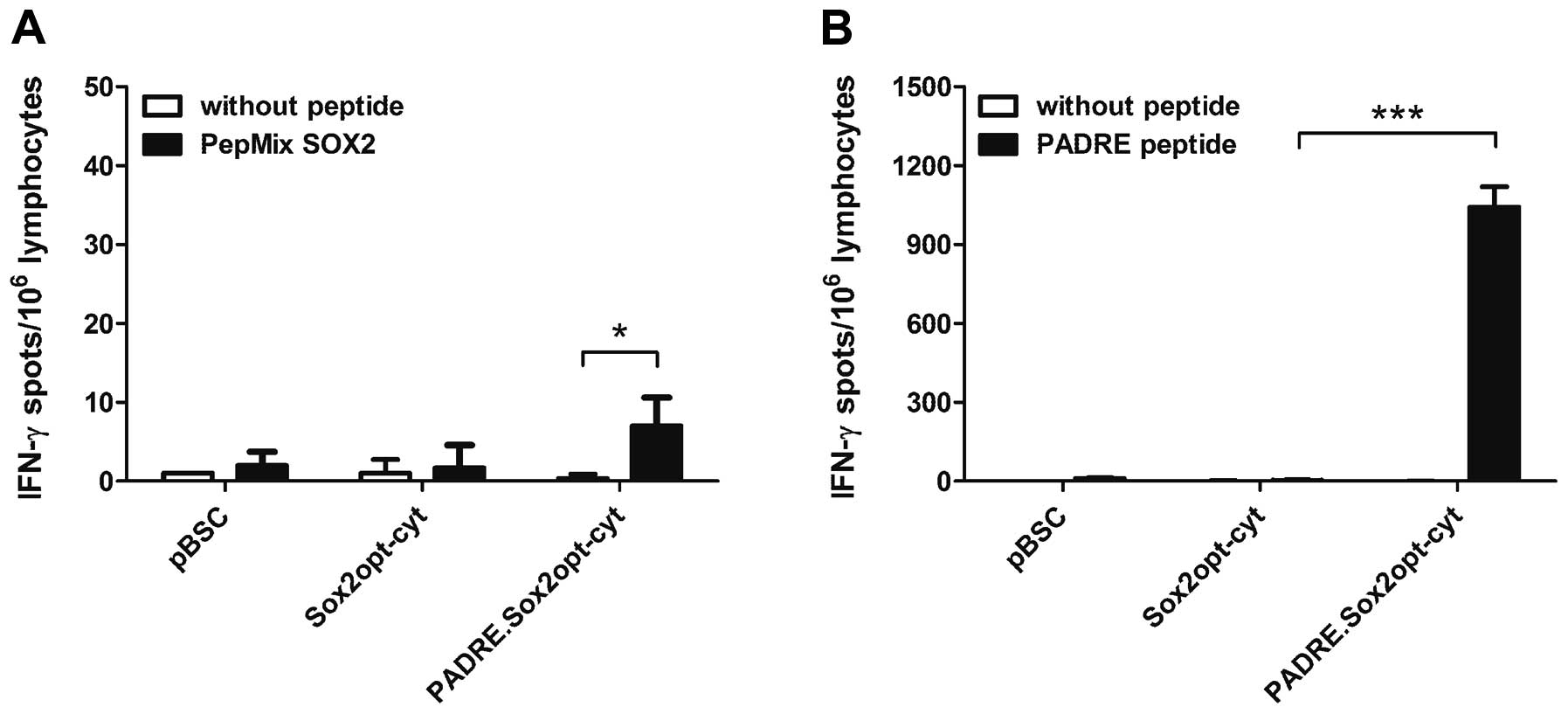
DNA. The IFN- γ -producing cells were detected by an ELISPOT assay
after incubation with a pool of SOX2 peptides (PepMix SOX2). While
no response was found after vaccination with the Sox2opt-cyt gene,
the PADRE.Sox2opt-cyt gene induced a weak Sox2-specific immunity
(Fig. 4A). The high response shown
after the stimulation with the PADRE peptide (Fig. 4B) suggests that activation of Th
cells enhanced immunity against Sox2.
Depletion of Treg cells enhanced the
efficacy of DNA vaccination
To further augment the weak Sox2-specific immunity,
we depleted Treg lymphocytes with anti-CD25 antibody delivered four
days before the first immunization. The stimulation of mononuclear
cells with the PepMix SOX2 showed higher response after the
application of anti-CD25 when compared to animals that did not
receive the antibody (Fig. 5A).
This effect was observed for both the PADRE.Sox2opt-cyt and
Sox2opt-cyt plasmids. Furthermore, an enhanced PADRE- specific
immune response was found after depletion of Treg cells in mice
immunized with the PADRE.Sox2opt-cyt construct (Fig. 5B).
The antitumor effect of DNA
vaccination
Our screening of mouse tumor cell lines (B16-F10,
NIH/3T3, TRAMP-C2, MK16/ABC, TC-1, A20, 12B1, and B210) by RT-PCR
revealed that only TC-1 cells expressed the Sox2 gene (data not
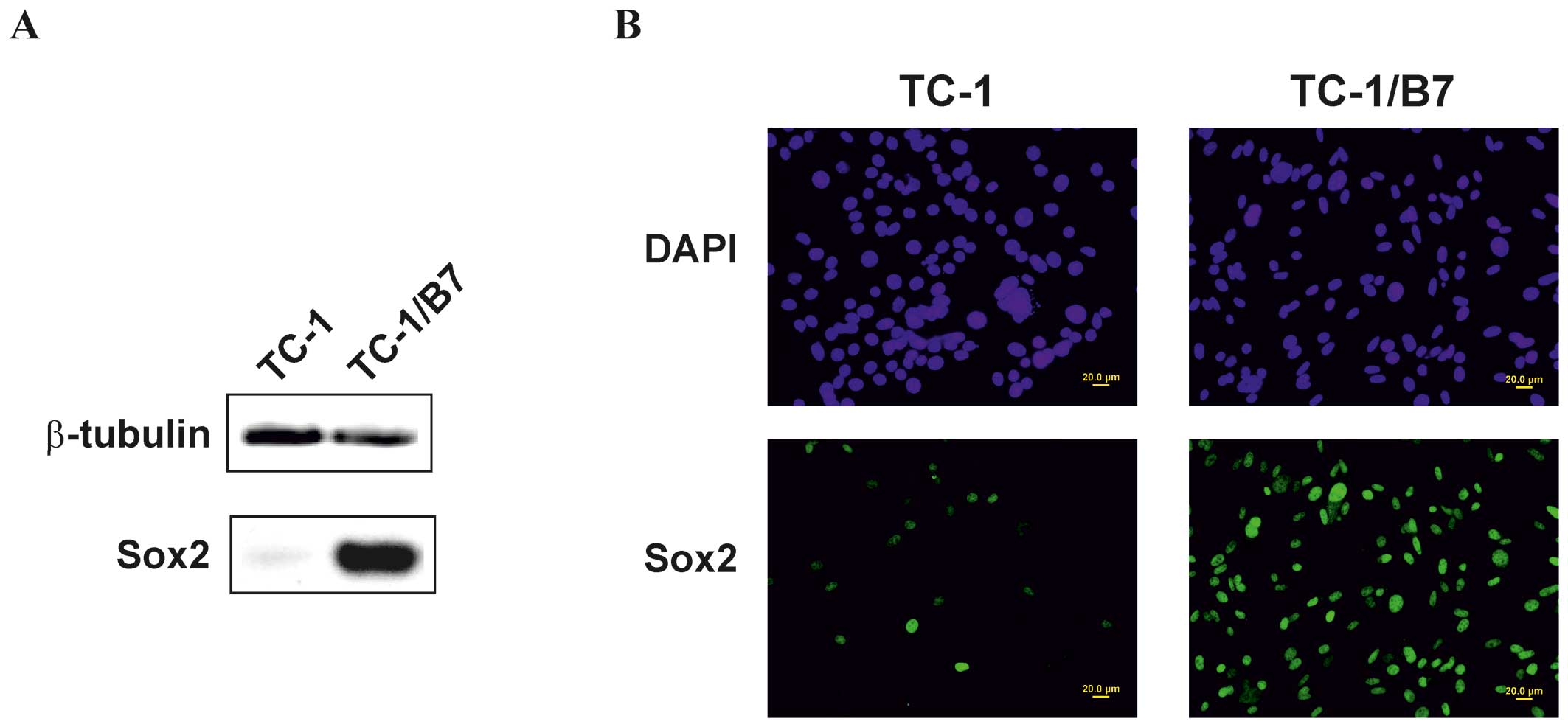
shown). The production of the Sox2 protein was confirmed by
immunoblot (Fig. 6A) and
immunofluorescence analyses (Fig.
6B). However, immunofluorescence staining found Sox2 in nuclei
of only ∼15% of TC-1 cells. Therefore, the production of the Sox2
protein was also detected in seven clones derived from tumors
induced by TC-1 cells in mice immunized against the HPV16 E7
oncoprotein (26,29). In five of them, including TC-1/B7
clone (Fig. 6B), the Sox2
production was substantially higher than in TC-1 cells and this
protein was stained in all nuclei. In subsequent immunization
experiments, TC-1/B7 cells were used to challenge mice, because
these cells were highly sensitive to immunization against the E7
antigen (26).
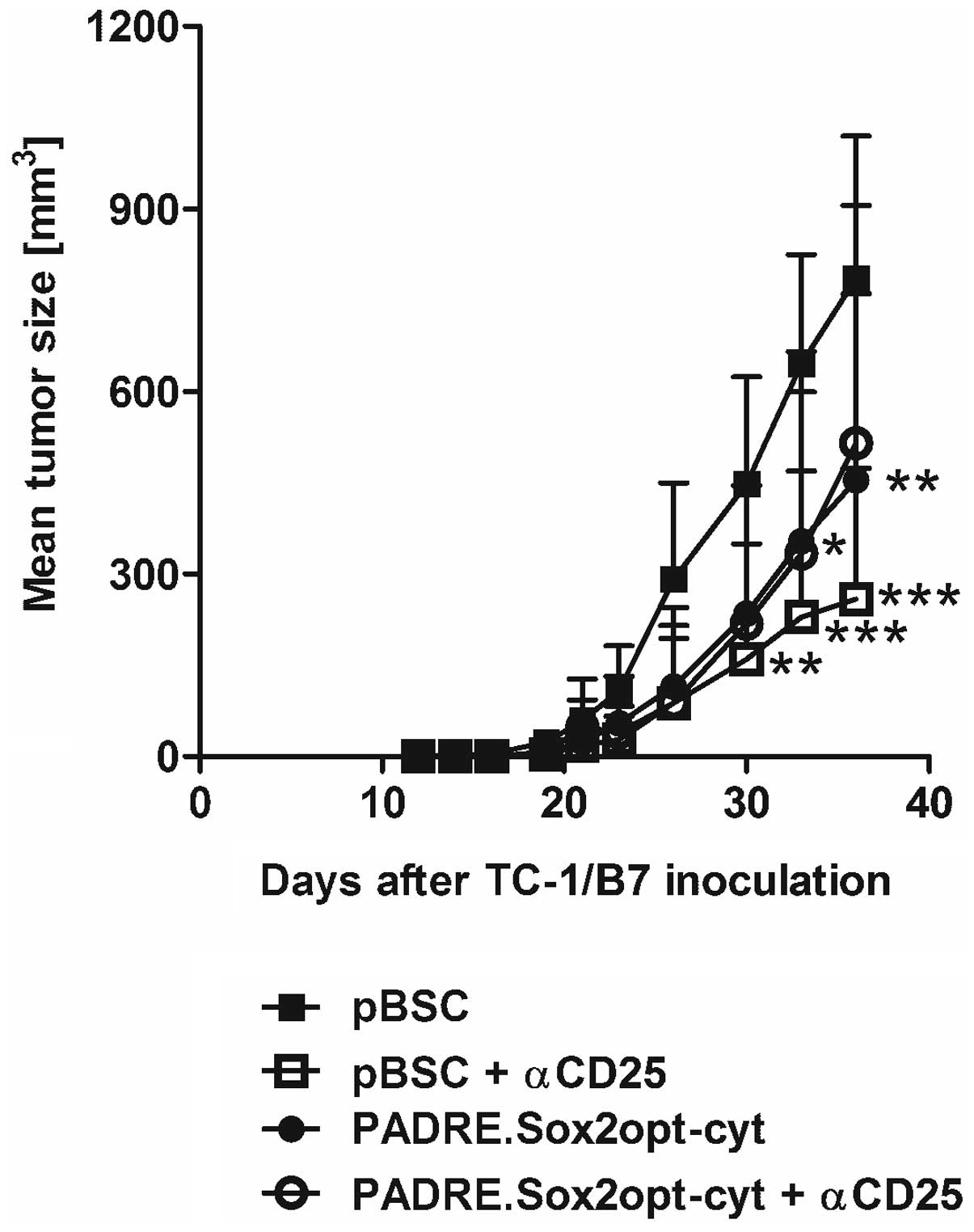
The antitumor effect of immunization against Sox2
was examined with the PADRE.Sox2opt-cyt plasmid. Simultaneously,
the impact of Treg depletion with anti-CD25 delivered before
immunization was tested. The PADRE.Sox2opt-cyt vaccine
significantly inhibited the growth of TC-1/B7 tumors (Fig. 7). This effect was also recorded
after the depletion of Treg cells (even at a higher level) but the
combination of immunization and Treg depletion did not further
enhance the antitumor response.
Discussion
The immunization against cancer cells could be a
supportive treatment to synergize with other anticancer therapies.
It can play a role especially in the elimination of CSCs, thus
preventing recurrences and curing minimal residual disease. To
target CSCs by vaccination, suitable antigens need to be
identified. However, seeking for markers specific for CSCs
suggested the difficulty of such efforts. The similarity of
transcriptional profiles between ESCs and CSCs pointed to
pluripotency transcription factors as candidate antigens.
In this study, we chose one of them, the Sox2
protein, for the development of an experimental DNA vaccine. To
enhance vaccine efficacy, we first ordered the synthesis of a
codon-optimized gene which usually results in increased production
of a protein. However, the steady-state level of the optimized Sox2
(Sox2opt) was similar to that of the wt Sox2. Such result is not
exceptional as of 50 proteins analyzed for codon optimization in a
study using the GeneOptimizer expert software, seven (14%) did not
exhibit augmented synthesis (30).
Nevertheless, we used the Sox2opt gene for further construction of
the DNA vaccine, because the modification of codons could prevent a
potential homologous recombination into the host genome. Next, as
Sox2 has oncogenic potential (20), we mutated both NLSs in Sox2 to
abolish its transcriptional activity. In accordance with the
previous study (19), these
mutations did not completely inhibit nuclear localization. However,
the mutagenesis of NLSs not only affected Sox2 cellular
localization, but also directly reduced its transcriptional
activity and inhibited its cooperation with Oct4 and wt Sox2
(19). Both our alterations of
Sox2, the codon optimization at the level of the DNA sequence, and
the NLS abolition at the level of the protein sequence, enhance the
safety of the DNA vaccine. Finally, we added the sequence encoding
the PADRE helper epitope to the 5’ terminus of the Sox2opt-cyt
gene. In our previous study (31),
this epitope was more potent than the universal p30 helper epitope
from the tetanus toxin and induced strong Th1 immunity that
markedly enhanced activation of CD8+ T lymphocytes.
To detect the immune response against mouse Sox2 in
an ELISPOT assay, we first performed computational prediction of
H-2b and H-2d epitopes and tested the
reactivity of five synthetic nonapeptides representing the
predicted epitopes (aa 42–50, 75–83, 91–99, 111–119 and 125–133)
with splenocytes obtained after immunization of C57BL/6 and BALB/c
mice. However, none of these peptides was active in these
examinations (data not shown). Therefore, we also tested the
stimulation of lymphocytes with PepMix SOX2 containing 77 peptides
derived from the human SOX2 protein. As homology between mouse and
human proteins is ∼97%, most mouse epitopes should be preserved in
PepMix SOX2. Indeed, we found significant Sox2-specific activation
of lymphocytes with PepMix SOX2, but only after immunization of
C57BL/6 mice with the PADRE.Sox2opt-cyt. This result suggests that
the Th1 immunity induced by the PADRE epitope was necessary for
breaking tolerance to the Sox2 self-antigen.
The depletion of Treg cells enhanced the efficacy of
DNA vaccination against various antigens (32–34)
and resulted in broken tolerance (34). Therefore, we used antibody against
CD25 for Treg depletion and showed the augmentation of both PADRE-
and Sox2-specific responses. In our analogous study with a DNA
vaccine against legumain overexpressed in tumor macrophages, we
found enhanced immunity against legumain only after vaccination
with the fusion gene carrying the sequence encoding the p30 helper
epitope (Smahel et al, unpublished data). However, the
response against Sox2 was also increased after immunization with
the Sox2opt-cyt gene without PADRE. These results propose that the
induction of Th immunity was necessary for the effect of Treg
depletion and that Sox2 contains a strong helper epitope. The
latter conclusion is supported by studies detecting SOX2-specific
immunity in patients with premalignant or malignant diseases,
because both CD8+ and CD4+ reactive T cells
were demonstrated (8,35).
Of eight mouse oncogenic cell lines of various
origins, Sox2 expression was only found in TC-1 cells derived from
the lung. When clones isolated from a tumor induced with TC-1 cells
in immunized mice were examined, five out of seven clones produced
a markedly higher amount of the Sox2 protein. Noh et al
(36) have also demonstrated Sox2
expression in TC-1 cells, but they found the same expression in
immunoresistant TC-1 P3 cells derived from tumors after 3 serial
passages in immunized mice.
For the examination of the antitumor effect of DNA
vacci-nation against Sox2, we used TC-1/B7 cells that were highly
sensitive to immunization against the HPV16 E7 oncoprotein
(26). However, despite strong
production of Sox2, DNA vaccination against this self-antigen did
not prevent tumor development. Otherwise, it significantly reduced
tumor growth, but this impact was low. Tumor growth of TC-1/B7
cells was also significantly inhibited by the depletion of Treg
cells, but the combination of immunization and depletion did not
further strengthen the antitumor effect. We obtained a similar
result after immunization against legumain. Treg lymphocytes
usually support tumor growth by their immunosupressive activities,
but in tumors with intense inflammation that is useful for tumor
progression, they can reduce this inflammation and thus inhibit
tumor development (37). It could
also be the case of tumors in immunized mice with a high Th1
response.
In conclusion, we constructed an experimental DNA
vaccine against Sox2 and demonstrated in vitro the
enhancement of the Sox2-specific response by the addition of the
PADRE helper epitope and depletion of Treg cells. However, this
depletion did not augment the antitumor effect. Our results support
the notion (37) that the
elimination of Treg cells, e.g. by antibodies against PD-1 or PD-L1
in clinical trials, may not always be beneficial in combination
with other types of immunotherapy.
Acknowledgements
We thank P. Vesela and K. Kernova for
technical assistance. This study was supported by grant
NT11541-4/2010 from the Ministry of Health of the Czech Republic
and grants CZ.2.16/3.1.00/24001 and CZ.2.16/3.1.00/28007 from the
European Regional Development Fund, Operational Programme
Prague-Competitiveness.
References
|
1.
|
Sugihara E and Saya H: Complexity of
cancer stem cells. Int J Cancer. 132:1249–1259. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Xu Q, Liu G, Yuan X, Xu M, Wang H, Ji J,
Konda B, Black KL and Yu JS: Antigen-specific T-cell response from
dendritic cell vaccination using cancer stem-like cell-associated
antigens. Stem Cells. 27:1734–1740. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Wong DJ, Liu H, Ridky TW, Cassarino D,
Segal E and Chang HY: Module map of stem cell genes guides creation
of epithelial cancer stem cells. Cell Stem Cell. 2:333–344. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Muller FJ, Laurent LC, Kostka D, Ulitsky
I, Williams R, Lu C, Park IH, Rao MS, Shamir R, Schwartz PH,
Schmidt NO and Loring JF: Regulatory networks define phenotypic
classes of human stem cell lines. Nature. 455:401–405. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Ben-Porath I, Thomson MW, Carey VJ, Ge R,
Bell GW, Regev A and Weinberg RA: An embryonic stem cell-like gene
expression signature in poorly differentiated aggressive human
tumors. Nat Genet. 40:499–507. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Boyer LA, Lee TI, Cole MF, Johnstone SE,
Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG,
Gifford DK, Melton DA, Jaenisch R and Young RA: Core
transcriptional regulatory circuitry in human embryonic stem cells.
Cell. 122:947–956. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Gure AO, Stockert E, Scanlan MJ, Keresztes
RS, Jager D, Altorki NK, Old LJ and Chen YT: Serological
identification of embryonic neural proteins as highly immunogenic
tumor antigens in small cell lung cancer. Proc Natl Acad Sci USA.
97:4198–4203. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Spisek R, Kukreja A, Chen LC, Matthews P,
Mazumder A, Vesole D, Jagannath S, Zebroski HA, Simpson AJ, Ritter
G, Durie B, Crowley J, Shaughnessy JD Jr, Scanlan MJ, Gure AO,
Barlogie B and Dhodapkar MV: Frequent and specific immunity to the
embryonal stem cell-associated antigen SOX2 in patients with
monoclonal gammopathy. J Exp Med. 204:831–840. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Schmitz M, Temme A, Senner V, Ebner R,
Schwind S, Stevanovic S, Wehner R, Schackert G, Schackert HK,
Fussel M, Bachmann M, Rieber EP and Weigle B: Identification of
SOX2 as a novel glioma-associated antigen and potential target for
T cell-based immunotherapy. Br J Cancer. 96:1293–1301. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Laga AC, Lai CY, Zhan Q, Huang SJ,
Velazquez EF, Yang Q, Hsu MY and Murphy GF: Expression of the
embryonic stem cell transcription factor SOX2 in human skin:
relevance to melanocyte and merkel cell biology. Am J Pathol.
176:903–913. 2010. View Article : Google Scholar
|
|
11.
|
Zhang X, Yu H, Yang Y, Zhu R, Bai J, Peng
Z, He Y, Chen L, Chen W, Fang D, Bian X and Wang R: SOX2 in gastric
carcinoma, but not Hath1, is related to patients’
clinicopathological features and prognosis. J Gastrointest Surg.
14:1220–1226. 2010.PubMed/NCBI
|
|
12.
|
Lengerke C, Fehm T, Kurth R, Neubauer H,
Scheble V, Muller F, Schneider F, Petersen K, Wallwiener D, Kanz L,
Fend F, Perner S, Bareiss PM and Staebler A: Expression of the
embryonic stem cell marker SOX2 in early-stage breast carcinoma.
BMC Cancer. 11:422011. View Article : Google Scholar
|
|
13.
|
Bass AJ, Watanabe H, Mermel CH, Yu S,
Perner S, Verhaak RG, Kim SY, Wardwell L, Tamayo P, Gat-Viks I,
Ramos AH, Woo MS, Weir BA, Getz G, Beroukhim R, O’Kelly M, Dutt A,
Rozenblatt-Rosen O, Dziunycz P, Komisarof J, Chirieac LR, Lafargue
CJ, Scheble V, Wilbertz T, Ma C, Rao S, Nakagawa H, Stairs DB, Lin
L, Giordano TJ, Wagner P, Minna JD, Gazdar AF, Zhu CQ, Brose MS,
Cecconello I, Jr UR, Marie SK, Dahl O, Shivdasani RA, Tsao MS,
Rubin MA, Wong KK, Regev A, Hahn WC, Beer DG, Rustgi AK and
Meyerson M: SOX2 is an amplified lineage-survival oncogene in lung
and esophageal squamous cell carcinomas. Nat Genet. 41:1238–1242.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Yuan P, Kadara H, Behrens C, Tang X, Woods
D, Solis LM, Huang J, Spinola M, Dong W, Yin G, Fujimoto J, Kim E,
Xie Y, Girard L, Moran C, Hong WK, Minna JD and Wistuba II: Sex
determining region Y-Box 2 (SOX2) is a potential cell-lineage gene
highly expressed in the pathogenesis of squamous cell carcinomas of
the lung. PLoS One. 5:e91122010. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Hussenet T, Dali S, Exinger J, Monga B,
Jost B, Dembele D, Martinet N, Thibault C, Huelsken J, Brambilla E
and du Manoir S: SOX2 is an oncogene activated by recurrent 3q26.3
amplifications in human lung squamous cell carcinomas. PLoS One.
5:e89602010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Freier K, Knoepfle K, Flechtenmacher C,
Pungs S, Devens F, Toedt G, Hofele C, Joos S, Lichter P and
Radlwimmer B: Recurrent copy number gain of transcription factor
SOX2 and corresponding high protein expression in oral squamous
cell carcinoma. Genes Chromosomes Cancer. 49:9–16. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Maier S, Wilbertz T, Braun M, Scheble V,
Reischl M, Mikut R, Menon R, Nikolov P, Petersen K, Beschorner C,
Moch H, Kakies C, Protzel C, Bauer J, Soltermann A, Fend F,
Staebler A, Lengerke C and Perner S: SOX2 amplification is a common
event in squamous cell carcinomas of different organ sites. Hum
Pathol. 42:1078–1088. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Wilbertz T, Wagner P, Petersen K, Stiedl
AC, Scheble VJ, Maier S, Reischl M, Mikut R, Altorki NK, Moch H,
Fend F, Staebler A, Bass AJ, Meyerson M, Rubin MA, Soltermann A,
Lengerke C and Perner S: SOX2 gene amplification and protein
overexpression are associated with better outcome in squamous cell
lung cancer. Mod Pathol. 24:944–953. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Li J, Pan G, Cui K, Liu Y, Xu S and Pei D:
A dominant-negative form of mouse SOX2 induces trophectoderm
differentiation and progressive polyploidy in mouse embryonic stem
cells. J Biol Chem. 282:19481–19492. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Liu K, Lin B, Zhao M, Yang X, Chen M, Gao
A, Liu F, Que J and Lan X: The multiple roles for Sox2 in stem cell
maintenance and tumorigenesis. Cell Signal. 25:1264–1271. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Dhodapkar MV and Dhodapkar KM: Spontaneous
and therapy-induced immunity to pluripotency genes in humans:
clinical implications, opportunities and challenges. Cancer Immunol
Immunother. 60:413–418. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Smahel M, Sima P, Ludvikova V and Vonka V:
Modified HPV16 E7 genes as DNA vaccine against E7-containing
oncogenic cells. Virology. 281:231–238. 2001. View Article : Google Scholar
|
|
23.
|
Alexander J, Sidney J, Southwood S,
Ruppert J, Oseroff C, Maewal A, Snoke K, Serra HM, Kubo RT and
Sette A: Development of high potency universal DR-restricted helper
epitopes by modification of high affinity DR-blocking peptides.
Immunity. 1:751–761. 1994. View Article : Google Scholar
|
|
24.
|
Jainchill JL, Aaronson SA and Todaro GJ:
Murine sarcoma and leukemia viruses: assay using clonal lines of
contact-inhibited mouse cells. J Virol. 4:549–553. 1969.PubMed/NCBI
|
|
25.
|
Lin KY, Guarnieri FG, Staveley OK,
Levitsky HI, August JT, Pardoll DM and Wu TC: Treatment of
established tumors with a novel vaccine that enhances major
histocompatibility class II presentation of tumor antigen. Cancer
Res. 56:21–26. 1996.PubMed/NCBI
|
|
26.
|
Smahel M, Smahelova J, Tejklova P, Tachezy
R and Marinov I: Characterization of cell lines derived from tumors
induced by TC-1 cells in mice preimmunized against HPV16 E7
oncoprotein. Int J Oncol. 27:731–742. 2005.PubMed/NCBI
|
|
27.
|
Kaufmann AM, Gissmann L, Schreckenberger C
and Qiao L: Cervical carcinoma cells transfected with the CD80 gene
elicit a primary cytotoxic T lymphocyte response specific for HPV
16 E7 antigens. Cancer Gene Ther. 4:377–382. 1997.
|
|
28.
|
Sudbeck P and Scherer G: Two independent
nuclear localization signals are present in the DNA-binding
high-mobility group domains of SRY and SOX9. J Biol Chem.
272:27848–27852. 1997. View Article : Google Scholar
|
|
29.
|
Smahel M, Sima P, Ludvikova V, Marinov I,
Pokorna D and Vonka V: Immunisation with modified HPV16 E7 genes
against mouse oncogenic TC-1 cell sublines with downregulated
expression of MHC class I molecules. Vaccine. 21:1125–1136. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Fath S, Bauer AP, Liss M, Spriestersbach
A, Maertens B, Hahn P, Ludwig C, Schafer F, Graf M and Wagner R:
Multiparameter RNA and codon optimization: a standardized tool to
assess and enhance autologous mammalian gene expression. PLoS One.
6:e175962011. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Smahel M, Polakova I, Duskova M, Ludvikova
V and Kastankova I: The effect of helper epitopes and cellular
localization of an antigen on the outcome of gene gun DNA
immunization. Gene Ther. 21:225–232. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Furuichi Y, Tokuyama H, Ueha S, Kurachi M,
Moriyasu F and Kakimi K: Depletion of
CD25+CD4+T cells (Tregs) enhances the
HBV-specific CD8+ T cell response primed by DNA
immunization. World J Gastroenterol. 11:3772–3777. 2005.PubMed/NCBI
|
|
33.
|
Chuang CM, Hoory T, Monie A, Wu A, Wang MC
and Hung CF: Enhancing therapeutic HPV DNA vaccine potency through
depletion of CD4+CD25+ T regulatory cells.
Vaccine. 27:684–689. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Jacob JB, Kong YC, Nalbantoglu I, Snower
DP and Wei WZ: Tumor regression following DNA vaccination and
regulatory T cell depletion in neu transgenic mice leads to an
increased risk for autoimmunity. J Immunol. 182:5873–5881. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Dhodapkar KM, Gettinger SN, Das R,
Zebroski H and Dhodapkar MV: SOX2-specific adaptive immunity and
response to immunotherapy in non-small cell lung cancer.
Oncoimmunology. 2:e252052013. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Noh KH, Lee YH, Jeon JH, Kang TH, Mao CP,
Wu TC and Kim TW: Cancer vaccination drives Nanog-dependent
evolution of tumor cells toward an immune-resistant and stem-like
phenotype. Cancer Res. 72:1717–1727. 2012. View Article : Google Scholar
|
|
37.
|
Whiteside TL: Regulatory T cell subsets in
human cancer: are they regulating for or against tumor progression?
Cancer Immunol Immunother. 63:67–72. 2014. View Article : Google Scholar : PubMed/NCBI
|