Introduction
Antitumor ribonucleases (RNases) are promising
family of small (10–28 kDa) basic proteins with strong anticancer
potential (1). As a whole this
superfamily of secretory enzymes is responsible for operating at
the crossroads of transcription and translation. After early
enthusiasm, their anticancer potential in terms of clinical utility
suffered a decline until quite recently, when they have attracted
attention again, due to discovery of their remarkable and complex
biological activities (2,3). Among various RNases described thus
far, only some of them were found to be toxic to cancer cells. The
best known and widely investigated is onconase (ONC), which has
reached the late-stage clinical trials for treatment of malignant
mesothelioma (4). More recently
described amphinase (Amph) as well as its recombinant form (r-Amph)
also display both cytotoxic and cytostatic effects (5).
ONC and Amph were discovered and sequenced by
Alfacell Corporation (currently TAmiR Biotechnology, Inc, Somerset,
NJ, USA), the first one more than two decades ago (6) while the latter more recently
(5). Both enzymes were isolated
from oocytes of leopard frog Rana pipiens. Amph is the
largest among frog homologues of RNase A and consists of 114 amino
acid residues (5) while ONC, on
the other hand, is the smallest of whole superfamily having only
104 amino acid residues (7).
The cytostatic and cytotoxic properties of ONC and
r-Amph depend on their enzymatic activity targeting the crossroads
between transcription and translation and catalyzing RNA
degradation (8). It was initially
considered, that the key target are rRNA and/or tRNA as their
degradation led to inhibition of protein synthesis. According to
recent findings, however, the suspected target of RNases can also
be the non-coding RNA (microRNAs), which is responsible for
regulation of gene expression through RNA interference (RNAi)
(9). Consistent with the latter,
they can modulate the process of cell differentiation in the early
embryos by targeting strictly RNAi.
Although a major advancement in antitumor treatment
has been observed, still several B cell-derived malignancies remain
incurable. A promising approach is the one involving targeting of
RNA. Of particular interest are numerous observations that ONC acts
strongly synergistic while combined with one of the several
different antitumor drugs. This was observed in vitro in
combination with the agents used during standard treatment such as
tamoxifen or trifluoroperazine (10) in solid tumors. Interestingly, in
combination with vincristine, ONC has shown high toxicity even
against multi-drug resistant cancer cells (11).
No previous report exists on the activity of ONC or
r-Amph in combination with standard cytostatic treatment against B
cell malignant disorders such as CLL or non-Hodgkin’s lymphoma. In
the present study we observed that both ONC and r-Amph, alone or in
combination with routinely used cytostatics has marked toxicity
against investigated cancer cells.
Materials and methods
Peripheral blood mononuclear cell (PBMC)
isolation
Peripheral blood samples were obtained from
untreated patients with chronic lymphocytic leukemia (CLL) and
acute lymphoblastic leukemia (ALL).
The CLL group consisted of 45 patients, 21 women and
34 men, median age 70.1 (range 51–85 years). There were 21 patients
in stage 0–I according to Rai classification, 11 patients in stage
II and 13 in stage III–IV. Twenty-seven patients were stable and 18
had progressive disease. Three out of 45 patients had 17p13
deletion, 5/45 had 11q region deletion or translocation. In 10/45
CLL cells were ZAP-70-positive and 14/45 showed
CD38-positivity.
The ALL group consisted of 15 patients, 10 women and
5 men, median age 37.5 (range 19–48 years). Two out of 15 patients
were Philadelphia-positive; hyperploid was found in 10/45 patients.
Median white blood cell count was 27.3 G/L (14–125 G/L).
Lymphocytes obtained from 31 healthy volunteers were
also treated with the studied drugs. PBMC were isolated from
heparinized blood samples from CLL and ALL patients by
centrifugation in Histopaque-1077 (Sigma Diagnostic, St. Louis, MO,
USA) density gradients. A 1:1 (v/v) mixture of blood and Hanks’
balanced salt solution (HBSS; Biomed, Lublin, Poland) was layered
on the top of the Histopaque media in centrifuge tubes and
centrifuged for 30 min at 200 × g. The interphase region containing
PBMC was collected and then washed twice, in HBSS and RPMI-1640
medium. Next, the cells were resuspended in RPMI-1640
(0.5×106 cells/ml); 1 ml aliquots of cell suspensions
were placed into 24-culture well dishes (Nunc, Roskilde, Denmark)
for further cultures.
Additionally, we tested in vitro activity of
ONC or r-Amph alone or in combination with doxorubicin (DOX) on
cell lines derived from Burkitt’s lymphoma (Raji), diffuse large B
cell lymphoma (DLBCL)-derived cells (Toledo) or with DEX on
multiple myeloma (MM)-derived cell line (RPMI 8226). All cell lines
were purchased from American Type Culture Collection (ATCC,
Manassas, VA, USA).
At the 0 h time point and after 72 h CLL or ALL
PBMCs or cells from the cell lines were subjected to a simultaneous
assessment of cytotoxicity and apoptosis. Cells were incubated for
72 h with ONC or r-Amph, the drugs kindly provided by TAmiR
Biotechnology, Inc, Somerset, NJ, USA (previously Alfacell
Corporation, Somerset, NJ, USA). At first the drugs were applied at
final concentration range between 1 μg/ml to 50
μg/ml, and subsequently the minimal concentrations that
exerted significant increase in cytotoxicity, compared to untreated
control samples, were selected for further studies (Table I). Experiments on ex vivo
cells were made in triplicates. In case of cell lines each
experiment was repeated at least 5 times.
 | Table I.Choosing of optimal onconase (ONC) and
r-amphinase (r-Amph) doses for further experiments in different
tumor cell models. The lowest drug doses exerting significant
cytotoxic effect compared to the untreated control samples were
chosen for further experiment as an optimal concentrations. |
Table I.
Choosing of optimal onconase (ONC) and
r-amphinase (r-Amph) doses for further experiments in different
tumor cell models. The lowest drug doses exerting significant
cytotoxic effect compared to the untreated control samples were
chosen for further experiment as an optimal concentrations.
| Cells | 1 μg/ml
| 5 μg/ml
| 10 μg/ml
| 20 μg/ml
| 30 μg/ml
| 40 μg/ml
| 50 μg/ml
|
|---|
| ONC | r-Amph | ONC | r-Amph | ONC | r-Amph | ONC | r-Amph | ONC | r-Amph | ONC | r-Amph | ONC | r-Amph |
|---|
| CLL | NS | NS | * | NS | → | * | → | → | → | → | → | → | → | → |
| ALL | NS | NS | NS | NS | NS | NS | * | NS | → | NS | → | * | → | → |
| Toledo | * | NS | → | NS | → | * | → | → | → | → | → | → | → | → |
| Raji | NS | NS | NS | NS | NS | NS | * | NS | → | NS | → | * | → | → |
| RPMI 8226 | NS | NS | NS | NS | NS | NS | NS | NS | * | NS | → | * | → | → |
Cell cultures were maintained in RPMI-1640 medium
containing 10% (v/v) heat inactivated fetal calf serum (FCS) and
antibiotics (streptomycin 50 mg/ml, penicillin 50 IU/ ml; Life
Technologies, Paisley, UK), at 37°C, 5% CO2, fully
humidified. Cells were incubated in 25-ml dishes (Nunc), in
concentration 0.2×106/ml. Viability of Toledo cells
before culturing was 95% or more.
Assessment of cytotoxicity
Cytotoxicity was assessed using the propidium iodide
(PI) exclusion test. Namely, after incubation with drugs, cells
were washed twice in PBS and stained in 0.5 ml of 10 μg/ml
PI solution in PBS (room temperature, in the dark). Then, the cell
fluorescence was measured by flow cytometry, using the FL3
fluorescence emission filter; PI-positive cells were defined as
non-viable.
Assessment of apoptosis
Drug-induced apoptosis was assessed finally after 72
h of incubation by the flow cytometry, using Annexin V (Ann V)
assay. After incubation with drugs cells were washed with PBS and
then re-suspended in 100 μl of binding buffer, containing 2
μl of FITC conjugated Ann V and 10 μg/ml of PI
(Becton-Dickinson, San Jose, CA, USA). Then, samples were incubated
for 15 min (room temperature, in the dark). The fluorescence was
measured by flow cytometer using FL1 (Ann V) and FL3 (PI) standard
emission filters. Apoptotic index (AI) was calculated as a
percentage Ann V-positive (Ann V+) cells.
Mechanisms of apoptosis activation
Activation of caspases -8, -9 and -3, a decline of
mitochondrial potential (ΔΨm) and expression of several
apoptosis-regulating proteins were investigated.
Detection of activated caspases
Caspase-3 activation was detected using
FITC-conjugated monoclonal rabbit anti-active caspase-3 antibody
(BD Pharmingen, San Diego, CA, USA). After incubation cells were
fixed and permeabilized using Cytofix/Cytoperm™ (BD Pharmingen)
solution (20 min on ice), then washed twice and re-suspended in the
Perm/Wash™ buffer (BD Pharmingen). The antibody was added as 60
μl per 300 μl of cell suspension (30-min incubation
at RT). The fluorescence was measured directly after staining and
washing in Perm/Wash buffer by flow cytometry using FL1 filter for
detecting the green fluorescence of anti-active caspase-3
antibody.
Activation of other caspases was detected using
FLICA methodology (12).
Commercially available FAM-LETD-FMK FLICA™ Caspase-8 assay kit and
FAM-LEHD-FMK Reagent-9 FLICA™ Caspase-9 assay kit were used for
assessment of caspase-8 and caspase-9 activation. Initially
prepared, 150X concentrated solution in dimethylsulphoxide (DMSO;
Sigma-Aldrich, St. Louis, MO, USA) were stored at −20°C, protected
from light. Directly before use, the aliquots were diluted in PBS
(1:5), then added to 300 μl of culture to obtain a 10
μM concentration of FAM-LETD-FMK (for caspase-8 detection)
or FAM-LEDH-FMK (for caspase-9 detection), respectively. Cultures
were terminated by washing the cells twice (5 min, 140 × g) with
the ‘wash buffer’. After centrifugation, the pellets were
re-suspended in 1 ml of wash buffer and the samples were placed on
ice. Cell fluorescence derived from FAM-LETD-FMK or FAM-LEDH-FMK
was measured during the next 15 min by flow cytometry.
Dissipation of mitochondrial potential
(ΔΨm)
MitoTracker Red 580 dye (Molecular Probes, Eugene,
OR, USA), which accumulates to active mitochondria of living cells,
was used as a probe of ΔΨm. The stock solution of MitoTracker Red
(1 mM) was diluted to concentration of 50 nM by adding to the
growth culture medium (20-min incubation, at room temperature). The
decline of ΔΨm was visualized by the decrease in red fluorescence
of the dye, as detected by flow cytometry.
Expression of apoptosis-regulating
proteins
Cellular expression of several apoptosis-regulating
proteins, including Bcl-2 family (Bax, Bak, Bad, Bcl-2, Mcl-1), and
inhibitor of apoptosis protein (IAP) family (cIAP1, cIAP2,
survivin, XIAP and Smac/Diablo), were also investigated. Cells were
fixed in 1% methanol-free paraformaldehyde and permabilized with
0.1% polysorbate-20 (Tween-20) in PBS. Anti-human Bax primary
rabbit Ab (Dako, Glostrup, Denmark) was used in the dilution 1:100
(60-min incubation). Anti-Bak, anti-Bid and anti-Bad (all Abcam,
Cambridge, UK) primary rabbit anti-human MoAbs were used in
dilutions 1:10 (60-min incubation). Anti-Bcl-2 MoAb (Dako) was used
in concentration 1:15 (30-min incubation). Mouse anti-Mcl-1 MoAb
(Abcam) was used in 1:30 concentration (30 min). Mouse anti-human
XIAP MoAb (Oncogene Res. Products, San Diego, CA, USA) was used in
dilution 1:100 (incubation for 60 min).
For assessment of IAPs and IAP antagonist expression
the following Abs were used: anti-cIAP1, anti-Smac/Diablo,
anti-survivin, rabbit polyclonal antibodies (PoAbs), anti-cIAP2,
anti-XIAP goat PoAbs (all R&D Systems, Minneapolis, MN, USA) in
concentration of 1:100. All Abs were diluted in PBS containing 1%
bovine serum albumin (BSA). Samples were incubated for 60 min, at
RT, then washed in PBS and subjected to centrifugation.
Subsequently, they were incubated with the swine FITC-conjugated Ab
anti-rabbit at 1:20 dilution for 60 min, at RT, in the dark. The
cells were then washed and re-suspended in 400 μl PBS and
analyzed by cytometry. The increase or decrease in expression of
the respective proteins measured as intensity of their
immunofluorescence (MFI) and compared to control, then defined as
up- or downregulation, respectively.
Fluorescence measurements
All fluorescence measurements were performed by flow
cytometry (FACScan; Becton-Dickinson, San Jose, CA, USA), using
standard emission filters: green (λ = 530±20 nm; FL1), orange (λ =
560–600 nm; FL2) and red (λ >600 nm; FL3), where necessary. Ten
thousand cells per sample were measured per each sample.
Statistics
The statistical analysis of data was performed using
statistical software (Statistica v.7.0, Tulsa, OK, USA). The range
of the measured variable, means and standard deviations (SD) as
well as medians and ranges were calculated. The differences between
values were evaluated with Student’s t-test or by Mann-Whitney U
test, as necessary. The Spearman’s rank test was used for assessing
correlations between some data. P-values <0.05 were considered
to indicate a statistically significant difference.
Results
Cytotoxicity of the drugs
Based on preliminary studies on different ex
vivo and in vitro experimental models, concentrations of
10–20 μg/ml of ONC and 20–60 μg/ml of r-Amph were
chosen for final studies as the lowest doses inducing significant
cytotoxic effect (Table I). In a
similar manner concentrations 1 μg/ml of FA, 10 μg/ml
of DOX and 20 μg/ml of DEX were selected.
Pro-apoptotic effects of ONC or r-Amph
alone and in combination with other drugs
The level of cytotoxicity assessed by PI staining
strongly correlated with AI evaluated by Ann V assay (R=0.93,
p<0.001). Thus, the mechanism of both ONC and r-Amph
cytotoxicity in the examined models was induction of
caspase-dependent apoptosis, with activation of both the external
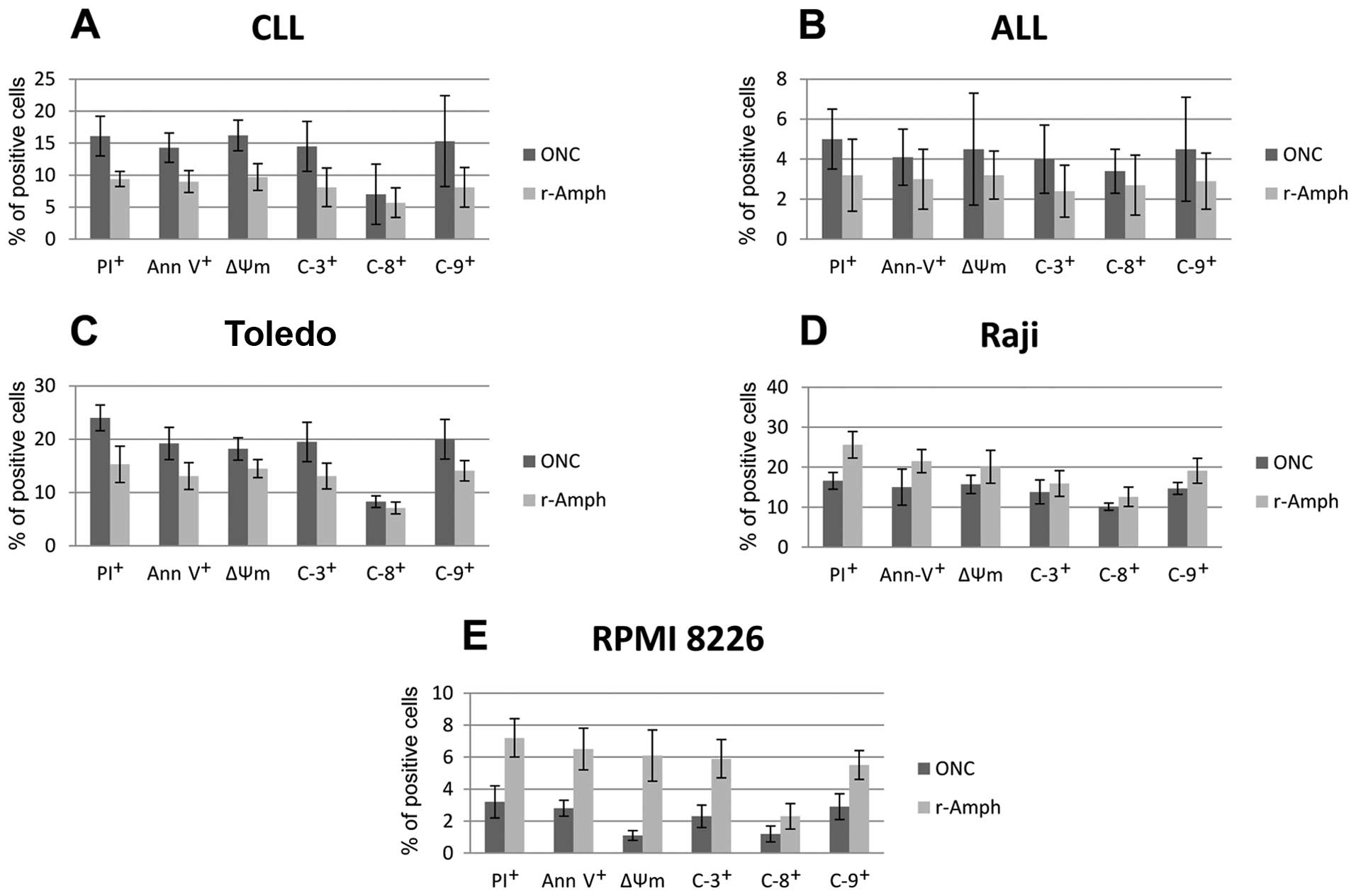
and internal caspase activation pathways (Fig. 1A).
In CLL cells, significant effect of both ONC and
r-Amph was evident after 72 h of treatment (median AI 38.1% and
31.5, respectively; vs. untreated control cells; p= 0.007 and
p=0.012, respectively). Combination of ONC, but not r-Amph, with FA
exerted significantly stronger effect than single drugs
(p<0.005) (Fig. 2A).
In case of ALL cells we did not observe any
significant cytotoxic activity of neither ONC nor r-Amph.
Interestingly, ONC+DOX combination, but not r-Amph, showed
significantly higher pro-apoptotic effect compared to the single
agents (p<0.005) (Fig. 2B).
Interestingly, both ONC and r-Amph were highly
active against the DLBCL-derived cell line Toledo, and even at the
lowest concentrations induced significant toxicity. Mean AI for ONC
27.8 and 19.1%, respectively; vs. untreated cells; p=0.001 and p=
0.008, respectively). Also, combinations of ONC or r-Amph with DOX
induced significantly higher pro-apoptotic effect than single
agents (p<0.003 and p<0.013, respectively) (Fig. 2C).
Both ONC and r-Amph showed significant antitumor
activity in the Burkitt’s lymphoma-derived cell line Raji (median
AI 23.1 and 30.3%, respectively; vs. control; p=0.007 and p= 0.001,
respectively). Combinations of either ONC or r-Amph with DOX
induced higher pro-apoptotic effects than single agents (p<0.005
and p<0.001, respectively) (Fig.
2D).
In MM cell line, RPMI 8226, only r-Amph alone
induced significant cytotoxity vs. control as a single drug (mean
AI 13.3%; vs. untreated control cells; p=0.036). However,
combination of both RNases with DEX exerted significantly higher
effects compared to single drugs (for ONC+DEX p<0.01; for
r-Amph+DEX p<0.0009) (Fig.
2E).
Mechanisms of RNase-induced
apoptosis
Apoptosis induced by both ONC and r-Amph was
evidently dependent on both, mitochondrial and external
caspase-activation pathways, inducing activation of caspases -9, -8
and -3; as well as decline of mitochondrial potential (Fig. 2).
Both ONC and r-Amph induced various effects on
apoptosis-regulating proteins (Table
II). In general, overexpression of Bax, except in ALL cells
treated with r-Amph.
 | Table II.Apoptosis-regulating proteins. |
Table II.
Apoptosis-regulating proteins.
A,
Apoptosis-regulating protein expression in response to onconase
(ONC).
|
| Cells | Bax | Bak | Bad | Bcl-2 | Mcl-1 | c-IAP1 | c-IAP2 | Survivin | XIAP | Smac-Diablo |
|
| CLL | ↑a | NS | NS | NS | ↓a | ↓a | NS | NS | ↓a | NS |
| ALL | ↑a | NS | NS | ↓a | NS | NS | NS | NS | ↓a | NS |
| Toledo | ↑a | NS | ↑a | NS | NS | NS | NS | NS | ↓a | NS |
| Raji | ↑a | NS | NS | ↓a | NS | NS | NS | NS | NS | NS |
| RPMI 8226 | ↑a | ↑a | NS | ↓a | NS | NS | NS | ↓a | NS | NS |
|
B,
Apoptosis-regulating protein expression in response to r-amphinase
(r-Amph).
|
| Cells | Bax | Bak | Bad | Bcl-2 | Mcl-1 | c-IAP1 | c-IAP2 | Survivin | XIAP | Smac-Diablo |
|
| CLL | ↑a | NS | NS | NS | ↓a | ↓a | NS | NS | ↓a | NS |
| ALL | NS | NS | NS | NS | NS | NS | NS | NS | ↓a | NS |
| Toledo | ↑a | NS | ↑a | ↓a | ↓a | NS | NS | NS | ↓a | NS |
| Raji | ↑a | ↑a | ↑a | NS | NS | NS | NS | NS | NS | ↑a |
| RPMI 8226 | ↑a | NS | NS | NS | NS | NS | NS | NS | NS | ↑a |
Other examined proteins show different level of
expression in response to RNase treatment. Namely, in CLL cells
treated with either ONC or r-Amph, despite the increase in Bax
expression, downregulation or Mcl-1, CIAP1 and XIAP proteins was
found.
In ALL cells treated with ONC, overexpression of
Bax, together with a decrease in Bcl-2 and XIAP expression was
found. In regard to r-Amph only downregulation of XIAP protein was
observed.
In Toledo cells ONC induced overexpression of Bax
and Bad, and downregulation of XIAP protein. R-Amph exerted more
effects on examined proteins, with increase of Bax and Bad, as well
as decrease in Bcl-2, Mcl-2 and XIAP protein expression.
In Raji cell line, ONC enhanced Bax and decreased
Bcl-2 levels, whereas r-Amph induced overexpression of Bax, Bak,
Bad and Smac/Diablo (IAP antagonist) proteins.
In RPMI 8226 cells, ONC triggered increased
expression of Bax and Bak, whereas decrease in Bcl-2 and survivin
levels. In r-Amph-treated cells overexpression of Bax and
Smac/Diablo proteins was noted (Table
II).
Effect of ONC and r-Amph on healthy
lymphocytes
Importantly, ONC and to some extent r-Amph showed
selective cytotoxicity on tumor cells, while sparing healthy cells.
Namely, all doses of ONC used in different experimental models did
not significantly affect survival of healthy lymphocytes.
Similarly, the lower doses of r-Amph, up to 30 μg/ml, did
not exert significant cytotoxic effect on the same cells (Table III).
 | Table III.Cytotoxic effectsa on healthy lymphocytes
treated for 72 h with onconase (ONC) or r-amphinase (r-Amph) vs.
untreated control samples (Ctrl). |
Table III.
Cytotoxic effectsa on healthy lymphocytes
treated for 72 h with onconase (ONC) or r-amphinase (r-Amph) vs.
untreated control samples (Ctrl).
| RNase | Drug doses | Statistics |
|---|
| ONC | 10
μg/ml | 20
μg/ml | 30
μg/ml | ONC vs. Ctrl |
| 5.1±2.5b | 7.0±2.9b | 9.7±4.1b |
| r-Amph | 10
μg/ml | 30
μg/ml | 60
μg/ml | r-Amph vs.
Ctrl |
| 4.8±1.1b | 6.75±2.1b | 17.7±4.7c |
Discussion
Various novel agents are investigated for treatment
of hemato-logic malignancies, as the current approaches are not
curative. Despite great advances, there is still a group of
patients, who achieve only partial response, or have refractory
disease after the initial treatment. In this context, innovative
and new treatment strategies are warranted. Great majority of the
new anticancer drugs are targeting either DNA, specific receptors
on cell surface or proteins with signal transduction properties
that are overly activated in cancer. RNases, by targeting the
post-transcriptional events, namely different species of RNA,
including microRNAs, can be considered as the anticancer drugs with
entirely new mechanism of action (7).
There is a growing body of evidence that prolonged
exposure of cancer cells to ONC or r-Amph leads to apoptosis
(13,14). Induction of apoptosis is considered
to be the predominant mechanism responsible for cytotoxic and
cytostatic activity of both RNases. Cell death is manifested by
typical morphologic changes and activation of caspases which
provide the mechanism leading to cell demise. Whereas both ONC and
Amph cause prolongation or arrest in G1 cycle phase and results in
apoptosis that involves classical activation of endonucleases,
caspases, transglutaminase and serine proteases (14). However in studies investigating
mechanism of antitumor activity of ONC the caspase independent
pathway was also recognized (15).
Our present study reveals strong pro-apoptotic activity of ONC and
r-Amph in both CLL and aggressive B cell lymphomas, with less
evident impact for surviving of ALL or MM cells. The mechanism
appears to depend on both the mitochondrial and external
caspase-activation pathways since caspases -9, -8, -3 are activated
concurrently with a decline of mitochondrial potential. It should
be noted, that specificity of FLICA reagents towards particular
caspases is not absolute (16) and
therefore activation of caspase-9 should be interpreted with
caution. Moreover, in our study we observed overexpression of Bax
and Bak proteins and downregulation of Bcl-2 expression in cells
treated with either of the RNases. Interestingly, the
concentrations of ONC used in different experimental models did not
significantly affect the viability of normal lymphocytes.
In this study we demonstrated that both ONC and
r-Amph have strong cytotoxic activity against cancer cells. Unlike
most chemotherapeutic agents that act rather rapidly the effects of
RNases can be observed after some delay (17). We observed significant effect of 72
h treatment of CLL cells with both RNases. In MM cells, only r-Amph
alone induced signifycant cytotoxity vs. control (p<0.05 both
in vitro and ex vivo). Both ONC and r-Amph showed
significant antitumor activity in Burkitt’s lymphoma-derived Raji
cell line (vs. control; p=0.007 and p=0,001, respectively). The
most sensitive was the DLBCL-derived cell line which responded to
ONC as well as r-Amph; even at the lowest concentrations. It was
crucial that, the same doses did not affect the survival of healthy
lymphocytes.
The major advantage of r-Amph and ONC over other
anticancer treatment is their apparent preference to tumor cells
(18). The mechanism responsible
for this effect is still not entirely elucidated. The most probable
explanation of this phenomenon may be related to cationic
properties of RNases that favor their preferential binding to a
negatively charged cell membrane. In cancer cells the level of
sialic acid, rich in gangliosides, is higher which leads to
electro-negativity of the plasma membrane. Because RNases are
strongly cationic they have greater electrostatic affinity to the
anionic surface and consequently are more avidly internalized by
tumor cells (19). This results in
their more effective attachment to cancer cells than to the normal
ones. After traversing through the plasma membrane by endocytosis
the RNases are released in the cytosol where RNA degradation occurs
(20).
Of particular interest is the observation that ONC
is strongly synergistic when combined with different
well-characterized anticancer agents. Initially the synergism was
observed on human lung carcinoma or pancreatic adenocarcinoma cell
lines, when ONC was used in combination with either tamoxifen or
trifluoroperazine (10).
Subsequently, a series of investigations were carried out revealing
a synergism or additive effects of ONC in combination with a
variety of other widely used agents such as lovastatin (21), interferons or vincristine (10), tumor necrosis factor α (22) as well as cepharantine (23). The striking feature is that the
observed synergisms were in combinations with different antitumor
agents, characterized by a distinct mechanism of action. Such
studies have not been performed with r-Amph thus far. It is likely
that the mechanism behind the synergistic effect of ONC with
respect to several antitumor drugs stems from the fact that ONC
targets microRNAi (8,24) and there is strong evidence that the
multidrug resistance provided by the (ABC)-transporter
P-glycoprotein is regulated by the RNA interference (25–27).
In the present study we observed that both ONC and
r-Amph were highly active against DLBCL-derived cell line.
Interestingly, the addition of DOX significantly amplified the
pro-apoptotic effect against DLBCL cells. Similar effect was also
observed for both ONC and r-Amph in Burkitt’s lymphoma-derived Raji
cell line in combinations with DOX compared to single agents. In
the case of ALL cells only ONC+DOX combination showed significantly
higher pro-apoptotic effect compared to the single agents. There
were no statistical differences when r-Amph was studied. Last but
not the least, in the case of cells from CLL patients the
combination of ONC, but not r-Amph, with 2-CdA or FA exerted
significantly stronger effect than single drugs.
It should be noted that whereas much has been
already learned about cytotoxic capabilities of RNases towards
cancer cells, the great majority of in vivo and in
vitro studies was made with particular emphasis on ONC
(28). The cytostatic, cytotoxic
and anticancer effects of Amph have not been extensively
investigated so far. Additional clinical trials and studies on
animal models are crucial to reveal the exact mechanisms of
antitumor activity and clinical potential of both RNases. Of
particular value would be in vivo studies of their
anticancer effectiveness carried on human tumors transplanted in
immunocompromised mice.
Acknowledgements
The study was supported by the grant
from Ministry of Science/National Science Centre, Poland (no.
507-18-010) and, in part, by the grant from Medical University of
Lodz, Poland (no. 503/8-093-01/503-01).
References
|
1.
|
Arnold U and Ulbrich-Hofmann R: Natural
and engineered ribonucleases as potential cancer therapies.
Biotechnol Lett. 28:1615–1622. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Leland PA and Raines RT: Cancer
chemotherapy - ribonucleases to the rescue. Chem Biol. 8:405–413.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Leland PA, Schultz LW, Kim BM, et al:
Ribonuclease A variants with potent cytotoxic activity. Proc Natl
Acad Sci USA. 95:10407–10112. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Turcotte RF and Raines RT: Interaction of
onconase with the human ribonuclease inhibitor protein. Biochem
Biophys Res Commun. 377:512–514. 2008. View Article : Google Scholar
|
|
5.
|
Singh UP, Ardelt W, Saxena SK, et al:
Enzymatic and structural characterisation of amphinase, a novel
cytotoxic ribonuclease from Rana pipiens oocytes. J Mol
Biol. 371:93–111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Ardelt W, Mikulski SM and Shogen K: Amino
acid sequence of an anti-tumor protein from Rana pipiens
oocytes and early embryos. Homology to pancreatic ribonucleases. J
Biol Chem. 266:245–251. 1991.PubMed/NCBI
|
|
7.
|
Dyer KD and Rosenberg HF: The RNase a
superfamily: generation of diversity and innate host defense. Mol
Divers. 10:585–597. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Ardelt W, Shogen K and Darzynkiewicz Z:
Onconase and amphinase, the antitumor ribonucleases from Rana
pipiens oocytes. Curr Pharm Biotechnol. 9:215–225. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Zhao H, Ardelt B, Ardelt W, Shogen K and
Darzynkiewicz Z: The cytotoxic ribonuclease onconase targets RNAi
(siRNA). Cell Cycle. 7:3258–3261. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Mikulski SM, Viera A, Ardelt W, et al:
Tamoxifen and trifluoroperazine (Stelazine) potentiate
cytostatic/cytotoxic effects of P-30 protein, a novel protein
possessing anti-tumor activity. Cell Tissue Kinet. 23:237–346.
1990.
|
|
11.
|
Rybak SM, Pearson JW, Fogler WE, et al:
Enhancement of vincristine cytotoxicity in drug-resistant cells by
simultaneous treatment with onconase, an antitumor ribonuclease. J
Natl Cancer Inst. 88:747–753. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Smolewski P, Bedner E, Du L, Hsieh TC, Wu
JM, Phelps DJ and Darzynkiewicz Z: Detection of caspases activation
by fluorochrome-labeled inhibitors: multiparameter analysis by
laser scanning cytometry. Cytometry. 44:73–82. 2001. View Article : Google Scholar
|
|
13.
|
Ardelt W, Ardelt B and Darzynkiewicz Z:
Ribonucleases as potential modalities in anticancer therapy. Eur J
Pharmacol. 625:181–189. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Zwolińska M and Smolewski P: Onconase: a
ribonuclease with antitumor activity. Post Hig Med Dosw. 64:58–66.
2009.
|
|
15.
|
Michaelis M, Cinatl J, Anand P, et al:
Onconase induces caspase-independent cell death in chemoresistant
neuroblastoma cells. Cancer Lett. 250:107–116. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Pozarowski P, Huang X, Halicka DH, Lee B,
Johnson G and Darzynkiewicz Z: Interactions of fluorochrome-labeled
caspase inhibitors with apoptotic cells. A caution in data
interpretation. Cytometry A. 55:50–60. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Ardelt B, Ardelt W, Pozarowski P, et al:
Cytostatic and cytotoxic properties of amphinase: a novel cytotoxic
ribonuclease from Rana pipiens oocytes. Cell Cycle.
6:3097–3102. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Matoušek J: Ribonucleases and their
antitumor activity. Comp Biochem Physiol. 129C:175–191. 2001.
|
|
19.
|
Fredman P: Glycosphingolipid tumor
antigens. Adv Lipid Res. 25:213–234. 1993.PubMed/NCBI
|
|
20.
|
Johnson RJ, Chao T-Y, Lavis LD, et al:
Biochemistry. 46:10308–10316. 2007. View Article : Google Scholar
|
|
21.
|
Mikulski SM, Viera A, Darzynkiewicz Z, et
al: Synergism between a novel amphibian oocyte ribonuclease and
lovastatin in inducing cytostatic and cytotoxic effects in human
lung and pancreatic carcinoma cell lines. Br J Cancer. 66:304–310.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Deptala A, Halicka HD, Ardelt B, et al:
Potentiation of tumor necrosis factor induced apoptosis by
onconase. Int J Oncol. 13:11–16. 1998.PubMed/NCBI
|
|
23.
|
Ita M, Halicka HD, Tanaka T, Kurose A,
Ardelt B, Shogen K and Darzynkiewicz Z: Remarkable enhancement of
cytotoxicity of onconase and cepharanthine when used in combination
on various tumor cell lines. Cancer Biol Ther. 7:1104–1108. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Ardelt B, Ardelt W and Darzynkiewicz Z:
Cytotoxic ribonucleases and RNA interference (RNAi). Cell Cycle.
2:22–24. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Ikemura K, Yamamoto M, Miyazaki S,
Mizutani H, Iwamoto T and Okuda M: MicroRNA-145
post-transcriptionally regulates the expression and function of
P-glycoprotein in intestinal epithelial cells. Mol Pharmacol.
83:399–405. 2013. View Article : Google Scholar
|
|
26.
|
Zhu X, Li Y, Shen H, Li H, Long L, Hui L
and Xu W: miR-137 restoration sensitizes multidrug-resistant
MCF-7/ADM cells to anticancer agents by targeting YB-1. Acta
Biochim Biophys Sin (Shanghai). 45:80–86. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Bao L, Hazari S, Mehra S, Kaushal D, Moroz
K and Dash S: Increased expression of P-glycoprotein and
doxorubicin chemo-resistance of metastatic breast cancer is
regulated by miR-298. Am J Pathol. 180:2490–2503. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Costanzi J, Sidransky D, Navon A, et al:
Ribonucleases as a novel pro-apoptotic anticancer strategy: Review
of the preclinical and clinical data for Ranpirnase. Cancer Invest.
23:643–650. 2005. View Article : Google Scholar : PubMed/NCBI
|