Introduction
Epithelial-mesenchymal transition (EMT) is a
biological process that allows a polarized epithelial cell to
undergo biological changes that enable it assume a mesenchymal cell
phenotype, for example, invasion, migration, and increased
production of metastasis related components. A general character of
EMT is degradation of the underlying basement membrane and the
formation of a mesenchymal cell that can migrate from its origin,
which is the epithelial layer. EMT is associated with implantation,
embryo formation, and organ development, and it has the ability for
wound healing and tissue regeneration (1–4);
however, in case cells have undergone genetic and epigenetic
changes such as an overexpressed oncogene or over-activation of
growth factor receptors, EMT occurs excessively and these effects
lead to abnormal metastasis (5–7).
A representative example of this phenomenon is
abnormal metastasis of cancer cells. Induction of EMT by cancer
cells is driven through the complex interplay between the tumor
environment and cells (8). Some
growth factors can promote EMT by triggering of specific
intracellular signaling pathways, and this is closely associated
with EGF, VEGF and TGF (9,10). Especially, activation of epidermal
growth factor receptor (EGFR) by EGF may play significant roles in
cancer EMT (11–13). A main point of these signaling
events is the loosening of the E-cadherin mediated cell to cell
interaction that results from the expression of several
transcriptional repressors such as Snail, Slug, Twist, and Zinc
finger E-box binding homeobox (ZEB) (14–17).
It has been reported previously that cells show an increase in
mesenchymal markers such as vimentin and N-cadherin in case of a
change in phenotype during the EMT process (18,19).
Furthermore, cancer cells committed to EMT exhibit increased
mobility by the activation of cell polarization related proteins
such as VEGF and p-VASP (10,20–23).
Blocking this process would be a therapeutic strategy to limit
abnormal metastasis of cancer cells.
A number of studies have investigated the beneficial
effects of diet on cancer prevention. In particular, the finding in
recent studies that cancers that were often seen in Western
countries, such as breast cancer or stomach cancer, are now
frequently diagnosed in eastern countries is thought to reflect a
change in dietary patterns (24,25).
For this reason, there has been an increase in using food
ingredients for the prevention and treatment of cancer. In
particular, attention has been paid to herbs that are used in
eastern medicine. Recently research has found that herb medicines
are effective in inhibiting cancer proliferation and invasion and
have fewer side-effects than existing drugs (26,27).
In this study, we investigated a specific herb for
anti-metastasis in MCF-7 breast cancer cells. The fruit of
Torilis japonica is used as a substitute for ‘She chuang
zi’, which is a principal Chinese medicament prescribed as an
anti-allergenic, anti-fungal, antibacterial and sedative agent. In
addition, several books state that this herb is effective against
tumors; however, these effects have not been examined
scientifically (28,29). Consequently, we investigated an
ethanol extract from the fruit of Torilis japonica (TJE) and
showed that it suppresses EGFR phosphorylation and its downstream
proteins. In addition, despite EGF-stimulation, TJE treated groups
showed induced E-cadherin expression levels and reduced
metastasis-related proteins such as VEGF and p-VASP. Also TJE
repressed expression of EMT markers. Taken together, the results
show that TJE suppresses cell polarity and loss of cell-cell
contact for the inhibition of abnormal metastasis.
Our results indicate that TJE is a new potential
food ingredient for EGFR-targeted therapy for anti-abnormal
metastasis in MCF-7 breast cancer cells.
Materials and methods
Plant material and preparation of
TJE
Dried whole fruit of Torilis japonica was
purchased from Na-num Pharmacy (Na-num Pharmacy, Kyung-buk, Korea).
Plant material (200 g) was extracted two times with 95% ethanol at
room temperature for 3 days and was subsequently filtered. The
combined filtrate was concentrated under vacuum at 60°C, and
completely dried by freeze drying. The yield was 10% and TJE powder
was dissolved in DMSO for in vitro studies.
Reagent
MTT and Phalloidin, EGF, PD 98059, LY 294002 are
purchased from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA).
Avastin are purchased from Roche (Roche Applied Science, Basel,
Switzerland). Specific antibodies that recognized p-EGFR, p-Akt,
p-Erk, Akt, Erk, β-actin are obtained from Cell Signaling
Technology (Beverly, MA, USA) and EGFR, VEGF, VASP and p-VASP are
purchased from Santa Cruz Biotechnology (Dallas, TX, USA).
Cell culture
MCF-7 cells were obtained from the American Type
Culture Collection (ATCC, Rockville, MD, USA). Cells were grown in
RPMI-1640 medium for MCF-7 (Hyclone, Waltham, MA, USA) containing
10% fetal bovine serum (Hyclone) and 1% antibiotics (100 mg/l
streptomycin, 100 U/ml penicillin) at 37°C in a 5% CO2
atmosphere. Cells were suspended by trypsin-EDTA (Hyclone) and
separated 1.5×105/ml at each plates, every 48
h.
Cell proliferation assay (MTT)
Cells were seeded at 4,000/ml each well in 96-well
plate, and incubated 24 h. After the incubation, treated with test
compound and incubate at 37°C in a 5% CO2 atmosphere.
After 24 h, cells were incubated with 20 μl MTT (5 mg/ml with PBS)
solution for 1 h. Optical densities of solution, in each well, were
determined by microplate reader (Bio-Rad Laboratories, Inc., Tokyo,
Japan) at 595 nm.
Observation of cellular morphology and
actin-cytoskeleton
Cells were seeded 1×105/ml in 12-well
plate with cover glasses. After treatment the indicated time and
dose at 37°C in a 5% CO2 atmosphere, the cells were
visualized with the use of bright field microscopy (Carl Zeiss,
Thornwood, NY, USA) for morphological changes. For the
actin-cytoskeleton observation, the cells, which were treated with
compound, were fixed with 3.7% formaldehyde for 20 min and
pemeabilized with 0.2% Triton X-100 for 20 min. Cells were washed
with PBS twice and reacted with E-cadherin antibody overnight at
4°C. Cells were washed with PBS twice and reacted with the
secondary antibody and stained with 0.1% Phalloidin-FITC for 40 min
and fluorescence was detected by fluorescence microscopy (Carl
Zeiss).
Wound healing assay
Cells were seeded 2.5×106/ml in a 6-well
plate, and incubated to 100% confluence at well. After the
incubation a wound was made in the cell monolayer at center of
well, and the cells were treated with TJE at the indicated dose for
24 h. The healing of the wound was detected with a bright field
microscope (Carl Zeiss).
Invasion assay
Quantitative cell invasion assays were performed
using a modified Boyden chamber (Costar-Corning, NY, USA) with an
8.0-μm pore polycarbonate membrane inserts with Matrigel in 24-well
plates in a routine manner. The lower chamber was filled with
complete medium for control and complete medium with TJE at the
indicated dose and EGF. The MCF-7 cells (5×104 cells/ml)
in serum-free medium were added into the upper chamber. The cells
were allowed to invade for 24 h at 37°C. The non-invasive cells
were removed from the upper surface of the membrane by scraping
with a cotton swab, and the invasive cells were stained with
crystal violet and photographed under a bright field
microscope.
Immunofluorescence staining
Cells were seeded at 1×105/ml in a
12-well plate with cover glasses. After treatment for the indicated
time and dose at 37°C in a 5% CO2 atmosphere, the cells
were fixed with 3.7% formaldehyde for 20 min and pemeabilized with
0.2% Triton X-100 for 20 min. Cells were washed with PBS twice and
reacted with E-cadherin antibody overnight at 4°C. Cells were
washed with PBS twice and reacted with the secondary antibody and
stained with 0.1% Phalloidin-FITC for 40 min. For counter staining,
cells were stained with Hoechst 33342 for 20 min and fluorescence
was detected by confocal microscopy (Carl Zeiss).
Transient transfection with small
interfering RNA
Small interfering RNA (siRNA) was purchased by
Dharmacon (Dharmacon, Chicago, IL, USA). For transient
transfection, cells were seeded at 5×103/ml on a 6-well
plate with antibiotic-free medium. After incubation overnight,
targeting siRNA was transfected using DharmaFECT1 transfection
reagent (Dharmacon) according to the manufacturer’s instructions.
After incubation for 72 h, cells were treated with TJE and EGF for
the indicated time.
Western blotting
Cells were seeded at 1×105/ml in a 6-well
plate and incubated for 24 h. After the incubation, cells were
treated with test compound for 6 h at 37°C in a 5% CO2
atmosphere. Cells were rinsed twice with ice-cold PBS and scraped
with lysis buffer (50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1% NP40,
0.5% sodium deoxycholate, 1 mM PMSF) and subjected to the western
blot analysis. First antibody was applied overnight at 4°C and the
second antibody for 75 min at room-temperature with slow
agitation.
Polymerase chain reaction (PCR)
Total RNA was extracted using RiboEx (GeneAll,
GeneAll biotechnology, Seoul, Korea) according to the
manufacturer’s instructions, and cDNA was generated using
ReverseAids cDNA synthesis kit (Thermo Scientific, Waltham, MA,
USA) according to the manufacturer’s instructions. RT-PCR was
performed with the following temperature profile: a
pre-denaturation step of 10 min at 95°C, followed by 35 cycles of
95°C for 30 sec, annealing temperature for 30 sec and 72°C for 30
sec and a final exposure at 72°C for 10 min. The specific primers
are listed in Table I.
 | Table IPrimer sequences for the amplification
of the target gene. |
Table I
Primer sequences for the amplification
of the target gene.
| Gene | Primer sequence
(5′-3′) | Amplification size
(bp) | Annealing temperature
(°C) |
|---|
| E-cadherin | For:
CGGACGATGATGTGAACACC | | |
| Rev:
TTGCTGTTGTGCTTAACCCC | 213 | 60.0 |
| N-cadherin | For:
GACAATGCCCCTCAAGTGTT | | |
| Rev:
CCATTAAGCCGAGTGATGGT | 179 | 59.5 |
| Vimentin | For:
GAGAACTTTGCCGTTGAAG | | |
| Rev:
TCCAGCAGCTTCCTGTAGGT | 170 | 59.5 |
| Snail | For:
CCCCAATCGGAAGCCTAACT | | |
| Rev:
ACAGAGTCCCAGATGAGCA | 157 | 60.0 |
| Slug | For:
CTTTTTCTTGCCCTCACTGC | | |
| Rev:
GCTTCGGAGTGAAGAAATGC | 224 | 59.0 |
| Twist | For:
GTCCGCAGTCTTACGAGGAG | | |
| Rev:
CCAGCTTGAGGGTCTGAATC | 159 | 57.5 |
| ZEB1 | For:
TGGACTGAGTGTGGAAAAGC | | |
| Rev:
TGGTGATGCTGAAAGAGACG | 237 | 60.0 |
| ZEB2 | For:
TTCCTGGGCTACGACCATAC | | |
| Rev:
GCCTTGAGTGCTCGATAAGG | 393 | 60.0 |
| GAPDH | For:
CAAGGTCATCCATGACAACTTTG | | |
| Rev:
GTCCACCACCCTGTTGCTGTAG | 496 | 58.0 |
Statistical analysis
Cell viability and invasive cells, EGFR activities
were statistically analyzed using unpaired t-test (SPSS, Chicago,
IL, USA). P<0.05 was considered statistically significant.
Results
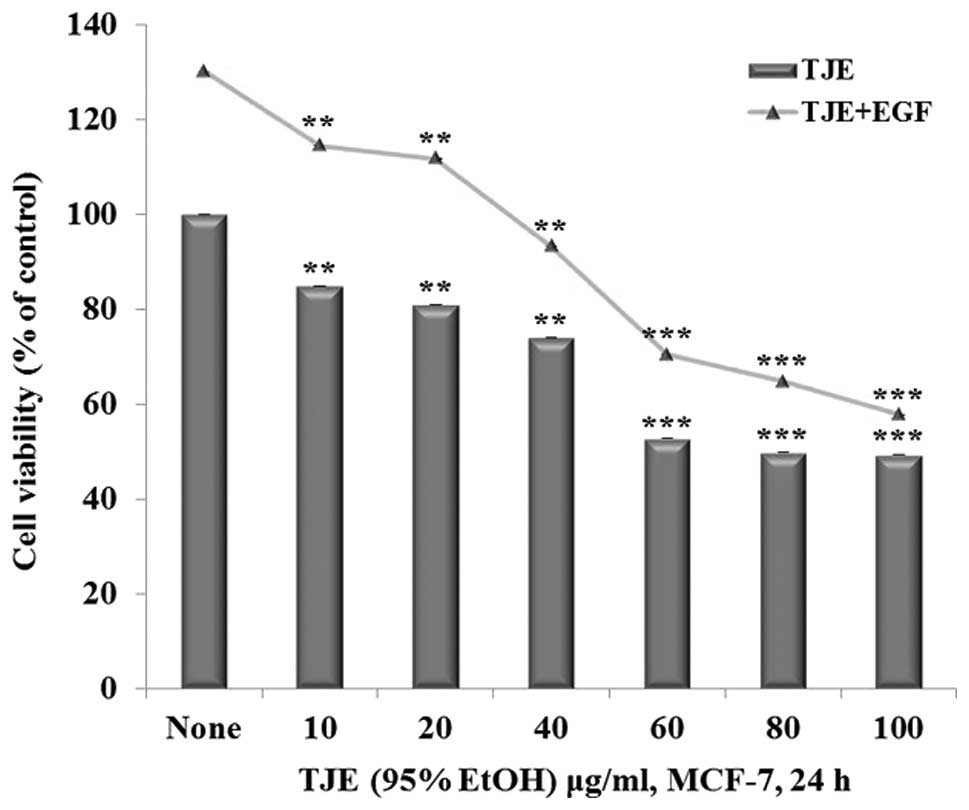
TJE reduces cell proliferation
The anti-proliferation effects of TJE were
investigated through regulation of EGFR activities by treating
cells with TJE (10–100 μg/ml) and co-treatment with TJE (10–100
μg/ml) and EGF (50 ng/ml) for 24 h. Then the cells were measured
for cell proliferation. It was shown that there was a decrease in
cell proliferation in both the TJE treated groups and TJE/EGF
co-treatment groups (Fig. 1).
TJE suppresses cell migration and
invasion through regulation of EGF-induced EMT
Morphological changes of MCF-7 breast cancer cells
after EGF treatment in the absence or presence of TJE were observed
by bright field and fluorescence microscopy. EGF induced
morphological changes were reversible 24 h after treatment. Cells
appeared to have lost cell to cell contact. Moreover, cells treated
with EGF showed changes in the actin-cytoskeleton architecture and
morphology with lamellipodia and filopodia becoming clearly
visible. A complete inhibition of these changes was observed in
groups with TJE treatment (Fig.
2A).
 | Figure 2Involvement of TJE in MCF-7 breast
cancer cell motility. (A) Morphological changes and cytoskeletal
polarization of cells after EGF treatment in the absence or
presence of TJE. Morphological changes of cells were observed using
a bright-field microscopy. Arrows indicate EMT cells, which
appeared to have lost cell to cell contact. In addition,
actin-cytoskeletal polarization was observed by fluorescence
microscopy. (B) Anti-migration abilities were detected by wound
healing assay. Representative images of the reduction of MCF-7
breast cancer cells by TJE treatment with EGF. (C) Invasion of
MCF-7 breast cancer cells with or without TJE treatment. Invasive
cells were determined by counting cells in four microscopic fields
per sample. Compared to control; *P<0.05,
**P<0.01, ***P<0.001 and compared to
EGF-treated group; ###P<0.001 (each experiment, n=3).
NS, not significant. (D) Invasion of MCF-7 breast cancer cells with
or without TJE treatment. Invasive cells were stained by crystal
violet for counting cells in microscopic fields per sample. a,
control; b, EGF 50 ng/ml; c, TJE 40 μg/ml in EGF; d, TJE 60
μg/ml in EGF. |
Also the anti-migration and anti-invasion abilities
of TJE were investigated via EGF regulation. Anti-migration ability
was investigated using a wound healing assay. While EGF treated
cells grew to confluence in a mono-layer, TJE treated groups did
not grow to confluence and the results were dose-dependent. EGF
induced cell invasive activity, but there was a decrease in
invasive cells dose-dependently in the TJE co-treated groups
(Fig. 2B–D).
Taken together, these data show that TJE suppresses
cell movement for abnormal metastasis through the regulation of
EGF-induced EMT.
TJE regulates EGFR activation and its
downstream proteins
To examine how TJE reduces cell metastasis, the
control by TJE of the activation of EGFR and its downstream
pathways such as Akt and Erk was investigated by using western
blotting to analyze changes in levels of p-EGFR, p-Akt, and p-Erk
after co-treatment with different concentrations of TJE and EGF.
The results show that TJE strongly suppresses EGFR activation and
its downstream pathways in a dose-dependent manner (Fig. 3A). Using a specific chemical
inhibitor, it was demonstrated that EGF-induced EMT is related to
Akt and Erk signaling pathways and TJE is able to repress both
these pathways (Fig. 3B).
TJE regulates expression of actin
polarization related proteins and EMT marker genes
Gene and protein expression were analyzed to
determine whether or not EGF treated MCF-7 cells show differences
in their expression of actin polarization proteins and EMT marker
genes with TJE treatment.
By western blotting, it was determined that actin
polarization related proteins such as VEGF and p-VASP were
upregulated by EGF stimulation. In addition, EGF treatment
increased the expression of E-cadherin transcriptional regulators,
ZEB1, ZEB2, and Snail and E-cadherin repressors such as Slug and
Twist as well as expression levels of mesenchymal markers
N-cadherin and vimentin. On the contrary, these changes were
inhibited in a dose-dependent manner in the EGF-stimulation groups
treated with TJE (Fig. 3C).
Using a specific chemical inhibitor, it was
demonstrated that EGF-stimulated expression of actin
polarization-related proteins and EMT markers is through Akt and
Erk signaling pathways and that TJE is able to repress EGF-induced
expression through reduced Akt and Erk activation. Groups treated
with Avastin (VEGF inhibitor) showed a decrease in VASP
phosphorylation as well as EMT markers, which indicates that
reduction of actin polarization and EMT by TJE was also through
control of VEGF expression (Fig.
3D).
Regulation of VEGF, p-VASP, and EMT
markers by TJE treatment through control of EGFR activation
It was confirmed that TJE controls actin
polarization and EMT through regulation of EGFR activation by
comparing groups treated with the specific EGFR inhibitor GW 2974,
and with TJE. The results show that, like the EFGR specific
inhibitor group, TJE downregulates EGFR phosphorylation. Moreover,
expression of actin polarization proteins and EMT markers is
reduced in both TJE and inhibitor treated groups (Fig. 4B). Based on the above data, EGFR
knock-down using siRNA transfection was performed to confirm that
TJE decreases EMT via the EGFR pathway. For the EGFR knock-down
group, there was no change in expression of VEGF, p-VASP, and EMT
markers with EGF-stimulation and the absence of TJE (Fig. 4A).
TJE regulates actin polarization and
E-cadherin expression by controlling EGFR activation
Previous studies have found a significant
correlation between E-cadherin and cell to cell contact. While
E-cadherin has been considered as an active suppressor of invasion
and migration in many epithelial cancer cells through induced
adhesion ability to the extracellular matrix, EGF induces invasion
and migration through the expression of an E-cadherin regulator and
suppressor (18,19,30).
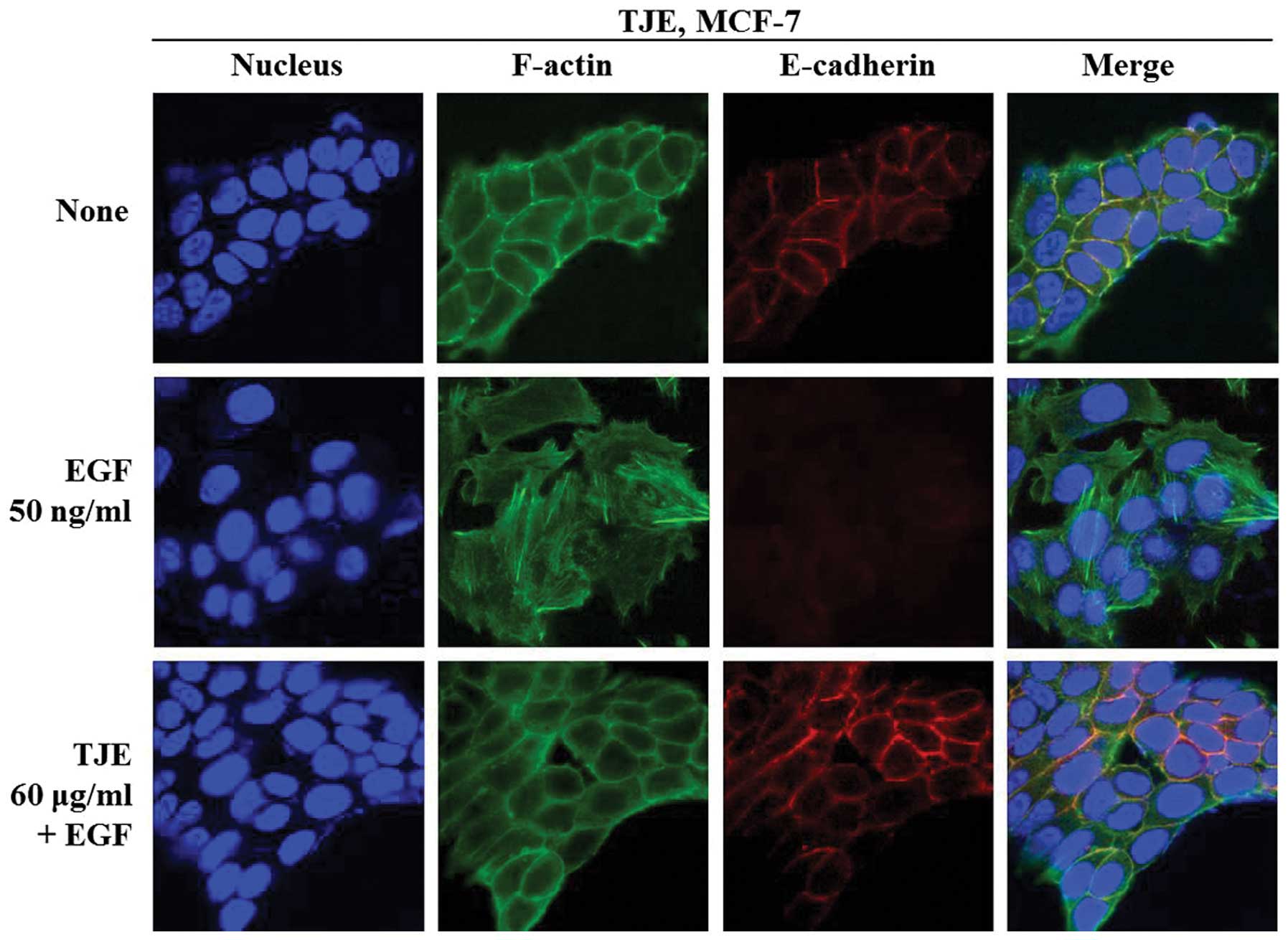
Immunofluorescence analysis of E-cadherin and
F-actin with or without TJE in EGF-stimulation shows that despite
EGF-stimulation, TJE upregulated expression of E-cadherin and
reduced cytoskeleton polarization (Fig. 5).
Discussion
Regulation of gene expression by EGF results in
modulation of EMT, and especially EMT is a key process in breast
cancer progression. Previous studies have found that breast cancer
cells and other carcinoma induce EMT when cells are exposed to EGF
(30–32); thus, modulating EGF is an obvious
strategy to regulate breast cancer progression and abnormal
metastasis. In this study, we showed that MCF-7 breast cancer cells
can induce EMT by EGF-stimulation and that treatment with TJE leads
to the repression of EGFR pathways. TJE induced the epithelial
phenotype and completely inhibited EGF-stimulated EMT.
EGF-exposed MCF-7 cells undergo EMT and acquire a
mesenchymal phenotype with increased mobility and cytoskeleton
polarization. In addition, EGF induces expression of actin
polarization-related proteins and EMT marker genes. This effect
leads to decreased expression of E-cadherin and is associated with
the tumor stage and clinicopathological features. These effects
usually activate several pathways through the EGF receptor
(30). The two major intracellular
signaling pathways activated by EGFR are the Erk MAP kinase pathway
and the PI3K/Akt pathway. Previous research has focused on an
anti-metastasis effect from one of these pathways, but not from
both. As a consequence, while it was possible to discover the
reduction in EMT from one of the EGFR downstream signaling
pathways, the effect of the other pathway was unclear (33–35);
on the other hand, in this study we found that TJE treatment led to
a decrease in EGF-induced EMT though blocking of both pathways. TJE
had a similar effect to the use of the inhibitors PD 98059 for Erk
inhibition and LY 294002 for Akt inhibition (Fig. 3). Moreover, using the EGFR specific
inhibitor GW 2974 and EGFR knock-down by siRNA transfection, it was
confirmed that TJE regulates EGFR activation and reduces
EGF-stimulated EMT through control of EGFR activation and its
downstream pathways (Fig. 4).
Breast cancer is the leading cause of cancer death
among females worldwide and accounted for 14% of total cancer
deaths in 2008 (24). Almost all
deaths were caused by abnormal metastasis such as invasion to other
organs or lymph nodes (36–38).
Moreover, recently breast cancer has been diagnosed frequently in
eastern countries and this is believed to reflect a change of
dietary patterns (25). For this
reason, researchers have been trying to find food ingredients that
have a beneficial effect for anti-metastasis in breast cancer.
This study describes for the first time the ability
of an extract from fruit of Torilis japonica to inhibit
EGF-stimulated EMT in MCF-7 breast cancer cells. TJE downregulates
EGFR activation and both its downstream pathways, the Erk MAP
kinase and Akt signaling pathways. Also TJE treatment results in
suppression of EGF-induced EMT by inducing the cell adherent
related gene E-cadherin and decreasing mesenchymal markers with the
E-cadherin suppressor gene. Taken together, these results suggest
that TJE has the potential to interfere with EGFR signaling and act
as a suppressor of abnormal metastasis in MCF-7 breast cancer.
Acknowledgements
This study was supported by the Korea Research
Foundation Grant (KRF-2010-0021402).
References
|
1
|
Hay ED: An overview of
epithelia-mesenchymal transformation. Acta Anat. 154:8–20. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kalluri R and Neilson EG:
Epithelial-mesenchymal transition and its implications for
fibrosis. J Clin Invest. 112:1776–1784. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kalluri R and Weinberg RA: The basic of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transition in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Acloque H, Adams MA, Fishwick K,
Bronner-Fraser M and Nieto MA: Epithelial-mesenchymal transitions:
the importance of changing cell state in development and disease. J
Clin Invest. 199:1438–1449. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Foroni C, Broggini M, Generali D and Damia
G: Epithelial-mesenchymal transition and breast cancer: role,
molecular emchanisms and clinical impact. Cancer Treat Rev.
38:689–697. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moreno-Bueno G, Portillo F and Cano A:
Transcriptional regulation of cell polarity in EMT and cancer.
Oncogene. 27:6958–6969. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Klymkikowsky MW and Savagner P:
Epithelial-mesenchymal transition: a cancer researcher’s conceptual
friend and foe. Am J Pathol. 174:1588–1593. 2009.PubMed/NCBI
|
|
9
|
Ahmed N, Maines-Bandiera S, Quinn MA,
Unger WG, Dedhar S and Auersperg N: Molecular patheways regulating
EGF-induced epithelia-mesenchymal transition in human ovarian
surface epithelium. Am J Physiol Cell Physiol. 290:C1532–C1542.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bhowmick NA, Chytil A, Plieth D, Gorska
AE, Dumont N, Shappell S, Washingtom MK, et al: TGF-β signaling in
fibroblasts modulation the oncogenic potential of adjacent
epithelia. Science. 6:848–851. 2004.
|
|
11
|
Suo Z, Risberg B, Karlsson MG, Villman K,
Skovlund E and Nesland JM: The expression of EGFR family ligands in
breast carcinoma. Int J Surg Pathol. 10:91–99. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ramapul RS, Pinder SE, Wencyk PM,
Nicholson RI, Blamey RW, Robertson JF and Ellis IO: Epidermal
growth factor receptor status operable invasive cancer: is it of
any prognostic value? Clin Cancer Res. 10:25782004.PubMed/NCBI
|
|
13
|
Sasaki T, Hiroki K and Yamashita Y: The
role of epidermal growth factor receptor in cancer metastasis and
microenviromental. Biomed Res Int. 2013:5463182013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nieto MA: The snail superfamily of
zinc-finger transcription factors. Nat Rev Mol Cell Biol.
3:155–166. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hajra KM, Chen SY and Fearon ER: The SLUG
zinc-finger protein represses E-cadherin in breast cancer. Cancer
Res. 62:1613–1618. 2002.PubMed/NCBI
|
|
16
|
Rosivatz E, Becker I, Specht K, Fricke E,
Luber B, Busch R, Höfler H, et al: Differential expression of the
epithelial-mesenchymal transition regulators Snail, SIP1, and twist
in gastric cancer. Am J Pathol. 161:1881–1891. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vandewalle C, Van Roy F and Berx G: The
role of the ZEB family of transcription factors in development and
disease. Cell Mol Life Sci. 66:773–787. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakajima S, Doi R, Toyoda E, Tsuji S, Wada
M, Koizumi M, Tulachan SS, et al: N-cadherin expression and
epithelial-mesenchymal transition in pancreatic carcinoma. Clin
Cancer Res. 10:4125–4133. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nijkamp MM, Span PN, Hoogsteen LJ, Van der
Kogel AJ, Kaanders JH and Bussink J: Expression of E-cadherin and
vimentin correlates with metastasis formation in head and neck
squamous cell carcinoma patients. Radiother Oncol. 99:344–348.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Benz PM, Blume C, Seifert S, Wilhelm S,
Waschke J, Schuh K, Gertler F, et al: Differential VASP
phosphorylation controls remodeling of the actin cytoskeleton. J
Cell Sci. 122:3954–3965. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Krause M, Dent EW, Bear JE, Loureiro JJ
and Gertler FB: Ena/VASP proteins: regulators of the actin
cytoskeleton and cell migration. Annu Rev Cell Dev Biol.
19:541–564. 2003.
|
|
22
|
Harbeck B, Hüttelmaier S, Schluter K,
Jockusch BM and Illenberger S: Phosphorylation of the
vasodilator-stimulated phosphoprotein regulates its interaction
with actin. J Biol Chem. 275:30817–30825. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He M, Cheng Y, Li W, Liu Q, Liu J, Huang J
and Fu X: Vascular endothelial growth factor C promotes cervical
cancer metastasis via up-regulation and activation of
RhoA/ROCK-2/moesin cascade. BMC Cancer. 10:1702010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
25
|
Colditz GA, Sellers TA and Trapido E:
Epidemiology - identifying cause and preventability of cancer? Nat
Rev Cancer. 6:75–83. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee SH, Kim GT, Kim JI, Lim EG, Kim IS and
Kim YM: The extract from Lysimachia foenum-graecum induce
apoptosis in MCF-7 breast cancer cells. KSSB J. 28:303–309.
2013.
|
|
27
|
Pan SY, Zhou SF, Gao SH, Yu ZL, Zhang SF,
Tang MK, Sun JN, et al: New perspectives on how to discover drugs
from herbal medicines: CAM’s outstanding contribution to modern
therapeutics. Evid Based Complement Alternat Med.
2013:62737582013.PubMed/NCBI
|
|
28
|
Shizhen Li: Ben Cao Gang Mu [Compendium of
Materia Medica]. China: 1593
|
|
29
|
Zhongjing Zhang: Shang han Lun [Treatise
on cold damage disorders]. China: pp. 220
|
|
30
|
Jones JL, Royall JE and Walker RA:
E-cadherin relates to EGFR expression and lymph node metastasis in
primary breast carcinoma. Br J Cancer. 74:1237–1241. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Holz C, Niehr F, Boyko M, Hristozova T,
Distel L, Budach V and Tinhofer I:
Epithelial-mesenchymal-transition induced by EGFR activation
interferes with cell migration and response to irradiation and
cetuximab in head and neck cancer cells. Radiother Oncol.
101:158–164. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cheng JC, Auersperg N and Leung PC:
EGF-induced EMT and invasiveness in serous borderline ovarian tumor
cells: a possible step in the transition to low-grade serous
carcinoma cells? PLoS One. 7:e340712012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vergara D, Valente CM, Tinelli A,
Siciliano C, Lorusso V, Acierno R, Giovinazzo G, et al: Resveratrol
inhibits the epidermal growth factor-induced epithelial mesenchymal
trasition in MCF-7 cells. Cancer Lett. 310:1–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jin Y, Iwata KK, Belldegrum A, Figlin R,
Pantuck A, Zhang ZF, Lieberman R, et al: Effect of an epidermal
growth factor receptor tyrosine kinase inhibitor in actin
remodeling in an in vitro bladder cancer carcinogenesis model. Mol
Cancer Ther. 5:1754–1765. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wallerand H, Cai Y, Wainberg ZA, Garraway
I, Lascombe I, Nicolle G, Thiery JP, et al: Phospho-Akt pathway
activation and inhibition depends on N-cadherin or phosphor-EGFR
expression in invasive human bladder cancer cell lines. Urol Oncol.
28:180–188. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Weigelt B, Peterse JL and van’t Veer LJ:
Breast cancer metastasis: markers and models. Nat Rev Cancer.
5:591–602. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pinder SE, Ellis IO, Galea M, O’Rouke S,
Blamey RW and Elston CW: Pathological prognostic factors in breast
cancer. III Vascular invasion: relationship with recurrence and
survival in a large study with long-term follow-up. Histopatology.
24:41–47. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
de Boer M, van Dijck JA, Bult P, Borm GF
and Tjan-Heijnen VC: Breast cancer prognosis and occult lymph node
metastases, isolated tumor cells, and micrometastases. J Natl
Cancer Inst. 102:410–425. 2010.PubMed/NCBI
|