Introduction
Malignant fibrous histiocytoma (MFH), which has
recently been classified as undifferentiated pleomorphic sarcoma
(UPS), is the most common soft tissue sarcoma in late adult life.
Advances in the treatment of MFH have led to multidisciplinary
treatments, including surgery, chemotherapy and radiation therapy.
However, the prognosis of patients with the disease is still
extremely poor because of local recurrence and distant metastases
(1).
Tumor hypoxia is a common feature of malignant
tumors, and contributes to their malignant progression, distant
metastasis and resistance to radiation therapy and chemotherapy
(2). The relevance of tumor
oxygenation to radiocurability was first proposed by Gray et
al (3). Since the role played
by oxygenation in radiosensitivity has been recognized, several
strategies including high-oxygen-content gas breathing,
radiosensitizers, and hypoxic cytotoxins, have been developed to
overcome hypoxia-mediated radioresistance (4). However, with these strategies an
increased tumor control rate is often accompanied by more severe
side effects (5,6).
Carbon dioxide (CO2) therapy in the form
of a carbonated spa has been historically used in Europe as an
effective treatment for cardiac diseases and skin problems
(7,8). It has been suggested that the
therapeutic effects of CO2 may be due to an increase in
blood flow and microcirculation, nitric oxide-dependent
neocapillary formation, and a partial increase in O2
pressure in the local tissues known as the Bohr effect (7–9). We
have previously demonstrated that the transcutaneous application of
CO2 increased local O2 pressure in treated
tissues, potentially causing an ‘artificial Bohr effect’ (10), and we have also shown that the
transcutaneous CO2 system has antitumor effects; it can
also enhance chemosensitivity through the improvement of hypoxia in
human MFH xenografts (11,12). In murine osteosarcoma cells, the
transcutaneous CO2 system has also been reported to
reduce hypoxic conditions (13).
Based on these findings, we hypothesized that the transcutaneous
application of CO2 could enhance the antitumor effects
of radiation therapy through the improvement of hypoxia in tumor
tissues.
In the presence of oxygen, ionizing radiation
generates reactive oxygen species (ROS) such as superoxide
radicles, hydrogen peroxide and hydroxyl radicals; these show high
reactivity to a variety of cellular macromolecules (14). ROS are implicated in playing
crucial roles in cellular responses to radiation therapy (14), and they also regulate a broad array
of signal transductions that control various biological processes
including apoptosis (15).
Mitogen-activated protein kinases (MAPKs) include three major
kinases: extracellular signal-regulated kinase (ERK), p38 kinase,
and c-Jun N-terminal kinase/stress-activated protein kinase
(JNK/SAPK). Among these kinases, p38 and JNK/SAPK are mainly
activated by extracellular stresses such as irradiation and
inflammatory cytokines (16).
Recent studies have shown that activation of p38 and JNK/SAPK
contributes to ROS-induced apoptosis (17,18).
Hypoxia is thought to induce apoptotic resistance by suppression of
caspase-3 and JNK/SAPK in cells (17).
Additionally, it is well documented that ROS are
involved in the induction of apoptosis in γ-irradiated cancer cells
as well as normal cells (18).
However, the effects of hypoxia on radiation induced apoptotic
activities in MFH cells are poorly understood, and the relationship
between ROS production and the induction of apoptosis by radiation
therapy in MFH has not been reported. In the current study, we
examined the in vitro effects of altering the oxygen
conditions on X-ray irradiation induced cell apoptosis and ROS
production, and the activation of ROS-mediated and apoptosis
related proteins in MFH cells. We also utilized a murine model of
human MFH to examine the antitumor effects of combined treatment
with CO2 therapy and X-ray irradiation. Additionally, we
investigated the effects of transcutaneous CO2
application on radiation therapy in terms of ROS production, the
induction of apoptosis, and the activation of ROS-mediated and
apoptosis- related proteins in human MFH.
Materials and methods
Cell cultures
Four human MFH cell lines (Nara-H, TNMY-1, Nara-F
and GBS-1) were used in our studies. Nara-H and Nara-F cell lines
were obtained from ScienStuff Co. (Nara, Japan) (19). TNMY-1 was previously established in
our laboratory (20). GBS-1 was
obtained from ACTT (21). Cells
were grown in culture medium consisting of Dulbecco’s modified
Eagle’s medium (DMEM: Sigma-Aldrich Co., St. Louis, MO, USA)
supplemented with 10% (v/v) fetal bovine serum (FBS: Sigma-Aldrich
Co.) and 100 U/ml penicillin/streptomycin solution (Sigma-Aldrich
Co.). Cells were maintained at 37°C in a humidified 5%
CO2 atmosphere.
X-ray irradiation
X-ray irradiation was performed at a dose rate of
0.64–0.66 Gy/min using a 150 kV X-ray generator unit operating at 5
mA with an external 0.1 mm aluminum filter (MBR-1505122: Hitachi
Medical Co., Tokyo, Japan).
Colony formation assay
We performed colony formation assays of X-ray
irradiated MFH cells to evaluate the response of the cells to X-ray
irradiation. Four MFH cell lines were treated by X-irradiation at
five different doses (0, 2, 4, 6 and 8 Gy). Immediately after
X-irradiation, cells were trypsinized and seeded at a density of
100–1000 cells/well in 6-well plates, and incubated in a humidified
atmosphere of 5% CO2 at 37°C. After 2 weeks of
incubation, cells were stained with Giemsa and the number of
colonies was counted.
In vitro experiments
To investigate the effects of altering the oxygen
conditions on the sensitivity of human MFH cells to X-ray
irradiation, Nara-H cells were incubated for 48 h under one of
three different oxygen conditions: normoxic (20% O2, 5%
CO2, and 75% N2), hypoxic (1% O2,
5% CO2, and 94% N2), or reoxygenated
conditions, as previously described (12). Under reoxygenated conditions, cells
were incubated under normoxia for 24 h followed by 24 h of
incubation under hypoxic conditions, as previously described
(12). Hypoxic conditions were
also obtained by exposing Nara-H cells to CoCl2, a well
known chemical used to induce of hypoxia (23). In particular, the CoCl2
powder was dissolved in DMEM and the resulting solution was
filtered and then added to cell cultures at final concentration of
150 μl (23). In the reoxygenated
condition, the medium without CoCl2 (normoxic condition)
was replaced after 24 h under CoCl2 hypoxic conditions.
We also examined the effects of altering the oxygen conditions on
the sensitivity of human MFH cells to X-ray irradiation. After 24 h
of incubation in one of three different oxygen conditions X-ray
irradiation (3.2 Gy) was performed, and incubation was continued
for a further 24 h.
Animal models
Male athymic BALB/c nude mice, aged 5–8 weeks, were
obtained from CLEA Japan Inc. (Tokyo, Japan). All animal
experiments were approved by the Institutional Animal Care and Use
Committee of Kobe University Graduate School of Medicine, and were
performed in accordance with the Guidelines for Animal
Experimentation of Kobe University Graduate School of Medicine, and
Kobe University Animal Experimentation Regulations (permission
number: P110201). To create a murine model of human MFH, Nara-H
cells were implanted into the dorsal subcutaneous area of male
athymic BALB/c nude mice at a concentration of 4.0×106
cells in 500 μl PBS as previously described (11). Transcutaneous application of
CO2 was carried out as previously described (11,12).
Each treatment was performed for 10 min. Control animals were
treated in a similar manner by replacing CO2 with room
air.
In vivo MFH tumor studies
We examined the in vivo effects of combined
treatments using transcutaneous CO2 therapy and X-ray
irradiation. Mice were randomly divided into four groups: the
control group (n=6), the CO2 group (n=6), the X-ray
irradiation group (n=6), and the combination (CO2 and
X-ray irradiation) group (n=6). Treatments commenced 3 days after
MFH cell implantation, and were performed twice weekly for 2 weeks,
four times in total. X-ray irradiation was given immediately after
CO2 or room air treatment at a dose of 0.8 Gy for each
treatment (3.2 Gy in total). Mouse body weight and tumor volume
were monitored twice weekly until the end of treatment. Tumor
volume (V) was calculated as previously described (11,12).
All tumors were excised at the end of treatment and apoptotic
activity, ROS production and their relationship in tumor tissue was
assessed.
Flow cytometric analysis
Flow cytometry was performed using a FACS Calibur™
flow cytometer (BD Pharmingen, Franklin Lakes, NJ, USA) as
previously described (11,12). We evaluated apoptotic activity in
MFH cells by means of the DNA fragmentation assay using the
Apo-Direct kit according to the manufacturer’s protocol (BD
Pharmingen). To evaluate ROS production we used 2′,
7′-dichlorofluorescin diacetate (DCFH-DA; Molecular Probes,
Invitrogen, Carlsbad, CA, USA) as an indicator (22).
Immunoblot analysis
Immunoblot analysis was performed as previously
described (11,12). We used the following antibodies:
anti-human caspase-3 antibody (1:1000) (Cell Signaling Technology,
Danvers, MA, USA), anti-human PARP antibody (1:1000) (Cell
Signaling Technology), anti-human p38 antibody (1:1000) (Cell
Signaling Technology), anti-human phospho p38 antibody (1:1000)
(Cell Signaling Technology), anti-human JNK/SAPK antibody (1:1000)
(Cell Signaling Technology), anti-human phospho-JNK/SAPK antibody
(1:1000) (Cell Signaling Technology), and anti-human α-tubulin
antibody (1:2000) (Sigma-Aldrich Co.).
Statistical analysis
All experiments were performed independently at
least three times, and data are presented as the mean ± standard
error (SE) unless otherwise indicated. Differences between groups
were evaluated using a two-tailed Student’s t-test, and also using
ANOVA with the post hoc test to compare for continuous values. All
tests were considered significant when the P-value was
<0.05.
Results
Effects of oxygen conditions on X-ray
induced apoptosis and ROS production in human MFH cells in
vitro
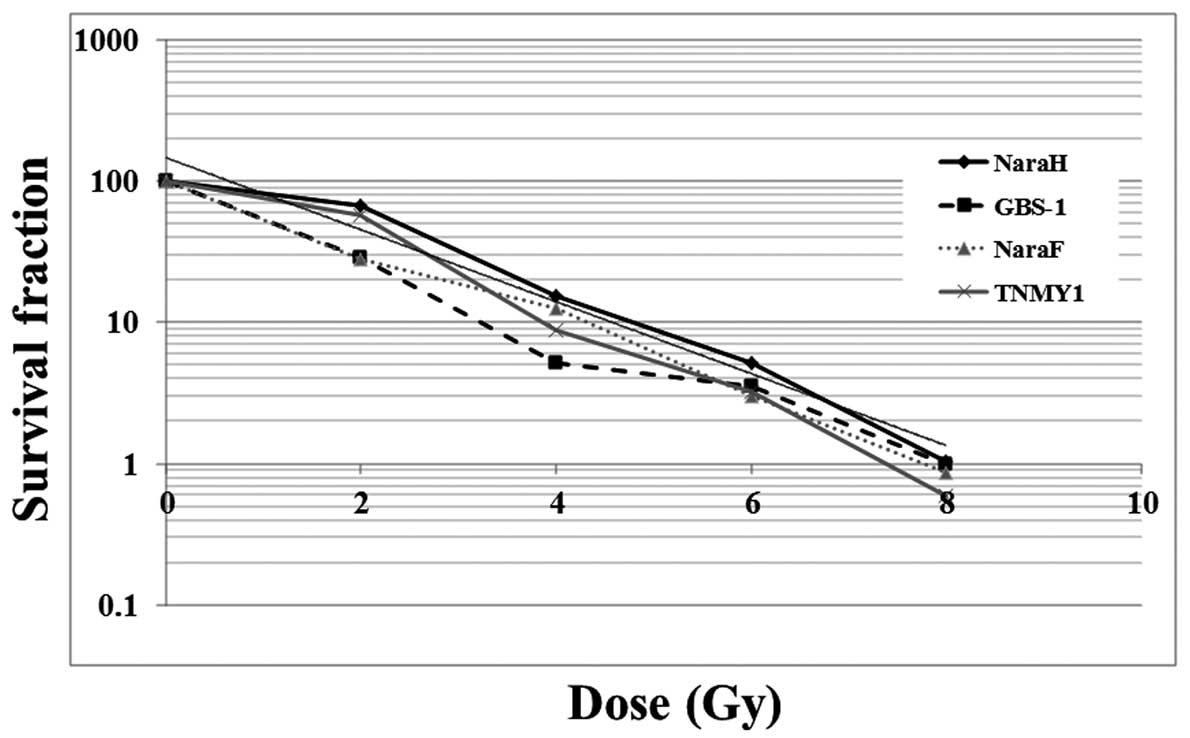
First, we performed colony formation assays using
four MFH cell lines (Nara-H, Nara-F, TNMY-1, and GBS-1) that were
treated with various doses of X-rays to evaluate the response of
the cells to radiation therapy. The number of MFH cell colonies
decreased in a dose-dependent manner after X-ray irradiation.
Nara-H cells were the most radioresistant of the four MFH cell
lines; a 50% decrease in colony formation was achieved at a dose of
3.2 Gy (Fig. 1).
Next, we studied the effects of altering the oxygen
conditions on X-ray induced cell apoptosis in Nara-H cells in
vitro. Three different oxygen conditions were used as
previously described (12).
Apoptotic activity and ROS production in the cells was assessed
using flow cytometric analysis and immunoblotting at 24 h after
X-ray irradiation (3.2 Gy) under three different oxygen conditions.
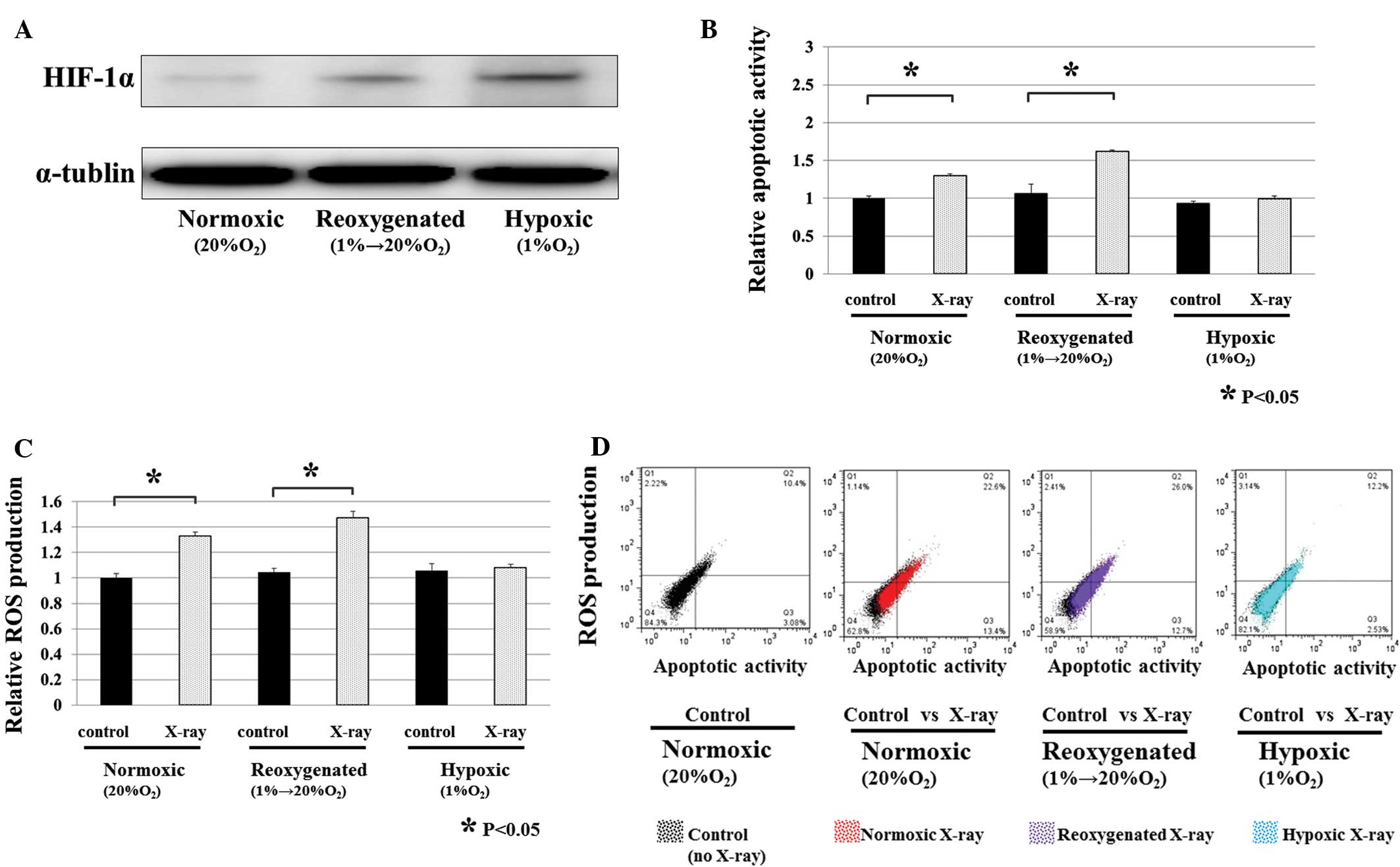
After 48 h of incubation, the expression of HIF-1α protein was
strongly increased in cells under hypoxic conditions, whereas the
expression of HIF-1α protein was decreased in cells cultured under
reoxygenated conditions (Fig. 2A).
It was found that both apoptotic activity and ROS production were
increased by X-ray irradiation in reoxygenated and normoxic
conditions (P<0.05) (Fig. 2B and
C). In addition, there was a correlation between apoptotic
activity and ROS production (Fig.
2D). However, neither the apoptotic activity or ROS production
was increased by X-ray irradiation under hypoxic conditions
(Fig. 2B and C). Hypoxic
conditions were also stimulated by exposure to CoCl2, a
well known chemical used to induce hypoxia (23). These results were consistent with
those obtained after the chemical induction (CoCl2) of
three different oxygen conditions (data not shown).
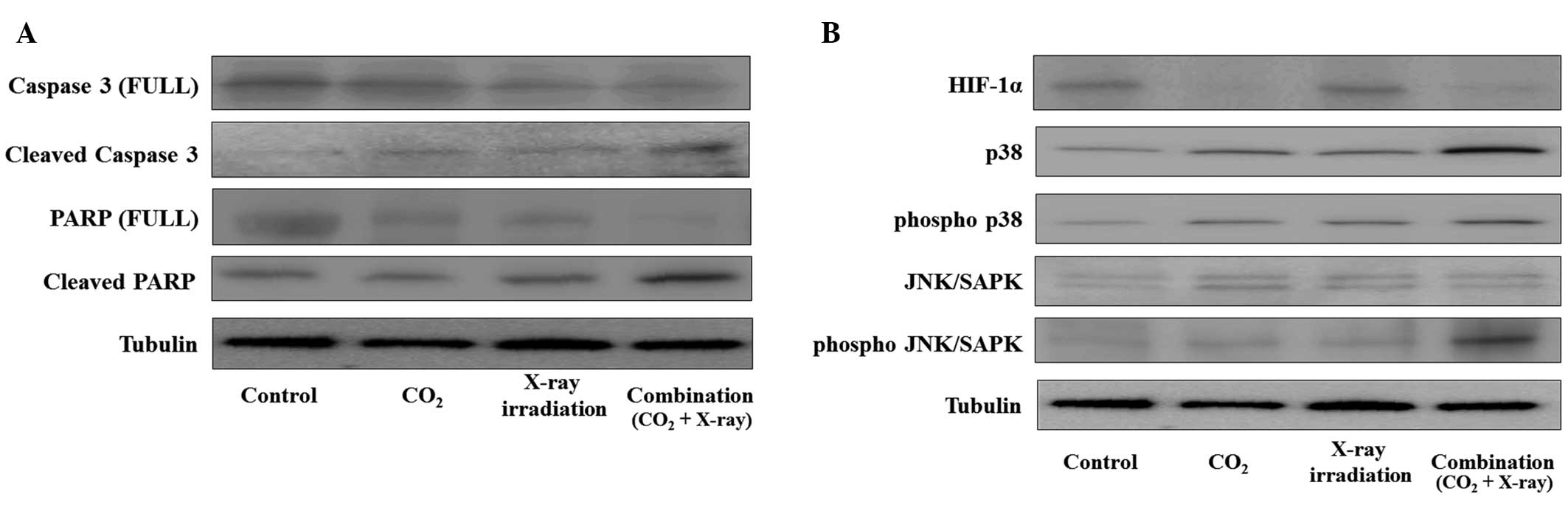
Immunoblot analyses revealed that the cleavage of
both caspase-3 and PARP was strongly increased in X-ray irradiated
MFH cells under normoxic and reoxygenated conditions (Fig. 3A). It also revealed that the
expression of phosphorylated forms of both of the pro-apoptotic
signaling molecules, p38 and JNK/SAPK, were increased after X-ray
irradiation, while the expression of total p38 and JNK/SAPK were
not changed under normoxic and reoxygenated conditions (Fig. 3B); however, under hypoxic
conditions the expression of protein was not changed (Fig. 3A and B). These results were
corroborated by chemical induction of three different oxygen
conditions, achieved by exposure to CoCl2 (data not
shown). They strongly indicated that the improvement in hypoxic
conditions enhanced X-ray irradiation-induced MFH cell apoptosis
and ROS production in vitro.
Effects of the transcutaneous application
of CO2 with X-ray irradiation on apoptosis and ROS
production in human MFH xenografts
To examine the effect of transcutaneous
CO2 therapy on X-ray irradiation in vivo, we used
the Nara-H cell line; the response of MFH tumors to this combined
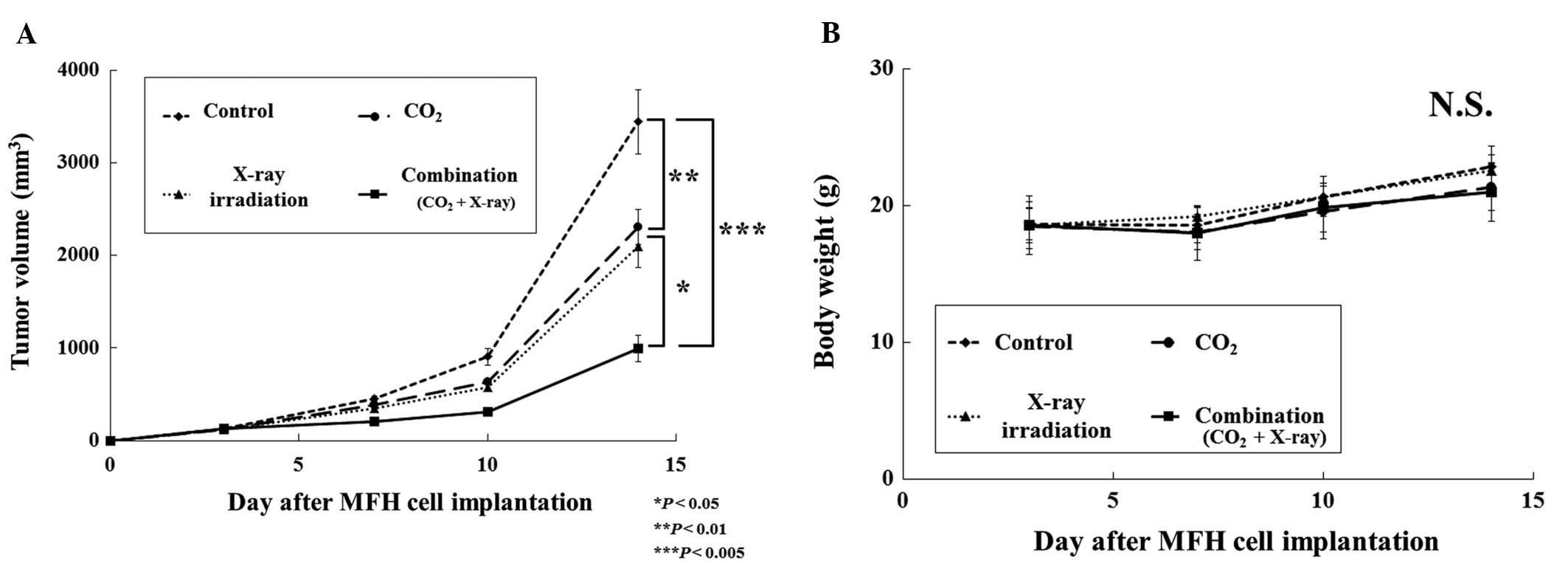
treatment was evaluated. We observed a significant decrease in
tumor volume in the CO2, X-ray irradiation and
combination groups when compared with the control group. At the end
of the experiment, tumor volume in the combination group
(CO2 and X-ray irradiation) was reduced to 28, 42 and
47% of that in the control, CO2 alone, and X-ray
irradiation alone groups, respectively (Fig. 4A). No significant differences in
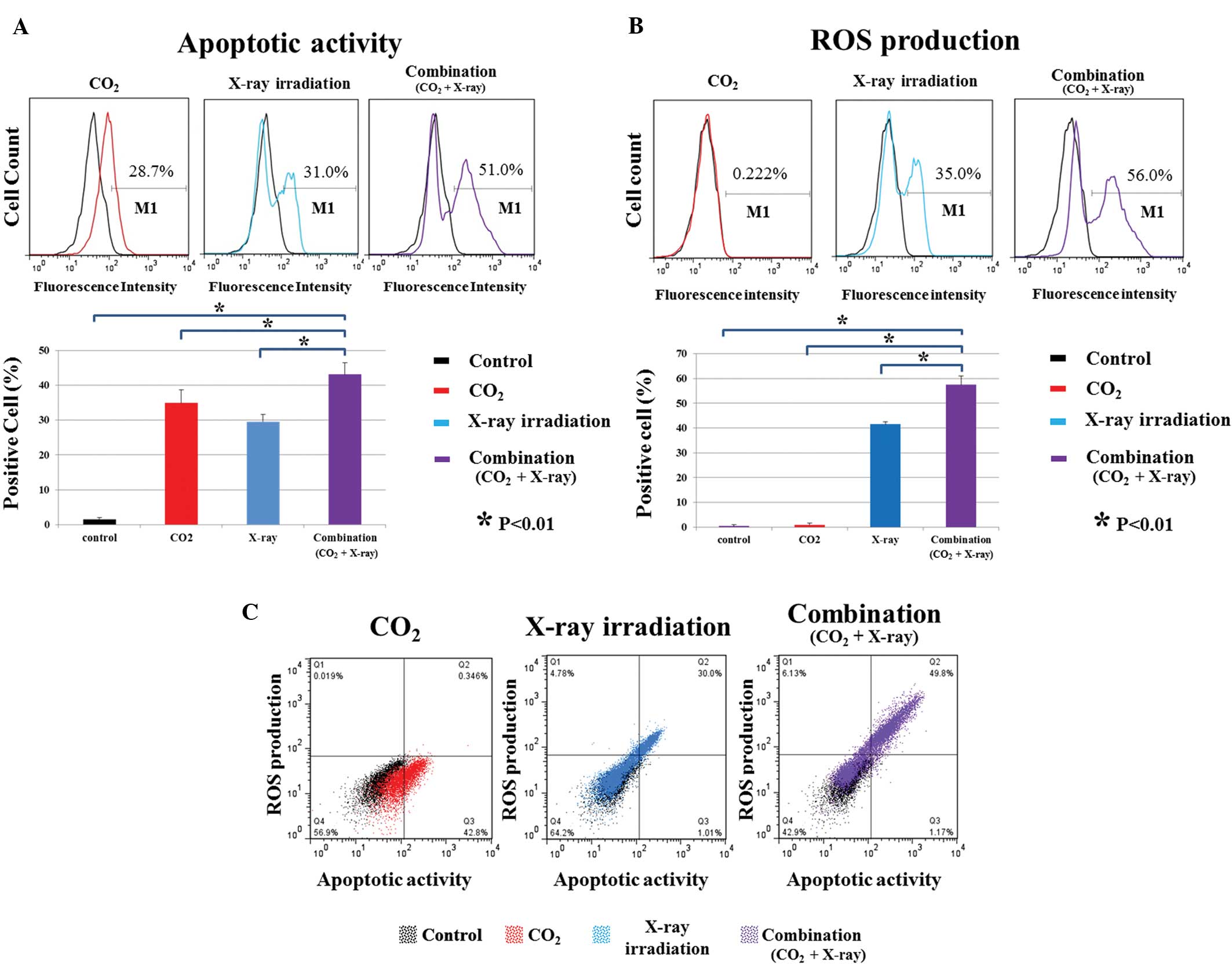
body weight were observed among the groups (Fig. 4B). FACS analyses showed that
apoptotic activity and ROS production in the combination group were
strongly increased relative to those in the control, CO2
alone, and X-ray irradiation alone groups (P<0.01) (Fig. 5A and B). Transcutaneous
CO2 therapy alone did not affect ROS production
(Fig. 5B). There was a correlation
between apoptotic activity and ROS production in the X-ray
irradiation alone and combination groups (Fig. 5C). In addition, we evaluated the
effect of combined treatment with transcutaneous CO2
therapy and X-ray irradiation on the ROS-mediated signaling
pathway. Immunoblot analyses showed that the expressions of the
cleavage of caspase-3, and PARP and phosphorylated forms of both
p38 and JNK/SAPK, were strongly increased with decreasing HIF-1α
expression in tumors that were treated by combined treatment
relative to the other treatment groups (Fig. 6). These results indicated that
combined treatment with transcutaneous CO2 therapy and
X-ray irradiation resulted in a significant antitumor effect in the
human MFH xenografts; in addition, transcutaneous CO2
therapy enhanced the antitumor effect of X-ray irradiation with an
improvement in hypoxic conditions through the activation of the
ROS-mediated signaling pathway.
Discussion
The presence of hypoxic cells in solid tumors has
been well documented, and tumor hypoxia is believed to be a major
cause of clinical radioresistance in various cancer types including
MFH (24–26). Previous studies dating back more
than 50 years have shown that well oxygenated cells are three times
more radiosensitive than hypoxic cells (3, 27).
Based on these strategies, several studies have been performed
using hyperbaric oxygen (28) and
the transfusion of red blood cells (29) to radiosensitize tumor tissues.
However, these therapies have proven to be of limited benefit and
can be harmful to patients (5,6).
We have previously demonstrated in human MFH
xenografts that the transcutaneous application of CO2
increased the local O2 pressure in treated tissue
(10), and that CO2
therapy enhanced chemosensitivity because of a reduction in hypoxia
(11). We have also previously
reported that CO2 therapy decreased the metastatic
potential of murine osteosarcoma (13). Therefore, we hypothesize that our
transcutaneous CO2 therapy can improve the response of
MFH cells to radiation therapy. There is little evidence to support
a possible relationship between CO2 therapy and
radiosensitivity. Carbogen, a hyperoxic gas containing a small
fraction of CO2, has been reported as being a more
effective and less toxic radiosensitizer (30). Carbogen was first used clinically
as a radiosensitizer in the early 1960s, and has now been
reintroduced into the clinic following the work of Rojas et
al (30). These authors
demonstrated the effectiveness of simple normobaric oxygen and
carbogen breathing in an animal tumor model (30). The gas has been shown to improve
tumor oxygenation by increasing the amount of dissolved oxygen in
plasma, and to increase tumor radiosensitivity (31). In the current study, we
demonstrated that the transcutaneous application of CO2
enhanced the antitumor effects of X-ray irradiation in human MFH
xenografts, with no observable side effects.
In addition, we found that a possible mechanism
involved in the induction of MFH cell death after combined
treatment with transcutaneous CO2 therapy and X-ray
irradiation, may depend on ROS production and the activation of
ROS-mediated signaling pathways. In the presence of oxygen,
ionizing radiation acts on biochemical systems via ROS production
(32). ROS play a major role in
radiation-induced cellular damage, and can induce apoptosis through
activation of a pro-apoptosis pathway (33). In the current in vivo study,
increased apoptotic activity along with increased ROS production
were observed in the combination group (CO2 and X-ray
irradiation), while ROS production was not increased by
transcutaneous CO2 therapy alone. The results suggest
that transcutaneous CO2 therapy can enhance
radiation-induced ROS production, but cannot increase ROS
production by itself. Our previous study revealed that
transcutaneous CO2 therapy improved hypoxic conditions
in MFH tumor tissues (11).
Therefore, we speculate that transcutaneous CO2 therapy
can enhance ROS production in X-ray irradiated MFH tumors through
increased local oxygenation. Pro-apoptotic signaling pathways, such
as p38 MAPK and JNK/SAPK, are stimulated by ROS production and
induce cell apoptosis (34,35).
It has been reported that p38 MAPK was efficiently activated by a
low dose of X-rays (36), and
radiation-induced upregulation of MAPKs and JNK/SAPK has also been
reported in cultured cells (37,38).
However, radiation-induced upregulation of MAPKs or JNK/SAPK in
musculoskeletal tumors has not been studied. In the present study,
we found that p38 and JNK/SAPK were activated in human MFH tumors
after X-ray irradiation in reoxygenated conditions in vitro;
in addition, the activation of both p38 and JNK/SAPK were strongly
increased with the improvement in hypoxic conditions in xenograft
bearing mice that were treated using combined treatment with
transcutaneous CO2 therapy and X-ray irradiation,
relative to CO2 therapy alone or X-ray irradiation
alone. These results suggest that the mechanism of enhancement of
the antitumor effects of radiation therapy by transcutaneous
CO2 therapy involves ROS production and ROS-mediated
activation of p38 and JNK/SAPK signaling pathways. However, the
ROS-related mehcanisms require further study.
In conclusion, the findings of the current study
strongly imply that CO2 therapy can enhance the
antitumor effect of radiation therapy for human MFH by increasing
ROS production as a consequence of reduced tumor hypoxia, with no
observable side effects. We believe that the current study is the
first to reveal the effect of transcutaneous CO2 therapy
on the radiocurability of a human sarcoma xenograft tumor, and
could represent a potent therapeutic breakthrough in the treatment
of radioresistant human malignancies.
Acknowledgements
We thank Minako Nagata, Maya Yasuda and Kyoko Tanaka
for their expert technical assistance. Mr. T. Ueha reports personal
fees from NeoChemir Inc., the others authors report grants and
non-financial support from NeoChemir Inc., during the conduct of
the study. In addition, Kobe University and NeoChemir Inc. have a
patent Antitumor Agent Containing Carbon Dioxide as Active
Ingredient issued to WO/2012/133114. This study was supported by
JSPS KAKENHI 24659565 grant number.
References
|
1
|
Le DV, Coindre JM, Leroux A, et al:
Prognostic factors for patients with localized primary malignant
fibrous histiocytoma: a multicenter study of 216 patients with
multivariate analysis. Cancer. 77:1823–1830. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ruan K, Song G and Ouyang G: Role of
hypoxia in the hallmarks of human cancer. J Cell Biochem.
107:1053–1062. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gray LH, Conger AD, Ebert M, Hornsey S and
Scott OC: The concentration of oxygen dissolved in tissues at the
time of irradiation as a factor in radiotherapy. Br J Radiol.
26:638–648. 1953. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moeller BJ, Richardson RA and Dewhirst MW:
Hypoxia and radiotherapy: opportunities for improved outcomes in
cancer treatment. Cancer Metastasis Rev. 26:241–248. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bennett M, Feldmeier J, Smee R and Milross
C: Hyperbaric oxygenation for tumour sensitisation to radiotherapy.
Cochrane Database Syst Rev. 19:CD0050072005.
|
|
6
|
Henke M, Laszig R, Rube C, et al:
Erythropoietin to treat head and neck cancer patients with anaemia
undergoing radiotherapy: randomised, double-blind,
placebo-controlled trial. Lancet. 362:1255–1260. 2003. View Article : Google Scholar
|
|
7
|
Resch KL and Just U: Possibilities and
limits of CO2balneotherapy. Wien Med Wochenschr.
144:45–50. 1994.(In German).
|
|
8
|
Hartmann BR, Bassenge E and Pittler M:
Effect of carbon dioxide-enriched water and fresh water on the
cutaneous microcirculation and oxygen tension in the skin of the
foot. Angiology. 48:337–343. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Riggs A: The nature and significance of
the Bohr effect in mammalian hemoglobins. J Gen Physiol.
43:737–752. 1960. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sakai Y, Miwa M, Oe K, et al: A novel
system for transcutaneous application of carbon dioxide causing an
‘artificial Bohr effect’ in the human body. PloS One.
6:e241372011.PubMed/NCBI
|
|
11
|
Onishi Y, Kawamoto T, Ueha T, et al:
Transcutaneous application of carbon dioxide (CO2)
enhances chemosensitivity by reducing hypoxic conditions in human
malignant fibrous histiocytoma. J Cancer Sci Ther. 4:174–181.
2012.
|
|
12
|
Onishi Y, Kawamoto T, Ueha T, et al:
Transcutaneous application of carbon dioxide (CO2)
induces mitochondrial apoptosis in human malignant fibrous
histiocytoma in vivo. PloS One. 7:e491892012.PubMed/NCBI
|
|
13
|
Harada R, Kawamoto T, Ueha T, et al:
Reoxygenation using a novel CO2 therapy decreases the
metastatic potential of osteosarcoma cells. Exp Cell Res.
319:1988–1997. 2013.PubMed/NCBI
|
|
14
|
Tominaga H, Kodama S, Matsuda N, Suzuki K
and Watanabe M: Involvement of reactive oxygen species (ROS) in the
induction of genetic instability by radiation. J Radiat Res.
45:181–188. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Engel RH and Evens AM: Oxidative stress
and apoptosis: a new treatment paradigm in cancer. Front Biosci.
11:300–312. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kyriakis JM and Avruch J: Mammalian
mitogen-activated protein kinase signal transduction pathways
activated by stress and inflammation. Physiol Rev. 81:807–869.
2001.PubMed/NCBI
|
|
17
|
Samuni AM, Kasid U, Chuang EY, et al:
Effects of hypoxia on radiation-responsive stress-activated protein
kinase, p53, and caspase 3 signals in TK6 human lymphoblastoid
cells. Cancer Res. 65:579–586. 2005.PubMed/NCBI
|
|
18
|
Mishra KP: Cell membrane oxidative damage
induced by gamma-radiation and apoptotic sensitivity. J Environ
Pathol Toxicol Oncol. 23:61–66. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kiyozuka Y, Nakagawa H, Uemura Y, et al:
Novel cell lines established from a human myxoid malignant fibrous
histiocytoma arising in the uterus. Cancer Genet Cytogenet.
127:7–15. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakatani T, Marui T, Yamamoto T, et al:
Establishment and characterization of cell line TNMY1 derived from
human malignant fibrous histiocytoma. Pathol Int. 51:595–602. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fang Z, Mukai H, Nomura K, et al:
Establishment and characterization of a cell line from a malignant
fibrous histiocytoma of bone developing in a patient with multiple
fibrous dysplasia. J Cancer Res Clin Oncol. 128:45–49. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sasaki H, Takayama K, Matsushita T, et al:
Autophagy modulates osteoarthritis-related gene expressions in
human chondrocytes. Arthritis Rheum. 64:1920–1928. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Indovina P, Rainaldi G and Santini MT:
Hypoxia increases adhesion and spreading of MG-63 three-dimensional
tumor spheroids. Anticancer Res. 28:1013–1022. 2008.PubMed/NCBI
|
|
24
|
Moulder JE and Rockwell S: Hypoxic
fractions of solid tumors: experimental techniques, methods of
analysis, and a survey of existing data. Int J Radiat Oncol Biol
Phys. 10:695–712. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Overgaard J: Sensitization of hypoxic
tumour cells - clinical experience. Int J Radiat Biol. 56:801–811.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Budach W, Budach V, Dinges S, Stuschke M
and Sack H: Correlation between primary chemo- and radiation
sensitivity in a panel of highly malignant human soft tissue
sarcoma xenografts. Radiother Oncol. 42:181–187. 1997. View Article : Google Scholar
|
|
27
|
Deschner EE and Gray LH: Influence of
oxygen tension on x-ray-induced chromosomal damage in Ehrlich
ascites tumor cells irradiated in vitro and in vivo. Radiat Res.
11:115–146. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Henk JM and Smith CW: Radiotherapy and
hyperbaric oxygen in head and neck cancer. Interim report of second
clinical trial. Lancet. 2:104–105. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Evans JC and Bergsjo P: The influence of
anemia on the results of radiotherapy in carcinoma of the cervix.
Radiology. 84:709–717. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rojas A: Radiosensitization with
normobaric oxygen and carbogen. Radiother Oncol. 20(Suppl 1):
65–70. 1991. View Article : Google Scholar
|
|
31
|
Rojas A, Joiner MC, Hodgkiss RJ, et al:
Enhancement of tumor radiosensitivity and reduced hypoxia-dependent
binding of a 2-nitroimidazole with normobaric oxygen and carbogen:
a therapeutic comparison with skin and kidneys. Int J Radiat Oncol
Biol Phys. 23:361–366. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Battino M, Ferri E, Gattavecchia E, et al:
Mitochondrial respiratory chain features after gamma-irradiation.
Free Radic Res. 26:431–438. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wallace SS: Enzymatic processing of
radiation-induced free radical damage in DNA. Radiat Res. 150(Suppl
5): S60–S79. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tobiume K, Matsuzawa A, Takahashi T, et
al: ASK1 is required for sustained activations of JNK/p38 MAP
kinases and apoptosis. EMBO Rep. 2:222–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim YS, Jhon DY and Lee KY: Involvement of
ROS and JNK1 in selenite-induced apoptosis in Chang liver cells.
Exp Mol Med. 36:157–164. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shimizu T, Kato T Jr, Tachibana A and
Sasaki MS: Coordinated regulation of radioadaptive response by
protein kinase C and p38 mitogen-activated protein kinase. Exp Cell
Res. 251:424–432. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kharbanda S, Saleem A, Shafman T, et al:
Ionizing radiation stimulates a Grb2-mediated association of the
stress-activated protein kinase with phosphatidylinositol 3-kinase.
J Biol Chem. 270:18871–18874. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Verheij M, Bose R, Lin XH, et al:
Requirement for ceramide-initiated SAPK/JNK signalling in
stress-induced apoptosis. Nature. 380:75–79. 1996. View Article : Google Scholar : PubMed/NCBI
|