Introduction
Nasopharyngeal carcinoma (NPC) is a human squamous
cell carcinoma arising in the epithelium lining the upper region of
the retronasal cavity. NPC has a remarkable geographic
distribution, being very prevalent in southern China, Southeast
Asia, and North Africa (1). The
annual incidence rate is ~30 per 100,000 in such regions, thus
50-fold higher than in the Western world. Radiotherapy is the
standard treatment for NPC, although such therapy is accompanied by
undesirable complications. The overall 5-year survival rate is
~60%, and is even higher if intensity-modulated radiation therapy
is employed (2,3). Chemotherapy is important to control
distant metastasis of chemoradiosensitive NPC, and thus plays an
important role. However, neither an optimal combination of
antitumor drugs, nor an ideal chemoradiotherapeutic regimen, has
been well-established (4).
BCL-2 proteins are critical in terms of cell
survival and are overexpressed in many tumors. To date, 25 members
of the Bcl-2 family have been identified. The family is subdivided
into three main groups based on differences in regions of the Bcl-2
homology (BH) domain, and the functions of these regions. One group
of proteins inhibits apoptosis and exhibits four distinct BH
domains; these proteins include Bcl-2, Bcl-xL, Bcl-w, Mcl-1, Bcl-B
and A1. The pro-apoptotic proteins are divided into two distinct
groups. These are the multidomain proteins (Bax, Bak and Bok)
containing three BH domains; and the (Bcl-2-homology domain 3-)
BH3-only proteins (Bad, Bid, Bim, Bmf, Bik, Hrk, Noxa and Puma),
which have a conserved BH3 domain that can bind to anti-apoptotic
Bcl-2 proteins to promote apoptosis (5–7).
Anti-apoptotic Bcl-2 family proteins (Bcl-2, Bcl-xL,
and Mcl-1) are commonly highly expressed in many types of cancer
(8). Lu et al detected
Bcl-2 in most (80%) samples of undifferentiated NPC9 cells
(9). Fan et al found that
the expression of Bcl-2 protein was significantly higher in NPC
tissues than in normal noncancerous nasopharyngeal epithelia (NPE)
and hyperplastic NPE1 (10). Sheu
et al immunohistochemically analyzed Bcl-2 expression levels
in biopsy specimens from 101 cases of NPC. The proportions of NPCs
(80%) and adjacent dysplastic lesions (71%) expressing Bcl-2 were
significantly higher than those of adjacent NPE (37%) and the NPE
of patients with chronic inflammation of the nasopharynx (30%)
(11). Yu et al explored
whether Bcl-2 was overexpressed in NPCs at the time of diagnosis.
The prevalence of Bcl-2 positivity in our material was 61% (31/51).
Expression of Bcl-2 in NPCs is significantly associated with
prognosis. Few patients with Bcl-2+ tumors survive for 5
years; most develop local recurrences or distant metastases
(12). Overexpression of
anti-apoptotic proteins (Bcl-2, Bcl-XL, Bcl-w and Mcl-1) reduces
the pro-apoptotic response and causes NPC cells to develop
resistance to traditional radiation and chemical therapies
(13).
Targeting of the anti-apoptotic Bcl-2 family of
proteins improves apoptosis and autophagy, thus overcoming drug
resistance developing during cancer chemotherapy (14–16).
Several groups have developed strategies to block the
anti-apoptotic activities of proteins of the Bcl-2 family. These
feature the use of a Bcl-2 antisense oligodeoxynucleotide,
peptides, and small-molecule inhibitors (17,18).
As Bcl-2, Bcl-xL and Mcl-1 are critical regulators of apoptosis,
being important anti-apoptotic molecules, it may be predicted that
pan-inhibition of such Bcl-2 family members by small-molecule
inhibitors would effectively induce cancer cell apoptosis. A Bcl-2
antisense oligodeoxynucleotide, oblimersen sodium (G3139,
Genasense) has shown promise when used as an anti-apoptotic agent
in tumor therapy, but G3139 targets only Bcl-2 mRNA, thus not mRNAs
encoding Mcl-1 and Bcl-xl, which are also overexpressed in many
cancer tissues (5,7).
ABT-737 (A-779024, Abbott Laboratories) is a small
molecule that targets anti-apoptotic Bcl-2 family proteins (Bcl-2,
Bcl-XL and Bcl-w), thereby sequestering pro-apoptotic proteins with
the BH3 domain, in turn promoting oligomerization of Bax and Bak
and, ultimately, programmed cell death of malignant cells (18–21).
ABT-737 is a potent small-molecule mimic of BH3 with high affinity
Bcl-2, Bcl-xL and Bcl-w, but low affinity for Mcl-1 and A1 (Ki ≤1
nM for Bcl-2, Bcl-XL and Bcl-w; Ki=0.46 nM for Bcl-B, Mcl-1 and
Bfl1/A-1) (19).Consistent with
the low affinity of ABT-737 for Mcl-1, several reports have
suggested that high basal levels of Mcl-1 are associated with
resistance to ABT-737. Combinations of ABT-737 with a
cyclin-dependent kinase inhibitor (flavopiridol), arsenic trioxide,
or fenretinide, are therapeutically synergistic, because the latter
agent inactivates Mcl-1. Such work is paving the way for
development of combination chemotherapies targeting the Bcl-2
family of proteins (22–25).
Gossypol is the first known compound to inhibit the
Bcl-2, Bcl-XL and Mcl-1. Gossypol is a potentially toxic phenolic
pigment found in the seed, stem, and root of the cotton plant, and
was initially (during the 1950s) identified as an antifertility
agent in China. Natural gossypol is a racemic mixture, and
levo-gossypol (AT-101, Ascenta) phase II clinical trials are
ongoing (in combination with rituximab) in chronic lymphocyte
leukemia (CLL) patients and (in combination with docetaxel) in
patients with hormone-refractory prostate cancer. In a fluorescence
polarization-based binding assay, (−)-gossypol bound to Bcl-2,
Bcl-xL, and Mcl-1 with Ki values of 320, 480 and 180 nM,
respectively. Gastrointestinal toxicity was dose-limiting in a
phase I/II clinical trial in prostate cancer patients (26,27).
Apogossypolone (ApoG2) is a promising semi-synthetic
derivative of gossypol, formed by removal of aldehyde groups, and
binds to Bcl-2 family proteins with Ki values of 35 and 25 nM for
Bcl-2 and Mcl-1, respectively, and a Ki of 660 nM for Bcl-XL
(13,18). ApoG2 exhibits a higher antitumor
activity and lower toxicity than gossypol and other derivatives
(29–31). Combinations of radiotherapy and
chemotherapy have become standard treatments for NPC. We previously
showed that ApoG2 indeed induced apoptosis by blocking the
anti-apoptotic functions of Bcl-2 family members and suppressed
tumor growth in NPC xenografts (13). ApoG2 disturbed the proliferation of
NPC cells by suppressing c-Myc signaling and inducing arrest at the
DNA synthesis stage in a large proportion of such cells (32), and suppressed growth of the human
lymphoma cell line U937 by inhibiting the actions of anti-apoptotic
proteins of the Bcl-2 family, and inducing mitochondrion-dependent
apoptotic cell death (30). In the
present study, we further investigated the radiosensitizing effects
of ApoG2 on NPC cells.
Materials and methods
Cell culture and reagents
Human NPC cell line CNE1 (a highly differentiated
line), the NPC, CNE2 and SUNE1 cell lines (which are poorly
differentiated), and the NP69 normal human nasopharyngeal
epithelial cell line, were cultured in RPMI-1640 with 10% (v/v)
fresh bovine serum, 1 U/ml penicillin, and 1 mg/ml streptomycin at
37°C under 5% (v/v) CO2. ApoG2 was provided by Dr Dajun
Yang, dissolved to 20 mM in dimethylsulfoxide (DMSO), and stored at
−20°C. The primary antibodies used for immunoblots and
immunoprecipitations were anti-Bcl-2; anti-LC3; anti-GAPDH;
anti-p62; and anti-Beclin 1 (Cell Signaling, Danvers, MA, USA).
MTT assay
Cell viability was measured using the MTT assay.
Mitochondria converted MTT from a soluble tetrazolium salt to an
insoluble colored formazan precipitate, which was dissolved in DMSO
and spectrophotometrically quantitated. NPC cells were plated in
96-well tissue culture plates (Costar, Cambridge, MA, USA) at a
density of 8×104 cells/ml. The ApoG2 stock solution was
serially diluted. All experiments were performed in duplicate or
triplicate. MTT (10 μl amounts of a solution of 10 mg/ml) was added
to each well and, 4 h later, the solution was removed, and DMSO
(100 μl) added to each well. Percentages of absorbance relative to
that of the control were plotted as linear functions of drug
concentration. The 50% inhibitory concentration (IC50)
was the concentration of drug required to inhibit growth by 50%,
relative to that of the control. Cell growth inhibition was
measured as the viable cell percentage relative to that of the
control.
Transmission electron microscopy
(TEM)
CNE2 cells were seeded in 10 cm-diameter dishes,
cultured for 24 h, and next treated with either DMSO [final
concentration, 0.1% (v/v)] or ApoG2 in DMSO (20 μM) for 24 h. Cells
were fixed in 4 ml of ice-cold 2.5% (v/v) glutaraldehyde of
electron microscopy grade, rinsed with PBS, fixed in 1% (w/v)
osmium tetroxide, dehydrated via passage through a graded series of
ethanol baths (50–90%, v/v), washed in 90% (v/v) acetone, and
embedded in Epon 812 resin. Ultrathin (50–60 nm) sections were cut
using an LKB NOVA ultramicrotome (LKB-NOVA, Bromma, Sweden). The
sections were stained with 2% (w/v) uranyl acetate and lead
citrate, and visualized using a Philips CN10 transmission electron
microscope at either ×6,000 or ×12,000 magnification.
Immunoblotting and
immunoprecipitation
CNE1 and CNE2 cells were treated with 5 μM ApoG2 for
0, 6, 12, 24 or 48 h; or with 0, 5, 10 or 20 μM ApoG2 for 24 h.
Whole-cell lysates were prepared in 3X SDS sample buffer. Equal
amounts of protein were electrophoresed on SDS-PAGE gels and
transferred to polyvinylidene difluoride membranes. Membranes were
next blocked for 60 min at room temperature with 5% (w/v) nonfat
dry milk/TBS-Tween-20, and reacted with antibodies detecting LC3,
GAPDH, or p62, overnight at 4°C with gentle rocking. Membranes were
next washed in TBS-Tween-20 and incubated with horseradish
peroxidase-conjugated secondary antibodies for 1 h at room
temperature. Proteins were visualized with the aid of reagents
detecting enhanced chemiluminescence, followed by exposure to
radiographic film.
When immunoprecipitation was planned, CNE2 cells
were treated with 20 μM ApoG2 for 24 h, harvested, and lysed in
ice-cold lysis buffer. Lysates were centrifuged at 12,000 × g for
15 min, and the supernatants incubated overnight at 4°C with
anti-Bcl-2 antibody. Antibody-protein complexes were pelleted with
the aid of protein A-agarose. Precipitates were harvested by brief
centrifugation and washed five times with ice-cold lysis buffer.
Immunoprecipitated proteins were eluted with 3X SDS sample buffer
and further analyzed by SDS-PAGE followed by immunoblotting with
either anti-Bcl-2 or anti-Beclin 1 antibody to determine the effect
of ApoG2 on the interaction of Beclin 1 and Bcl-2.
Plasmids and transfection
We used a retroviral construct (pSUPER puro, a gift
of Professor Musheng Zeng, Cancer Center, Sun Yat-sen University,
Guangzhou, China) encoding stable ATG5 siRNA with hairpin
sequences. CNE2 cells stably expressing Atg5 shRNA were established
by infection with retrovirus-containing supernatants, as described
previously (33). After 24 h of
transfection, cells were used in further experiments.
GFP-LC3 punctate staining assay
CNE2 cells were grown on glass coverslips and
transfected with the pGFP-LC3 vector using Lipofectamine 2000
(Invitrogen Life Technologies, Carlsbad, CA, USA). Twenty-four
hours later, the cells were divided into four groups: a control
group, an ApoG2 group (20 μM ApoG2), an irradiation group (2 Gy
irradiation), and an ApoG2-plus-irradiation group (20 μM ApoG2 for
4 h and irradiation with 2 Gy). After 24 h, all cells were fixed in
4% (v/v) paraformaldehyde in PBS for 30 min at room temperature.
Coverslips were mounted in anti-fade solution and stored at 4°C
prior to examination using laser-scanning confocal microscopy
(Olympus FV-1000).
Colony formation assay
CNE2 cells were irradiated with 0–6 Gy, as
indicated, at a dose rate of 1.8 Gy/min, using a 137[Cs]
irradiator (Shepherd and Associates, Glendale, CA, USA). Irradiated
cells were seeded in 12-well plates in RPMI-1640 with 10% (v/v)
FBS, and incubated at 37°C for 12–14 days. Cells were fixed for 15
min in 3:1 (v/v) methanol:acetic acid and stained for 15 min with
0.5% (w/v) crystal violet (Sigma, St. Louis, MO, USA) in methanol.
After staining, colonies were counted using a cut off of 50 viable
cells/colony. A ‘surviving fraction’ was calculated as (mean colony
counts)/(cells inoculated) × [plating efficiency (PE)], where PE
was defined as (mean colony counts)/(cells inoculated) for
non-irradiated controls. Experiments were performed in triplicate
and mean, SD and P-values calculated.
In vivo treatment and
immunohistochemistry
Athymic nude (nu/nu) mice from the Animal Center of
Guangzhou University of Traditional Chinese Medicine (Guangdong,
China) received subcutaneous injections of 2×106 CNE2
cells into each axilla. When the weights of subcutaneous tumors
attained >1,500 mg, mice were euthanized, and tumors dissected
and mechanically dissociated into equally sized pieces prior to
transplantation into the flanks of a new group of mice. When
xenograft tumors became palpable (~0.1 mm3 in volume),
mice were randomly divided into a control group [receiving a 0.5%
(w/v) sodium carboxymethylcellulose solution]; an ApoG2 group (120
mg ApoG2 per kg of body weight intragastrically daily for 7
continuous days); a 2 Gy group (irradiation with 2 Gy of
60[Co] γ-rays divided into five equal fractions given
over 5 consecutive days); and an ApoG2-plus-2 Gy combination group.
Each group had four mice, and tumor size did not differ among
groups. Tumor volumes were calculated every 3 days, using the
formula V=ab2 π/6, where a was the greatest tumor
diameter and b the shortest. After treatment for 20 days, mice were
euthanized and tumors were dissected and weighed.
Immunohistochemical analysis was performed on sections of CNE2
xenografts from the four groups. All samples were stained with
hematoxylin and eosin and microscopically examined to confirm that
the tumors originated from CNE2 cells. Sections were next incubated
with anti-LC3 antibody at 4°C overnight and visualized using
diaminobenzidine (DAB) as a peroxidase substrate. The experiment
protocol was approved by Sun Yat-sen University Cancer Center
ethics committee for animal experiment.
Statistical analysis
The significance of between-group differences were
compared using the unpaired t-test of SPSS version 15.0
software.
Results
ApoG2 inhibits the proliferation of NPC
cells
The chemical structures of gossypol and the
optimized derivative ApoG2 (34),
an oxidation product of gossypol, lacking two aldehyde groups are
shown in Fig. 1A. The NPC cell
lines CNE1, CNE2 and SUNE1, and the immortalized NP69 cell line,
were exposed to different concentrations of ApoG2 for 72 h. As
shown in Fig. 1B, ApoG2
dose-dependently inhibited the viability of CNE1, CNE2 and SUNE1
cells, but not that of NP69 cells. At 100 μM, ApoG2 reduced
proliferation of CNE1, CNE2 and SUNE1 cells by ~90% over 72 h. In
contrast, when NP69 cells were exposed to different concentrations
of ApoG2, no obvious inhibition was observed even at ≤50 μM ApoG2
(Fig. 1B). At 72 h, the
IC50 values of ApoG2 acting on CNE1, CNE2, and SUNE1
cells were 2.84±0.19, 2.18±0.38 and 5.64±0.65 μM, respectively.
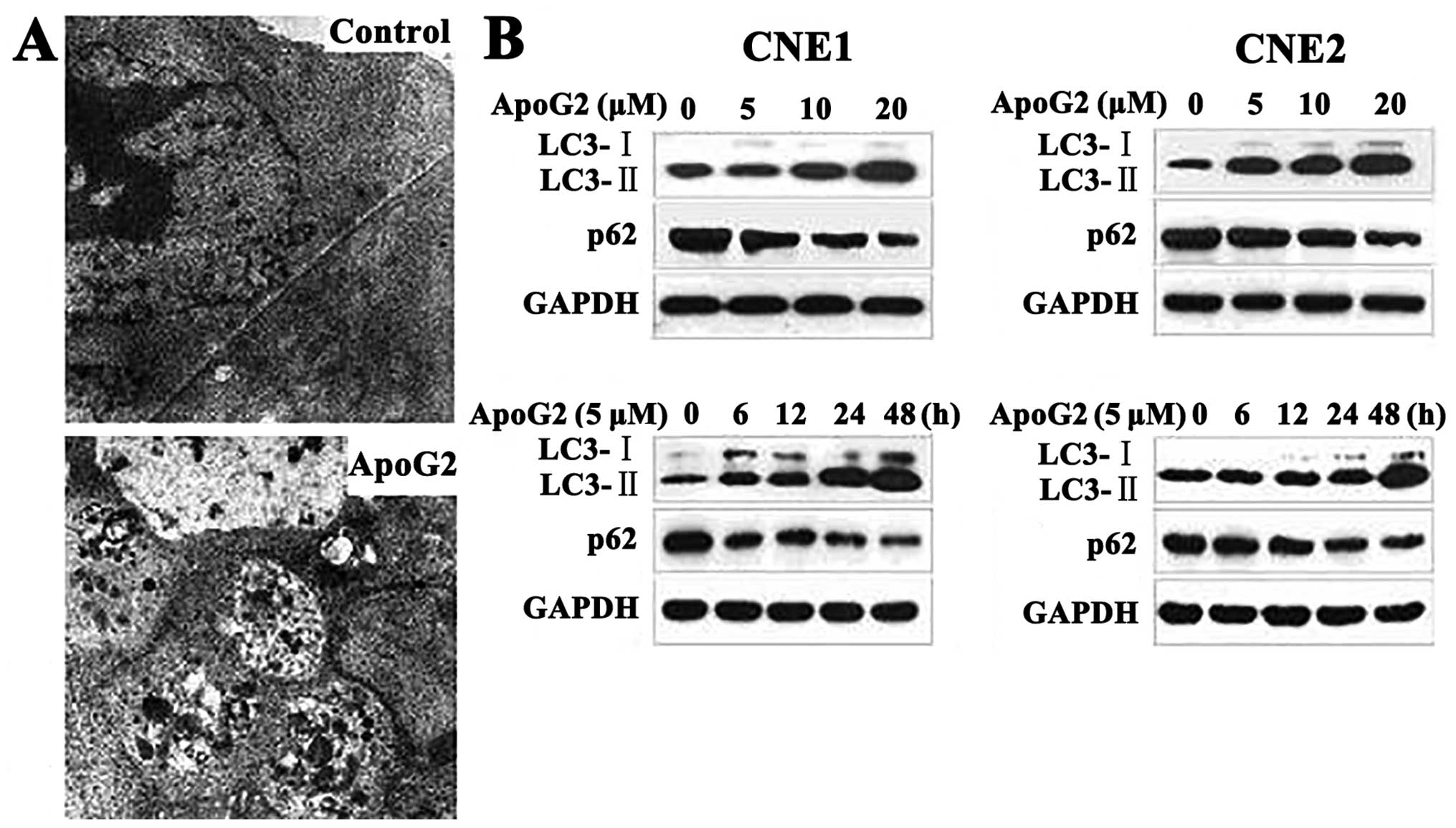
ApoG2 induces autophagy of NPC cells
Autophagy is an evolutionarily conserved process of
protein degradation associated with tumor promotion and tumor
suppression in different situations. To determine whether ApoG2
could induce autophagy of NPC cells, we monitored morphological
changes in such cells via transmission electron microscopy. As
shown in Fig. 2A, no autophagic
vacuoles were observed in DMSO-treated CNE2 cells. CNE2 cells
incubated with 20 μM ApoG2 for 24 h developed large cytoplasmic
vacuoles with membrane bilayers. The vacuoles resembled
autophagosomes and contained remnants of degraded organelles.
These results were confirmed by western blotting.
The microtubule-associated protein 1 light chain 3 (LC3), a homolog
of yeast Atg8, is present on isolated autophagosomal membranes. The
amount of LC3-II correlates well with the number of autophagosomes.
Enhancement of conversion of LC3-I to LC3-II, and upregulation of
LC3 expression, occurs when autophagy is induced (35). Protein p62 (also known as SQSTM1)
has a short region that interacts with LC3. p62 participates in
autophagy and is degraded in autolysosomes (36). Conversion of LC3-I to LC3-II and
p62 degradation are two reliable markers of autophagy. After CNE1
and CNE2 cells were treated with ApoG2 at different concentrations
or for different times, LC3 and p62 expression levels were measured
by immunoblotting. Expression of LC3-II increased whereas that of
p62 protein decreased in CNE1 and CNE2 cell lines treated with
ApoG2, in a dose- and time-dependent manner (Fig. 2B).
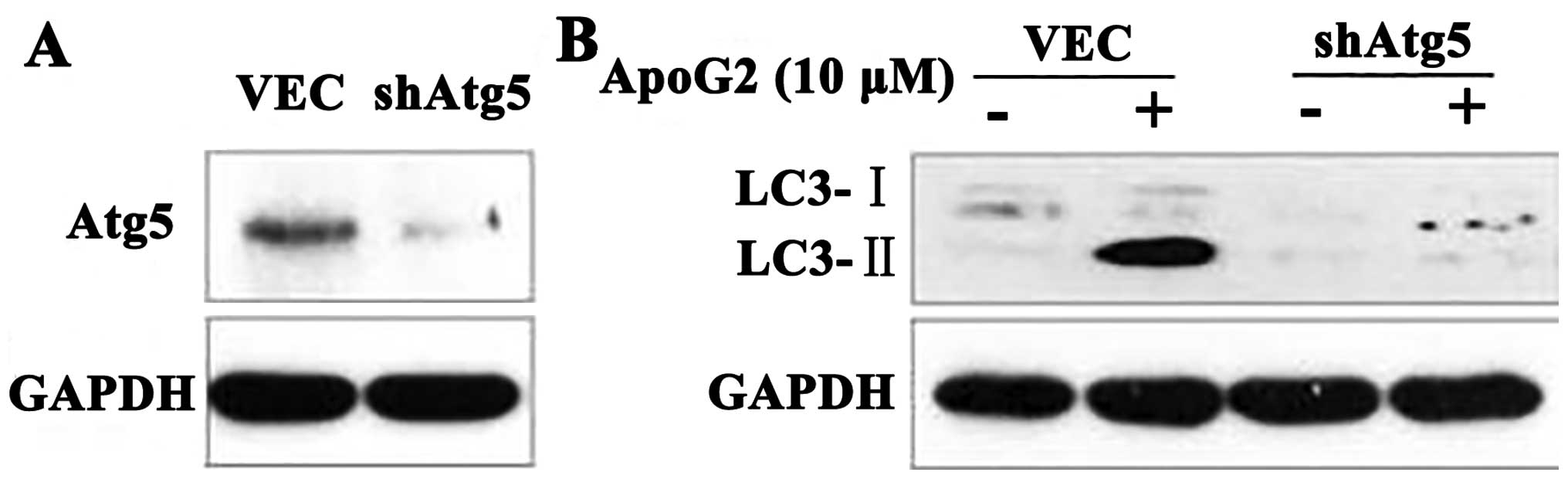
Atg5 is required for ApoG2-induced
autophagy
Atg5 is required for formation of autophagosomes,
and Atg5-deficient mouse embryonic stem cells exhibited
significantly reduced numbers of autophagic vesicles (37). To confirm the role played by
autophagy in this process, we used CNE2-shAtg5 cells. We found that
CNE2-shAtg5 cells lacked Atg5 expression (Fig. 3A). When CNE2 cells treated with 10
μM ApoG2 were additionally transfected with a plasmid expressing
shAtg5, or not, expression of LC3-II in CNE2-shAtg5 cells decreased
over 24 h, compared with control cells (Fig. 3B). This indicated that Atg5 was
required for ApoG2-induced autophagy.
ApoG2 blocks binding of Bcl-2 to Beclin
1
We earlier reported that ApoG2 blocked the
anti-apoptotic functions of Bcl-2 family proteins without affecting
the expression levels of these proteins, rather inhibiting the
binding of Bcl-2 and Bcl-xL to Bax in CNE2 cells (13). and that of Bcl-2 to Bax in U937
cells (30). The BH3 mimetic
ABT-737 competitively dissociated Beclin 1 from the pro-survival
Bcl-2/Bcl-xL complex, thereby inducing autophagy (38). Gossypol, another mimic of the Bcl-2
homology domain 3 induced autophagy in both MCF-7 and HeLa cells,
and inhibited the binding of Bcl-2 to Beclin 1 in MCF-7 cells, but
not HeLa cells. ABT-737 inhibited the binding of Bcl-2 to Beclin1
in HeLa cells (39). We speculated
that ApoG2 might trigger autophagy of CNE2 cells by influencing the
interaction of an anti-apoptotic protein (Bcl-2) with a
pro-autophagic and pro-apoptotic protein (Beclin 1). To verify this
hypothesis, whole lysates from treated and untreated CNE2 cells
were collected, immunoprecipitated with an anti-Bcl-2 specific
antibody, and next probed with an anti-Beclin 1 specific antibody.
As shown in Fig. 4, Bcl-2 and
Beclin 1 bound to each other in untreated CNE2 cells. However, when
cells were exposed to 10 μM of ApoG2 or 20 μM ABT-737 for 24 h,
such binding was eliminated. These results showed that ApoG2
inhibited heterodimerization of Bcl-2 and Beclin 1 in CNE2
cells.
ApoG2-mediated autophagy contributes to
the radiation sensitivity of NPC cells
We used the colony-forming test to demonstrate the
radiosensitizing effect of ApoG2. CNE2 cells were treated with
ApoG2 in combination with ionizing radiation. Surviving colonies
were counted 14 days later. Survival decreased, in a dose-dependent
manner, when cells were treated with 0.5, 1 and 2 μM of ApoG2 for
14 days. All DEFs (dose enhancement factors) were greater than
unity. The DEFs of radiation-plus-continuous ApoG2 at 2, 1 or 0.5
μM were 1.92, 1.12 and 1.12, respectively. The SFs (survival
fractions) were 0.81, 0.83 and 0.86, respectively. Thus, ApoG2
enhanced radiosensitization of CNE2 cells in a dose-dependent
manner (Fig. 5A and B).
 | Figure 5ApoG2 enhanced radiation-mediated
autophagy and apoptosis of NPC cells. (A) Colony-forming test
results. CNE2 cells were incubated with ApoG2 (0, 0.5, 1, or 2 μM)
with or without radiation (0, 2, 4, or 6 Gy). Cells were irradiated
to various extents and seeded into 12-well plates with different
concentrations of ApoG2. After 14 days of incubation, cells were
stained with crystal violet, and the numbers of colonies containing
>50 cells were counted. Values are the means ± SD of data from
triplicate experiments. (B) SFs (survival fractions) were
calculated. (C) Expression and localization of GFP-LC3 in CNE2
cells treated with ApoG2, 2 Gy, or the combination, for 24 h. (D)
Autophagy was induced by ApoG2 combined with radiation. CNE1 and
CNE2 cells were treated (or not) with 5 μM of ApoG2 for 4 h and
next irradiated with 2 Gy. After 24 h, cells were subjected to
immunoblotting using anti-LC3 and -p62 antibodies. |
Autophagy is an evolutionarily conserved process of
protein degradation associated with both tumor promotion and
suppression in different situations. Autophagy contributes to cell
killing following radiation. We explored whether ApoG2 could
enhance radiosensitivity by stimulating autophagy. First, CNE1 and
CNE2 cells were transfected with a plasmid expressing LC3 fused to
green fluorescent protein (GFP-LC3). Cells exhibiting punctate GFP
signaling were considered to be autophagic because the reporter
protein was lysosomally located, as is characteristic of LC3 during
autophagy. In control cells, GFP-LC3 was evenly distributed
throughout the entire cytoplasm. After cells were exposed to 20 μM
ApoG2, ring-shaped structures were easily detectable in the
cytosol, indicating that GFP-LC3 protein associated with
autophagosomal membranes, demonstrating induction of autophagy.
After treatment with ApoG2 and radiation, more ring-shaped
structures were evident in the cytosol than after treatment with
ApoG2 alone (Fig. 5C).
These results were confirmed by measurement of the
levels of processed LC3 and p62. After treatment with ApoG2 and
radiation, LC3 and p62 expression levels were measured via
immunoblotting. LC3-II expression in both CNE1 and CNE2 cell lines
treated with ApoG2 (5 μM) and radiation (2 Gy) increased compared
with the control, ApoG2-alone (5 μM), and radiation-alone (2 Gy)
groups, and the p62 expression level fell (Fig. 5D).
ApoG2 enhances the radiosensitivity of
CNE2 xenografts in nude mice
The most prominent antitumor effect was observed
when ApoG2 and radiation were given in combination. ApoG2 was well
tolerated by the nude mice. The maximal tolerated dose (MTD) was
previously found to be over 480 mg/kg, upon daily intragastric
administration. Fig. 6A shows the
tumor volumes of mice given ApoG2 (120 mg/kg intragastrically daily
for 7 days), mice irradiated with 2 Gy (using 60[Co] γ-rays for 5
continuous days), and the ApoG2-plus-2 Gy combination group,
compared with the control group. The tumor volumes of the
combination group decreased significantly compared those of the
other two test groups (P<0.05). When it was estimated that all
tumors in the control group had grown to weigh over 1,000 mg, the
mice were sacrificed and xenografts removed for weighing (Fig. 6B). Antitumor activity (inhibitory
rate) measurements for the ApoG2, 2 Gy, and combination groups
[measured by calculation of (C-T)/C percentage ratios] were 46.89,
19.34, and 61.64%, respectively (Table
I).
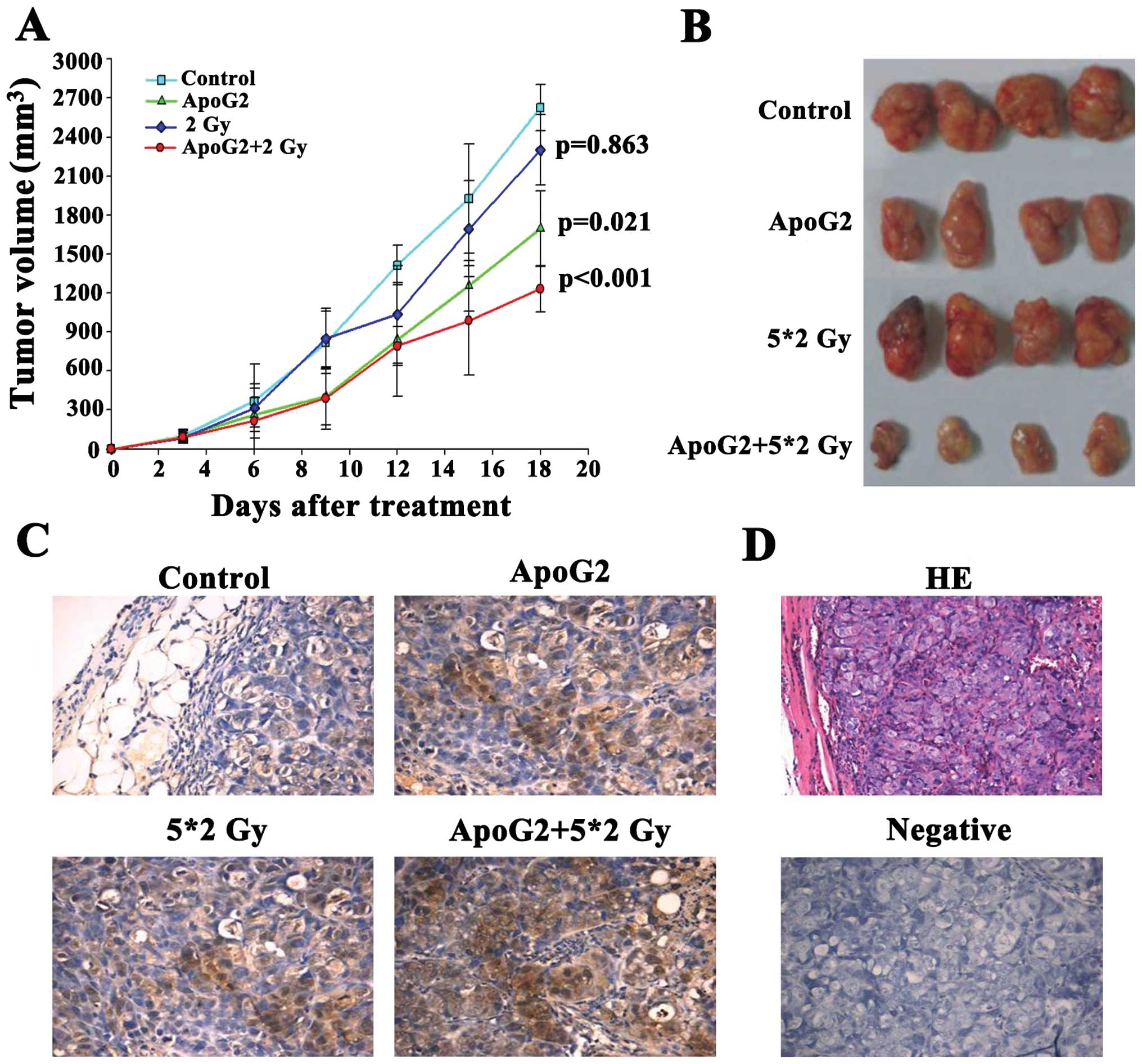 | Figure 6ApoG2 enhanced the radiosensitivity
of CNE2 xenografts in nude mice. We measured the inhibition of
tumor growth by ApoG2, 2 Gy of radiation, or the combination, in
CNE2 xenograft-bearing nude mice. Maximal and minimal tumor
diameters (the a and b values, respectively) were measured every 3
days (A). Tumor volumes were calculated as v=ab2π/6. Control and
test data were compared. After 18 days, the experiment was
terminated. (B) The tumor growth inhibition ratios [100x(1-T/C) (%
values)] were calculated by dividing the average tumor volume of
the treatment group by that of the control group.
(*P<0.05, **P<0.01; Table I). Tumor tissues from each group
(control, ApoG2, 2 Gy and the ApoG2-plus-2 Gy combination) were
obtained at the end of treatment (C). Immunohistochemical staining
for LC3 of CNE2 xenograft tumor sections after treatment. (D)
H&E staining, and staining of the negative control.
Magnification, ×400. |
 | Table ITumor growth inhibition ratios
(%). |
Table I
Tumor growth inhibition ratios
(%).
| Group | n | Weight of tumor
(g) | Inhibitory rate
(%) |
|---|
| Control | 4 | 3.05±0.63 | - |
| ApoG2 | 4 | 1.62±0.89 | 46.89±5.57a |
| 2 Gy | 4 | 2.64±0.43 | 19.43±1.82 |
| ApoG2+2 Gy | 4 | 1.17±0.87 | 61.64±5.79b |
To determine whether ApoG2 or radiation induced
autophagy of NPC cells in vivo, tumor sections from CNE2
xenografts were incubated with anti-LC3 antibody. When the weights
of control xenografts exceeded 1,000 mg, all mice were euthanized
and tumors were dissected, weighed, and fixed for immunochemical
examination. As shown in Fig. 6C,
treatment with a combination of ApoG2 and 2 Gy of radiation
stimulated a significant rise in LC3 expression in CNE2 xenografts
compared to controls.
Discussion
Nasopharyngeal carcinoma (NPC) is the most common
malignancy in China. The annual morbidity rate is 10–25/100,000. Of
all Chinese NPC patients, 60% live in Guangdong province. The
primary treatment for NPC is radiotherapy. The 5-year survival rate
of early-stage NPC patients treated with radiotherapy is 80–90%. In
patients with recurrent NPC who receive a second course of
radiation therapy, and who experience severe radiation-induced
damage, the 5-year survival rate drops to 12–40%. Further, a third
course of radiation is even less effective (4). Therefore, it is very important to
explore new ways to enhance the sensitivity of NPC cells to
radiation.
Autophagy is one response of cancer cells to various
therapies, including ionizing radiation. Radiation induces
autophagy, but not apoptosis, of various malignant tumor cell
lines. Beclin 1, an essential autophagic protein, was recently
identified as a BH3 domain-only protein that binds to members of
the Bcl-2 anti-apoptotic family (40). Dissociation of Beclin 1 from Bcl-2
inhibitors is essential if the former protein is to exhibit
autophagic activity. Apogossypolone (ApoG2) is a novel derivative
of gossypol, a small-molecule inhibitor of anti-apoptotic Bcl-2
family proteins (39). We
hypothesized that ApoG2 was both a Bcl-2 inhibitor and exerted a
radiosensitizing effect by activating autophagy.
In the current study, we used human NPC cell lines
(CNE1, CNE2, and SUNE1), and a normal human nasopharyngeal
epithelial cell line (NP69), to study the inhibitory effect of
ApoG2. We incubated cells with ApoG2 (at different concentrations)
for 72 h. Remarkable inhibitory effects were observed. The
IC50 values for CNE1, CNE2 and SUNE1 cells were 2.84,
2.18 and 5.64 μM, respectively. However, ApoG2 had no obvious
effect on normal NP69 cells.
The adaptor protein p62, also termed sequestosome 1
(SQSTM1), binds to both LC3 and to ubiquitinated proteins to
facilitate autophagic clearance. p62 accumulates when autophagy is
inhibited, and falls when autophagy is induced. Therefore, p62 can
be used as a marker of autophagic flux (41). LC3 is an autophagosomal ortholog of
yeast Atg8. A lipidated form of LC3, LC3-II, has been shown to
serve as an autophagosomal marker in mammals (35). We earlier found that ApoG2 induced
apoptosis of human lymphoma U937 and NPC cells (30). In the present study, we found that
expression of LC3-II increased and that of p62 decreased, in a
dose- and time-dependent manner, when CNE1 and CNE2 cells were
treated with ApoG2. This means that ApoG2 induced autophagy in
these two cell types (Fig. 2).
Autophagosomes were evident in transmission electron micrographs of
CNE2 cells treated with ApoG2 for 24 h (Fig. 2). When CNE2 cells were transfected
with the GFP-LC3 plasmid, ring-shaped structures were detectable in
the cytosol of the ApoG2 group, indicating that autophagy was in
play (Fig. 2). Thus, three
different experimental approaches showed that ApoG2 induced
autophagy of NPC cells.
To further confirm that ApoG2 induced autophagic
death of NPC cells, CNE2 cells were treated with shRNA targeting
Atg5 expression. The Atg5 protein is required for formation of
autophagosomes and plays an important role in vesicle expansion and
completion (42). In the present
study, western blotting indicated that shRNA targeting Atg5
significantly reduced expression of both Atg 5 as well as LC3-II in
cells treated with ApoG2. This means that ApoG2 induced autophagy
of CNE2 cells and that Atg 5 was involved in this process.
How does ApoG2 induce autophagy? It is known that
Bcl-2 and close homologs thereof, including Bcl-xL and Mcl-1,
interact with the evolutionarily conserved autophagic protein
Beclin 1, and inhibit Beclin 1-dependent autophagy in yeast and
mammalian cells. BH3-only proteins stimulate autophagy by
competitively disrupting the interaction between Beclin 1 and
Bcl-2/Bcl-xL, thus releasing Beclin 1 from its inhibition (40). ABT-737 disrupts the interaction
between Beclin 1 and Bcl-2 in HeLa cells, thereby liberating Beclin
1 from an inhibitory complex (38,39).
Lian et al found that a small-molecule inhibitor of Bcl-2,
(−)-gossypol, abolished Bcl-2/Beclin 1 interaction in prostate CL-1
cancer cells (43). Gao et
al used MCF-7 and HeLa cells, which express detectable
endogenous levels of both Beclin 1 and Bcl-2, to investigate the
effect of gossypol on dissociation of these proteins (39). Co-immunoprecipitation experiments
showed that when gossypol induced autophagy, dissociation of the
Beclin 1/Bcl-2 complex was observed in MCF-7 cells, but not HeLa
cells, as was also the case when cells were starved. In a previous
study, we found that ApoG2, the oxidation product of apogossypol,
blocked formation of the Bcl-2/Bax (Bcl-xL/Bak) complex, triggering
activation of the mitochondrial apoptotic pathway in NPC CNE2
(13) and human lymphoma U937
cells (30). Thus, we hypothesized
that ApoG2 might inhibit the binding of Bcl-2 to Beclin 1.
We used ABT-737 as a positive control, and found
that when CNE2 cells were exposed to 10 μM ApoG2 or 20 μM ABT-737
for 24 h, the binding of Bcl-2 to Beclin 1 was inhibited. Thus,
ApoG2 inhibited the heterodimerization of Bcl-2 with Beclin 1 in
CNE2 cells, triggering release of the BH3-only pro-autophagic
protein Beclin 1, in turn triggering the autophagic cascade.
NPC is highly radiosensitive. Thus, radiotherapy
(RT) plays a central role in the treatment of all stages of NPC
that lack distant metastases. As RT achieves good local control,
distant metastatic failure has become the most common pattern of
recurrence, especially among those with locoregionally advanced
disease, which is difficult to control using conventional 2D RT. It
is thus important to improve NPC radiosensitivity. The rationale of
induction chemotherapy is to reduce the tumor load of locoregional
disease prior to RT commencement, and to prescribe early systemic
treatment for eradication of micro-metastases. The International
Nasopharyngeal Carcinoma Study Group reported that a combination of
bleomycin, epirubicin, and cisplatin significantly improved
disease-free, but not overall, survival. This may be attributable
to elevated numbers of treatment-related deaths among patients on
induction chemotherapy compared to RT alone (8% vs. 1%) (4).
Radiotherapy induced apoptosis and autophagy of NPC
cells but the cells continued to express the Bcl-2 protein at a
high level, Bcl-2 mediates resistance to apoptosis and autophagy
(44). Thus, we sought a new
approach toward inhibition of Bcl-2 function, to improve the
radiosensitivity of NPC cells. Earlier, we showed that ApoG2
induced apoptosis in NPC cells by blocking the binding of Bcl-2 to
Bax. In the present study, we found that ApoG2 induced autophagy of
NPC cells.
We found that ApoG2 could radiosensitize the human
NPC CNE2 cell line in vitro. The clone-forming assay
revealed that the DEFs of radiation given in the continuous
presence of 2, 1 or 0.5 μM ApoG2 were 1.92, 1.12 and 1.12,
respectively. The SFs of groups given radiation in the continuous
presence of 2 μM ApoG2 was 0.81, and those of groups given 1 and
0.5 μM of ApoG2 0.83 and 0.86. These results indicated that ApoG2
enhanced radiosensitization of CNE2 cells in a time-dependent
manner. We next tested the extent of autophagy of CNE1 and CNE2
cells by western blotting and confocal microscopy.
ApoG2-plus-radiation induced more autophagy, increased punctate GFP
signaling, raised the expression level of LC3-II, and reduced that
of P62, compared to the ApoG- or radiation-alone groups, and
controls.
Next, we explored the anticancer effects of ApoG2 in
nude mice, We found that ApoG2 combined with radiation suppressed
the growth of CNE2 tumor xenografts in nude mice more effectively
that did ApoG2 or radiation alone. ApoG2 radiosensitized NPC cells
in vivo as revealed by the (C-T)/C ratios (in percentages).
The images for the ApoG2, radiation, and combination groups were
46.89, 19.34, and 61.64%, respectively (Fig. 5C). Immunohistological staining
showed that LC3-II levels became gradually upregulated in the
ApoG2-plus-radiation group (Fig.
5D).
In conclusion, we have shown that the BH3-mimetic
ApoG2 induced autophagic death of human NPC cells. ApoG2
radiosensitized CNE1 and CNE2 cells via induction of autophagy,
triggered by inhibition of the binding of Bcl-2 to Beclin 1. This
is the potential mechanism by which ApoG2 acts in NPC cells. ApoG2
exhibited anticancer and radiosensitizing effects on CNE2
xenografts in nude mice. These findings suggest that autophagy is
one mechanism triggered by ApoG2 and that enhancement of autophagy
can be used to complement combination therapy with ionizing
radiation when NPC is to be treated.
Acknowledgements
This study was supported by the National Nature
Science Foundation of China (30873085), 973 Program (2011CB504300),
the Nature Science Foundation of Guangdong (10451008901004533) and
the technical New Star of Zhujiang, Pan Yu districts, Guangzhou
(2013-special-15-6.09).
Abbreviations:
|
NPC
|
nasopharyngeal carcinoma
|
|
ApoG2
|
apogossypolone
|
|
BH3
|
Bcl-2-homology domain 3
|
|
DMSO
|
dimethylsulfoxide
|
|
IC50
|
50% inhibitory concentration
|
|
GFP-LC3
|
LC3 fused to green fluorescent
protein
|
References
|
1
|
McDermott AL, Dutt SN and Watkinson JC:
The aetiology of nasopharyngeal carcinoma. Clin Otolaryngol Allied
Sci. 26:82–92. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bei JX, Jia WH and Zeng YX: Familial and
large-scale case-control studies identify genes associated with
nasopharyngeal carcinoma. Semin Cancer Biol. 22:96–106. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee AW, Lin JC and Ng WT: Current
management of nasopharyngeal cancer. Semin Radiat Oncol.
22:233–244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yoshizaki T, Ito M, Murono S, Wakisaka N,
Kondo S and Endo K: Current understanding and management of
nasopharyngeal carcinoma. Auris Nasus Larynx. 39:137–144. 2012.
View Article : Google Scholar
|
|
5
|
Kang MH and Reynolds CP: Bcl-2 inhibitors:
targeting mitochondrial apoptotic pathways in cancer therapy. Clin
Cancer Res. 15:1126–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou FF, Yang Y and Xing D: Bcl-2 and
Bcl-xL play important roles in the crosstalk between autophagy and
apoptosis. FEBS J. 278:403–413. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Levine B, Sinha S and Kroemer G: Bcl-2
family members: dual regulators of apoptosis and autophagy.
Autophagy. 4:600–606. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Green DR and Evan GI: A matter of life and
death. Cancer Cell. 1:19–30. 2002. View Article : Google Scholar
|
|
9
|
Lu QL, Elia G, Lucas S and Thomas JA:
Bcl-2 proto-oncogene expression in Epstein-Barr-virus-associated
nasopharyngeal carcinoma. Int J Cancer. 53:29–35. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fan SQ, Ma J, Zhou J, Xiong W, Xiao BY,
Zhang WL, Tan C, Li XL, Shen SR, Zhou M, Zhang QH and Ou YJ:
Differential expression of Epstein-Barr virus-encoded RNA and
several tumor-related genes in various types of nasopharyngeal
epithelial lesions and nasopharyngeal carcinoma using tissue
microarray analysis. Hum Pathol. 37:593–605. 2006. View Article : Google Scholar
|
|
11
|
Sheu LF, Chen A, Meng CL, Ho KC, Lin FG
and Lee WH: Analysis of bcl-2 expression in normal, inflamed,
dysplastic nasopharyngeal epithelia, and nasopharyngeal carcinoma:
association with p53 expression. Hum Pathol. 28:556–562. 1997.
View Article : Google Scholar
|
|
12
|
Yu Y, Dong W, Li X, Yu E, Zhou X and Li S:
Significance of c-Myc and Bcl-2 protein expression in
nasopharyngeal carcinoma. Arch Otolaryngol Head Neck Surg.
129:1322–1326. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu ZY, Zhu XF, Zhong ZD, Sun J, Wang J,
Yang D and Zeng YX: ApoG2, a novel inhibitor of antiapoptotic Bcl-2
family proteins, induces apoptosis and suppresses tumor growth in
nasopharyngeal carcinoma xenografts. Int J Cancer. 123:2418–2429.
2008. View Article : Google Scholar
|
|
14
|
Del Poeta G, Venditti A, Del Principe MI,
et al: Amount of spontaneous apoptosis detected by Bax/Bcl-2 ratio
predicts outcome in acute myeloid leukemia (AML). Blood.
101:2125–2131. 2003.PubMed/NCBI
|
|
15
|
Minn AJ, Rudin CM, Boise LH and Thompson
CB: Expression of bcl-xL can confer a multidrug resistance
phenotype. Blood. 86:1903–1910. 1995.PubMed/NCBI
|
|
16
|
Yoshino T, Shiina H, Urakami S, et al:
Bcl-2 expression as a predictive marker of hormone-refractory
prostate cancer treated with taxane-based chemotherapy. Clin Cancer
Res. 12:6116–6124. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lacy J, Loomis R, Grill S, Srimatkandada
P, Carbone R and Cheng YC: Systemic Bcl-2 antisense
oligodeoxynucleotide in combination with cisplatin cures EBV1
nasopharyngeal carcinoma xenografts in SCID mice. Int J Cancer.
119:309–316. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oltersdorf T, Elmore SW, Shoemaker AR,
Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges
J, Hajduk PJ, Joseph MK and Kitada S: An inhibitor of Bcl-2 family
proteins induces regression of solid tumours. Nature. 435:677–681.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kang MH, Wan Z, Kang Y, Sposto R and
Reynolds CP: Mechanism of synergy of N-(4-hydroxyphenyl) retinamide
and ABT-737 in acute lymphoblastic leukemia cell lines:
Mcl-1inactivation. J Natl Cancer Inst. 100:580–595. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Konopleva M, Contractor R, Tsao T, et al:
Mechanisms of apoptosis sensitivity and resistance to the BH3
mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 10:375–388.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chauhan D, Velankar M, Brahmandam M, et
al: A novel Bcl-2/Bcl-X(L)/Bcl-w inhibitor ABT-737 as therapy in
multiple myeloma. Oncogene. 26:2374–2380. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Van Delft MF, Wei AH, Mason KD, et al: The
BH3 efficiently induces apoptosis via Bak/Bax if Mcl-1 is
neutralized. Cancer Cell. 10:389–399. 2006.PubMed/NCBI
|
|
23
|
Del Gaizo Moore V, Brown JR, Certo M, Love
TM, Novina CD and Letai A: Chronic lymphocytic leukemia requires
BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2
antagonist ABT-737. J Clin Invest. 117:112–121. 2007.PubMed/NCBI
|
|
24
|
Lin X, Morgan-Lappe S and Huang X: ‘Seed’
analysis of off-target siRNAs reveals an essential role of Mcl-1 in
resistance to the small-molecule Bcl-2/Bcl-X(L) inhibitorABT-737.
Oncogene. 26:3972–3979. 2007.
|
|
25
|
Hann CL, Daniel VC, Sugar EA,
Dobromilskaya I, Murphy SC, Cope L, Lin X, Hierman JS, Wilburn DL,
Watkins DN and Rudin CM: Therapeutic efficacy of ABT-737, a
selective inhibitor of BCL-2, in small cell lung cancer. Cancer
Res. 68:2321–2328. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
MacVicar GR, Kuzel TM and Curti BD: An
open label, multicenter, phase I/II study of AT-101 in combination
with docetaxel (D) and prednisone (P) in men with hormone
refractory prostate cancer (HRPC). J Clin Oncol. 26:160482008.
|
|
27
|
Yang D, Chen J, Xu L, Gao W, Guo J, Qiu S,
Holmlund J, Sorensen M and Wang S: AT-101 and ApoG2, highly potent
and orally active small molecule inhibitors of Mcl-1 protein and
potential application for apoptosis-targeted anticancer therapy.
AACR-NCI-EORCT International Conference on Molecular Targets and
Cancer Therapeutics; pp. 255abs. 223. Nov 14–18 2005; http://www.aacr.org/Uploads/DocumentRepository/pdf_files/2005MTCT/MT05_abstracts_C.pdf.
|
|
28
|
Arnold AA, Aboukameel A, Chen J, Yang D
and Wang S: Preclinical studies of Apogossypolone: a new
nonpeptidic pan small-molecule inhibitor of Bcl-2, Bcl-XL and Mcl-1
proteins in Follicular Small Cleaved Cell Lymphoma model. Mol
Cancer. 7:202008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dao VT, Dowd MK, Martin MT, Gaspard C,
Mayer M and Michelot RJ: Cytotoxicity of enantiomers of gossypol
Schiff’s bases and optical stability of gossypolone. Eur J Med
Chem. 39:619–624. 2004.PubMed/NCBI
|
|
30
|
Sun J, Li ZM, Hu ZY, Lin XB, Zhou NN, Xian
LJ, Yang DJ and Jiang WQ: ApoG2 inhibits antiapoptotic Bcl-2 family
proteins and induces mitochondria-dependent apoptosis in human
lymphoma U937 cells. Anticancer Drugs. 19:967–974. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang XQ, Huang XF, Hu XB, Zhan YH, An QX,
Yang SM, Xia AJ, Yi J, Chen R, Mu SJ and Wu DC: Apogossypolone, a
novel inhibitor of antiapoptotic Bcl-2 family proteins, induces
autophagy of PC-3 and LNCaP prostate cancer cells in vitro. Asian J
Androl. 12:697–708. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hu ZY, Sun J, Zhu XF, Yang D and Zeng YX:
ApoG2 induces cell cycle arrest of nasopharyngeal carcinoma cells
by suppressing the c-Myc signaling pathway. J Transl Med. 7:742009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou WJ, Deng R, Zhang XY, Feng GK, Gu LQ
and Zhu XF: G-quadruplex ligand SYUIQ-5 induces autophagy by
telomere damage and TRF2 delocalization in cancer cells. Mol Cancer
Ther. 8:3203–3213. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Adams R, Morris RC, Geissman TA, et al:
Structure of gossypol. XV An interpretation of its reactions. J Am
Chem Soc. 60:21931938. View Article : Google Scholar
|
|
35
|
Tanida I, Ueno T and Kominami E: LC3
conjugation system in mammalian autophagy. Int J Biochem Cell Biol.
36:2503–2518. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pankiv S, Clausen TH, Lamark T, Brech A,
Bruun JA, Outzen H, Øvervatn A, Bjørkøy G and Johansen T:
p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of
ubiquitinated protein aggregates by autophagy. J Biol Chem.
282:24131–24145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pyo JO, Nah J, Kim HJ, Lee HJ, Heo J and
Lee H: Compensatory activation of ERK1/2 inAtg5-deficient mouse
embryo fibroblasts suppresses oxidative stress-induced cell death.
Autophagy. 4:315–321. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Maiuri MC, Criollo A, Tasdemir E, Vicencio
JM, Tajeddine N and Hickman JA: BH3-only proteins and BH3 mimetics
induce autophagy by competitively disrupting the interaction
between Beclin 1 and Bcl-2/ Bcl-X(L). Autophagy. 3:374–376. 2007.
View Article : Google Scholar
|
|
39
|
Gao P, Bauvy C, Souquère S, Tonelli G, Liu
L, Zhu Y, Qiao Z, Bakula D, Proikas-Cezanne T, Pierron G, Codogno
P, Chen Q and Mehrpour M: The Bcl-2 homology domain 3 mimetic
gossypol induces both Beclin 1-dependent and Beclin 1-independent
cytoprotective autophagy in cancer cells. J Biol Chem.
285:25570–25581. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Maiuri MC, Le Toumelin G, Criollo A, Rain
JC, Gautier F, Juin P, Tasdemir E, Pierron G, Troulinaki K and
Tavernarakis N: Functional and physical interaction between
Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J. 26:2527–2539.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bjørkøy G, Lamark T and Brech A:
p62/SQSTM1 forms protein aggregates degraded by autophagy and has a
protective effect on huntingtin-induced cell death. J Cell Biol.
171:603–614. 2005.PubMed/NCBI
|
|
42
|
Boland B and Nixon RA: Neuronal
macroautophagy: from development to degeneration. Mol Aspects Med.
27:503–519. 2006. View Article : Google Scholar
|
|
43
|
Lian J, Wu X, He F, Karnak D, Tang W, Meng
Y, Xiang D, Ji M, Lawrence TS and Xu L: A natural BH3 mimetic
induces autophagy in apoptosis-resistant prostate cancer via
modulating Bcl-2-Beclin1 interaction at endoplasmic reticulum. Cell
Death Differ. 8:60–71. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pattingre S, Tassa A, Qu X, et al: Bcl-2
antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell.
122:927–939. 2005. View Article : Google Scholar : PubMed/NCBI
|