Introduction
The incidence and mortality of gastric cancer have
fallen dramatically in USA and elsewhere over the past several
decades. Nonetheless, gastric cancer remains a major public health
issue as the fourth most common cancer and the second leading cause
of cancer death worldwide. For the past few decades, gastric cancer
mortality has decreased markedly in most areas of the world
(1). However, gastric cancer
remains a disease of poor prognosis and high mortality, second only
to lung cancer as the leading cause of cancer-related death
worldwide. Gastric cancer is a multifactorial disease. The marked
geographic variation, time trends and the migratory effect on
gastric cancer incidence suggest that environmental or lifestyle
factors are major contributors to the etiology of this disease
(2). The well-established
histopathological factors that influence disease outcome are tumor
size, histological type and subtype, the presence of signet ring
morphology, the degree of differentiation, the presence of
lymphovascular invasion and lymph node involvement (3,4).
Further understanding of the molecular mechanisms underlying the
pathophysiology of metastatic processes will not only help us to
identify those patients at greatest risk of recurrence but also
find novel molecular targets for the development of treatment
strategies for gastric cancer. A preliminary study on the
epithelial membrane protein 1 (EMP1) gene found that EMP1 is
closely linked to tumor development and progression (5,6).
Activation of the EMP1 gene in particular can prevent tumor
proliferation, and it may be a new target for tumor therapy
(7,8). However, to date there is no
information available regarding the relationship between EMP1 and
gastric cancer. We studied EMP1 expression in gastric cancer using
immunohistochemistry and western blot and analyzed the effect of
EMP1 overexpression in vitro in the gastric cancer cell line
SGC-7901 (9,10).
Materials and methods
Clinical data
All patients enrolled in this study provided
informed consent in advance. There were 39 males and 26 females,
aged from 21 to 78 years old, with a median age of 54 years. Of the
65 cases of gastric cancer, 31 had T1 and T2 stage cancers and 34
had T3 and T4 stage cancer. Twenty-seven patients presented with no
lymph node metastasis (N0), whereas 38 presented with identified
lymph node involvement (N+). As for the clinical stage, 28 cases
had stage I–II gastric cancers and 37 had stage III–IV gastric
cancer. Regarding grade of differentiation, 29 had Grade I (well
differentiated) tumors and 36 had Grade II or III (moderately to
poorly differentiated) tumors. Samples were taken immediately after
the endoscopic biopsy, and either fixed in 4% paraformaldehyde
solution and embedded in paraffin for immunohistochemistry or
stored in liquid nitrogen for western blot analysis.
Cell culture and gene transfection
Human gastric cancer SGC-7901 cells were maintained
in RPMI-1640 medium (Gibco BRL, Carlsbad, CA, USA) supplemented
with 10% fetal bovine serum (Gibco BRL). Medium was changed every
2–3 days; when the cultures reached confluence, the cells were
subcultured with 0.25% trypsin and 1% ethylene diaminetetraacetic
acid (EDTA). Cells were tested every 3 months for mycoplasma and
mycoplasma removal agent (MRA) (MP Biomedicals Co., Ltd., Shanghai,
China) was used to maintain mycoplasma-free cultures. EMP1 cDNA was
cloned into the BamHI and AscI sites of the
plenti6/V5-DEST vector (Invitrogen, Carlsbad, CA, USA). After
amplification and DNA sequence confirmation, this vector was used
to overexpress EMP1 in SGC-7901 cells. Briefly, SGC-7901 cells were
grown and stably transfected with pLenti6-EMP1 or plenti6/V5-DEST
for control using Lipofectamine 2000 (Invitrogen) and grown in
Blastidicin (5 μg/ml)-containing RPMI-1640 medium.
Immunohistochemistry
Immunohistochemistry was performed as previously
described (11). Briefly, 4-μm
sections were prepared from a paraffin-embedded block and
dehydrated, incubated in 3% hydrogen peroxide for 10 min and
incubated in trypsin for 20 min. Sections were blocked with 10%
goat serum at room temperature for 20 min and treated with rabbit
anti-human EMP1 polyclonal antibody (1:100; Abcam, Cambridge, UK)
overnight at 4°C. After rinsing, sections were treated with
biotin-conjugated antibodies (4A Biotech Co., Ltd., Beijing, China)
for 20 min, and biotin-immune complexes were identified with a
diaminobenzidine (DAB) substrate immunochemistry kit (4A Biotech
Co., Ltd.) and hematoxylin stain. Sections were mounted and
dehydrated with the coverslip sealed. For the negative control,
sections were treated identically except primary antibody was
replaced with PBS. Two pathologists blinded to patient and tissue
status assessed the results. Three slides for each specimen were
counted, with five fields of view randomly selected for evaluation
per section. EMP1 expression level was based on the percentage of
positive cells and staining intensity. The percentage of positive
cells was divided into four levels: 0 points, ≤5% of positive
cells; 1 point, 5–25%; 2 points, 25–50%; and 3 points, >50%. The
intensity of staining was classified as: 0 points, no staining; 1
point, weak staining (light yellow); 2 points, moderate staining
(yellowish-brown); and 3 points, strong staining (brown). The final
score of EMP1 expression was the product of the EMP1 expression
rate (percentage score) and intensity: − for 0 points, + to +++ for
positive (+ for 1–3 points, ++ for 4–6 points and +++ for 7–9
points).
Quantitative real-time (q) reverse
transcription (RT)-PCR
Total RNA was extracted from SGC-7901 cells using
TRIzol reagent (Invitrogen) according to the manufacturer’s
protocol (12). Total RNA (500 ng)
was reverse transcribed using Takara Reverse Transcriptase Reagents
(Takara, Shiga, Japan). qRT-PCR was performed with an ABI PRISM
7300 (Applied Biosystems, Inc., Carlsbad, CA, USA) according to the
standard protocol for SYBR-Premix ExTaq (Perfect Real-Time;
Takara). Primers for EMP1 and β-actin for normalization were as
follows: EMP1 sense 5′-CCCTCCTGGTCTTCGTGT, antisense
5′-AATAGCCGTGGTGATA; β-actin sense 5′-ATC GTCCACCGCAAATGCTTCTA,
antisense 5′-AGCCAT GCCAATCTCATCTTGTT. Thermal cycling conditions
were 95°C for 1 min, 95°C for 15 sec and 40 cycles at 60°C for 1
min. The relative expression was calculated using the
2−ΔΔCt method in SDS 1.3 software (Applied Biosystems,
Inc.).
Western blot analysis
Western blot was performed as previously described
(13). Samples were lysed in lysis
buffer containing 1% NP-40, 0.1% SDS, 25 mmol/l HEPES, 134 mmol/l
NaCl, 1 mmol/l vanadate, 100 mmol/l NaF and 0.5% Na-deoxycholate.
After centrifugation at 12000 r/min for 20 min at 4°C, the
supernatant was stored at −20°C. Protein concentration was detected
with the BCA Protein Assay kit (Tiangen Biotech Co., Ltd., Beijing,
China). Protein (50 mg) was resolved on a 10% SDS-PAGE and
transferred to nitrocellulose membrane. For EMP1, blots were
blocked for 2 h with 5% skim milk and incubated overnight at 4°C
with rabbit anti-human EMP1 (1:1000), caspase-9 (1:1000; Abcam) and
VEGFC (1:1000; Abcam). For β-actin, blots were blocked in 5%
non-fat dry milk for 1 h at room temperature and incubated
overnight in mouse anti-β-actin (Sigma, St. Louis, MO, USA)
overnight at 4°C. After washing, membranes were either incubated
with goat anti-mouse fluorescent secondary antibody (1:20000;
IRDye800, LI-COR Bioscience, Inc., Lincoln, NE, USA) or DyLight
Fluor conjugated goat anti-rabbit secondary antibody (LI-COR
Bioscience, Inc.) in the dark for 1 h at room temperature. The
blots were scanned and analyzed using the Odyssey Infrared Imaging
System (LI-COR Bioscience, Inc.). Western blot data were quantified
by normalizing the signal intensity of each sample to that of
β-actin (13).
MTT assay
Cell viability was determined using the tetrazolium
salt MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide] assay, as previously described (14). Briefly, cells were plated into
96-well culture plates at an optimal density of 5×103
cells/ml in 200 μl of culture medium/well. After 24–96 h of
culture, 20 μl of 5 mg/ml MTT was added to each well and incubated
at 37°C for 4 h. The medium was then gently aspirated and 150 μl of
dimethyl sulfoxide (DMSO) was added to each well to solubilize the
formazan crystals. The optical density of each sample was
immediately measured using a microplate reader (Bio-Rad, Hercules,
CA, USA) at 570 nm.
Flow cytometry assay
An Annexin V-FITC-flow cytometry assay (4A Biotech
Co., Ltd.) was used to detect the apoptosis rate in the cells after
EMP1 transfection, as previously described (15). Cells were seeded into 60-mm dishes
for 48 h and grown to 70–75% confluence. After quick detachment
from the plate, cells were collected, washed with ice-cold PBS, and
resuspended at a cell density of 1×106/ml in a binding
buffer from the Annexin V-FITC apoptosis detection kit (4A Biotech
Co., Ltd.). Cells were then stained with 5 μl of Annexin V-FITC and
10 μl of propidium iodide (PI, 20 μg/ml). The cells were incubated
in the dark at 25°C for 15 min before 10,000 cells were analyzed by
a FACScan flow cytometer (BD Immunocytometry Systems, San Jose, CA,
USA) and Cellquest software (BD Immunocytometry Systems) for
apoptosis rate determination.
Invasion and migration assays
Invasion and migration assays were performed as
previously described (16). For
the invasion assay, Costar Transwell 8 μm inserts were coated with
50 μg reduced serum Matrigel (BD Biosciences, Bedford, MA, USA).
Invasion Chambers (BD China, Shanghai, China) were coated with
Matrigel and 7×105 cells were added per chamber. Medium
supplemented with 10% FBS was used in the lower chamber. For
migration assays, the same procedure was used excluding the
Matrigel. After 12 h, non-invading cells and media were removed,
and cells on the lower surface of the membrane were fixed with
polyoxymethylene (Sigma) and stained with 0.1% crystal violet
(Sigma) for 0.5 h. Stained cells were counted under a microscope in
four randomly selected fields, and the average was used to indicate
cell migration and invasion.
Statistical analysis
All statistical analyses were performed using SPSS
16.0 software (IBM, Chicago, IL, USA), as previously described
(17). For the clinicopathological
features, P-values were calculated using the χ2 test.
Student’s t-test was used to analyze the difference between groups.
Survival distributions were estimated with the Kaplan-Meier method
and compared with the log-rank test. A P-value <0.05 was
considered to be statistically significant.
Results
EMP1 protein expression in gastric cancer
and normal tissues
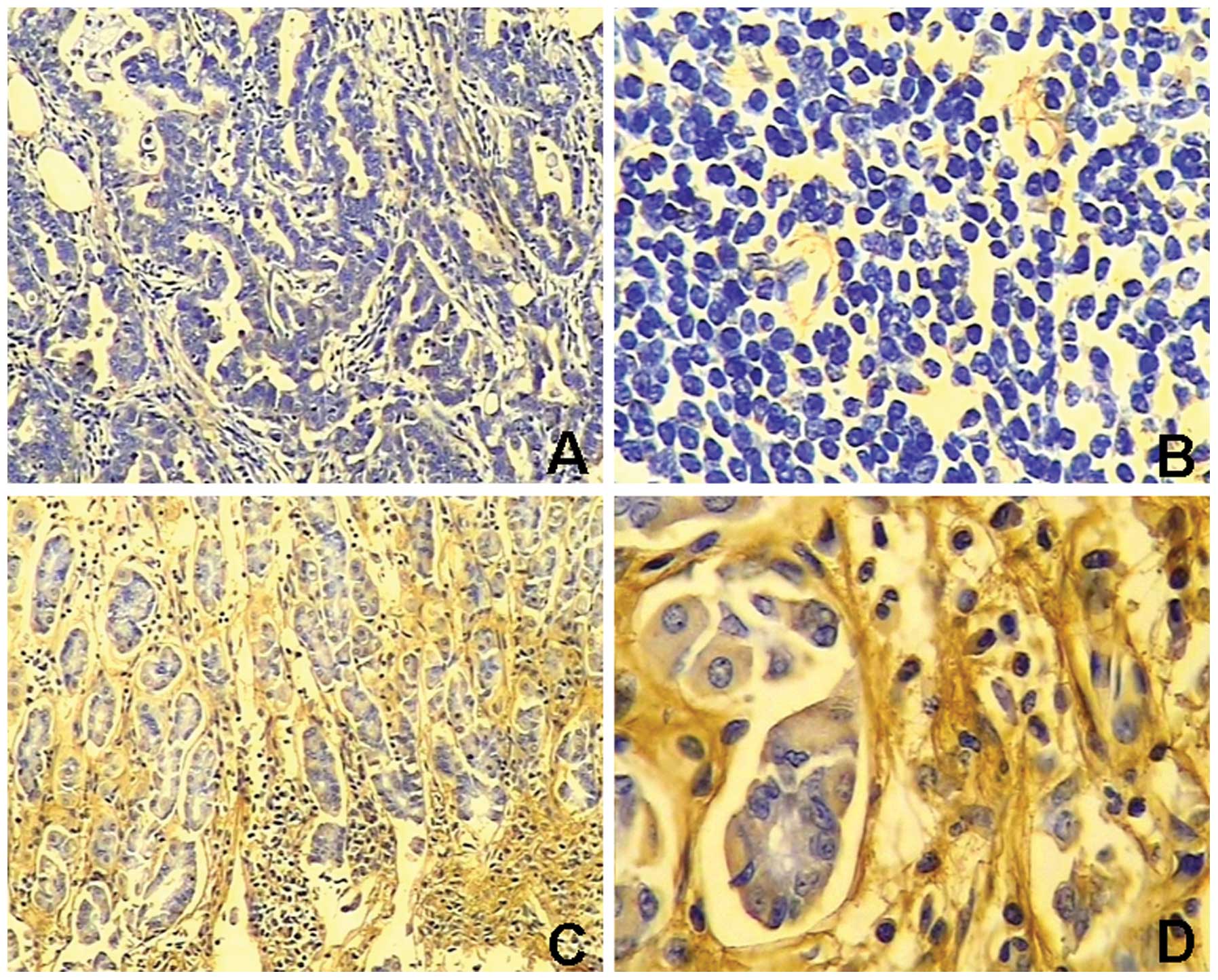
EMP1 staining in gastric cancer tissue was negative
or weak relative to normal adjacent gastric tissues that exhibited
light yellow to brown staining. EMP1 expression was significantly
lower (P<0.05) in gastric cancer tissue (expressed in 41.5%,
27/65) than normal tissue (expressed in 70.4%, 19/27) (Table I, Fig.
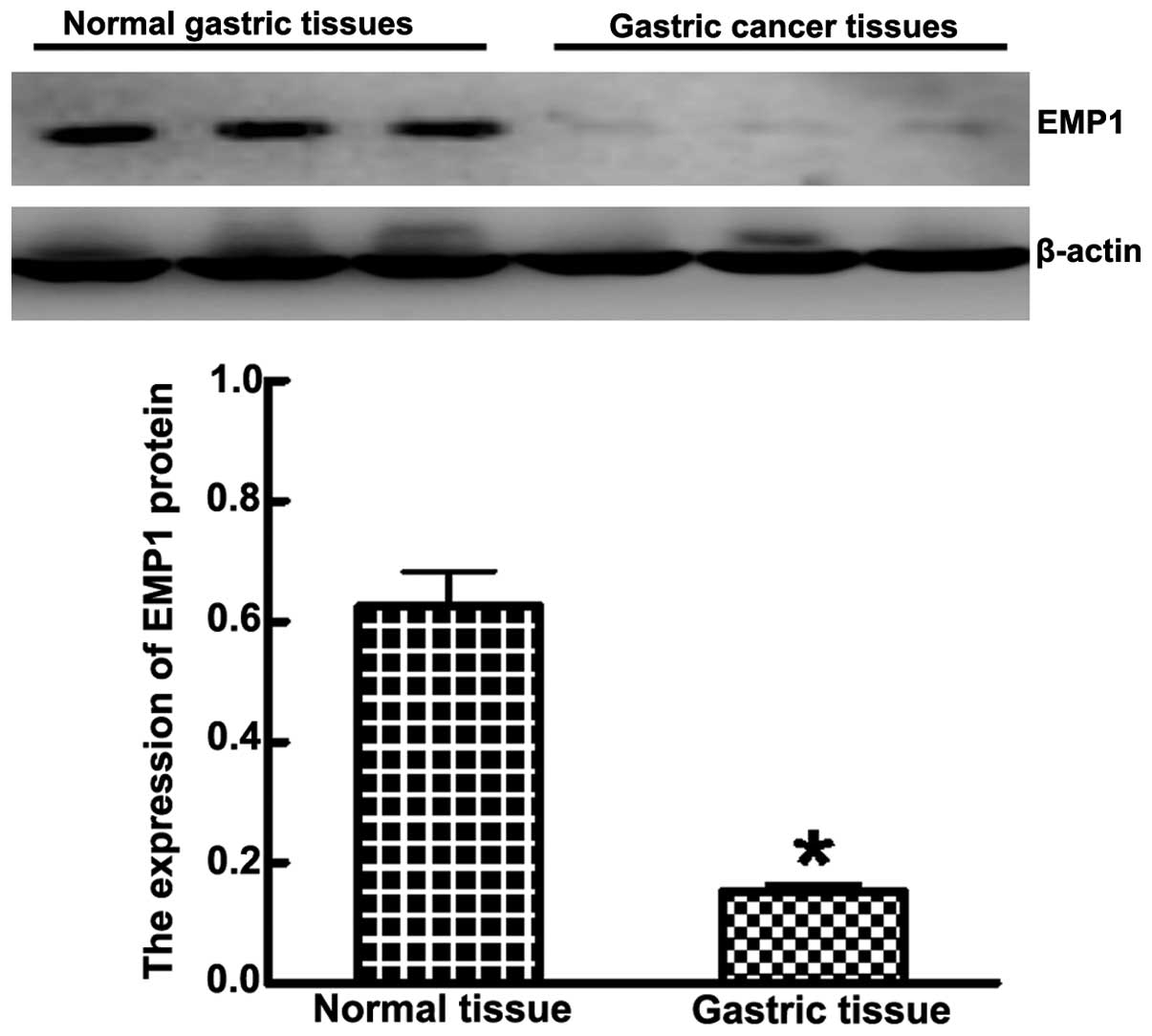
1). Western blot analysis showed that the expression of EMP1 in
cancer lesions was significantly less than adjacent normal tissue
(0.153±0.012 and 0.626±0.058, respectively; P<0.05) (Fig. 2). The expression of EMP1 negatively
correlated with tumor invasion, lymph node metastasis, clinical
stages and pathological differentiation (P<0.05, Table II).
 | Table IExpression of EMP1 in gastric cancer
tissue and normal tissue. |
Table I
Expression of EMP1 in gastric cancer
tissue and normal tissue.
| | Expression of EMP1
protein |
|---|
| |
|
|---|
| Groups | Case | − | + | ++ | +++ | χ2 | P-value |
|---|
| Normal tissue | 27 | 8 | 4 | 7 | 8 | 9.587 | 0.022 |
| Cancer tissue | 65 | 38 | 11 | 10 | 6 | | |
 | Table IIRelationship between EMP1 expression
and clinical characteristics in gastric cancer tissue. |
Table II
Relationship between EMP1 expression
and clinical characteristics in gastric cancer tissue.
| Expression of EMP1
protein |
|---|
|
|
|---|
| Groups | Case | − | + to +++ | χ2 | P-value |
|---|
| Tumor invasion | | | | | |
| T1+T2 | 31 | 21 | 20 | 6.412 | 0.011 |
| T3+T4 | 34 | 27 | 7 | | |
| Clinical
stages | | | | | |
| I–II | 28 | 11 | 17 | 4.800 | 0.028 |
| III–IV | 37 | 27 | 10 | | |
| Histological
grade | | | | | |
| III | 29 | 10 | 19 | 4.593 | 0.032 |
| I–II | 36 | 27 | 8 | | |
| Lymph node
metastasis | | | | | |
| N0 | 27 | 11 | 16 | 5.972 | 0.015 |
| N+ | 38 | 27 | 11 | | |
EMP1 expression and prognosis
Patients were followed up for 60 months for survival
analysis. At the end of the study in 2013, 37 of 63 patients had
survived. Patients were divided into two groups according to
expression level of EMP1. Of the 27 patients with positive levels
of EMP1 expression, 19 were still alive, yielding a survival rate
of 70.3%. Of the 38 patients with undetectable levels of EMP1
expression, only 18 were still alive, yielding a survival rate of
47.4%. Patients with high levels of EMP1 had a significantly higher
5-year survival rate than those with low levels of EMP1 (P<0.05)
(Fig. 3).
Stable transfection of EMP1 cDNA in
gastric cancer cells
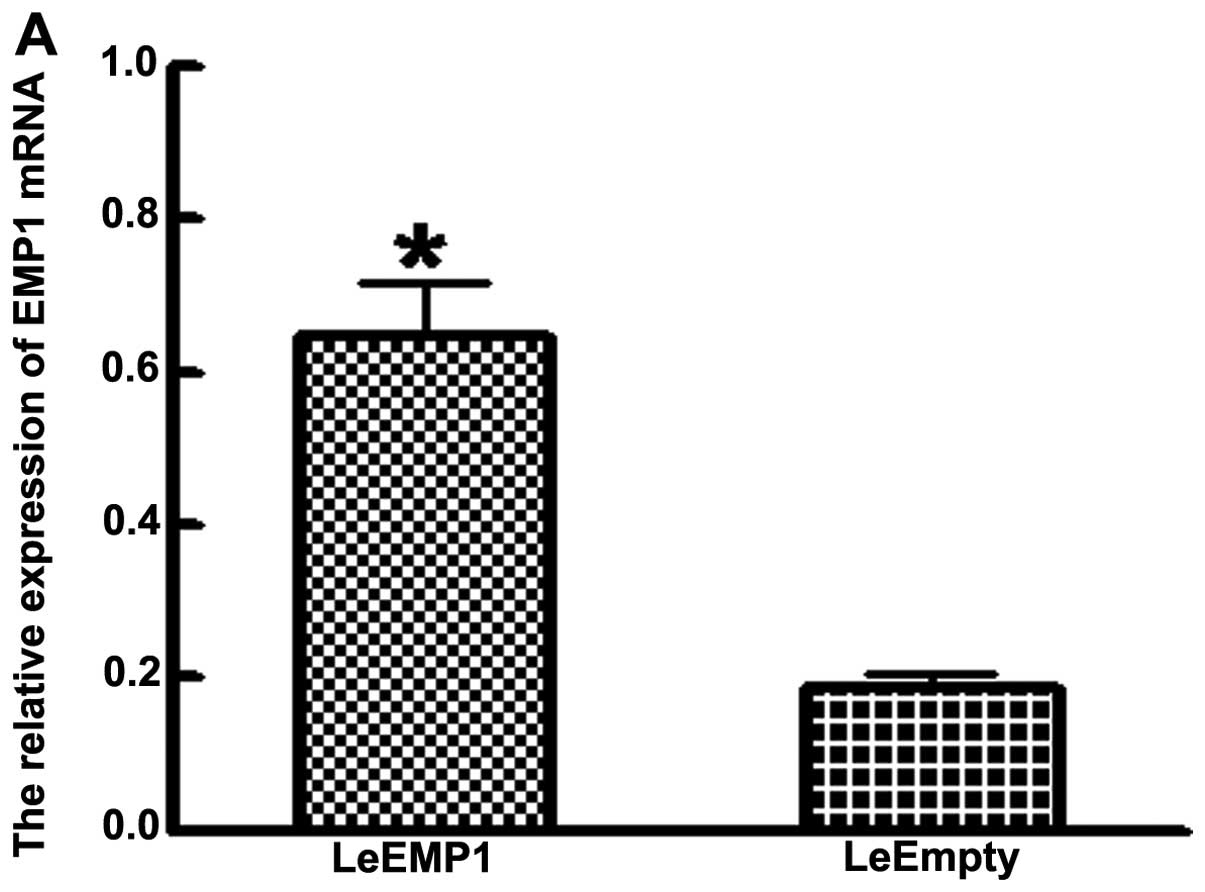
SGC-7901 cells stably transfected with EMP1
overexpressed EMP1 (named as LeEMP1 cells). Control SGC-7901 cells
were transfected with an empty vector (named as LeEmpty cells). The
expression of EMP1 mRNA and protein was significantly elevated in
LeEMP1 cells relative to control cells (P<0.05). EMP1 mRNA
levels detected by RT-PCR was significantly higher in LeEMP1 cells
(0.626±0.058) than LeEmpty cells (0.188±0.018) (P<0.05; Fig. 4A). Western blot analysis found that
the level of immunoreactive protein was significantly higher in
EMP1 transfected cells (0.731±0.070) relative to controls cells
(0.244±0.019) (P<0.05; Fig.
4B).
Effects of EMP1 overexpression on gastric
cancer cells
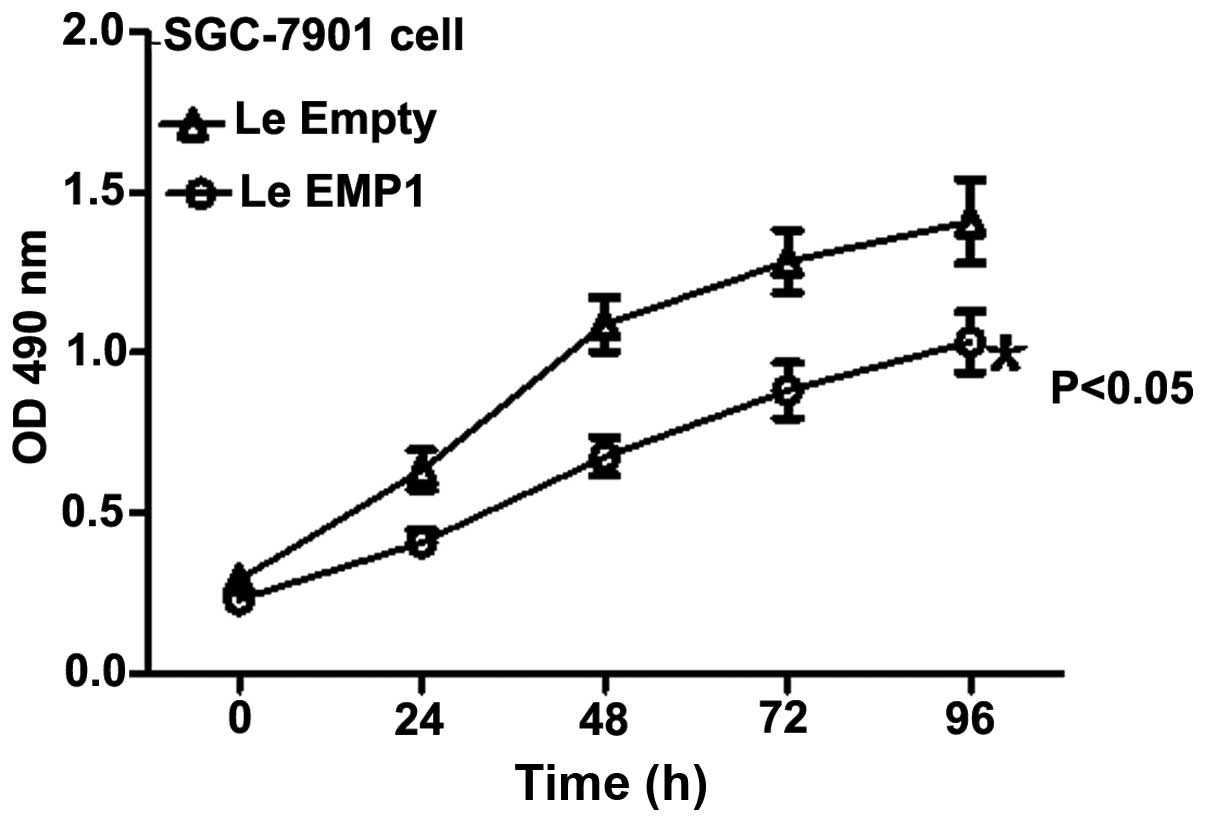
Next, we assessed the effect of EMP1 expression on
the regulation of gastric cancer cell viability. MTT assay showed
that relative proliferative capacity of LeEMP1 cells grew
significantly slower at 24, 48, 72 and 96 h relative to LeEmpty
cells (P<0.05; Fig. 5).
Meanwhile, there was a significant increase in the early apoptosis
rate in LeEMP1 cells (13.2±1.5%) relative to control cells
(2.2±0.5%) (P<0.05; Fig. 6).
SGC-7901 cells transfected with EMP1 or empty vector were
transferred to transwell chambers or Matrigel-coated transwell
chambers to evaluate the effect of EMP1 on cell invasion potential.
Overexpression of EMP1 clearly significantly decreased cell
migration and invasion of SGC-7901 cells (157.0±16.0 and
112.0±12.0, respectively) relative to control cells (243.0±21.0 and
203.0±19.0, respectively) (P<0.05; Fig. 7).
To further study the mechanisms by which EMP1
inhibited gastric cancer cell proliferation, cell apoptosis,
migration and invasion, we analyzed the expression of two proteins
with critical roles in these processes, caspase-9 and VEGFC.
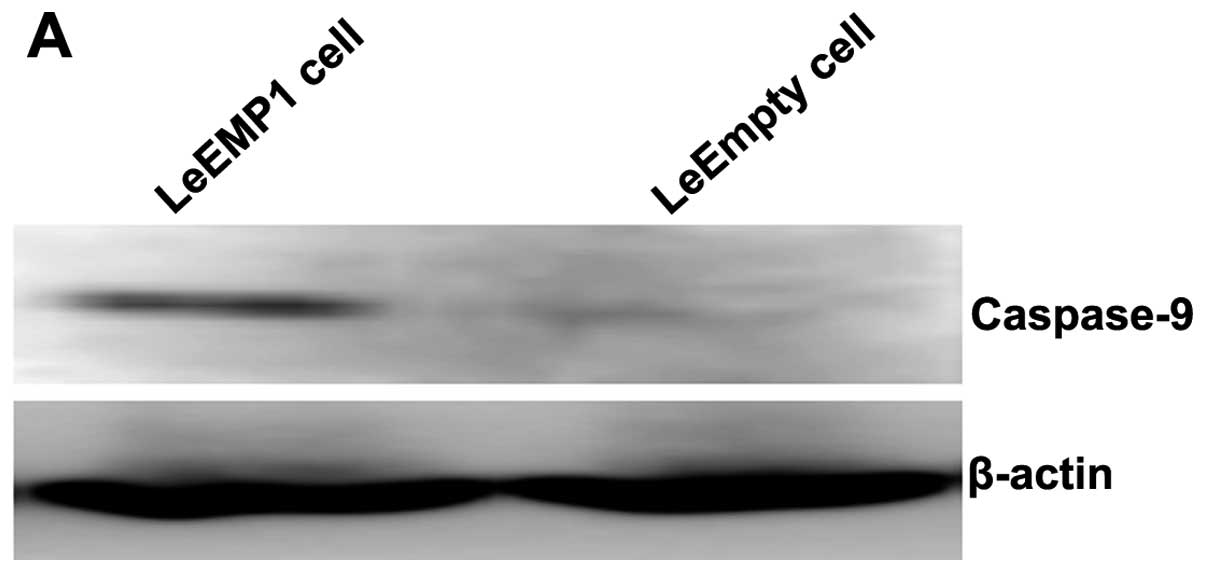
Western blot analysis revealed that overexpression of EMP1 in
SGC-7901 cells significantly upregulated caspase-9 protein
expression (0.501±0.050) relative to control cells (0.114±0.010)
(P<0.05; Fig. 8). In contrast,
the level of VEGFC protein expression was significantly lower in
SGC-7901 cells overexpressing EMP1 (0.135±0.011) than control cells
(0.619±0.074) (P<0.05; Fig.
8).
Discussion
Several studies have shown that the EMP1 gene is
expressed in a number of normal tissues (7,18–23).
In this study, we localized and quantified for the first time EMP1
protein expression in gastric cancer tissue and normal gastric
tissue using immunohistochemistry and immunoblotting. EMP1 protein
levels were significantly lower in gastric carcinoma than in normal
tissue and EMP1 protein levels correlated with tumor invasion,
lymph node metastasis and clinical stage of gastric cancer. Since
dedifferentiation is a hallmark of tumor cells, our findings
suggest that a decline in EMP1 level is a factor in the development
and progression of gastric cancer. In a study evaluating several
types of human breast cancer cells with different metastatic
characteristics, EMP1 gene expression was correlated with cell
invasion and other properties of metastasis (24). EMP1 gene expression was
downregulated in oral squamous cell carcinoma and this
downregulation was correlated with lymph node metastasis (25). Therefore, the EMP1 gene may be an
important factor for the regulation of cell signaling, cell
communication and adhesion (26).
Currently an effective treatment paradigm for
gastric cancer is surgical extended lesion resection, accompanied
by chemotherapy and/or radiotherapy before and after surgery.
However, the survival rate with this strategy is not very
satisfactory (27,28). Therefore, efforts should be
directed toward early detection of gastric cancer and the
refinement of individual-based treatment strategies. Conventional
treatment and prognosis of gastric cancer rely mainly on TNM
classification (29). This system
is subjective and not informative for early gastric cancer, and
offers limited information on the disease severity, prognosis and
response to treatment. Early detection of gastric cancer is the
most effective way to improve survival (30). Using survival analysis, we found
that EMP1 expression-positive patients had a significantly higher
5-year overall survival rate than patients with undetectable EMP1
expression. Thus, combining information from the TNM classification
system and EMP1 expression scores may provide valuable information
for clinicians regarding prognosis, prediction of disease severity
and selection of treatment regimens.
Furthermore, in vitro experiments
demonstrated for the first time that gastric cancer cells with high
EMP1 expression had significantly weakened proliferation,
significantly increased apoptosis, markedly increased caspase-9 and
reduced VEGFC protein levels. Previously, overexpression of EMP1 in
an esophageal cancer cell line slowed esophageal cancer cell growth
and yielded fewer S-phase cells and more G1-phase cells (26). Together with our findings, these
data suggest that low levels of EMP1 affect cellular processes that
are abnormally regulated in cancer. Mitochondria are not only the
site of cellular respiration and oxidative phosphorylation, but
also the regulation center of apoptosis. Cytochrome C released from
mitochondria to the cytoplasm associates with apoptotic protease
activating factor (Apaf-1) to form a multiservice complex in the
presence of deoxyribonucleotide triphosphate (dNTP) (31). This complex interacts with
pro-caspase-9 to form an apoptosome and, following dimerization,
results in autoactivation of caspase-9. This activated caspase-9
stimulates other caspases, such as caspase-3 and caspase-7,
culminating in apoptosis via signaling cascades (32–34).
We found in this study that high expression of EMP1 is associated
with significantly higher expression of caspase-9 protein,
implicating a mitochondrial apoptosis pathway in EMP1-induced
apoptosis.
VEGF is a member of the platelet-derived growth
factor (PDGF) family and is the most important vascular endothelial
growth-stimulating factor during tumor angiogenesis. VEGFC is a
recently identified member of the VEGF family, which promotes the
proliferation of endothelial cells, increases vascular
permeability, and functions as a key factor in tumor angiogenesis,
invasion and metastasis (35,36).
We found in this study that overexpression of EMPl is associated
with a significant decrease in VEGFC expression. This finding
suggests that EMP1 may inhibit tumor angiogenesis by suppressing
VEGFC expression and hence tumor metastasis.
In summary, we demonstrated that EMP1 protein levels
were significantly reduced in gastric carcinoma and were associated
with tumor invasion, lymph node metastasis, clinical stage and cell
differentiation. EMP1 is involved in a number of biological
processes including proliferation, apoptosis, invasion and
metastasis of gastric cancer. Given the complexity of
carcinogenesis, further research is needed to understand the
molecular mechanism underlying EMP1 regulation of this process. Our
findings identify a novel potential therapeutic target for gastric
cancer and suggest EMP1 may be a reliable biomarker for prognosis
of gastric cancer.
References
|
1
|
Hofmockel G: Molecular genetic principles
of tumor development and progression. Urologe A. 39:212–213.
2000.PubMed/NCBI
|
|
2
|
de Martel C, Forman D and Plummer M:
Gastric cancer: epidemiology and risk factors. Gastroenterol Clin
North Am. 42:219–240. 2013.PubMed/NCBI
|
|
3
|
Dimofte G, Tarcoveanu E, Taraşi M, Panait
C, Lozneanu G, Nicolescu S, Porumb V and Grigoraş O: Mean number of
lymph nodes in colonic cancer specimen: possible quality control
index for surgical performance. Chirurgia (Bucur). 106:759–764.
2011.
|
|
4
|
Fleming M, Ravula S, Tatishchev SF and
Wang HL: Colorectal carcinoma: pathologic aspects. J Gastrointest
Oncol. 3:153–173. 2012.
|
|
5
|
Lai S, Wang G, Cao X, Li Z, Hu J and Wang
J: EMP-1 promotes tumorigenesis of NSCLC through PI3K/AKT pathway.
J Huazhong Univ Sci Technolog Med Sci. 32:834–838. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang HT, Liu ZH, Wang XQ and Wu M: Effect
of EMP-1 gene on human esophageal cancer cell line. Ai Zheng.
21:229–232. 2002.PubMed/NCBI
|
|
7
|
Lee HS, Sherley JL, Chen JJ, Chiu CC,
Chiou LL, Liang JD, Yang PC, Huang GT and Sheu JC: EMP-1 is a
junctional protein in a liver stem cell line and in the liver.
Biochem Biophys Res Commun. 334:996–1003. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kasher R, Bajayo A, Gabet Y, Nevo N,
Fridkin M, Katchalski-Katzir E, Kohen F and Bab I: Restrain of bone
growth by estrogen-mimetic peptide-1 (EMP-1): a micro-computed
tomographic study. Peptides. 30:1181–1186. 2009. View Article : Google Scholar
|
|
9
|
Bozec A, Peyrade F and Milano G: Molecular
targeted therapies in the management of head and neck squamous cell
carcinoma: recent developments and perspectives. Anticancer Agents
Med Chem. 13:389–402. 2013.PubMed/NCBI
|
|
10
|
Suzuki K, Nakamura K, Kato K, Hamada H and
Tsukamoto T: Exploration of target molecules for prostate cancer
gene therapy. Prostate. 67:1163–1173. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Turashvili G, Bouchal J, Ehrmann J,
Fridman E, Skarda J and Kolar Z: Novel immunohistochemical markers
for the differentiation of lobular and ductal invasive breast
carcinomas. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub.
151:59–64. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Muller PY, Janovjak H, Miserez AR and
Dobbie Z: Processing of gene expression data generated by
quantitative real-time RT-PCR. Biotechniques. 32:1372–1379.
2002.PubMed/NCBI
|
|
13
|
Ranganathan V and De PK: Western blot of
proteins from Coomassie-stained poly-acrylamide gels. Anal Biochem.
234:102–104. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van Meerloo J, Kaspers GJ and Cloos J:
Cell sensitivity assays: the MTT assay. Methods Mol Biol.
731:237–245. 2011.PubMed/NCBI
|
|
15
|
Rasola A and Geuna M: A flow cytometry
assay simultaneously detects independent apoptotic parameters.
Cytometry. 45:151–157. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kramer N, Walzl A, Unger C, Rosner M,
Krupitza G, Hengstschläger M and Dolznig H: In vitro cell migration
and invasion assays. Mutat Res. 752:10–24. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Richards RJ: Responsibility for
statistical analyses. Endocr Pract. 9:3292003.PubMed/NCBI
|
|
18
|
Taylor V, Welcher AA, Program AE and Suter
U: Epithelial membrane protein-1, peripheral myelin protein 22, and
lens membrane protein 20 define a novel gene family. J Biol Chem.
270:28824–28833. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lobsiger CS, Magyar JP, Taylor V, Wulf P,
Welcher AA, Program AE and Suter U: Identification and
characterization of a cDNA and the structural gene encoding the
mouse epithelial membrane protein-1. Genomics. 36:379–387. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wulf P and Suter U: Embryonic expression
of epithelial membrane protein 1 in early neurons. Brain Res Dev
Brain Res. 116:169–180. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zoidl G, Blass-Kampmann S, D’Urso D,
Schmalenbach C and Müller HW: Retroviral-mediated gene transfer of
the peripheral myelin protein PMP22 in Schwann cells: modulation of
cell growth. EMBO J. 14:1122–1128. 1995.PubMed/NCBI
|
|
22
|
Jetten AM and Suter U: The peripheral
myelin protein 22 and epithelial membrane protein family. Prog
Nucleic Acid Res Mol Biol. 64:97–129. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu XM, Li CW, Li YY, Liu J, Lin ZB, Li TY,
Zhao L, Pan XL, Shi L and Wang de Y: Down-regulation of EMP1 is
associated with epithelial hyperplasia and metaplasia in nasal
polyps. Histopathology. 63:686–695. 2013.PubMed/NCBI
|
|
24
|
Gnirke AU and Weidle UH: Investigation of
prevalence and regulation of expression of progression associated
protein (PAP). Anticancer Res. 18:4363–4369. 1998.PubMed/NCBI
|
|
25
|
Zhang J, Cao W, Xu Q and Chen WT: The
expression of EMP1 is downregulated in oral squamous cell carcinoma
and possibly associated with tumour metastasis. J Clin Pathol.
64:25–29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang HT, Kong JP, Ding F, Wang XQ, Wang
MR, Liu LX, Wu M and Liu ZH: Analysis of gene expression profile
induced by EMP-1 in esophageal cancer cells using cDNA Microarray.
World J Gastroenterol. 9:392–398. 2003.PubMed/NCBI
|
|
27
|
Isobe T, Hashimoto K, Kizaki J, Miyagi M,
Aoyagi K, Koufuji K and Shirouzu K: Characteristics and prognosis
of gastric cancer in young patients. Oncol Rep. 30:43–49.
2013.PubMed/NCBI
|
|
28
|
Lee HS, Lee HK, Kim HS, Yang HK and Kim
WH: Tumour suppressor gene expression correlates with gastric
cancer prognosis. J Pathol. 200:39–46. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen Y and Mou L: A risk score system to
preoperatively predict TNM stages in gastric cancer. Am J Clin
Oncol. 34:130–134. 2011.PubMed/NCBI
|
|
30
|
Jiexian J, Xiaoqin X, Lili D, Baoguo T,
Ting S, Xianwen Z and Cunzhi H: Clinical assessment and prognostic
evaluation of tumor markers in patients with gastric cancer. Int J
Biol Markers. 28:192–200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sen S, Kawahara B and Chaudhuri G:
Mitochondrial-associated nitric oxide synthase activity inhibits
cytochrome c oxidase: implications for breast Cancer. Free Radic
Biol Med. 57:210–220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang JM, Wang HC, Wang HX, Ruan LH, Zhang
YM, Li JT, Tian S and Zhang YC: Oxidative stress and activities of
caspase-8, -9, and -3 are involved in cryopreservation-induced
apoptosis in granulosa cells. Eur J Obstet Gynecol Reprod Biol.
166:52–55. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Johnson CR and Jarvis WD: Caspase-9
regulation: an update. Apoptosis. 9:423–427. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fearnhead HO, Rodriguez J, Govek EE, Guo
W, Kobayashi R, Hannon G and Lazebnik YA: Oncogene-dependent
apoptosis is mediated by caspase-9. Proc Natl Acad Sci USA.
95:13664–13669. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xie LX, Zhai TT, Yang LP, Yang E, Zhang
XH, Chen JY and Zhang H: Lymphangiogenesis and prognostic
significance of vascular endothelial growth factor C in
gastro-oesophageal junction adenocarcinoma. Int J Exp Pathol.
94:39–46. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang Y, Meng X, Zeng H, Guan Y, Zhang Q,
Guo S, Liu X and Guo Q: Serum vascular endothelial growth factor-C
levels: a possible diagnostic marker for lymph node metastasis in
patients with primary non-small cell lung cancer. Oncol Lett.
6:545–549. 2013.PubMed/NCBI
|