Introduction
Lung cancer is the leading cause of cancer-related
death worldwide. Approximately, 85% of lung cancer cases are
non-small cell lung cancer (NSCLC), which has a more variable
behavior and depends on histological type (1). Additionally, more than 40% of these
patients will have distant metastasis outside of the chest at the
time of diagnosis. Circulating tumor cells (CTCs) are cells that
have shed into the vasculature from a primary tumor, circulate in
the bloodstream and are seeds for the subsequent growth of
additional tumors, also called metastasis to vital distant organs.
CTCs trigger a mechanism that is responsible for the vast majority
of cancer-related deaths (2–5).
Their role in the metastatic pathway has proven to be
essential.
However, CTCs are rare, representing as few as one
cell per 109 hematologic cells in the blood of patients
with metastatic cancer; hence, their isolation presents a
tremendous technical challenge (6,7).
Microfluidic-based devices (called the CTC-chips) provide unique
opportunities to isolate, quantify and analyze circulating tumor
cells from a blood sample (8–10).
An average of 132 EpCAM-positive circulating tumor cells per
milliliter (median, 67 cells/ml) are isolated at high purity from
virtually all tested patients with metastatic cancers, but not from
healthy controls (10,11). The FDA-approved CellSearch system
has set the standard for the use of EpCAM in the enrichment of CTCs
using a magnetic ferrofluid approach (12–15).
EpCAM is also used as a main capture component in other
immunomagnetic bead-based systems as well as microfluidic systems
(16,17). A number of studies using the
CellSearch system have shown a good correlation between the numbers
of these circulating EpCAM-positive cells and the prognosis for
cancer survival (18,19).
However, the development of metastasis depends on
multiple factors that determine overall tumor cell growth,
survival, angiogenesis and invasion (20). For epithelial malignancies, the
epithelial-mesenchymal transition (EMT) is considered the crucial
event in the metastatic process, which involves the disruption of
epithelial cell homeostasis and the acquisition of a migratory
mesenchymal phenotype allowing these cells to travel to the site of
metastasis formation without being affected by conventional
treatment (21). Accordingly,
through mesenchymal-to-epithelial transition (MET), the opposite of
the EMT, a metastasis occurs followed by a micrometastasis
(22,23). The EMT appears to be controlled by
signal-transduction pathways such as the Wnt (24), transforming growth factor β (TGF-β)
(25) and HGF/cMET (26) pathways, all of which can be
aberrantly activated during neoplasia (27–29).
Gemcitabine is a nucleoside analog (30). As a chemical drug, gemcitabine
replaces one of the nucleic acids during DNA replication to arrest
the tumor growth as only one additional nucleoside can be attached
to the ‘faulty’ nucleoside, resulting in apoptosis (31). Gemcitabine has been used in various
carcinomas: non-small cell lung cancer, pancreatic cancer, bladder
cancer, breast cancer and other tumor types (32–35).
GemCarbo chemotherapy, consisting of a combination of gemcitabine
and carboplatin, is used to treat several different types of
cancer, but is most commonly used to treat later period non-small
cell lung cancer (36,37). However, the therapy indication of
gemcitabine for NSCLC with micrometastasis is still unknown. The
mechanism of action of gemcitabine as a chemotherapy drug for NSCLC
is also not fully understood.
Here, we detected the EpCAM-positive CTCs of NSCLC
patients before and after the gemcitabine treatment. Then, other
common clinical parameters and survival rates were followed up. The
mechanism study showed that gemcitabine targeted the EpCAM-positive
CTCs, inhibiting metastasis and invasion by inverting the EMT
features induced by the HGF/cMET pathway in NSCLC. These results
suggested that gemcitabine chemotherapy can effectively inhibit
metastasis and circulating tumor cells in non-small cell lung
cancer.
Materials and methods
Patient eligibility and study design
Forty patients aged 45–75 years, pathologically
diagnosed, without obvious distant metastases and in clinical
stages II and III of NSCLC regardless of surgical treatment were
enrolled in this study. Patients were randomly divided into two
groups of experimental and control. Eligibility criteria included
hemoglobin level ≥8 g/dl. Patients were ineligible if enrolled in a
concurrent treatment protocol in which the total weekly blood draw
would exceed 150 ml. The negative control samples were obtained
from healthy adults. Patients provided written informed consent and
the protocol was approved by the Cancer Research Center of Shaanxi
Province.
All patients were required to have disease
evaluations at 3-week intervals. The experimental group patients
underwent systemic gemcitabine therapy for 63 days in 3 periods.
The control group was treated with a palliative or curative
resection. Twenty milliliters of peripheral blood were obtained
from patients at study entry and at 3-week intervals to correspond
with points of disease evaluation by computed tomography (CT) scan.
After chemotherapy, all patients were rechecked and followed for 3
years.
During the period of recruitment, each subject was
scheduled for an interview after written informed consent was given
and a structured questionnaire was administered by the interviewer
to collect information about demographics and risk factors such as
smoking status and alcohol use. The population study was approved
by the institutional review board ‘Ethics Committee of Shaanxi
Province Tumor Hospital’ in Shannxi, China. The Ethics Committee of
Shaanxi Province Tumor Hospital approved the design of the NSCLC
study including samples collection.
Cell culture and drug
The human non-small cell lung cancer cell lines
A549, NCI-H460, HCC827 and NCI-H1299 were purchased from ATCC
(American Type Culture Collection, Manassas, VA, USA). Cells were
cultivated in RPMI-1640 medium (Sigma-Aldrich, St. Louis, MO), with
the appropriate amount of heat-inactivated fetal bovine serum (FBS,
Invitrogen, Carlsbad, CA) at 37°C in an atmosphere of 5%
CO2. All of the experiments were performed during the
cell growing to exponential phase and after a culture confluence of
80–90%.
For the first line of chemotherapy, all the patients
received platinum-based combination chemotherapy. The
chemotherapeutic agent added to the platinum was gemcitabine
(Lilly, Suresnes, France) at the recommended dose of 1250
mg/m2 for the 28 experimental group patients. In one
treatment period of 21 days, the treatment was administered to
patients in the first and eighth day for three periods. For the
cell line, gemcitabine was added at a concentration of 50 nmol/l
and the culture media was replaced every day. HGF agent was
purchased from Sigma Co. and added to the A549 cells at a
concentration of 50 μg/l.
Isolation and enrichment of CTC
To isolate CTCs from NSCLC patients, whole
peripheral blood cells (10 ml) were centrifuged with
Ficoll-Hypaque, a solution with a density of 1.077 g/ml. Because
red blood cells and granulocytes have densities >1.077 g/ml and
mononuclear cells have a density <1.077 g/ml, centrifugation
utilizing Ficoll-Hypaque helps create a layered separation of these
cell types. Mononuclear cells, lymphocytes, platelets and
granulocytes were collected after centrifugation and washed twice
with PBS. Cells were then incubated at 4°C for 30 min with EpCAM
immunomagnetic fluid. Following incubation, the sample was placed
in a magnetic field, selected and washed with PBS. Isolated NSCLC
CTCs were then divided and cultured with RPMI-1640 medium.
Flow cytometry
To determine the percentage of CTCs in the
peripheral blood of NSCLC patients, flow cytometry was performed.
Blood samples (2–3 ml) were drawn into Cell Save tubes, which were
maintained at room temperature and processed within 72 h of
collection. CTCs were defined as nucleated EpCAM-positive cells,
lacking CD45 (Becton-Dickinson, Franklin Lakes, NJ). All CTC
evaluations were performed by flow cytometry
(Becton-Dickinson).
Reverse-transcriptase PCR and real-time
PCR
Total RNA from cells was extracted using the TRIzol
Reagent (Invitrogen). Total cDNA was used as a template for
amplification at 95°C for 5 min followed by 30 cycles for EpCAM and
25 cycles for β-actin as a control. Real-time quantitative PCR was
performed in triplicate for each primer set and in each cell sample
using an iQ5 multicolor real-time PCR Detection System (Bio-Rad,
Hercules, CA). The protocol for real-time PCR was 1 cycle of 95°C
for 30 sec, 40 cycles of 95°C for 5 sec, 60°C for 30 sec and then a
dissociation stage. The cycle threshold (CT) value was determined
as the point at which the fluorescence exceeded a limit preset by
the instrument’s software. PCR primer sequences for EpCAM, CK8,
CK18, CK19 and β-actin are as follows: EpCAM F:
TACACTGCCCAGGAGCCAGA, R: TG GCACCAGTGTCCGGATTA; CK8 F: GCTTCTCCGCTC
CTTCTAGGATCT, R: GACACCTTGTAGGACTTCTGG GTCA; CK18 F:
AAATCTCAGGACCTCGCCAAG, R: GTC TCAGCAGCTCCAACCTCA; CK19 F:
CTGAGTGACA TGCGAAGCCAATA, R: CAGTAACCTCGGACCTGCTC ATC; β-actin F:
CTAAGTCATAGTCCGCCTAGAAGCA, R: TGGCACCCAGCACAATGAA.
Western blot analysis
Western blot analyses were performed as previously
described with the lysates from cells. Rabbit polyclonal antibodies
against human p-cMET and cMET (1:500 dilution; Santa Cruz
Biotechnology, Santa Cruz, CA) and mouse monoclonal antibody
against human β-actin (1:500 dilution; Santa Cruz Biotechnology)
were incubated with the membranes at 4°C overnight, followed by a
secondary incubation using horseradish peroxidase (HRP)-conjugated
anti-rabbit or anti-mouse IgG (Thermo Fisher Scientific, Inc., New
York, NY). Proteins were briefly incubated with an enhanced
chemiluminescence reagent (Millipore, Billerica, MA) and then
visualized on X-ray film.
Transwell analysis
For the migration assay, 5×105 cells were
plated onto a 6-well plate (Corning, Lowell, MA, USA) with an 8-μm
properly carbonate membrane. For the invasion assay,
5×105 cells were placed on plates pre-coated with 20 μg
Matrigel. In both assays, cells were plated in medium without
serum, and medium containing 10% FBS in the lower chamber served as
a chemoattractant. After 24 h, cells that did not migrate or invade
through the pores were removed by cotton swabs. The inserts were
fixed, stained and three random fields for each insert were
counted. The results were averaged among three independent
experiments.
Statistical analysis
All clinical data were collected independently by
two physicians. The survival analysis was calculated using the
Kaplan-Meier product limit method. The log-rank test was used to
compare survival between groups, according to the CTC count and the
type of systemic therapy. The t-test and the Pearson’s
χ2 test were used to determine significant differences
in patient characteristics according to the baseline CTC count. All
statistical analyses, performed using the SPSS 16.0 software (SPSS,
Inc., Chicago, IL, USA), were two-sided, and P<0.05 was
considered statistically significant.
Results
Patient characteristics
Forty patients were enrolled on this study. Eighteen
patients underwent surgery One patient was deemed ineligible after
enrollment and did not contribute any blood specimens. Twenty-one
patients had previously received chemotherapy, with eighteen having
been treated with gemcitabine. Additional patient characteristics
are listed in Table I.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| No. of
patients | 39 |
| Age (mean ± SD)
(years) | 63.5±9.4 |
| Cender |
| Female | 16 |
| Male | 23 |
| TNM stage |
| II | 21 |
| III | 18 |
| Grade |
| I | 2 |
| II | 23 |
| III | 14 |
| Surgical
specimen |
| Surgery | 18 |
| Non-surgery | 21 |
Detection of EpCAM-positive CTCs in
non-small cell lung cancer patients
To date, antigen-based CTC isolation strategies have
made use of the transmembrane protein EpCAM for cell capture,
followed by staining for cytoplasmic keratins (CKs) ubiquitously
expressed by all epithelial cell types. In our work, the expression
of EpCAM and CD45 was first detected in the 4 NSCLC cell lines
A549, NCI-H460, HCC827 and NCI-H1299 by flow cytometry. Nearly all
cancer cells were EpCAM positive and CD45 negative (Fig. 1A). Furthermore, quantitative
analysis of EpCAM staining counts in NSCLC-derived CTCs was
performed (Fig. 1B). Both male and
female healthy controls had insignificant counts (median, 5.2
CTCs/ml; range, 0–16; mean, 6.6±1.1) (Fig. 1B,b2). For consistent analysis of
CTC counts in patients with NSCLC, we set a threshold of detection
in patients that was higher than any count noted in any healthy
donor. Thirty-two of 37 (86%) patients (median, 65 CTCs/ml; range,
18–690; mean, 151±31) had detectable concentrations of CTCs
relative to 0 of 16 healthy controls (Fig. 1B,b1). Compared with the healthy
controls, the CTCs in the NSCLC patients were almost 23-fold
(Fig. 1B,b3, P<0.05).
Next, the EpCAM-expressing CTCs in the NSCLC
patients were isolated with EpCAM immunomagnetic fluid. Following
isolation, CTCs were divided and cultured with RPMI-1640 medium
(Fig. 1C). Then, we detected the
expression of EpCAM in isolated CTCs by RT-PCR. The results showed
that all of the CTCs isolated by EpCAM beads expressed a
considerable amount of EpCAM transcript (Fig. 1D).
Significant decrease of EpCAM-expressing
CTCs due to gemcitabine
Gemcitabine has been used in various carcinomas,
including non-small cell lung cancer, especially later period
NSCLC. However, the therapy indication for gemcitabine in NSCLC
with micrometastasis is still unknown and the mechanism of action
of gemcitabine as a chemotherapy drug in NSCLC is not fully
understood. Here, we compared the EpCAM-expressing CTC counts of
two groups treated with systemic gemcitabine therapy or a
palliative or curative resection. When the EpCAM-expressing CTCs
were evaluated at 3 intervals of 63 days, there was a gradual,
significant decrease of these cells in the gemcitabine group (mean:
from 99.28±29.00 to 13.00±2.84 CTCs/ml, P<0.05); however, in the
non-gemcitabine group, the EpCAM-expressing CTCs counts showed no
significant change (mean: from 102.23±19.00 to 87.00±2.84 CTCs/ml,
P>0.05, Fig. 2A).
Across different patients with different
characteristics of the disease (see Table I for patient characteristics), the
change in the number of EpCAM-positive CTCs/ml was positively
correlated with the TNM stage and differentiation grade, especially
in the gemcitabine group. However, it was poorly correlated with
surgery (Fig. 2B). Additionally,
the expression of cytokeratin, a protein marker for epithelial
cells, was used to evaluate the relative CTC yield. Here, the
evaluation of the expression levels of selected genes (CK8, CK18
and CK19) based on the RNA extracted from the CTC-enriched baseline
blood samples was feasible using quantitative real-time PCR
(Fig. 2C). After 3 treatment
periods, the gemcitabine group patients with 13.00±2.84 CTCs/ml
peripheral blood expressed higher CK8, CK18 and CK19 genes compared
with the non-gemcitabine group patients with 77.26±24.51 CTCs/ml
(Fig. 2C). These results suggested
that treatment with gemcitabine in NSCLC patients significantly
decreased the EpCAM-positive CTCs and cytokeratin gene expression
levels.
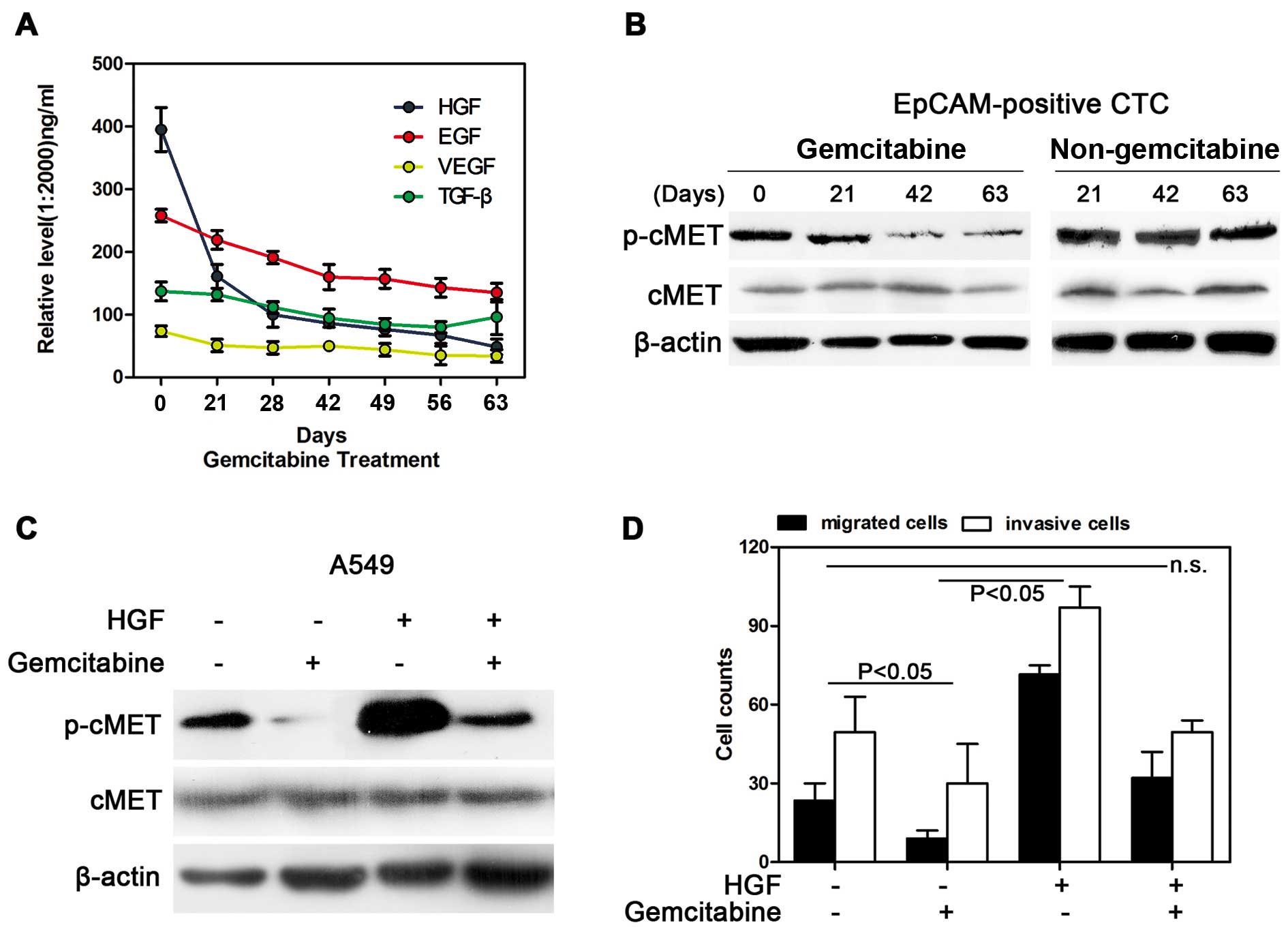
HGF/cMET pathway was significantly
inhibited by gemcitabine in NSCLC
To explore the pathway involved in the
micrometastasis of tumor cells, the HGF level in the peripheral
blood serum was gradually decreased along with the treatment of
gemcitabine (Fig. 3A). As the
receptor for the HGF factor, the activated p-cMET was inhibited by
gemcitabine in the EpCAM-positive CTCs; however, for the
non-gemcitabine group, neither HGF nor p-cMET were shown to be
inhibited (Fig. 3B). Additionally,
the HGF/c-MET pathway was indeed inhibited by gemcitabine in the
NSCLC cell line A549. The p-cMET gene was inactivated when the
cells were treated with gemcitabine, and gemcitabine also inhibited
the increase of p-cMET binding to HGF (Fig. 3C). Furthermore, the cell migration
and invasion abilities were inhibited after treatment with
gemcitabine, which was consistent with the decreased activity of
the HGF/cMET pathway (P<0.05). The migrated and invasive cell
count was restored when the cells were treated with gemcitabine and
HGF, suggesting that gemcitabine effectively inhibited the
HGF/c-MET pathway and the cell migration and invasion
abilities.
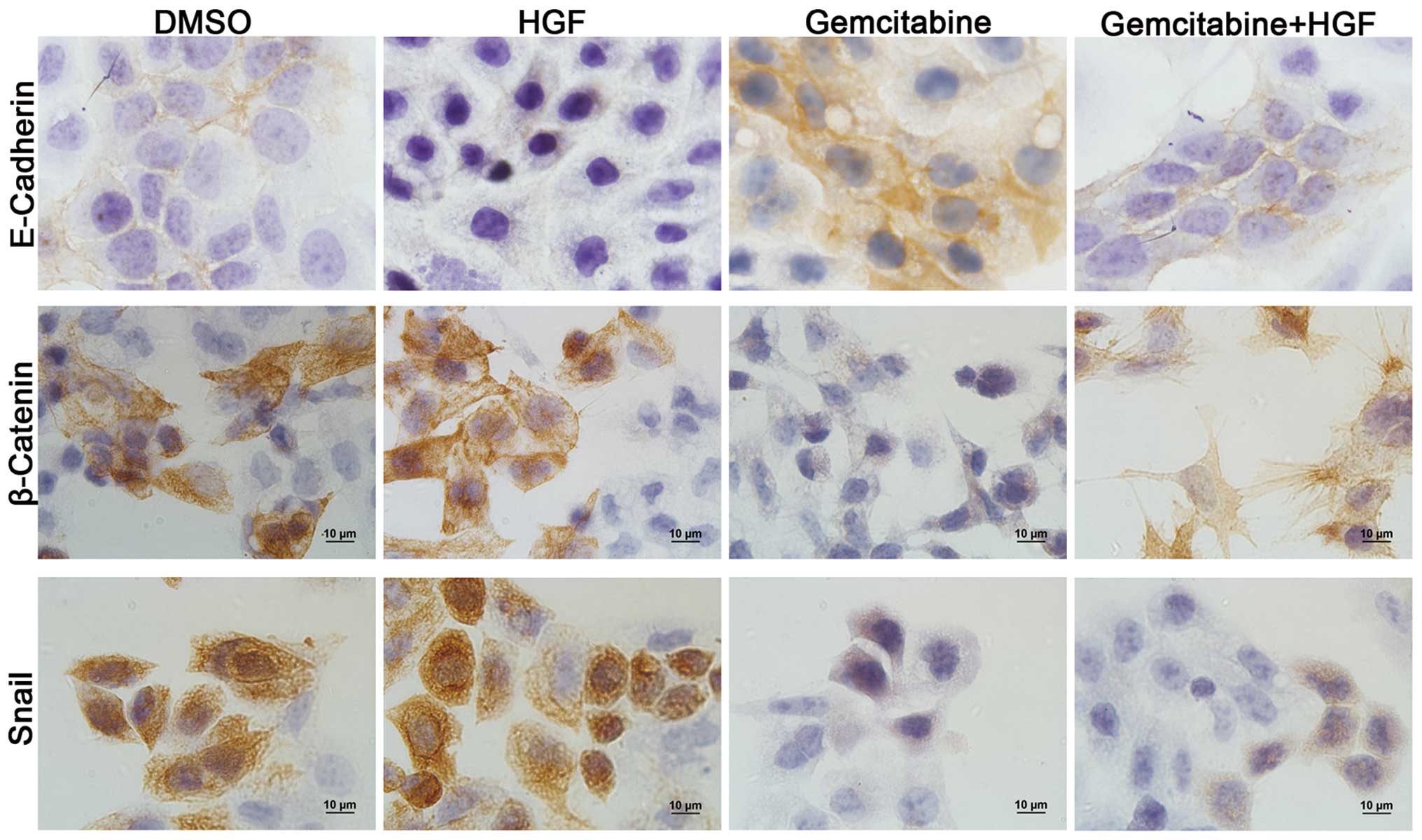
Gemcitabine inhibited cell migration and
invasion by epithelial-mesenchymal transition
For epithelial malignancies, the EMT is considered
to be the crucial event in the metastatic process and appears to be
controlled by signal-transduction pathways such as the HGF/cMET
pathways. To confirm this, the EMT related markers E-Cadherin,
β-Catenin and Snail were detected in the NSCLC cell line A549 with
different treatment by IHC (Fig.
4). The staining results showed that the epithelial marker
E-Cadherin was downregulated and mesenchymal markers β-Catenin and
Snail were markedly upregulated at the protein level when cells
were cultured in the medium containing HGF. Cells treated with
gemcitabine were shown to be undergoing epithelialization with high
expression of E-Cadherin and low expression of β-Catenin and Snail.
Gemcitabine could effectively reverse the EMT forced by HGF. This
result suggested that gemcitabine may inhibit the EMT, resulting in
decreased cell migration and invasion abilities.
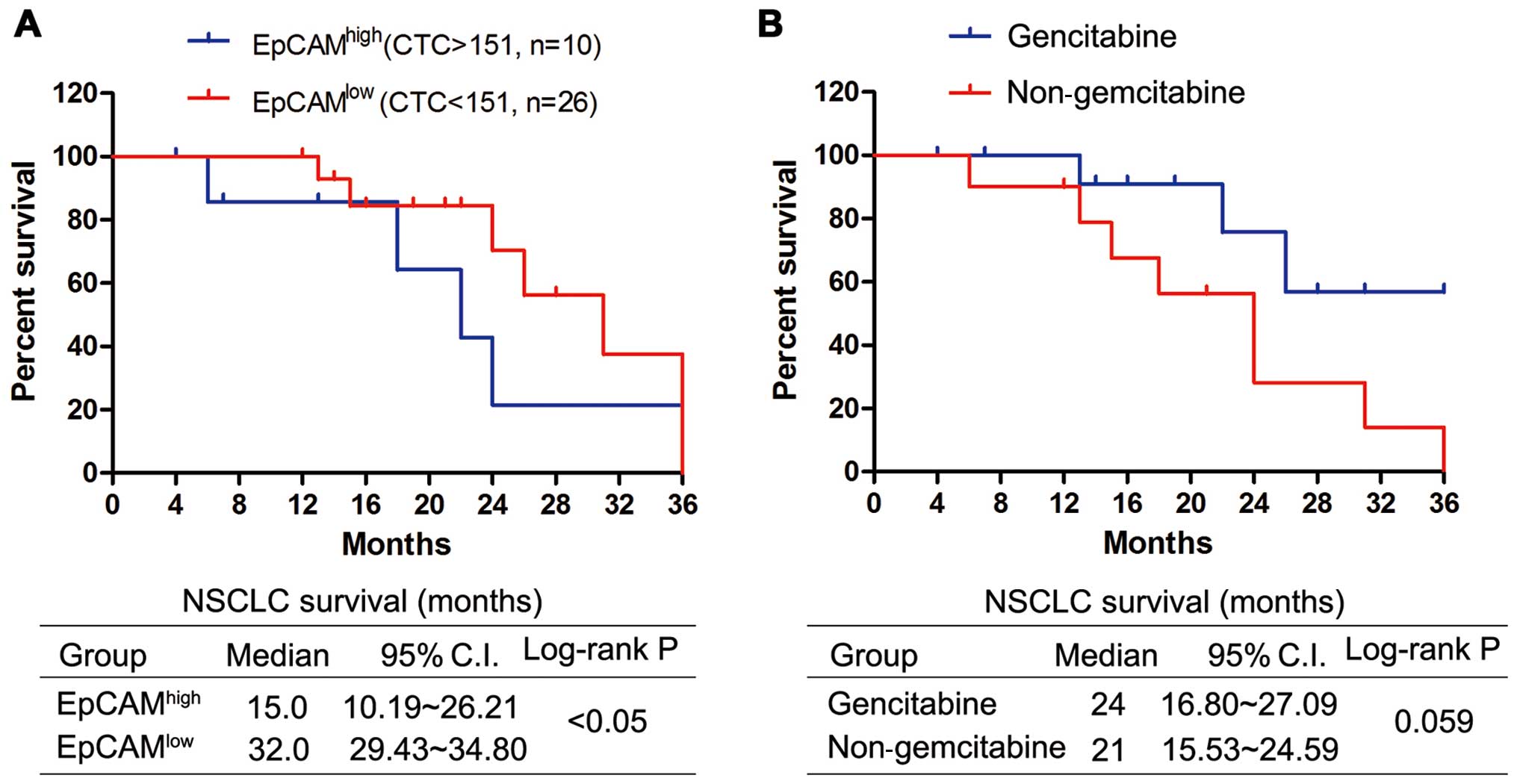
Prognostic value of circulating tumor
cells and gemcitabine treatment in NSCLC patients
The median follow-up time for all patients was three
years; 20 patients (55.56%) had died at the time of analysis. We
found a remarkable correlation between the baseline value of CTCs
and the outcome of all patients. The median survival was 15.0
months (95% CI 10.19–26.21) for EpCAMhigh patients with
CTCs >151 and 32.0 months (95% CI 29.43–34.80) for those
EpCAMlow patients with CTCs <151 (Fig. 5A, log-rank P<0.05).
Additionally, the differential ability of each modality of
treatment to reduce the CTC number led us to evaluate whether the
gemcitabine therapy could impact the prognostic value associated
with a high count of CTCs. We evaluated the survival value in the
two treatment groups, including the common chemotherapy
(non-gemcitabine group) and common chemotherapy plus gemcitabine
(Gemcitabine group). The median survival rate was 21 months (95% CI
15.53–24.56) for patients treated without gemcitabine and 24 months
(95% CI 16.80–27.09) for those treated with gemcitabine (log-rank
P=0.059, Fig. 5B). These results
suggested a therapeutic benefit for the NSCLC patient survival with
the common therapy plus gemcitabine.
Discussion
The study of CTCs is essential to understanding the
vascular spread of cancer to distant sites and for determining the
higher risk of cancer progression. Up until now, multistep batch
purification strategies such as immunomagnetic bead capture have
been used, but microfluidic approaches have the advantage of
simpler processing steps and allow the isolation of viable cells at
higher sensitivity and purity. Here, we applied the epithelial cell
adhesion molecule-associated antigen EpCAM, which is expressed in
the vast majority of carcinomas, to isolate the CTCs in NSCLC
patient peripheral blood. We detected the EpCAM-positive CTCs and
determined the survival rates for the NSCLC patients treated with
gemcitabine, exploring the clinical significant and mechanism of
action of gemcitabine on micrometastasis in non-small cell lung
cancer.
Recurrence and metastasis are the key factors that
determine treatment effectiveness and survival time associated with
drug resistance or insensitivity of tumor cells, EMT,
micrometastasis and so on. In the present study, the rare CTCs in
the peripheral blood play an important role in recurrence and
metastasis through the formation of distant micrometastases
(38). Several studies have
confirmed that the number of peripheral blood CTCs was much higher
in patients with cancers such as breast, colon and prostate cancer
than in healthy patients (3).
Furthermore, the CTC level was correlated with the metastasis
degree and survival, especially in breast cancer (11). Here, we detected the NSCLC CTCs by
staining for EpCAM expression. The threshold CTC number chosen to
optimally distinguish between patients with cancer and cancer-free
patients (6.6 CTCs/ml) may underestimate the CTCs in some cancer
patients with few true EpCAM-positive cells, but it ensures a low
likelihood of false positives. Thirty-two of 37 (86%) patients
(median, 65 CTCs/ml; range, 18–690; mean, 151±31) had detectable
concentrations of CTCs compared to 0 of 16 healthy controls.
Compared with the healthy controls, the CTCs in NSCLC patients were
almost 23-fold. Although the isolation of CTCs based on EpCAM
expression has been established for the detection of breast cancer,
colon cancer and NSCLC CTCs, other equally sensitive and specific
antibodies could in theory be used for the detection of circulating
cells from other cancers, such as PSA staining in prostate cancer
(39) and HER2 staining in subsets
of breast cancer (40) or neural
crest marker staining in melanoma (41).
Gemcitabine is the agent designed with a known
molecular target to receive FDA approval for the treatment of lung
cancer, yet its activity in micrometastasis is unclear, especially
in the subgroup of patients with non-small cell lung cancer. We
have identified a significant decrease of the EpCAM-positive CTCs
as the molecular correlation to the dramatic responses to
gemcitabine by NSCLC patients. The HGF/cMET pathway was shown to be
inactivated in the isolated EpCAM-positive CTCs of NSCLC patients
and A549 cells that responded to the gemcitabine treatment; the
non-gemcitabine CTCs may have had the detected HGF/cMET activity.
These results, together with the finding of decreased
EpCAM-positive CTCs in the peripheral blood of NSCLC patients who
received the gemcitabine treatment, suggest that EpCAM-positive
CTCs account for the majority of the responses to gemcitabine
reported in clinical studies. Additionally, the mechanism of
gemcitabine as an anticancer agent for NSCLC by inhibiting the
HGF/cMET pathways revealed that the EMT feature was effectively
reversed and the cell migration and invasion abilities were
decreased in NSCLC cells.
Our data also suggested a potential role for
enumeration of CTCs and gemcitabine treatment in predicting disease
progression. An increased number of EpCAM-positive CTCs was seen in
poor disease progression and a change in the CTC number might
predict clinical responses. Since we initiated our pilot study, the
results of a multi-center study of CTCs in metastatic breast cancer
have been published. Patients with ≥151 CTCs/ml peripheral blood at
the baseline and follow-up assessments had shorter overall survival
compared with patients with <151 CTCs/ml peripheral blood. The
patients with gemcitabine treatment had longer survival time
compared with the non-gemcitabine group. These results led to the
USA Food and Drug Administration approval of this technology in
metastatic breast cancer, with high reproducibility of the test
across multiple laboratories. Thus, proof of CTC enumeration
predicting clinical outcome and gemcitabine treatment prolonging
the survival time have been demonstrated and supports further
clinical evaluation of CTCs and gemcitabine in NSCLC.
This pilot study investigated the EpCAM-positive
CTCs in NSCLC patients before and after the treatment with
gemcitabine. The mechanism study showed that gemcitabine targeted
the EpCAM-positive CTCs, inhibiting metastasis and invasion through
reversal of the EMT features induced by the HGF/cMET pathway in
NSCLC. These results suggested that gemcitabine chemotherapy can
effectively inhibit metastasis and circulating tumor cells in
non-small cell lung cancer.
Acknowledgements
This study was supported by a grant to Z.J.L.
[2006K09-G4 (4)] from the science
and technology project of Shaanxi province: the clinical study of
gemcitabine on resistant non-small cell lung cancer
micrometastasis.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ni X, Zhuo M, Su Z, et al: Reproducible
copy number variation patterns among single circulating tumor cells
of lung cancer patients. Proc Natl Acad Sci USA. 110:21083–21088.
2013. View Article : Google Scholar
|
|
3
|
Plaks V, Koopman CD and Werb Z: Cancer.
Circulating tumor cells. Science. 341:1186–1188. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li P, Stratton ZS, Dao M, Ritz J and Huang
TJ: Probing circulating tumor cells in microfluidics. Lab Chip.
13:602–609. 2013. View Article : Google Scholar
|
|
5
|
Young R, Pailler E, Billiot F, et al:
Circulating tumor cells in lung cancer. Acta Cytol. 56:655–660.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Y, Qian J, Feng JG, et al: Detection
of circulating tumor cells in peripheral blood of colorectal cancer
patients without distant organ metastases. Cell Oncol (Dordr).
36:43–53. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hou HW, Warkiani ME, Khoo BL, et al:
Isolation and retrieval of circulating tumor cells using
centrifugal forces. Sci Rep. 3:12592013.PubMed/NCBI
|
|
8
|
Huang SB, Wu MH, Lin YH, et al:
High-purity and label-free isolation of circulating tumor cells
(CTCs) in a microfluidic platform by using
optically-induced-dielectrophoretic (ODEP) force. Lab Chip.
13:1371–1383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Diamond E, Lee GY, Akhtar NH, et al:
Isolation and characterization of circulating tumor cells in
prostate cancer. Front Oncol. 2:1312012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dong Y, Skelley AM, Merdek KD, et al:
Microfluidics and circulating tumor cells. J Mol Diagn. 15:149–157.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Green TL, Cruse JM and Lewis RE:
Circulating tumor cells (CTCs) from metastatic breast cancer
patients linked to decreased immune function and response to
treatment. Exp Mol Pathol. 95:174–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin HK, Zheng S, Williams AJ, et al:
Portable filter-based microdevice for detection and
characterization of circulating tumor cells. Clin Cancer Res.
16:5011–5018. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alunni-Fabbroni M and Sandri MT:
Circulating tumour cells in clinical practice: Methods of detection
and possible characterization. Methods. 50:289–297. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ross JS and Slodkowska EA: Circulating and
disseminated tumor cells in the management of breast cancer. Am J
Clin Pathol. 132:237–245. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Amadori A, Rossi E, Zamarchi R, Carli P,
Pastorelli D and Jirillo A: Circulating and disseminated tumor
cells in the clinical management of breast cancer patients:
unanswered questions. Oncology. 76:375–386. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wen CY, Wu LL, Zhang ZL, et al:
Quick-response magnetic nanospheres for rapid, efficient capture
and sensitive detection of circulating tumor cells. ACS Nano.
8:941–949. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen Q, Ge F, Cui W, et al: Lung cancer
circulating tumor cells isolated by the EpCAM-independent
enrichment strategy correlate with Cytokeratin 19-derived CYFRA21-1
and pathological staging. Clin Chim Acta. 419:57–61. 2013.
View Article : Google Scholar
|
|
18
|
Pantel K and Alix-Panabieres C:
Circulating tumour cells in cancer patients: challenges and
perspectives. Trends Mol Med. 16:398–406. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sieuwerts AM, Kraan J, Bolt J, et al:
Anti-epithelial cell adhesion molecule antibodies and the detection
of circulating normal-like breast tumor cells. J Natl Cancer Inst.
101:61–66. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fidler IJ: The pathogenesis of cancer
metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003.
|
|
21
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hugo H, Ackland ML, Blick T, et al:
Epithelial-mesenchymal and mesenchymal-epithelial transitions in
carcinoma progression. J Cell Physiol. 213:374–383. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chaffer CL, Thompson EW and Williams ED:
Mesenchymal to epithelial transition in development and disease.
Cells Tissues Organs. 185:7–19. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vincan E and Barker N: The upstream
components of the Wnt signalling pathway in the dynamic EMT and MET
associated with colorectal cancer progression. Clin Exp Metastasis.
25:657–663. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zavadil J: New TGF-beta and Ras crosstalk
in EMT. Cell Cycle. 8:1842009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thiery JP: Epithelial-mesenchymal
transitions in development and pathologies. Curr Opin Cell Biol.
15:740–746. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Polakis P: The many ways of Wnt in cancer.
Curr Opin Genet Dev. 17:45–51. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Akhurst RJ, Fitzpatrick DR, Fowlis DJ,
Gatherer D, Millan FA and Slager H: The role of TGF-beta s in
mammalian development and neoplasia. Mol Reprod Dev. 32:127–135.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou HY, Pon YL and Wong AS: HGF/MET
signaling in ovarian cancer. Curr Mol Med. 8:469–480. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gesto DS, Cerqueira NM, Fernandes PA and
Ramos MJ: Gemcitabine: a critical nucleoside for cancer therapy.
Curr Med Chem. 19:1076–1087. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Muggia F, Diaz I and Peters GJ: Nucleoside
and nucleobase analogs in cancer treatment: not only sapacitabine,
but also gemcitabine. Expert Opin Investig Drugs. 21:403–408. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cok G, Goksel T, Soyer S and Aysan T:
Effectiveness of gemcitabine as second-line chemotherapy in
non-small cell lung cancer. Tuberk Toraks. 56:74–80.
2008.PubMed/NCBI
|
|
33
|
Noble S and Goa KL: Gemcitabine. A review
of its pharmacology and clinical potential in non-small cell lung
cancer and pancreatic cancer. Drugs. 54:447–472. 1997.PubMed/NCBI
|
|
34
|
Galsky MD, Hahn NM, Powles T, et al:
Gemcitabine, Cisplatin, and sunitinib for metastatic urothelial
carcinoma and as preoperative therapy for muscle-invasive bladder
cancer. Clin Genitourin Cancer. 11:175–181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Martin M, Ruiz A, Munoz M, et al:
Gemcitabine plus vinorelbine versus vinorelbine monotherapy in
patients with metastatic breast cancer previously treated with
anthracyclines and taxanes: final results of the phase III Spanish
Breast Cancer Research Group (GEICAM) trial. Lancet Oncol.
8:219–225. 2007. View Article : Google Scholar
|
|
36
|
Reaume MN, Leighl NB, Mittmann N, et al:
Economic analysis of a randomized phase III trial of gemcitabine
plus vinorelbine compared with cisplatin plus vinorelbine or
cisplatin plus gemcitabine for advanced non-small-cell lung cancer
(Italian GEMVIN3/NCIC CTG BR14 trial). Lung Cancer. 82:115–120.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gridelli C, Gallo C, Shepherd FA, et al:
Gemcitabine plus vinorelbine compared with cisplatin plus
vinorelbine or cisplatin plus gemcitabine for advanced
non-small-cell lung cancer: a phase III trial of the Italian GEMVIN
Investigators and the National Cancer Institute of Canada Clinical
Trials Group. J Clin Oncol. 21:3025–3034. 2003.
|
|
38
|
Perlikos F, Harrington KJ and Syrigos KN:
Key molecular mechanisms in lung cancer invasion and metastasis: a
comprehensive review. Crit Rev Oncol Hematol. 87:1–11. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Stott SL, Lee RJ, Nagrath S, et al:
Isolation and characterization of circulating tumor cells from
patients with localized and metastatic prostate cancer. Sci Transl
Med. 2:25ra232010.PubMed/NCBI
|
|
40
|
Wallwiener M, Hartkopf AD, Baccelli I, et
al: The prognostic impact of circulating tumor cells in subtypes of
metastatic breast cancer. Breast Cancer Res Treat. 137:503–510.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ma J, Lin JY, Alloo A, et al: Isolation of
tumorigenic circulating melanoma cells. Biochem Biophys Res Commun.
402:711–717. 2010. View Article : Google Scholar : PubMed/NCBI
|