Introduction
Mammalian hepatitis B X-interacting protein (HBXIP)
is a conserved 18 kDa protein, which was originally identified
because of its interaction with the C-terminus of hepatitis B virus
X protein (HBx) (1,2). HBXIP sequences are well conserved
among mammalian species, with close orthologues found in all
vertebrate species where sequence data exist. HBXIP formed a
complex with survivin, an anti-apoptotic protein that is
overexpressed in most human cancers, resulting in the suppression
of cell apoptosis through the mitochondrial/cytochrome pathway.
HBXIP also regulates centrosome duplication, causing excessive
centrosome production and multipolar mitotic spindles in HeLa cells
(3,4). HBXIP is involved in mTORC1 pathway
regulating cell growth (5). Our
previous studies reported that HBXIP was able to promote cell
proliferation and migration through S100A4 and IL-8 in breast
cancer cells (6,7). However, the mechanism by which HBXIP
enhances migration of ovarian cancer cells is poorly
understood.
S-phase kinase-associated protein 2 (Skp2) belongs
to the family of the F-box proteins. Skp2, which was originally
discovered by Zhang et al in 1995, because of its ability to
interact with the cell cycle protein cyclin A, is necessary for DNA
replication (8). The Skp2 protein
levels changes during the cell cycle, which is low in early G1
phase, while it is high during G1/S transition (9). This alteration in the Skp2 protein
level during cell cycle progression is partly due to a change in
its gene expression and protein stability (10). A previous report showed that Skp2
overexpression in prostate cancer cells markedly promoted cancer
cell growth and tumorigenesis in a xenograft tumor model (11). And other groups showed that Skp2
deficiency displayed a defect in cell migration and metastasis,
while Skp2 overexpression promoted cell migration and invasion
(11,12). Inuzuka et al have shown that
acetylation of Skp2 enhanced cellular migration through
ubiquitination and destruction of E-cadherin (13). Subsequent experiments revealed that
Skp2 was involved in cell cycle progression. Cardozo and Pagano
have shown that Skp2 plays an important role in governing cell
cycle progression and cell survival by promoting the destruction of
numerous tumor suppressor proteins, including p27, p21, p57, p130
and FOXO1 (14). Aberrant Skp2
signaling has been implicated as a driving event in tumorigenesis.
Overexpression of Skp2 was frequently observed in numerous human
cancers, such as ovarian, colorectal, gastric, prostate, lung,
sarcoma, breast and other cancers (15–26).
These observations suggest that Skp2 may contribute to the
development of human cancers. Accumulated evidence suggests that
Skp2 displays a proto-oncogenic role in vitro and in
vivo. Previous report showed that repamycin, an mTOR inhibitor,
could downregulate the expression of Skp2 in breast cancer
(27). Thus, we speculate Skp2 may
play an important role in the function mediated by HBXIP.
In the present study, we investigated the role of
HBXIP and Skp2 in migration of ovarian cancer cells, with the hope
that such associations might provide insight into the causal
mechanisms by which HBXIP enhances the migration of ovarian cancer
cells.
Materials and methods
Immunohistochemistry
The ovarian carcinoma tissue micro-arrays were
obtained from the Xi’an Aomei Biotechnology Co., Ltd. (Xi’an,
China). These microarrays (catalog no. C1026) were composed of 80
ovarian carcinoma tissue samples (average age 39), which included
duplicate core biopsies (1 mm in diameter) from fixed,
paraffin-embedded tumors. Immunohistochemical staining of samples
were performed as previously reported (28) and the primary antibody of rabbit
anti-HBXIP (1:100, Proteintech Group, Chicago, IL, USA) or the
primary antibody of rabbit anti-Skp2 (1:30, Boster Group, Wuhan,
China) was used. Immunostained slides were evaluated under a
microscope. Categorization of immunostaining intensity was
performed by three independent observers. The staining levels of
HBXIP and Skp2 were classified into three groups using a modified
scoring method based on the intensity of staining (0, negative; 1,
low; and 2, high) and the percentage of stained cells (0, 0%
stained; 1, 1–49% stained; and 2, 50–100% stained). A multiplied
score (intensity score x percentage score) lower than 1 was
considered to be a negative staining (−), 1 and 2 were considered
to be moderate staining (+), and 4 was considered to be intense
staining (++).
Cell lines and cell culture
Ovarian cancer cell lines, SKOV3 cells (29) were cultured in RPMI-1640 medium
(Gibco-BRL, Grand Island, NY, USA), 10% fetal bovine serum (FBS);
CAOV3 cells (29) were cultured in
DMEM medium (Gibco-BRL), 10% FBS. 100 U/ml penicillin and 100
μg/ml streptomycin in humidified 5% CO2 at
37°C.
Plasmid construction and small
interference RNA (siRNA)
pCMV-tag2B, pGL3-Basic vectors (Promega, Madison,
WI, USA), pCMV-HBXIP was maintained in our laboratory (6). The 5′-flanking region (from -1309 to
+235 nt) of Skp2 gene was inserted into the KpnI/XhoI
site upstream of the luciferase gene in the pGL3-basic vector,
termed pGL3-Skp2 promoter. Mutant construction of Skp2 promoter,
termed as pGL3-Skp2 promoter mut, carried a series substitution of
nucleotides within Sp1 binding site. The complete human Skp2
(GenBank accession no. NC 000005.9) gene was subcloned into
pCMV-tag2B vector to generate the pCMV-Skp2 construct. siRNA
duplexes targeting human HBXIP (or Skp2) gene and siRNA duplexes
with non-specific sequences using as negative control (NC) were
synthesized by RiboBio (Guangzhou, China) (3,30).
All primers and siRNA sequences are listed in Table I.
 | Table I.The primers and sequences usxed in
this study. |
Table I.
The primers and sequences usxed in
this study.
| Gene | Primer | Sequence
(5′→3′) |
|---|
| Primers for Skp2
promoter | | |
| −1309 | Forward |
CGGGGTACCCCGTCCCTTCTTTACACCAATCTC |
| +235 | Reverse |
CCGCTCGAGCGGCGTTTACCTGTGCATAGCG |
| Sp-1-MUT | Forward |
CACGCTCGGAGCAGCTGTGCGCCAAAGCGG |
| Reverse |
CCGCTTTGGCGCACAGCTGCTCCGAGCGTG |
| Primers for
qRT-PCR | | |
| Skp2 | Forward |
CTTTCTGGGTGTTCTGGATTCTC |
| Reverse |
TGGAAGTTCTGTATGTTTGAGGG |
| HBXIP | Forward |
ATGGAGCCAGGTGCAGGTC |
| Reverse |
TGGAGGGATTCTTCATTGTG |
| GAPDH | Forward |
CATCACCATCTTCCAGGAGCG |
| Reverse |
TGACCTTGCCCACAGCCTTG |
| Primers for
ChIP | | |
| −640 | Forward |
GCGGGACGGAAACTACAA |
| −443 | Reverse |
TGCATTAACTGCAGAGCTGC |
| siRNA duplexes | | |
| HBXIP siRNA | Sense |
CGGAAGCGCAGUGAUGUUUdTdT |
| Antisense | AAACAUCACUGCGCUUCCG
dTdT |
| Skp2 siRNA | Sense |
GCAAAGGGAGTGACAAAdTdT |
| Antisense |
TTTGTCACTCCCTTTGCdTdT |
| Control siRNA | Sense |
UUCUCCGAACGUGUCACGUdTdT |
| Antisense |
ACGUGACACGUUCGGAGAAdTdT |
Transfection
One day before transfection, cells were harvested
and seeded into 6- or 24-well plates. Cells were transfected with
plasmid or siRNAs using Lipofectamine 2000 reagent (Invitrogen,
Carlsbad, CA, USA) according to the manufacturer’s protocol.
Wound-healing assay and Transwell
migration assay
Cells transfected with plasmid of HBXIP, plasmid of
HBXIP and siRNAs of Skp2, siRNAs of HBXIP, siRNAs of HBXIP and
pCMV-Skp2 were seeded in a 6-well plate and cultured for 24 h to
form confluent monolayers. A wound was created by dragging a
pipette tip through the monolayer, and plates were washed using
pre-warmed PBS to remove cellular debris. Wound images were
photographed at 0, 24, 48 and 72 h after wounding. The wound gaps
were measured at each time point. For Transwell migration assay
using SKOV3 and CAOV3 cells with indicated treatment,
5×105 cells were plated on 8 μm Transwell filters
(Corning Incorporated, Corning, NY, USA). The cells were induced to
migrate towards medium containing 10% FBS for 20 h. Non-migrating
cells were removed with a cotton swab. The remaining cells were
fixed, stained with hematoxylin and eosin, and analysed by a
bright-field microscope.
RNA extraction and reverse-transcription
polymerase chain reaction (RT-PCR)
Total RNA of cells was extracted using TRIzol
reagent (Invitrogen). First-strand cDNA was synthesized by
PrimeScript reverse transcriptase (Takara Bio, Dalian, China) and
oligo (dT) following the manufacturer’s instructions. The primers
are listed in Table I.
Western blot analysis
Western blot analysis was carried out with standard
protocols. Primary antibodies used were rabbit anti-Skp2 (1:300,
Boster Group), rabbit anti-HBXIP (1:1,000, Proteintech Group), and
mouse anti-β-actin (1:800, Sigma-Aldrich, St. Louis, MO, USA). All
experiments were repeated 3 times.
Luciferase reporter gene assays
For luciferase reporter gene assays, the ovarian
cancer cells were transfected with plasmids encoding HBXIP by
Lipofectamine 2000. The luciferase activities were determined 48 h
after transfection, and the results are the average of 3
independent repeats. The luciferase activities in the cell lysates
were measured by a dual luciferase reporter assay kit (Promega),
and the luciferase activity was normalized with renilla luciferase
activity.
Chromatin immunoprecipitation (ChIP) and
ReChIP assay
The ChIP assay was performed using the EpiQuik™
chromatin immunoprecipitation kit from Epigentek Group Inc
(Farmingdale, NY, USA) according to the published methods (6,31).
Protein-DNA complexes were immunoprecipitated with HBXIP
antibodies, whereas rabbit preimmune serum served as a control. DNA
from input or immunoprecipitated samples was assayed using
SYBR-Green-based quantitative PCR with specific primers designed to
amplify the Skp2 promoter around the SREs. ChIP/ReChIP: ChIP was
performed as above, binding complexes from the first
immunoprecipitation were eluted from the sepharose beads using
Re-ChIP buffer. The eluted protein-DNA complexes were diluted in
radioimmunoprecipitation buffer and resubjected to ChIP using a
different antibody.
Statistical analysis
Each experiment was repeated at least three times.
Statistical significance was assessed by comparing mean values (±
SD) using a Student’s t-test for independent groups or pairing
χ2 for dependent groups and was assumed for
*P<0.05, **P<0.01 and
***P<0.001.
Results
The expression of HBXIP is positively
associated with that of Skp2 in clinical ovarian cancer
tissues
Our previous reports showed that HBXIP was
overexpressed in breast cancer and other cancer cells. However,
there is no report concerning the expression of HBXIP in ovarian
cancer. In this study, we examined the expression of HBXIP in
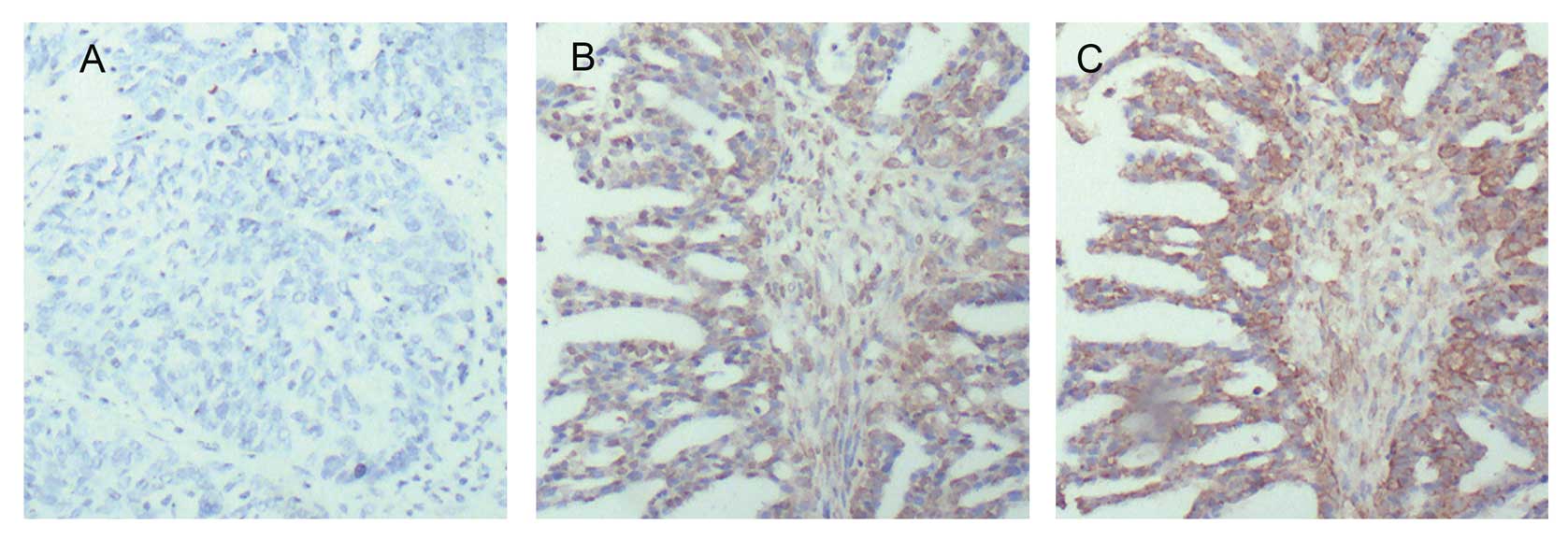
ovarian cancer. The data revealed that HBXIP was overexpressed in
ovarian cancer tissue (Fig. 1). It
has been reported that Skp2 is also over-expressed in ovarian
cancer tissues (23). Thus, we
supposed that overexpression of Skp2 might be correlated with
enhanced HBXIP in ovarian cancer. Then, we investigated the
expression correlation between HBXIP and Skp2 by IHC using tissue
microarrays from the same tissue paraffin block. Our data showed
that the positive rate of HBXIP was 75% (60/80) in clinical ovarian
cancer tissue samples, and the positive rate of Skp2 was 81.67%
(49/60) in the HBXIP-positive specimens (Fig. 1). Pairing χ2 analysis
showed that there was no significant difference between the
positive rate of HBXIP and that of Skp2 in the tissues (P>0.05,
Table II), suggesting that the
expression of Skp2 is relevant to that of HBXIP in ovarian cancer
tissues. Additionally, in this study, IHC staining showed that the
expression of HBXIP could be observed in both cytoplasm and nucleus
in ovarian cancer tissues (Fig.
1B). We also observed that Skp2 was expressed in both cytoplasm
and nucleus in ovarian cancer tissues (Fig. 1C), which is consistent with a
previous study (32). Thus, we
speculated that HBXIP might be involved in the transcriptional
regulation of Skp2.
 | Table II.Cross tabulation analysis of HBXIP
and Skp2 in ovarian cancer tissues. |
Table II.
Cross tabulation analysis of HBXIP
and Skp2 in ovarian cancer tissues.
| HBXIP |
|---|
|
|---|
| Total | Negative | Positive |
|---|
| Skp2 | | | |
| Negative | 20 | 5 (25.00%) | 15 (75.00%) |
| Positive | 60 | 11 (18.33%) | 49 (81.67%) |
| Total | 80 | 16 (20.00%) | 64 (80.00%) |
Skp2 is responsible for HBXIP-enhanced
migration of ovarian cancer cells in vitro
Cell migration is an essential process in cancer
metastasis and HBXIP can promote cell migration in breast cancer
cells (6,7). Other reports showed that Skp2 can
modulate cell migration of breast cancer, prostate cancer and
myxofibrosarcoma (18,21,22,24).
Thus, we supposed that Skp2 might be involved in the migration
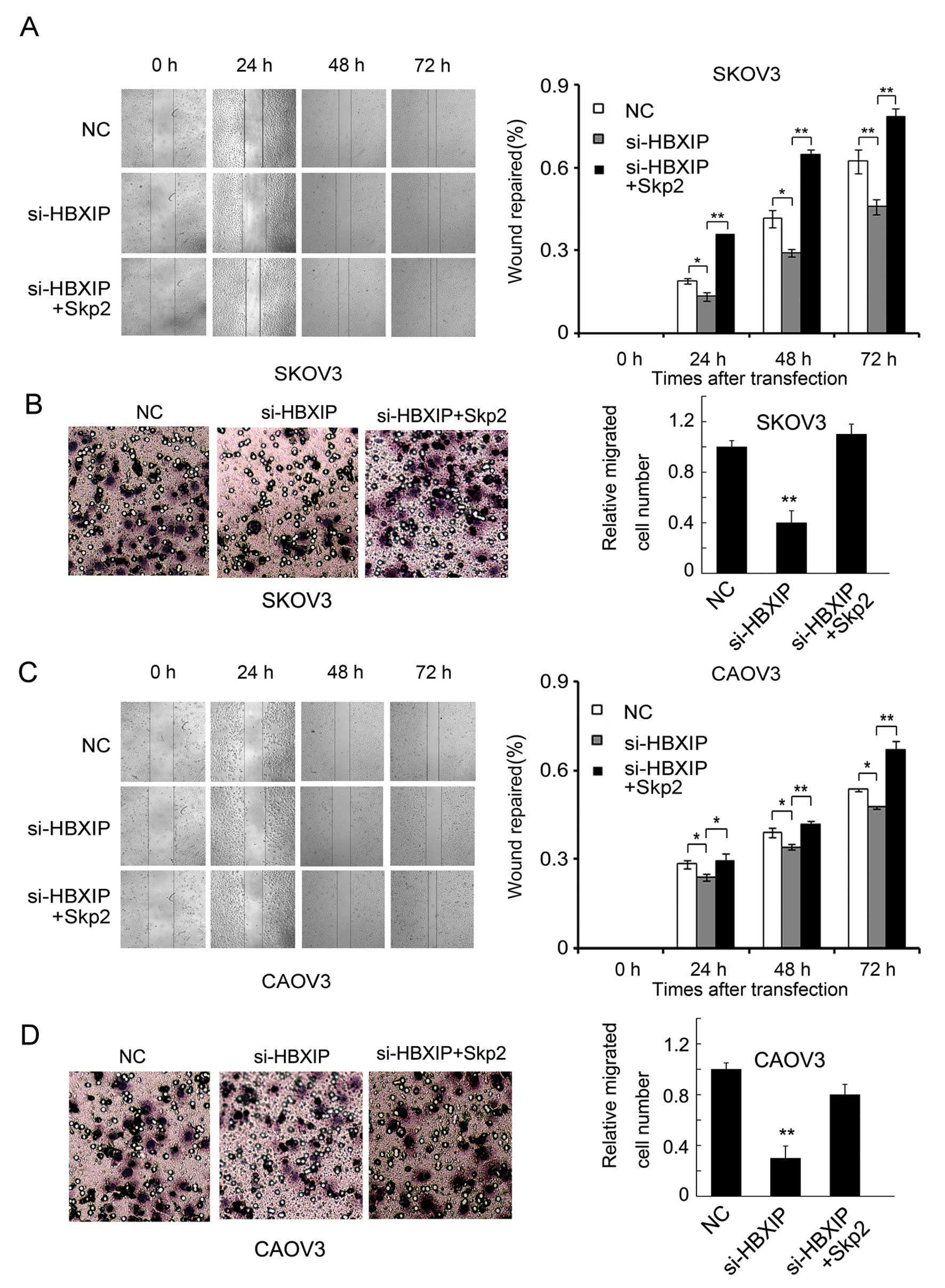
enhanced by HBXIP. Wound-healing and Transwell assays showed that
treatment with plasmid encoding HBXIP enhanced the migration of
SKOV3 cells, while additionally treated with Skp2 siRNAs abolished
the effect (Fig. 2A and B).
Furthermore, we performed the same assay using another ovarian
cancer cell line CAOV3, and obtained similar results (Fig. 2C and D). We found that the
inhibited migration of SKOV3 ovarian cancer cells induced by HBXIP
siRNAs, was rescued by the overexpression of Skp2 (Fig. 3A and B). Similar results occurred
in CAOV3 ovarian cancer cell line (Fig. 3C and D). Furthermore, as shown in
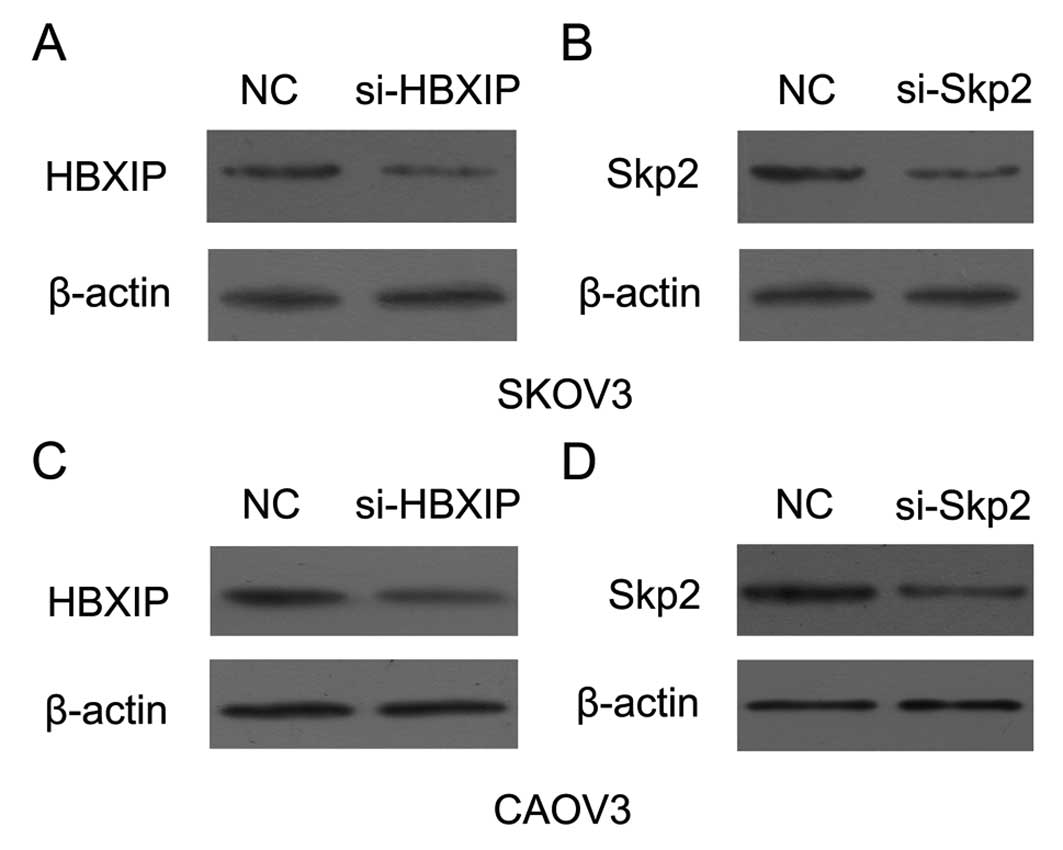
Fig. 4, the RNA interference of
HBXIP (or Skp2) decreased the expression of protein levels in SKOV3
and CAOV3 cells. Thus, our data suggest that HBXIP promotes the
migration of ovarian cancer cells through Skp2 in vitro.
HBXIP upregulates the expression of Skp2
in ovarian cancer cells
Next, we evaluated whether HBXIP was able to
upregulate Skp2 in ovarian cancer cell lines. After transfection
with plasmid encoding HBXIP, we observed that the levels of mRNA
and protein of Skp2 were upregulated by HBXIP in SKOV3 and CAOV3
cell lines in a dose-dependent manner (Fig. 5). Thus, we verified that HBXIP was
able to upregulate Skp2 in ovarian cancer cells.
HBXIP activates Skp2 promoter via
transcription factor Sp1
Furthermore, to explore the mechanism by which HBXIP
upregulated Skp2, we cloned the promoter region of Skp2
(-1309/+235) into pGL3-Basic plasmid. Luciferase reporter gene
assays showed that HBXIP could increase the promoter activities of
Skp2 in SKOV3 or CAOV3 ovarian cancer cells in a dose-dependent
manner (Fig. 6A and B), suggesting
that HBXIP is capable of activating the Skp2 promoter in the
ovarian cancer cells. Next, we investigated the underlying
mechanism by which HBXIP activates Skp2 promoter. To map the HBXIP
binding site in Skp2 promoter, we designed a series of primers of
Skp2 promoter fragments in 5′-flanking region, including the
fragments −776/−567, −640/−443 and −464/−250. Interestingly, ChIP
assays showed that HBXIP was able to occupy the Skp2 promoter
fragment −640/−443 (Fig. 6C),
suggesting that the −640/−443 region of Skp2 promoter is the
regulatory target sequence of HBXIP. Then, we used online promoter
analysis tool Search Promoter Site (http://alggen.lsi.upc.es/cgibin/promo_v3/promo/promoinit.cgi?dir-DB=TF8.3)
to predict the putative transcription factor binding sites in the
−640/−443 promoter region of Skp2. Strikingly, we observed a
putative Sp1 binding site in the region (Fig. 6D). ChIP/ReChIP assay showed that
HBXIP and Sp1 were able to form a transcriptional complex on the
Skp2 promoter (Fig. 6E).
Luciferase reporter gene assays showed that HBXIP failed to work
when the Sp1 binding site in Skp2 promoter was mutated (Fig. 6F and G). Thus, we conclude that
HBXIP activates Skp2 promoter activity through the transcription
factor Sp1.
Discussion
Our studies have showed that HBXIP is a novel
oncoprotein. HBXIP was highly expressed in breast cancer tissues
and metastatic lymph node tissues and significantly associated with
the growth and metastasis of breast cancer cells (6,7,33).
However, the expression and role of HBXIP in ovarian cancer cells
is poorly understood. Many studies have shown that over-expression
of Skp2 is observed in a variety of human cancers, including
ovarian cancer, gastric cancer, colorectal cancer, prostate cancer,
sarcoma, breast cancer, lung cancer, pancreatic cancer and other
cancers (15,26). In addition, Skp2 has an established
role in the migration of cancer cells. Therefore, we are interested
in the effect of HBXIP on cell migration in ovarian cancer and the
role Skp2 plays in the signaling pathway.
Latest study showed that HBXIP was a regulator
components that is required for mTORC1 activation by amino acids
(5). Cross-talk between mTOR
pathway and Skp2 pathway has been reported recently. Shapira et
al showed that repamycin, an mTOR inhibitor, could downregulate
the expression of Skp2 in breast cancer (27). Shigemasa et al proved that
Skp2 was expressed in nearly half of the 91 ovarian adenocarcinomas
(23). In this study, we first
observed that HBXIP is overexpressed in ovarian cancer tissues. We
noted that the expression of HBXIP was significantly correlated
with Skp2 in ovarian cancer tissues.
Skp2 overexpression has been correlated with tumor
progression such as stage and survival in ovarian cancer and other
human cancers (16), indicating
that Skp2 may be important in cancer cell migration, invasion and
metastasis. Previous reports showed that Skp2 deficiency displayed
a defect in cell migration and metastasis, while Skp2
overexpression promoted cell migration and invasion (11,12).
It has also been shown that acetylation of Skp2 enhanced cellular
migration (13). Consistent with
this notion, we found that Skp2 is responsible for the enhanced
migration of ovarian cancer cells mediated by HBXIP.
We observed that HBXIP was able to upregulate the
mRNA and protein levels of Skp2 in ovarian cancer cells. Next, we
sought to elucidate the underlying mechanism by which HBXIP
upregulates Skp2. We previously observed the nuclear localization
of HBXIP in MCF-7 cells (6), we
found a similar phenomenon in ovarian cancer tissues, implying that
HBXIP may be involved in the transcriptional regulation of Skp2.
Then, we predicted the putative transcription factor binding sites
in the -640/-443 promoter region of Skp2. Strikingly, we found a
Sp1 binding site in the region. Sp1 is a transcription factor that
either enhance or repress the activity of promoters of genes
involved in differentiation, cell cycle progression and oncogenesis
(34). In comparison to normal
tissues or cells, Sp1 level is greater in breast carcinomas,
thyroid cancer, hepatocellular carcinomas, pancreatic cancer,
colorectal cancer, gastric cancer and lung cancer (34–37).
Sp1 is also overexpressed in ovarian cancer (38) and plays an important role in the
process of cancer. We found that HBXIP was able to bind to the Skp2
promoter region through interacting with Sp1 by ChIP/ReChIP assays.
We further demonstrated that HBXIP activated Skp2 promoter through
the transcription factor Sp1. Thus, we report that the
transcription factor Sp1 plays a role in regulating Skp2 mediated
by HBXIP in ovarian cancer cells.
In summary, our finding indicates that HBXIP
promotes the migration of ovarian cancer cells through upregulating
Skp2, in which HBXIP activates the transcription of Skp2 through
interaction with transcription factor Sp1. HBXIP may act as a
co-activator of transcription factors to upregulate many genes in
the development of cancer. Therefore, our finding provides new
insight into the mechanism of HBXIP in promotion of migration of
ovarian cancer cells.
Acknowledgements
This study was supported by grants
from the National Basic Research Program of China (973 Program,
nos. 2011CB512113 and 2009CB521702) and National Natural Science
Foundation of China (nos. 81071623, 81071624 and 81272217).
References
|
1.
|
Melegari M, Scaglioni PP and Wands JR:
Cloning and characterization of a novel hepatitis B virus x binding
protein that inhibits viral replication. J Virol. 72:1737–1743.
1998.PubMed/NCBI
|
|
2.
|
Lok AS: Hepatitis B infection:
pathogenesis and management. J Hepatol. 32:89–97. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Fujii R, Zhu C, Wen Y, Marusawa H,
Bailly-Maitre B, Matsuzawa S, Zhang H, Kim Y, Bennett CF, Jiang W
and Reed JC: HBXIP, cellular target of hepatitis B virus
oncoprotein, is a regulator of centrosome dynamics and cytokinesis.
Cancer Res. 66:9099–9107. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Wen Y, Golubkov VS, Strongin AY, Jiang W
and Reed JC: Interaction of hepatitis B viral oncoprotein with
cellular target HBXIP dysregulates centrosome dynamics and mitotic
spindle formation. J Biol Chem. 283:2793–2803. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Bar-Peled L, Schweitzer LD, Zoncu R and
Sabatini DM: Ragulator is a GEF for the Rag GTPases that signal
amino acid levels to mTORC1. Cell. 150:1196–1208. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Liu S, Li L, Zhang Y, Zhang Y, Zhao Y, You
X, Lin Z, Zhang X and Ye L: The oncoprotein HBXIP uses two pathways
to up-regulate S100A4 in promotion of growth and migration of
breast cancer cells. J Biol Chem. 287:30228–30239. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Hu N, Zhang J, Cui W, Kong G, Zhang S, Yue
L, Bai X, Zhang Z, Zhang W, Zhang X and Ye L: miR-520b regulates
migration of breast cancer cells by targeting hepatitis B
X-interacting protein and interleukin-8. J Biol Chem.
286:13714–13722. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Zhang H, Kobayashi R, Galaktionov K and
Beach D: p19Skp1 and p45Skp2 are essential elements of the cyclin
A-CDK2 S phase kinase. Cell. 82:915–925. 1995. View Article : Google Scholar
|
|
9.
|
Kurland JF and Tansey WP: Crashing waves
of destruction: the cell cycle and APC(Cdh1) regulation of
SCF(Skp2). Cancer Cell. 5:305–306. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Suzuki S, Fukasawa H, Misaki T, Togawa A,
Ohashi N, Kitagawa K, Kotake Y, Liu N, Niida H, Nakayama K,
Nakayama KI, Yamamoto T and Kitagawa M: The amelioration of renal
damage in Skp2-deficient mice canceled by p27 Kip1 deficiency in
Skp2−/−p27−/−mice. PLoS One. 7:e362492012.PubMed/NCBI
|
|
11.
|
Lin HK, Wang G, Chen Z, Teruya-Feldstein
J, Liu Y, Chan CH, Yang WL, Erdjument-Bromage H, Nakayama KI, Nimer
S, Tempst P and Pandolfi PP: Phosphorylation-dependent regulation
of cytosolic localization and oncogenic function of Skp2 by
Akt/PKB. Nat Cell Biol. 11:420–432. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Chan CH, Lee SW, Li CF, Wang J, Yang WL,
Wu CY, Wu J, Nakayama KI, Kang HY, Huang HY, Hung MC, Pandolfi PP
and Lin HK: Deciphering the transcriptional complex critical for
RhoA gene expression and cancer metastasis. Nat Cell Biol.
12:457–467. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Inuzuka H, Gao D, Finley LW, Yang W, Wan
L, Fukushima H, Chin YR, Zhai B, Shaik S, Lau AW, Wang Z, Gygi SP,
Nakayama K, Teruya-Feldstein J, Toker A, Haigis MC, Pandolfi PP and
Wei W: Acetylation-dependent regulation of Skp2 function. Cell.
150:179–193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Cardozo T and Pagano M: The SCF ubiquitin
ligase: insights into a molecular machine. Nat Rev Mol Cell Biol.
5:739–751. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Bretones G, Acosta JC, Caraballo JM,
Ferrándiz N, Gómez-Casares MT, Albajar M, Blanco R, Ruiz P, Hung
WC, Albero MP, Perez-Roger I and León J: SKP2 oncogene is a direct
MYC target gene and MYC down-regulates p27(KIP1) through SKP2 in
human leukemia cells. J Biol Chem. 286:9815–9825. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Einama T, Kagata Y, Tsuda H, Morita D,
Ogata S, Ueda S, Takigawa T, Kawarabayashi N, Fukatsu K, Sugiura Y,
Matsubara O and Hatsuse K: High-level Skp2 expression in pancreatic
ductal adenocarcinoma: correlation with the extent of lymph node
metastasis, higher histological grade, and poorer patient outcome.
Pancreas. 32:376–381. 2006. View Article : Google Scholar
|
|
17.
|
Hung WC, Tseng WL, Shiea J and Chang HC:
Skp2 overexpression increases the expression of MMP-2 and MMP-9 and
invasion of lung cancer cells. Cancer Lett. 288:156–161. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Li CF, Wang JM, Kang HY, Huang CK, Wang
JW, Fang FM, Wang YH, Wu WR, Li SH, Yu SC, Lee JC, Lan J, Shiue YL,
Wu LC and Huang HY: Characterization of gene amplification-driven
SKP2 overexpression in myxofibrosarcoma: potential implications in
tumor progression and therapeutics. Clin Cancer Res. 18:1598–1610.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Lim MS, Adamson A, Lin Z, Perez-Ordonez B,
Jordan RC, Tripp S, Perkins SL and Elenitoba-Johnson KS: Expression
of Skp2, a p27(Kip1) ubiquitin ligase, in malignant lymphoma:
correlation with p27(Kip1) and proliferation index. Blood.
100:2950–2956. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Lu M, Zhao Y, Xu F, Wang Y, Xiang J and
Chen D: The expression and prognosis of FOXO3a and Skp2 in human
ovarian cancer. Med Oncol. 29:3409–3415. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Masuda TA, Inoue H, Sonoda H, Mine S,
Yoshikawa Y, Nakayama K, Nakayama K and Mori M: Clinical and
biological significance of S-phase kinase-associated protein 2
(Skp2) gene expression in gastric carcinoma: modulation of
malignant phenotype by Skp2 overexpression, possibly via p27
proteolysis. Cancer Res. 62:3819–3825. 2002.
|
|
22.
|
Shapira M, Ben-Izhak O, Linn S, Futerman
B, Minkov I and Hershko DD: The prognostic impact of the ubiquitin
ligase subunits Skp2 and Cks1 in colorectal carcinoma. Cancer.
103:1336–1346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Shigemasa K, Gu L, O’Brien TJ and Ohama K:
Skp2 over-expression is a prognostic factor in patients with
ovarian adenocarcinoma. Clin Cancer Res. 9:1756–1763.
2003.PubMed/NCBI
|
|
24.
|
Sonoda H, Inoue H, Ogawa K, Utsunomiya T,
Masuda TA and Mori M: Significance of skp2 expression in primary
breast cancer. Clin Cancer Res. 12:1215–1220. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Traub F, Mengel M, Luck HJ, Kreipe HH and
von Wasielewski R: Prognostic impact of Skp2 and p27 in human
breast cancer. Breast Cancer Res Treat. 99:185–191. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Wang Z, Gao D, Fukushima H, Inuzuka H, Liu
P, Wan L, Sarkar FH and Wei W: Skp2: a novel potential therapeutic
target for prostate cancer. Biochim Biophys Acta. 1825:11–17.
2012.PubMed/NCBI
|
|
27.
|
Shapira M, Kakiashvili E, Rosenberg T and
Hershko DD: The mTOR inhibitor rapamycin down-regulates the
expression of the ubiquitin ligase subunit Skp2 in breast cancer
cells. Breast Cancer Res. 8:R462006. View
Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Zhang X, Dong N, Yin L, Cai N, Ma H, You
J, Zhang H, Wang H, He R and Ye L: Hepatitis B virus X protein
upregulates survivin expression in hepatoma tissues. J Med Virol.
77:374–381. 2005. View Article : Google Scholar
|
|
29.
|
Al-Alem L, Southard RC, Kilgore MW and
Curry TE: Specific thiazolidinediones inhibit ovarian cancer cell
line proliferation and cause cell cycle arrest in a PPARgamma
independent manner. PLoS One. 6:e161792011. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Liang M, Liang YY, Wrighton K,
Ungermannova D, Wang XP, Brunicardi FC, Liu X, Feng XH and Lin X:
Ubiquitination and proteolysis of cancer-derived Smad4 mutants by
SCFSkp2. Mol Cell Biol. 24:7524–7537. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Nelson JD, Denisenko O and Bomsztyk K:
Protocol for the fast chromatin immunoprecipitation (ChIP) method.
Nat Protoc. 1:179–185. 2006. View Article : Google Scholar
|
|
32.
|
Hu D, Liu W, Wu G and Wan Y: Nuclear
translocation of Skp2 facilitates its destruction in response to
TGFbeta signaling. Cell Cycle. 10:285–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Wang FZ, Sha L, Ye LH and Zhang XD:
Promotion of cell proliferation by HBXIP via upregulation of human
telomerase reverse transcriptase in human mesenchymal stem cells.
Acta Pharmacol Sin. 29:83–89. 2008. View Article : Google Scholar
|
|
34.
|
Davie JR, He S, Li L, Sekhavat A, Espino
P, Drobic B, Dunn KL, Sun JM, Chen HY, Yu J, Pritchard S and Wang
X: Nuclear organization and chromatin dynamics - Sp1, Sp3 and
histone deacetylases. Adv Enzyme Regul. 48:189–208. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Zheng Y, Ritzenthaler JD, Sun X, Roman J
and Han S: Prostaglandin E2 stimulates human lung carcinoma cell
growth through induction of integrin-linked kinase: the involvement
of EP4 and Sp1. Cancer Res. 69:896–904. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Kong LM, Liao CG, Chen L, Yang HS, Zhang
SH, Zhang Z, Bian HJ, Xing JL and Chen ZN: Promoter hypomethylation
up-regulates CD147 expression through increasing Sp1 binding and
associates with poor prognosis in human hepatocellular carcinoma. J
Cell Mol Med. 15:1415–1428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Chuang JY, Wu CH, Lai MD, Chang WC and
Hung JJ: Overexpression of Sp1 leads to p53-dependent apoptosis in
cancer cells. Int J Cancer. 125:2066–2076. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Previdi S, Malek A, Albertini V, Riva C,
Capella C, Broggini M, Carbone GM, Rohr J and Catapano CV:
Inhibition of Sp1-dependent transcription and antitumor activity of
the new aureolic acid analogues mithramycin SDK and SK in human
ovarian cancer xenografts. Gynecol Oncol. 118:182–188. 2010.
View Article : Google Scholar : PubMed/NCBI
|