Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignancies and the fifth leading cause of cancer-related
death worldwide. Despite recent advances in diagnostic technology
and new therapeutic modalities for HCC, the prognosis for patients
with advanced-stage HCC is still poor (1). Thus, it is crucial to find novel
cancer-related genes that may serve as diagnostic markers and
molecular targets in HCC therapy, especially after curative
treatment.
Hypoxia is a central feature of solid tumors, and it
regulates the expression of a diverse group of genes that promote
tumor growth, invasion, angiogenesis and cell survival (2–5). In
tumor cells under hypoxic conditions, the hypoxia-inducible
factor-1 (HIF-1) pathway is activated and leads to upregulation of
many hypoxia-response genes, which are associated with an
aggressive tumor phenotype (5–7). We
previously reported that these hypoxia-related genes include
several angiogenic factors that play important roles in cancer
biology (3,8–10).
The anti-VEGF antibody bevacizumab is used clinically for treatment
of several human cancers (11),
and the multi-tyrosine kinase inhibitor sorafenib was shown to have
survival benefits for patients with advanced HCC in two phase III
clinical trials (12,13). These findings support the use of
hypoxia-induced genes as clinically relevant therapeutic
targets.
Ephrin-A1 (EFNA1) is known as an angiogenesis factor
and is induced through an HIF-1-dependent pathway (14,15).
EFNA1 was originally isolated as a secreted protein in conditioned
media from cultures of human umbilical vein endothelial cells
treated with tumor necrosis factor-α (16,17).
Binding of EFNA1 ligand to its receptor EPHA2 promotes
autophosphorylation, which triggers downstream signals that
regulate cell growth and migration. EFNA1 expression has been
observed in tumor cells and in endothelial cells and has been shown
to induce endothelial cell migration (18), capillary assembly in vitro
and corneal angiogenesis in vivo (19). EFNA1 and EPHA2 expression is
associated with carcinogenesis, angiogenesis (18,20–22),
and tumorigenesis in various types of cancer (23–28).
We previously reported that HIF1A expression is
correlated with tumor angiogenesis in HCC and that high nuclear
expression of HIF-1 is a significant predictive factor for
recurrence after curative resection in HCC patients (9). Previously, we detected several
potential prognostic factors and therapeutic targets in hypoxic
tumor cells from hepatic metastases of CRC in vivo (8). Of the 3,000 genes ranked in the
microarray data, the top 30 were identified as hypoxia-inducible
genes. Among these hypoxia-inducible genes, Jumonji domain
containing 1A (JMJD1A, also known as KDM3A) and
procollagen-lysine, 2-oxoglutarate 5-dioxygenase 2 (PLOD2)
were novel prognostic factors of HCC (3,10).
In these experiments, EFNA1 expression was highly induced in
hypoxic regions of liver metastases. Thus, we hypothesized that
EFNA1 expression may be a novel prognostic factor in
patients with HCC. In the present study, we examined the
correlation between EFNA1 expression and prognosis in HCC
patients and analyzed the biological significance of EFNA1
expression in human HCC.
Materials and methods
Cell culture
The human hepatoma cell lines PLC/PRF/5, HuH7, and
HpeG2 were obtained from the Japanese Cancer Research Resources
Bank (Tokyo, Japan), and the Hep3B cell line was obtained from the
Institute of Development, Aging and Cancer, Tohoku University
(Sendai, Japan). All cell lines were maintained in Dulbecco’s
modified Eagle’s medium (DMEM) plus 10% fetal bovine serum, 100
U/ml penicillin, and 100 μg/ml streptomycin at 37°C in a humidified
incubator with 5% CO2. For hypoxic conditions, cells
were maintained in a continuously monitored atmosphere of 1%
O2, 5% CO2, and 94% N2 in a
multigas incubator (model 9200; Wakenyaku Company, Kyoto,
Japan).
Patients and clinical sample
collection
A total of 139 HCC patients who underwent
hepatectomy at Osaka University Hospital and its associated
hospitals were enrolled in this study. All aspects of our study
protocol were approved by the ethics committee of the Graduate
School of Medicine, Osaka University. All patients provided written
informed consent to use their surgical specimens and
clinicopathological data for research purposes. Clinical staging
was based on the TNM classification of the Union for International
Cancer Control (UICC), and histological grading was based on World
Health Organization classification.
Immediately after surgical resection, a tissue
sample was collected from the fresh specimens and stored in RNA
Stabilization Reagent (RNA Later; Ambion, Inc., Austin, TX, USA) at
−80°C until RNA extraction.
RNA extraction and real-time quantitative
RT-PCR analysis
Total RNA was extracted by a single-step method with
TRIzol reagent (Life Technologies, Inc., Gaithersburg, MD, USA) at
Osaka University. Complementary DNA (cDNA) was generated by using
avian myeloblastosis virus reverse transcriptase (Promega, Madison,
WI, USA), as described previously (3). Real-time monitoring of PCR reactions
was performed with the LightCycler system (Roche Applied Science,
Indianapolis, IN, USA) for quantification of mRNA expression, as
described previously (29). The
housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH)
was used as an internal standard. The sequences of the GAPDH
primers were as follows: sense primer, 5′-CAACTACATGGTTTACATGTTC-3′
and antisense primer, 5′-GCCAGTGGACTCCACGAC-3′. EFNA1 primer
sets were designed to flank one intron and were tested to ensure
amplification of only cDNA to avoid amplification of possible
contaminating genomic DNA. The sequences of these PCR primers were
as follows: EFNA1 sense primer, 5′-TGCC GTCCGGACGAGACAGGC-3′
and antisense primer, 5′-CTG GAGCCAGGACCGGGACTG-3′.
Microarray experiment
Microarray results were evaluated in accordance with
previously described methods (30). Briefly, total RNA was extracted
with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to
the instructions supplied by the manufacturer. The integrity of RNA
was assessed with Agilent 2100 Bioanalyzer and RNA 6000 LabChip
kits (Yokokawa Analytical Systems, Tokyo, Japan). Only high-quality
RNA was used for analysis. Seven RNA extractions from different
normal liver tissue samples were mixed and used as the control
reference. Next, 2 μg of total RNA was used to synthesize
double-stranded cDNA that contained a promoter for T7 RNA
polymerase. Amplified antisense RNA was synthesized by in
vitro transcription of the cDNA templates using the Amino Allyl
MessageAmp aRNA kit (Ambion, Austin, TX, USA). The reference and
test samples were labeled with Cy3 and Cy5, mixed, and hybridized
on a microarray covering 30,336 human probes (AceGene Human 30K;
DNA Chip Research Inc. and Hitachi Software Engineering Company,
Yokohama, Japan). The microarrays were scanned using ScanArray
Lite, and signal values were calculated using DNASIS array software
(Hitachi Software Engineering Company). The local background was
subtracted from each spot, and the ratio of the intensity of
fluorescence from the Cy5 channel to the intensity of fluorescence
from the Cy3 channel was calculated for each spot. The ratio of
expression levels of each gene was converted to a logarithmic scale
(base 2), and the data matrix was normalized.
Statistical analysis
For clinicopathological analyses, study samples were
divided into high- and low-expression groups based on the median
EFNA1 mRNA expression levels in tumor tissue. All
statistical analyses were carried out using the StatView J-5.0
program (Abacus Concepts, Inc., Berkeley, CA), USA. The
post-operative period was measured from the date of surgery to the
date of the last follow-up or death. Differences were estimated
using Fisher’s exact probability test. Survival curves were
calculated by the Kaplan-Meier method and compared statistically
using the log-rank test. To estimate relative risk (RR) and 95%
confidence intervals (95% CI), univariate and multivariate analyses
were performed using the Cox proportional hazards regression model.
Data are reported as mean ± standard deviation. Mean values were
compared using the Mann-Whitney test. A probability value of
<0.05 was deemed to be statistically significant.
Results
Expression of EFNA1 under hypoxic
conditions
First, we evaluated expression of EFNA1 under
hypoxic conditions. EFNA1 was expressed in all four hepatoma
cell lines and gradually increased under hypoxia in HuH7, HepG2 and
Hep3B cell lines, but not in PLC/PRF/5 cells (Fig. 1). This result suggests that hypoxic
conditions are associated with increased EFNA1 expression in
HCC.
Patient profiles
Next, we evaluated the expression of EFNA1 in
clinical samples by using microarray analysis. The patients
selected for microarray analysis included 113 (81.3%) men and 26
(18.7%) women. Twenty-six patients had hepatitis B virus infection,
and 85 patients were positive for hepatitis C virus antibody. A
total of 102 patients had a single tumor in the liver, and 65
patients had a tumor <3 cm in diameter. Macroscopic vascular
invasion was seen in 15 patients. With regard to TNM staging, 96
patients (69.1%) were stage I, 31 patients (22.3%) were stage II,
and 12 patients (8.6%) were stage III. The characteristics of the
139 patients are summarized in Table
I.
 | Table IAssociation between
clinicopathological factors and EFNA1 expression. |
Table I
Association between
clinicopathological factors and EFNA1 expression.
|
Characteristics | Low expression
(n=69) | High expression
(n=70) | p-value |
|---|
| Age (years) | | | 0.9999 |
| <65 | 31 | 32 | |
| ≥65 | 38 | 38 | |
| Gender | | | 0.1271 |
| Male | 60 | 53 | |
| Female | 9 | 17 | |
| HBV infection | | | 0.5150 |
| Present | 11 | 15 | |
| Absent | 58 | 55 | |
| HCV infection | | | 0.9999 |
| Present | 42 | 43 | |
| Absent | 27 | 27 | |
| Child-Pugh
grade | | | 0.0761 |
| A | 53 | 62 | |
| B | 16 | 8 | |
| Cirrhosis | | | 0.4955 |
| Absent | 41 | 37 | |
| Present | 28 | 33 | |
| α-fetoprotein
(ng/ml) | | | 0.1519 |
| <100 | 42 | 51 | |
| ≥100 | 27 | 19 | |
| PIVKA-II
(mAU/ml) | | | 0.2829 |
| <40 | 26 | 20 | |
| ≥40 | 43 | 50 | |
| Tumor size
(cm) | | | 0.9999 |
| <3 | 32 | 33 | |
| ≥3 | 37 | 37 | |
| Tumor
multiplicity | | | 0.2500 |
| Single | 54 | 48 | |
| Multiple | 15 | 22 | |
| Macroscopic portal
invasion | | | 0.1829 |
| Absent | 59 | 65 | |
| Present | 10 | 5 | |
| Stage (TNM) | | | 0.7055 |
| I/II | 64 | 63 | |
| IIIA/IIIB | 5 | 7 | |
| Histological
grade | | | 0.2796 |
|
Well/moderately | 42 | 40 | |
| Poorly | 27 | 30 | |
| Microscopic portal
vein invasion | | | 0.292 |
| Absent | 47 | 41 | |
| Present | 22 | 28 | |
| Microscopic
intrahepatic metastasis | | | 0.8469 |
| Absent | 51 | 53 | |
| Present | 18 | 17 | |
Microarray analysis of EFNA1 mRNA
expression
We examined the correlation between expression
levels of EFNA1 and EPHA2 and the clinicopathological
factors of the 139 HCC patients who had undergone hepatic
resection. The 139 patients were divided into two groups, a
high-expression group (n=70) and a low-expression group (n=69),
based on median expression levels from the microarray data for each
gene in Table II. There was no
correlation between EFNA1 expression and clinicopathological
factors including tumor size, vascular invasion and number of
tumors. EPHA2 expression was not significantly correlated
with any clinicopathological factors, except for microscopic portal
invasion. Tumors with high expression of EPHA2 had a
tendency to have microscopic vascular invasion, although this
result was not statistically significant (p=0.0786) (Tables I and II).
 | Table IIAssociation between
clinicopathological factors and EphA2 expression. |
Table II
Association between
clinicopathological factors and EphA2 expression.
|
Characteristics | Low expression
(n=69) | High expression
(n=70) | p-value |
|---|
| Age (years) | | | 0.2349 |
| <65 | 35 | 34 | |
| ≥65 | 34 | 42 | |
| Gender | | | 0.8283 |
| Male | 57 | 56 | |
| Female | 12 | 14 | |
| HBV infection | | | 0.6689 |
| Present | 14 | 12 | |
| Absent | 55 | 58 | |
| HCV infection | | | 0.999 |
| Present | 42 | 43 | |
| Absent | 27 | 27 | |
| Child-Pugh
grade | | | 0.6596 |
| A | 56 | 59 | |
| B | 13 | 11 | |
| Cirrhosis | | | 0.8650 |
| Absent | 38 | 40 | |
| Present | 31 | 30 | |
| α-fetoprotein
(ng/ml) | | | 0.2829 |
| <100 | 43 | 50 | |
| ≥100 | 26 | 20 | |
| PIVKA-II
(mAU/ml) | | | 0.1519 |
| <40 | 27 | 19 | |
| ≥40 | 42 | 51 | |
| Tumor size
(cm) | | | 0.8656 |
| <3 | 33 | 32 | |
| ≥3 | 36 | 38 | |
| Tumor
multiplicity | | | 0.2500 |
| Single | 54 | 48 | |
| Multiple | 15 | 22 | |
| Macroscopic portal
invasion | | | 0.9999 |
| Absent | 62 | 62 | |
| Present | 7 | 8 | |
| Stage (TNM) | | | 0.3472 |
| I/II | 65 | 62 | |
| IIIA/IIIB | 4 | 8 | |
| Histological
grade | | | 0.3309 |
|
Well/moderately | 45 | 37 | |
| Poorly | 24 | 33 | |
| Microscopic portal
vein invasion | | | 0.0786 |
| Absent | 49 | 39 | |
| Present | 20 | 31 | |
| Microscopic
intrahepatic metastasis | | | 0.4353 |
| Absent | 54 | 50 | |
| Present | 15 | 20 | |
Correlation between EFNA1 and EPHA2
expression levels
We next evaluated the correlation between
EFNA1 and EphA2 expression levels using microarray
data. We found that EFNA1 expression levels were
significantly correlated with those of EPHA2 (Fig. 2).
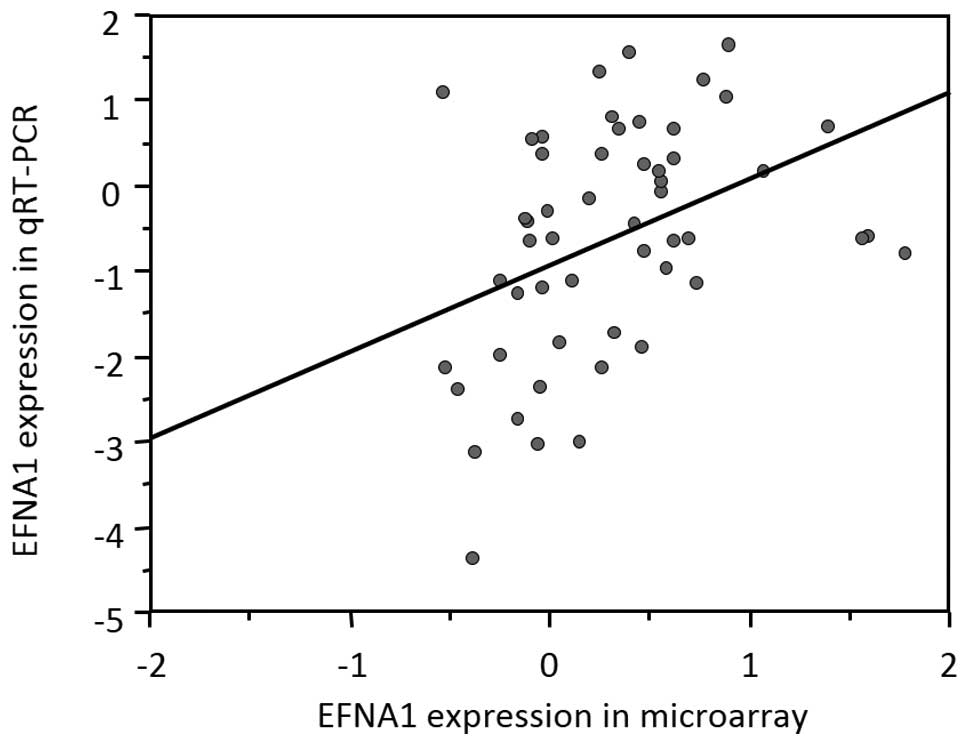
EFNA1 expression measured by quantitative
RT-PCR correlated with microarray data
We next examined the correlation between expression
data from the microarray and quantitative RT-PCR (qRT-PCR) analysis
of EFNA1 to validate the microarray data. qRT-PCR analysis
was performed on 53 HCC tissue samples that were randomly selected
from among the 139 HCC tissue specimens. Individual mRNA levels
were normalized to GAPDH. In the 53 samples, qRT-PCR data for
EFNA1 were significantly correlated with the results
obtained from the microarray data (Fig. 3).
Survival analysis stratified by EFNA1 and
EPHA2 mRNA expression
Kaplan-Meier survival curves demonstrated that
patients with high EFNA1 expression had significantly
shorter disease-free survival (DFS) than those with low
EFNA1 expression based on both microarray (Fig. 4A) and qRT-PCR (Fig. 4B) data. EPHA2 expression was
not correlated with the prognosis for HCC after curative resection
(Fig. 4C). Univariate analysis for
survival revealed that tumor number, microscopic vascular invasion,
microscopic intrahepatic metastasis, and EFNA1 expression
were significantly associated with DFS based on microarray data
(Table III). Multivariate Cox
regression analysis clarified that only EFNA1 expression
remained an independent prognostic factor (Table IV).
 | Table IIIUnivariate analysis of disease-free
survival. |
Table III
Univariate analysis of disease-free
survival.
|
Characteristics | n | Hazard ratio | p-value |
|---|
| Age (years) | | | 0.2987 |
| <65 | 63 | Ref. | |
| ≥65 | 76 | 1.239 | |
| Gender | | | 0.3658 |
| Male | 113 | Ref. | |
| Female | 26 | 0.785 | |
| HBV infection | | | 0.3692 |
| Present | 26 | Ref. | |
| Absent | 113 | 0.798 | |
| HCV infection | | | 0.2871 |
| Present | 85 | Ref. | |
| Absent | 54 | 1.244 | |
| Child-Pugh
grade | | | 0.5886 |
| A | 115 | Ref. | |
| B | 24 | 0.8681 | |
| Cirrhosis | | | 0.3820 |
| Absent | 78 | Ref. | |
| Present | 61 | 1.194 | |
| α-fetoprotein
(ng/ml) | | | 0.1128 |
| <100 | 93 | Ref. | |
| ≥100 | 46 | 1.395 | |
| PIVKA-II
(mAU/ml) | | | 0.4261 |
| <40 | 46 | Ref. | |
| ≥40 | 93 | 1.193 | |
| Tumor size
(cm) | | | 0.5323 |
| <3 | 65 | Ref. | |
| ≥3 | 74 | 0.881 | |
| Tumor
multiplicity | | | 0.0143 |
| Single | 102 | Ref. | |
| Multiple | 37 | 1.736 | |
| Macroscopic portal
invasion | | | 0.2075 |
| Absent | 124 | Ref. | |
| Present | 15 | 1.477 | |
| Stage (TNM) | | | 0.3271 |
| I/II | 127 | Ref. | |
| IIIA/IIIB | 12 | 1.410 | |
| Histological
grade | | | 0.1678 |
|
Well/moderately | 82 | Ref. | |
| Poorly | 57 | 1.321 | |
| Microscopic portal
vein invasion | | | 0.0042 |
| Absent | 88 | Ref. | |
| Present | 51 | 1.801 | |
| Microscopic
intrahepatic metastasis | | | 0.0007 |
| Absent | 104 | Ref. | |
| Present | 35 | 2.185 | |
| EFNA1 | | | 0.0113 |
| Low
expression | 69 | Ref. | |
| High
expression | 70 | 1.701 | |
| EPHA2 | | | 0.4044 |
| Low
expression | 69 | Ref. | |
| High
expression | 70 | 1.185 | |
 | Table IVMultivariate analysis of disease-free
survival. |
Table IV
Multivariate analysis of disease-free
survival.
|
Characteristics | n | Hazard ratio | 95% CI | p-value |
|---|
| Tumor
multiplicity | | | | 0.8701 |
| Single | 102 | Ref. | | |
| Multiple | 37 | 1.053 | 0.565–1.965 | |
| Microscopic portal
invasion | | | | 0.0574 |
| Absent | 88 | Ref. | | |
| Present | 51 | 1.505 | 0.987–2.295 | |
| Microscopic
intrahepatic metastasis | | | | 0.0611 |
| Absent | 104 | Ref. | | |
| Present | 35 | 1.848 | 0.972–3.516 | |
| EFNA1
expression | | | | 0.0277 |
| Low
expression | 69 | Ref. | | |
| High
expression | 70 | 1.605 | 1.054–2.451 | |
Discussion
EFNA1 expression was previously reported to
be associated with prognosis in early squamous cell cervical
carcinoma (31) and colorectal
cancer (32). However, the
prognostic impact of EFNA1 in HCC patients remains unknown.
The present study evaluated the correlation between EFNA1
mRNA expression levels and prognosis in patients with HCC by
microarray analysis of 139 HCC samples and qRT-PCR analysis of 53
samples. The most important finding was that patients with high
EFNA1 expression had a poorer prognosis than those with low
EFNA1 expression. Furthermore, multivariate analysis
demonstrated that EFNA1 expression was an independent
prognostic factor for HCC.
HCC is generally known to occur as a hypervascular
tumor, but the rapid proliferation of tumor cells continuously
induces local hypoxia in advanced stages. Angiogenesis is an
essential process in carcinogenesis and progression, and several
angiogenic factors play important roles in HCC. We previously
reported that the expression of vascular endothelial growth factor
(VEGF) and angiopoietin-2 is associated with microvascular density
in HCC. We also found that high nuclear expression of HIF1A is a
significant predictive factor for recurrence after curative
resection in HCC patients (9).
HIF1A is one of the key transcription factors induced by hypoxic
conditions. In the absence of oxygen, it binds to hypoxia-response
elements, which activates the expression of numerous
hypoxia-response genes, such as VEGF, glucose transporter-1,
erythropoietin and EFNA1 (33).
Two previous studies evaluated the association
between expression of EFNA1 and clinical features in
patients with HCC. One report revealed that expression of
EFNA1 and AFP was strongly associated and that they
induced the expression of genes related to the cell cycle,
angiogenesis and cell-cell interactions (34). The other report showed that
EFNA1 mRNA was overexpressed in 90% of HCC cells and
EPHA2 expression was significantly correlated with poor
survival in HCC patients (35).
Both reports indicated that EFNA1 and its receptor, EphA2, promote
proliferation and invasiveness in HCC.
Our result show that EFNA1 mRNA expression
was significantly associated with EPHA2 mRNA expression,
based on microarray data. Moreover, EFNA1 was a novel
independent prognostic factor for HCC. However, EPHA2 was
not a significant prognostic factor. EPHA2 is a transmembrane
receptor tyrosine kinase that is frequently overexpressed in
various cancers and is stimulated and phosphorylated by EFNA1
(36–38). Overexpression of EPHA2 is
associated with aggressive phenotypes and decreased differentiation
(37,39). Our results also show EPHA2
expression tends to correlate with microscopic portal invasion.
However, there was no association between EPHA2 and prognosis in
HCC.
In the present study, we used tissue microarrays to
analyze not only tumor cells, but also many vascular endothelial
cells. EFNA1 ligand and its receptor, EPHA2, were expressed and
upregulated in both tumor cells and tumor vessels. In line with the
results of the present study, hypoxic conditions are known to
upregulate the expression of EFNA1 in hepatoma cells in
vitro (34,35). We previously reported that
silencing of EFNA1 in tumor cells inhibits the migration,
invasion and proliferation of tumor cells themselves and also
inhibits the migration of endothelial cells in coculture experiment
(32). Thus, EFNA-mediated
interactions between the endothelium and surrounding cells may be
critical for vascular sprouting and the penetration of vessels into
tumor tissues.
In conclusion, the present findings strongly suggest
that EFNA1 expression is a useful marker for predicting a
high risk of recurrence in HCC patients who have undergone curative
resection. Anticancer treatments that target EFNA1 and EPHA2 may be
particularly effective, because they could both suppress tumor
neovascularization and directly affect tumor cells. It will be
critical to the development of novel anticancer therapies to
distinguish the effects of inhibiting EFNA1/EPHA2 activity on tumor
vasculature versus tumor cells.
Abbreviations:
|
RT-PCR
|
reverse transcription PCR
|
References
|
1
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar
|
|
2
|
Semenza GL: Hypoxia and cancer. Cancer
Metastasis Rev. 26:223–224. 2007. View Article : Google Scholar
|
|
3
|
Noda T, Yamamoto H, Takemasa I, et al:
PLOD2 induced under hypoxia is a novel prognostic factor for
hepatocellular carcinoma after curative resection. Liver Int.
32:110–118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maxwell PH and Ratcliffe PJ: Oxygen
sensors and angiogenesis. Semin Cell Dev Biol. 13:29–37. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pugh CW and Ratcliffe PJ: Regulation of
angiogenesis by hypoxia: role of the HIF system. Nat Med.
9:677–684. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar
|
|
7
|
Zhong H, De Marzo AM, Laughner E, et al:
Overexpression of hypoxia-inducible factor 1alpha in common human
cancers and their metastases. Cancer Res. 59:5830–5835.
1999.PubMed/NCBI
|
|
8
|
Uemura M, Yamamoto H, Takemasa I, et al:
Jumonji domain containing 1A is a novel prognostic marker for
colorectal cancer: in vivo identification from hypoxic tumor cells.
Clin Cancer Res. 16:4636–4646. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wada H, Nagano H, Yamamoto H, et al:
Expression pattern of angiogenic factors and prognosis after
hepatic resection in hepatocellular carcinoma: importance of
angiopoietin-2 and hypoxia-induced factor-1 alpha. Liver Int.
26:414–423. 2006. View Article : Google Scholar
|
|
10
|
Yamada D, Kobayashi S, Yamamoto H, et al:
Role of the hypoxia-related gene, JMJD1A, in hepatocellular
carcinoma: clinical impact on recurrence after hepatic resection.
Ann Surg Oncol. 19(Suppl 3): S355–S364. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hurwitz H, Fehrenbacher L, Novotny W, et
al: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for
metastatic colorectal cancer. N Engl J Med. 350:2335–2342. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheng AL, Kang YK, Chen Z, et al: Efficacy
and safety of sorafenib in patients in the Asia-Pacific region with
advanced hepatocellular carcinoma: a phase III randomised,
double-blind, placebo-controlled trial. Lancet Oncol. 10:25–34.
2009. View Article : Google Scholar
|
|
13
|
Llovet JM, Ricci S, Mazzaferro V, et al:
Sorafenib in advanced hepatocellular carcinoma. N Engl J Med.
359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vihanto MM, Plock J, Erni D, Frey BM, Frey
FJ and Huynh-Do U: Hypoxia up-regulates expression of Eph receptors
and ephrins in mouse skin. FASEB J. 19:1689–1691. 2005.PubMed/NCBI
|
|
15
|
Yamashita T, Ohneda K, Nagano M, et al:
Hypoxia-inducible transcription factor-2alpha in endothelial cells
regulates tumor neovascularization through activation of ephrin A1.
J Biol Chem. 283:18926–18936. 2008. View Article : Google Scholar
|
|
16
|
Bartley TD, Hunt RW, Welcher AA, et al:
B61 is a ligand for the ECK receptor protein-tyrosine kinase.
Nature. 368:558–560. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Holzman LB, Marks RM and Dixit VM: A novel
immediate-early response gene of endothelium is induced by
cytokines and encodes a secreted protein. Mol Cell Biol.
10:5830–5838. 1990.PubMed/NCBI
|
|
18
|
Brantley DM, Cheng N, Thompson EJ, et al:
Soluble Eph A receptors inhibit tumor angiogenesis and progression
in vivo. Oncogene. 21:7011–7026. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pandey A, Shao H, Marks RM, Polverini PJ
and Dixit VM: Role of B61, the ligand for the Eck receptor tyrosine
kinase, in TNF-alpha-induced angiogenesis. Science. 268:567–569.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheng N, Brantley DM, Liu H, et al:
Blockade of EphA receptor tyrosine kinase activation inhibits
vascular endothelial cell growth factor-induced angiogenesis. Mol
Cancer Res. 1:2–11. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deroanne C, Vouret-Craviari V, Wang B and
Pouyssegur J: EphrinA1 inactivates integrin-mediated vascular
smooth muscle cell spreading via the Rac/PAK pathway. J Cell Sci.
116:1367–1376. 2003. View Article : Google Scholar
|
|
22
|
Ogawa K, Pasqualini R, Lindberg RA, Kain
R, Freeman AL and Pasquale EB: The ephrin-A1 ligand and its
receptor, EphA2, are expressed during tumor neovascularization.
Oncogene. 19:6043–6052. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abraham S, Knapp DW, Cheng L, et al:
Expression of EphA2 and Ephrin A-1 in carcinoma of the urinary
bladder. Clin Cancer Res. 12:353–360. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brantley-Sieders DM, Fang WB, Hwang Y,
Hicks D and Chen J: Ephrin-A1 facilitates mammary tumor metastasis
through an angiogenesis-dependent mechanism mediated by EphA
receptor and vascular endothelial growth factor in mice. Cancer
Res. 66:10315–10324. 2006. View Article : Google Scholar
|
|
25
|
Liu DP, Wang Y, Koeffler HP and Xie D:
Ephrin-A1 is a negative regulator in glioma through downregulation
of EphA2 and FAK. Int J Oncol. 30:865–871. 2007.PubMed/NCBI
|
|
26
|
Nakamura R, Kataoka H, Sato N, et al:
EPHA2/EFNA1 expression in human gastric cancer. Cancer Sci.
96:42–47. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nasreen N, Mohammed KA, Lai Y and Antony
VB: Receptor EphA2 activation with ephrinA1 suppresses growth of
malignant mesothelioma (MM). Cancer Lett. 258:215–222. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Noblitt LW, Bangari DS, Shukla S, et al:
Decreased tumorigenic potential of EphA2-overexpressing breast
cancer cells following treatment with adenoviral vectors that
express Ephrin-A1. Cancer Gene Ther. 11:757–766. 2004. View Article : Google Scholar
|
|
29
|
Yamamoto H, Kondo M, Nakamori S, et al:
JTE-522, a cyclooxygenase-2 inhibitor, is an effective
chemopreventive agent against rat experimental liver fibrosis1.
Gastroenterology. 125:556–571. 2003.PubMed/NCBI
|
|
30
|
Yoshioka S, Takemasa I, Nagano H, et al:
Molecular prediction of early recurrence after resection of
hepatocellular carcinoma. Eur J Cancer. 45:881–889. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Holm R, de Putte GV, Suo Z, Lie AK and
Kristensen GB: Expressions of EphA2 and EphrinA-1 in early squamous
cell cervical carcinomas and their relation to prognosis. Int J Med
Sci. 5:121–126. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yamamoto H, Tei M, Uemura M, et al:
Ephrin-A1 mRNA is associated with poor prognosis of colorectal
cancer. Int J Oncol. 42:549–555. 2013.PubMed/NCBI
|
|
33
|
Harris AL: Hypoxia - a key regulatory
factor in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Iida H, Honda M, Kawai HF, et al:
Ephrin-A1 expression contributes to the malignant characteristics
of {alpha}-fetoprotein producing hepatocellular carcinoma. Gut.
54:843–851. 2005. View Article : Google Scholar
|
|
35
|
Cui XD, Lee MJ, Yu GR, et al: EFNA1 ligand
and its receptor EphA2: potential biomarkers for hepatocellular
carcinoma. Int J Cancer. 126:940–949. 2010.PubMed/NCBI
|
|
36
|
Miyazaki T, Kato H, Fukuchi M, Nakajima M
and Kuwano H: EphA2 overexpression correlates with poor prognosis
in esophageal squamous cell carcinoma. Int J Cancer. 103:657–663.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zelinski DP, Zantek ND, Stewart JC,
Irizarry AR and Kinch MS: EphA2 overexpression causes tumorigenesis
of mammary epithelial cells. Cancer Res. 61:2301–2306.
2001.PubMed/NCBI
|
|
38
|
Zeng G, Hu Z, Kinch MS, et al: High-level
expression of EphA2 receptor tyrosine kinase in prostatic
intraepithelial neoplasia. Am J Pathol. 163:2271–2276. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Walker-Daniels J, Riese DJ 2nd and Kinch
MS: c-Cbl-dependent EphA2 protein degradation is induced by ligand
binding. Mol Cancer Res. 1:79–87. 2002.PubMed/NCBI
|