Introduction
CSCs have been studied in terms of their
self-renewal capability and pluripotency, as well as their
resistance to anticancer therapy and ability to metastasize to
distant organs (1,2). Conventional chemotherapies and
radiation therapies were initially developed targeting the
cancer-cell population. However, these treatments have no efficacy
against CSCs, which have been shown to be resistant to standard
chemotherapeutic agents (3–5).
Pancreatic cancer is the 5th most common cause of cancer death in
Japan (Center for Cancer Control and Information Services, National
Cancer Center, Japan). The overall 5-year survival rate worldwide
is <10% (6). The prognosis for
pancreatic cancer patients with hepatic metastases is dismal
because these patients cannot have radical surgery. Thus, novel and
effective treatments against pancreatic CSCs are greatly needed.
CSCs can be identified and isolated by different methodologies,
including isolation by CSC-specific cell surface marker expression,
detection of side population phenotype by Hoechst 33342 exclusion,
and assessment of their ability to grow as floating spheres
(7–13). However, the population of CSCs in
tumor specimens is quite low; therefore, it is difficult to obtain
purified CSCs in adequate numbers for effective study. To overcome
this problem, we established a culture method to induce a
P-CSLC-enriched population from human pancreatic cancer cell lines.
In long-term culture, these induced cells maintained their
stem-like phenotype as characterized by: i) the ability to survive
under harsh conditions created by the media without serum and with
EGF, bFGF, LIF, and NSF-1, in which non-stem-like cancer cells are
not able to survive; ii) sphere-shaped morphology; and iii) longer
survival in laminin-coated dishes. This method is stable and
durable and will support the establishment of CSC-targeting therapy
by consistently providing abundant CSCs.
Materials and methods
Culture of human pancreatic cancer cell
lines
The human cancer cell lines used in the experimental
study were pancreatic cancer cell lines YPK2 and YPK5, which were
established in our department (14). Cell lines were maintained in
DMEM-F12 (Sigma-Aldrich, Tokyo, Japan) containing 10%
heat-inactivated FBS (Life Technologies, Tokyo, Japan) at 37°C in
5% CO2.
Induction and culture of CSLC-enriched
population
Cells were initially cultured in serum-free medium
which is based on neural stem cell medium. The basal medium for the
sphere induction is DMEM-F12 supplemented with 10 mM HEPES
(Sigma-Aldrich), 1× antibiotic antimycotic solution
(Sigma-Aldrich), 0.6% glucose (Sigma-Aldrich), 1 mg/ml transferrin,
250 μg/ml insulin (Sigma-Aldrich), 0.6 mM putrescine
(Sigma-Aldrich), 0.3 μM sodium selenite (Sigma-Aldrich), and 0.2 μM
progesterone (Sigma-Aldrich). Complete sphere induction medium was
prepared by adding 2 μg/ml heparin (Sigma-Aldrich), 20 ng/ml EGF
(Sigma-Aldrich), 20 ng/ml bFGF (Merck Millipore, Tokyo, Japan), 10
ng/ml LIF (Merck Millipore), 1/50 Vol NSF-1 (Lonza, Tokyo, Japan),
and 60 μg/ml N-acetyl-L-cysteine (Sigma-Aldrich). Upon the
formation of spheres, the sphere cells (YPK2-Sp and YPK5-Sp) were
collected. YPK2-Sp or YPK5-Sp were then transferred to a
laminin-coated dish with the sphere culture medium containing 20
μl/ml B27 supplement (Life Technologies), 1× antibiotic antimycotic
solution, 75 μg/ml BSA (Sigma-Aldrich), 10 ng/ml EGF, and 10 ng/ml
bFGF. Medium was renewed by a 50% change every 7 days. Cells became
attached and gradually divided and increased in number (YPK2-Lm and
YPK5-Lm).
Flow cytometry analysis and sorting
Dissociated cells were counted and transferred to a
5-ml tube, washed twice with PBS containing 2% heat-inactivated
FBS, and resuspended in PBS with 2% FBS at a concentration of
106 cells per 100 μl. Antibodies at the appropriate
dilution were added to the cells, and the mixture was incubated for
20 min on ice. Then, the sample was washed twice with PBS
containing 2% FBS. The antibodies were anti-CD44 allophycocyanin
(APC) (eBioscience, San Diego, CA, USA), anti-CD24 phycoerythrin
(PE) (Beckman Coulter, Brea, CA, USA), anti-ESA-FITC (GeneTex,
Irvine, CA, USA), and anti-CD44v, which was kindly provided by Dr
Hideyuki Saya (Keio University, Tokyo, Japan). Flow cytometry
analysis was performed by using a MACSQuant analyzer (Miltenyi
Biotec, Gladbach, Germany), and results were analyzed with FlowJo
software (TreeStar, OR, USA). CD24high/CD44high cells were then
isolated and sorted from YPK-Lm by FACSAria III (BD Immunocytometry
Systems, Franklin Lakes, NJ, USA). The sorted CD24high/CD44high
cells were referred to as YPK2-SortLm and YPK5-SortLm.
Analysis of ALDH activity
To assess the cellular ALDH activity, the Aldefluor
assay kit (StemCell Technologies, Vancouver, BC, Canada) was used
according to the manufacturer’s guidelines. Briefly, cells were
harvested, placed in Aldefluor assay buffer (1×106/ml),
and incubated with Aldefluor substrate for 45 min at 37°C to allow
substrate conversion. As a negative control for all experiments, an
aliquot of Aldefluor-stained cells was immediately quenched with
1.5-mM diethylamino-benzaldehyde (DEAB), a specific ALDH inhibitor.
Cells were analysed by using the green fluorescence channel (FL1)
on a MACSQuant analyzer, and results were analyzed with FlowJo
software. Cells that fell within the closed area were considered to
represent subpopulations of cells with enhanced ALDH activity as
compared with the rest of the cell population.
Cell cycle phase distribution
analysis
We performed the cell cycle analysis according to
company recommendations (BD Bioscience, Franklin Lakes, NJ, USA).
Briefly, cells were trypsinized and centrifuged at 1500 rpm for 5
min, washed twice with PBS, and then fixed with 70% cold ethanol.
Fixed cells were stained by using PI/RNase Staining Buffer (BD
Bioscience) and incubated for 15 min at room temperature before
analysis. Analysis was performed with the MACSQuant analyzer, and
results were analyzed with FlowJo software.
Xenograft model
Rag−/−IL-2 common gamma
chain−/−mice were purchased from the Jackson Laboratory
(Bar Harbor, ME, USA) and bred and maintained in a HEPA-filtered
environment with autoclave-sterilized cages, food, and bedding. All
animal studies were conducted in accordance with the Institutional
Animal Care and Use Committee of Yamaguchi University and conformed
to the Guide for the Care and Use of Laboratory Animals published
by the US National Institutes of Health. Mice were inoculated with
103 or 104 cells in each experiment. All mice
were inoculated subcutaneously in the left lower abdominal quadrant
with a 27-gauge needle.
Semi-quantitative real-time RT-PCR
The expression levels of stemness genes (KIT,
ALDH1A1, NANOG) and epithelial-mesenchymal transition (EMT)-related
genes (CDH1, CDH2, VIM, FN1, SNAI1, SNAI2, ZEB1, ZEB2) were
examined by RT-PCR. Semi-quantitative real-time RT-PCR was
performed as described previously with minor modifications
(15,16). RNAs were extracted from cells by
using TRIzol reagent (Life Technologies). Reverse transcription was
performed with the PrimeScript RT reagent kit (Takara Bio, Shiga,
Japan). Real-time PCR amplification was performed by using
LightCycler 480 Probe Master (Roche Diagnostics, Tokyo, Japan) and
Universal ProbeLibrary probes (Roche Diagnostics) in a LightCycler
System Version 3 (Roche Diagnostics). Primers and probes are listed
in Table I. Amplification was
performed according to a 2-step cycle procedure consisting of 45
cycles of denaturation at 95°C for 10 sec and annealing/elongation
at 60°C for 30 sec. We measured mRNA levels semi-quantitatively by
the Δ/Δ threshold cycle (Ct) method. Both the GAPDH and β-actin
(ACTB) genes were used as reference genes. The values are expressed
as relative to the parental cells.
 | Table IPrimers and probes. |
Table I
Primers and probes.
| Symbol | Name | UPL probe no. | Sequence
(5′-3′) |
|---|
| KIT (C-Kit,
CD117) | KIT-S | 71 |
ctttcctcgcctccaagaat |
| KIT-AS | |
gtgatccgaccatgagtaagg |
| ALDH1A1 | ALDH1A1-S | 14 |
tttggtggattcaagatgtctg |
| ALDH1A1-AS | |
cactgtgactgttttgacctctg |
| NANOG | NANOG-S | 31 |
agatgcctcacacggagact |
| NANOG-AS | |
tttgcgacactcttctctgc |
| CDH1
(E-cadherin) | CDH1-S | 35 |
cccgggacaacgtttattac |
| CDH1-AS | |
gctggctcaagtcaaagtcc |
| CDH2
(N-cadherin) | CDH2-S | 80 |
agtatccggtccgatctgc |
| CDH2-AS | |
ctgtggggtcattgtcagc |
| VIM (vimentin) | VIM-S | 13 |
tacaggaagctgctggaagg |
| VIM-AS | |
accagagggagtgaatccag |
| FN1 | FN1-S | 60 |
aagagcgagcccctgatt |
| FN1-AS | |
atgaagattggggtgtggaa |
| SNAI1 | SNAI1-S | 10 |
catgtccggacccacact |
| SNAI1-AS | |
tggcactggtacttcttgaca |
| SNAI2 (SLUG) | SNAI2-S | 7 |
tggttgcttcaaggacacat |
| SNAI2-AS | |
gttgcagtgagggcaagaa |
| ZEB1 | ZEB1-S | 36 |
cctaaaagagcacttaagaattcacag |
| ZEB1-AS | |
catttcttactgcttatgtgtgagc |
| ZEB2 (SIP1) | ZEB2-S | 68 |
aagccagggacagatcagc |
| ZEB2-AS | |
ccacactctgtgcatttgaact |
Measurements of cytokine and chemokine
levels
Frozen aliquots of YPK2 and YPK5 were thawed and
cultured for 2 weeks prior to harvesting culture supernatant from
sub-confluent cultures (Sup-YPK2 and Sup-YPK5). YPK2-Lm and YPK5-Lm
supernatant was harvested when cells were sub-confluent 1 month
after transfer to laminin-coated dishes in the sphere culture
medium (Sup-Lm2 and Sup-Lm5). The Bioplex assay (Bio-Rad, Marne la
Coquette, France) was performed according to the manufacturer’s
instructions to evaluate the levels of cytokines and chemokines in
the supernatant. Samples were analyzed in triplicate. Experimental
data were analyzed by using five-parametric curve fitting. We
measured the protein level of the following 28 cytokines and
chemokines: TGF-β, IL-1b, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-7,
IL-8, IL-9, IL-10, IL-12, IL-13, IL-17, eotaxin, bFGF, G-CSF,
GM-CSF, interferon (IFN)-γ, immune protein (IP)-10, monocyte
chemotactic protein (MCP)-1, macrophage inflammatory proteins
(MIP)-1α, MIP-1β, platelet-derived growth factor (PDGF)-BB,
regulated on activation, normal T-cell expressed and secreted
(RANTES), tumor necrosis factor (TNF)-α, and vascular endothelial
growth factor (VEGF).
Statistical analysis
The results are presented as means ± SD. Statistical
differences were determined using the Mann-Whitney U tests.
P-values of <0.05 were considered significant.
Results
Induction and culture of CSLC-enriched
population
When YPK2 or YPK5 were initially cultured in the
CSC-inducing media, cells began to attach on the plate, and a
portion of cells formed spheres in suspension culture within a few
hours (YPK2-Sp and YPK5-Sp) (Fig. 1A
and D). These spheres grew to become larger sphere clusters
within a week. YPK2-Sp or YPK5-Sp were harvested on day 7 and
transferred to laminin-coated dishes. Cells began to attach to the
dishes within a few hours; then, they gradually divided and the
number of spheres and attached cells increased for 2 months
(YPK2-Lm and YPK5-Lm, Fig. 1B, C, E
and F). The surviving cells displayed both attached and
cluster-formatted morphology. When these cells were grown in
culture for >3 months, they became apoptotic without
proliferation. Fig. 1G and H show
YPK2 and YPK5 cultured in DMEM containing 10% FBS. These cells were
attached and proliferated quickly.
Cell surface markers
In general, a
CD44+/CD24+/ESA+ phenotype has
stem-cell properties in pancreatic cancer cells (7). In the present study, the ratio of the
expression of CD24high/CD44high in YPK2 (Fig. 2A) and YPK5 (Fig. 2D) was ~0.1%, while the ratios (mean
± SD) in YPK2-Lm (Fig. 2B) and
YPK5-Lm (Fig. 2E) were
significantly increased to 7.5±2.6% (P=0.0211) and 11.1±2.8%
(P=0.0211), respectively. Expression of ESA in YPK2-SortLm (23.2%)
and YPK5-SortLm (36.2%) was higher than that of YPK2 (0.1%) and
YPK5 (0.1%) (Fig. 2C and F).
Recently, some studies have focused on the role of CD44v in CSCs
(17,18). We also focused on the expression of
CD44v. Fig. 2G shows that
YPK2-SortLm expressed a higher ratio of CD44v than YPK2-Lm and
YPK2. The ratio of CD44v in YPK2 was only 0.2%; however, this ratio
in YPK2-Lm and YPK2-SortLm was 16.7 and 99.8%, respectively.
Expression of CD44v in YPK5-SortLm was also high compared to YPK5
(Fig. 2H). When NSF-1 or LIF was
omitted from CSC-inducing media, CD24low/CD44low cells were
dominant; these cells were also dominant in parent cancer cells
(Fig. 2I and J).
 | Figure 2Expression of CD24, CD44, CD44v and
ESA. The ratio of the expression of CD24high/CD44high in YPK2 and
YPK5 was 0.1% [(A) YPK2; (D) YPK5]. The ratios of the expression of
CD24high/CD44high in YPK2-Lm and YPK5-Lm were 7.5±2.6 and
11.1±2.8%, respectively [(B) YPK2-Lm; (E) YPK5-Lm]. Expression of
ESA in YPK2-SortLm [(C) black line, 23.2%] and YPK5-SortLm [(F)
black line, 36.2%] was higher than that of YPK2 [(C) tinted line,
0.1%] and YPK5 [(F) tinted line, 0.1%]. (G) The ratio of CD44v in
YPK2 (tinted line) was only 0.2%, but the ratios in YPK2-Lm (dotted
line) and YPK2-SortLm (black line) were increased to 16.7 and
99.8%, respectively. (H) The ratio of CD44v in YPK5 (tinted line)
was only 0.2%, however these ratios of CD44v in YPK5-Lm (dotted
line) and YPK5-SortLm (black line) were 16.7 and 99.8%,
respectively. (I) YPK 2-Lm was cultured in the CSC-inducing media
without NSF-1. (J) YPK 2-Lm was cultured in the CSC-inducing media
without LIF. When NSF-1 or LIF were omitted from the CSC-inducing
media, cells expressed cancer cell-like patterns of surface markers
such as CD24low/CD44low. Incidences in (B) and (E) were evaluated
by Mann-Whitney U tests; *P<0.05. |
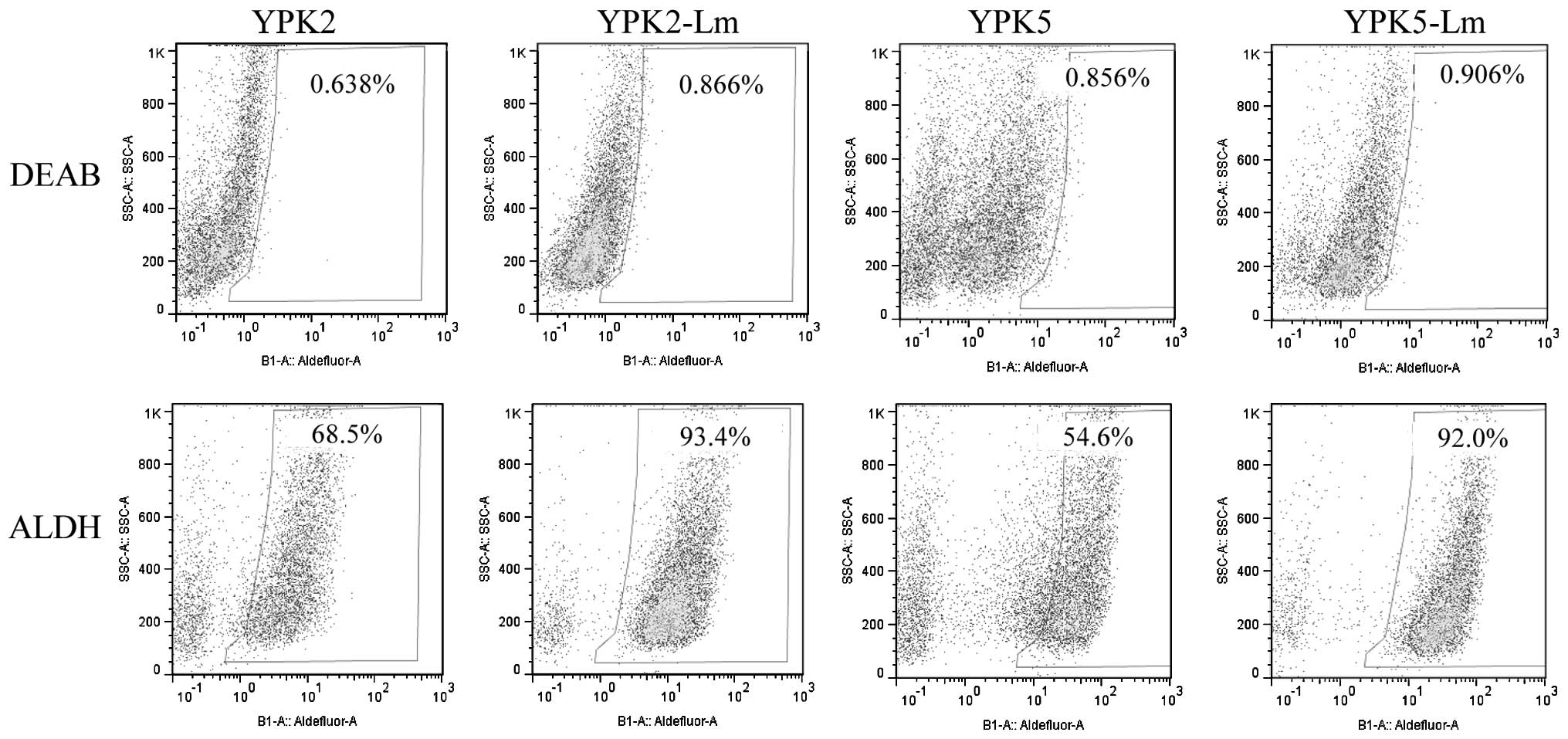
ALDH activity and cell cycle
analysis
A functional mechanism for chemo-resistance has been
associated with ALDH activity (19). In the present study, YPK2 and YPK5
expressed high levels of ALDH activity (YPK2, 68.5%; YPK5, 54.5%),
however, YPK2-Lm and YPK5-Lm expressed much higher levels of ALDH
activity (YPK2-Lm, 93.4%; YPK5-Lm, 92.0%) than parental cells
(Fig. 3).
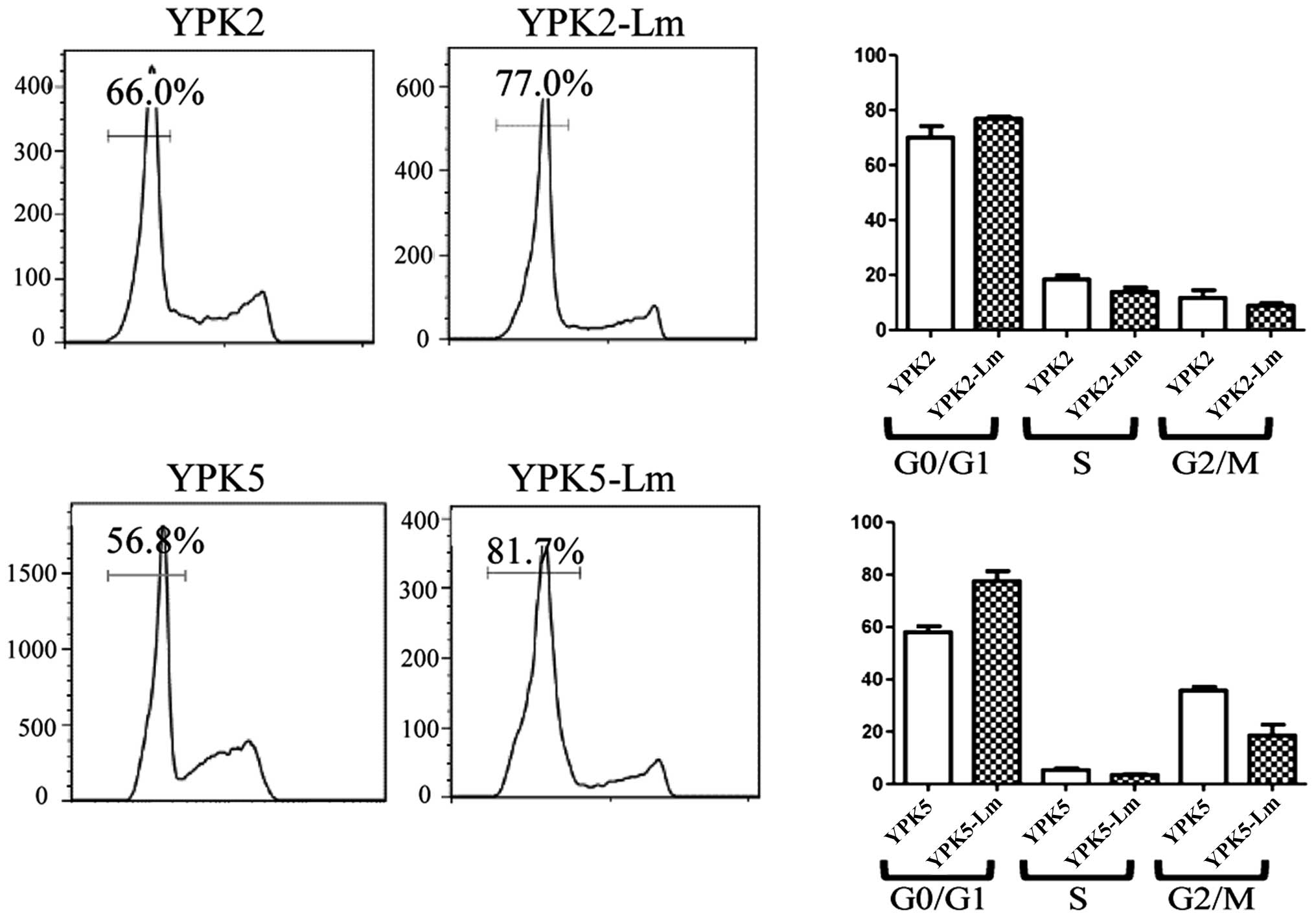
Stem cell quiescence is also highly relevant for
chemotherapy against cancer, as it is retained and contributes to
relapse following discontinuation of therapy (20). Many CSCs are non-cycling G0 cells
and would not be susceptible to cell cycle-specific chemotherapy
agents. Many of YPK2-Lm and YPK5-Lm are relatively quiescent
compared to the YPK2 and YPK5, however, these were not
statistically significant (Fig.
4).
Tumorigenicity
YPK2-SortLm cells gave rise to new tumors in 3 of 3
mice, ≥103 cells were injected. In contrast, no tumors
formed when 103 YPK2 cells were injected, which
demonstrates the much higher tumorigenicity of YPK2-SortLm
cells.
mRNA expression of stem-cell and
mesenchymal markers
The theory of the relationship between EMT and CSCs
has been supported recently by the fact that cancer cells with
migratory and invasive capabilities associated with metastatic
competence are caused through EMT (21–23).
Recent studies have established a crucial link between passage
through EMT and the acquisition of the molecular and functional
properties of stem cells (24,25).
We therefore confirmed whether YPK-Lm have EMT properties (Fig. 5). RT-PCR resulted in significantly
higher expression levels of stemness genes such as KIT and ALDH1A1
in both YPK2-Lm (P=0.0095 and 0.0022, respectively) and YPK5-Lm
(P=0.0022 and 0.0049, respectively) (Fig. 5A). The expression level of NANOG
was significantly high in only YPK2-Lm (YPK2-Lm; P=0.005, YPK-5Lm;
P=0.9361). The expression levels of mesenchymal genes such as CDH2,
VIM, SNAI1, SNAI2, ZEB1, ZEB2, and FN1 were significantly higher in
YPK2-Lm than in YPK2 (P=0.0022, respectively) (Fig. 5C). In YPK5-Lm, the expression
levels of SNAI1 and ZEB2 were significantly higher than in YPK5
(P=0.026 and 0.0087, respectively). The expression level of CDH1
was significantly higher in YPK2-Lm than YPK2, but was not
statistically significant between YPK5 and YPK5-Lm (Fig. 5B).
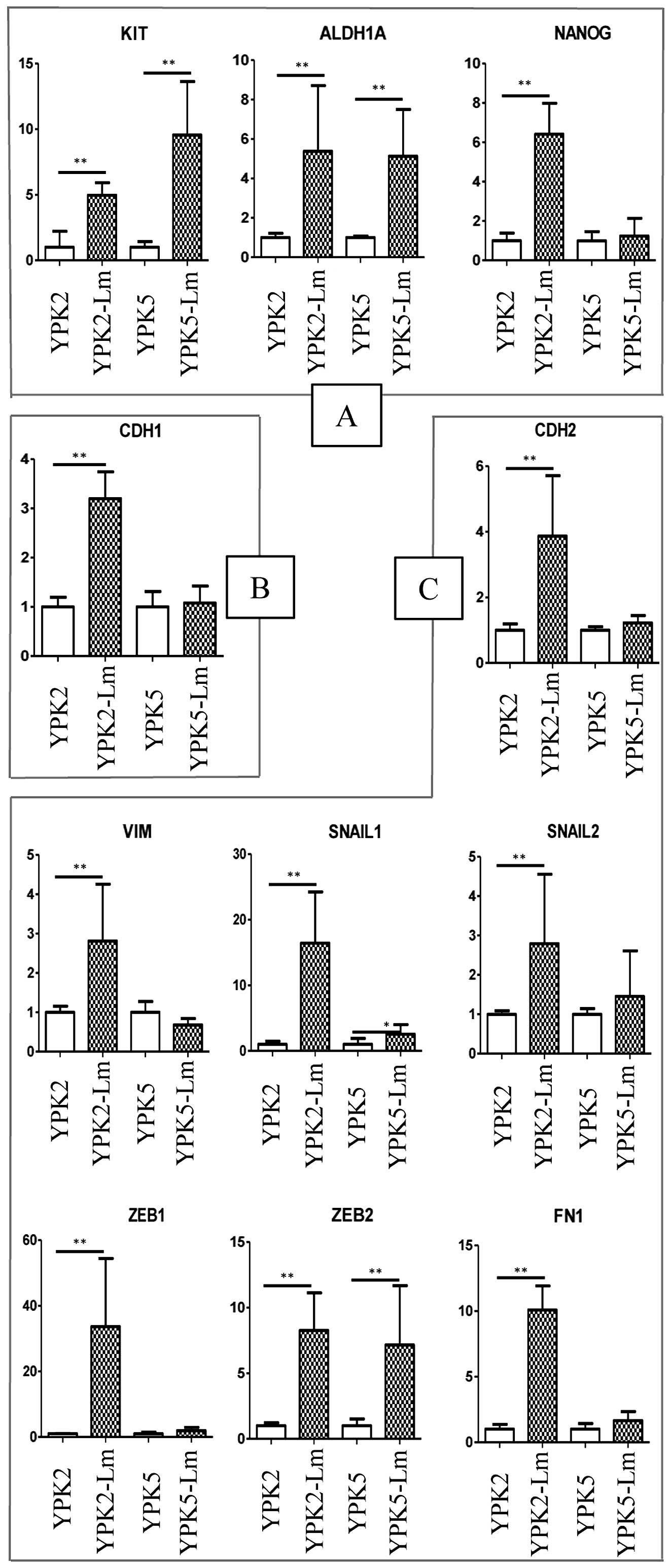 | Figure 5mRNA expression of stemness and
EMT-related markers. (A) The expression levels of stemness genes.
RT-PCR resulted in significantly higher expression levels of KIT
and ALDH1A1 in both YPK2-Lm (P=0.0095 and 0.0022, respectively) and
YPK5-Lm (P=0.0022 and 0.0049, respectively). (B) The expression
levels of epithelial genes. The expression level of CDH1 was
significantly higher in YPK2-Lm than YPK2, but was not
statistically significant between YPK5 and YPK5-Lm. (C) The
expression levels of mesenchymal genes. The expression levels of
CDH2, VIM, SNAI1, SNAI2, ZEB1, ZEB2, and FN1 were significantly
higher in YPK2-Lm than in YPK2 (P=0.0022, respectively). In
YPK5-Lm, the expression levels of SNAI1 and ZEB2 were significantly
higher than in YPK5 (P=0.026 and 0.0087, respectively). Evaluated
by the Mann-Whitney U tests; *P<0.05;
**P<0.01. |
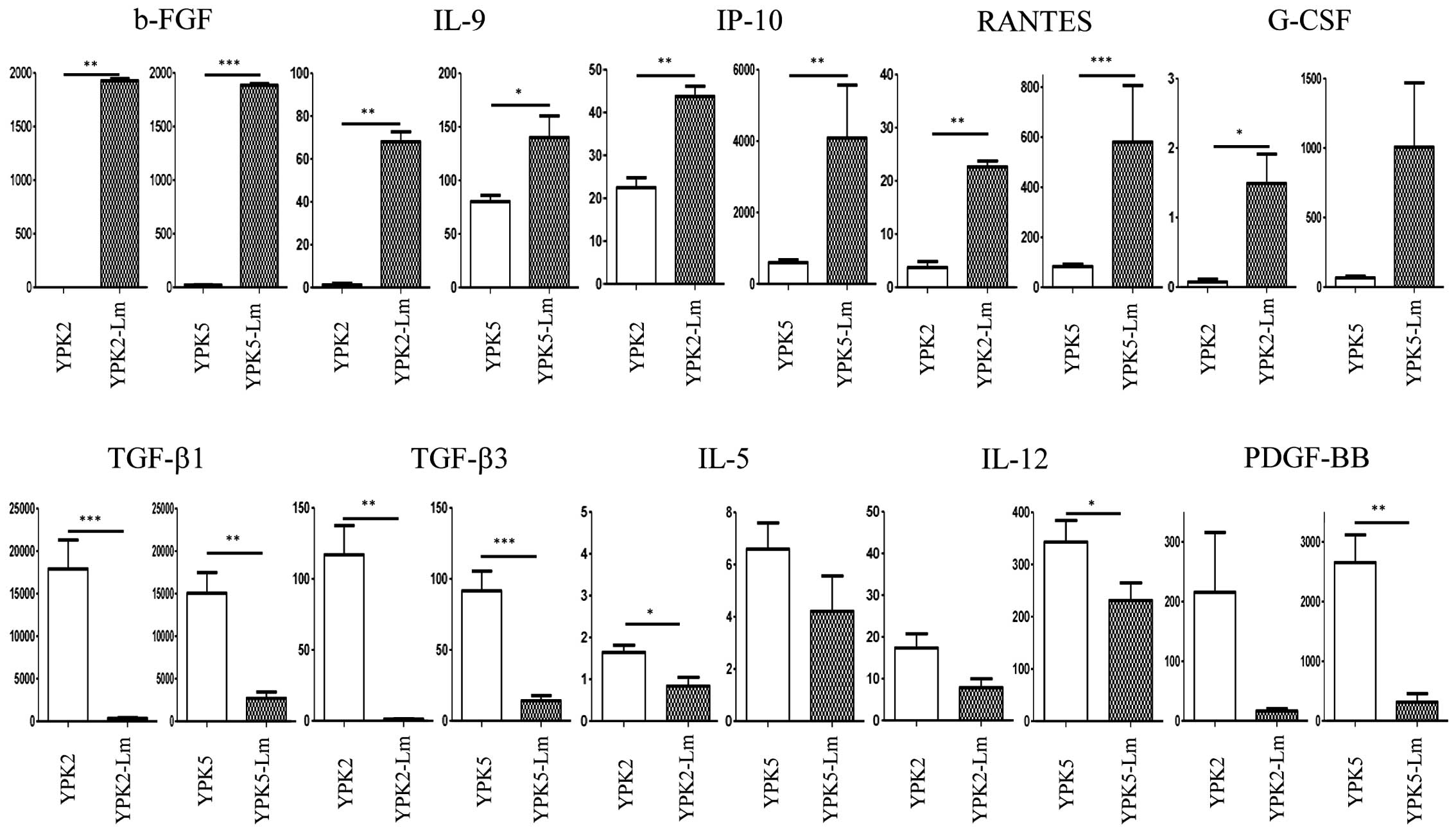
Cytokine analysis in the culture
media
To question the interaction of microenvironment
between cancer and CSLC, we performed multiple cytokine assays with
their culture media (Fig. 6). The
levels of b-FGF, IL-9, IP-10, and RANTES were significantly
detected as higher concentrations in the Sup-Lm2 and Sup-Lm5
compared to the Sup-YPK2 and Sup-YPK5 (P<0.05). The level of
G-CSF was also significantly detected as higher concentrations in
the Sup-Lm2 compared to the Sup-YPK2 (P=0.02), and also higher
trend in Sup-Lm5 than in Sup-YPK5 (P=0.06). The levels of TGF-β1
and TGF-β3 were detected as higher concentrations in the Sup-YPK2
and Sup-YPK5 compared to the Sup-Lm2 and Sup-Lm5 (P<0.01). The
level of IL-5 was significantly detected as higher concentrations
in the Sup-YPK2 compared to the Sup-Lm2 (P=0.015), and also higher
trend in the Sup-YPK5 than in the Sup-Lm5 (P=0.07). The levels of
IL-12 and PDGF-BB were also significantly detected as higher
concentrations in the Sup-YPK5 compared to the Sup-Lm5 (P=0.04 and
0.0027), although these were not statistically significant between
the Sup-YPK2 and Sup-Lm2 (P=0.0939 and 0.0926).
Discussion
We established a novel culture method to induce a
P-CSLC-enriched population from human pancreatic cancer cell lines.
As a first step, human pancreatic cancer cell lines were cultured
and induced to form spheres/aggregates within a week. As a second
step, these sphere cells were transferred to a laminin-coated dish
with the medium, attached and the population of these induced cells
expanded within a few months. In the present study, the ratio of
CD24high/CD44high cells in YPK-Lm was enriched (Fig. 2B and E). Almost all of YPK-Lm
expressed CD44v and also expressed high levels of ALDH activity
(Figs. 2G and 3). Cell-cycle analysis showed that many
YPK-Lm preferentially stayed in the G0/G1 phase (Fig. 4). mRNA levels of mesenchymal
markers such as SNAI1 and ZEB2 were expressed in YPK-Lm as we
expected (Fig. 5C). Similarly,
RT-PCR resulted in higher expression levels of stemness marker such
as KIT and ALDH1A1 from YPK-Lm (Fig.
5A). These results suggest that YPK-Lm acquired stemness
properties through the EMT. The expression level of CDH1, which is
an epithelium-related gene expected to be high in YPK based on this
EMT theory, was high in YPK-Lm (Fig.
5B). Thus, this theory of CSC induction by passage through the
EMT still has room for argument. Based on the facts described
above, we confirmed that these induced cells have CSCs
characteristics.
A prominent feature of CSCs is their ability to form
floating spheroids in serum-free culture conditions (26). Several studies have suggested that
CSCs can be enriched in spheres when cultured in serum-free medium
supplemented with adequate mitogens, such as bFGF and EGF (27–30).
However, culture cells kept in the sphere formation for >10 days
forfeit not only their stemness properties but also viability. The
most problematic issue is the spontaneous differentiation and cell
death that accompany stem cell divisions in the sphere environment
(31). In contrast, most
individual cells in adherent culture conditions are uniformly
exposed to defined growth factors and oxygen tension, which allows
most CSCs to maintain their stemness properties without spontaneous
differentiation and cell death. The laminins are an important and
biologically active part of the basal lamina, influencing cell
differentiation, migration, and adhesion, as well as survival
(32,33). To overcome the limitations of the
neurosphere culture paradigm, Pollard et al cultured glioma
tumor-initiating cells as adherent cell lines by using
laminin-coated dishes (31). In
our experiment, the modified stem cell medium with NSF-1, and LIF
induced a P-CSLC-enriched population, however, the medium without
NSF-1 and/or LIF failed to induce this population (Fig. 2I, and J). In addition, this induced
population did not divide and the number of cells did not increase
in this condition without transferring to laminin-coated dishes.
This population has to be transferred to laminin-coated dishes
approximately one week after sphere formation. Then, this
population is able to maintain the stemness properties and
viability with self-renewing properties. We suggest that the
process of CSLC induction demands the neural stimulation factors
with some adequate cytokines and chemokines, such as bFGF and EGF.
Based on our data of cytokines from the supernatant, it was
established that induced and maintained conditions between CSCs and
cancer cells are drastically different in terms of cytokines
profile in the culture (Fig. 6).
As typical examples, b-FGF, IL-9, IP-10, RANTES, and G-CSF were
higher in supernatant of CSCs culturing, while TGF-β1, TGF-β3,
IL-5, IL-12, and PDGF-BB were higher in supernatant of cancer cells
culturing. Needless to say, this part of the study is immature and
weak. Further analysis and study will be required to reveal the
mechanism inducing CSLCs in the culture.
Currently, CSC-targeting therapy has been attempted
to be established (34,35), because conventional anticancer
treatments do not target CSCs and have no efficacy against CSCs.
However, one of the difficulties in the quest to characterize the
CSC population from tumor specimens is the rarity of this
population. Using the method as established in this study, we can
easily enrich the CSLC population without special instruments.
Although this method is potentially able to be applied to freshly
harvested cancer tissue, further investigations in this area are
needed. We are planning to use these induced cells to establish a
novel immunotherapy targeting CSCs through proteomics. For
screening the ability of the immune effector cells to eradicate
their target-CSCs, an appropriate number of CSCs can be used with
this novel technology.
In conclusion, we established a culture method to
induce a CSLC-enriched population from human pancreatic cancer cell
lines. This method may be useful to analyze CSC characteristics in
detail, and to help in the establishment of novel therapies against
CSCs.
Acknowledgements
We thank Hirokazu Sadahiro and Moeko Inoue for
technical support. This study was supported by Japan Society for
the Promotion of Science KAKENHI grants 24390317 (to M.O.) and
Yamaguchi University research grant Project of Priming Water
(Strategic Research Promotion Project) (to K.Y.).
References
|
1
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Costello RT, Mallet F, Gaugler B, et al:
Human acute myeloid leukemia CD34+/CD38−
progenitor cells have decreased sensitivity to chemotherapy and
Fas-induced apoptosis, reduced immunogenicity, and impaired
dendritic cell transformation capacities. Cancer Res. 60:4403–4411.
2000.PubMed/NCBI
|
|
4
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar
|
|
5
|
Guzman ML, Swiderski CF, Howard DS, et al:
Preferential induction of apoptosis for primary human leukemic stem
cells. Proc Natl Acad Sci USA. 99:16220–16225. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
7
|
Li C, Heidt DG, Dalerba P, et al:
Identification of pancreatic cancer stem cells. Cancer Res.
67:1030–1037. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hermann PC, Huber SL, Herrler T, et al:
Distinct populations of cancer stem cells determine tumor growth
and metastatic activity in human pancreatic cancer. Cell Stem Cell.
1:313–323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eramo A, Lotti F, Sette G, et al:
Identification and expansion of the tumorigenic lung cancer stem
cell population. Cell Death Differ. 15:504–514. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kondo T, Setoguchi T and Taga T:
Persistence of a small subpopulation of cancer stem-like cells in
the C6 glioma cell line. Proc Natl Acad Sci USA. 101:781–786. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu C and Alman BA: Side population cells
in human cancers. Cancer Lett. 268:1–9. 2008. View Article : Google Scholar
|
|
12
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
et al: Identification and expansion of human
colon-cancer-initiating cells. Nature. 445:111–115. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dontu G, Abdallah WM, Foley JM, et al: In
vitro propagation and transcriptional profiling of human mammary
stem/progenitor cells. Genes Dev. 17:1253–1270. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamamoto K, Yahara N, Gondo T, Ishihara T
and Oka M: Establishment and characterization of a new human
pancreatic cancer cell line, YPK-1. Bull Yamaguchi Med Sch.
49:33–42. 2002.
|
|
15
|
Tsunedomi R, Iizuka N, Tamesa T, et al:
Decreased ID2 promotes metastatic potentials of hepatocellular
carcinoma by altering secretion of vascular endothelial growth
factor. Clin Cancer Res. 14:1025–1031. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsunedomi R, Iizuka N, Harada S and Oka M:
Susceptibility of hepatoma-derived cells to histone deacetylase
inhibitors is associated with ID2 expression. Int J Oncol.
42:1159–1166. 2013.PubMed/NCBI
|
|
17
|
Nagano O, Okazaki S and Saya H: Redox
regulation in stem-like cancer cells by CD44 variant isoforms.
Oncogene. 32:5191–5198. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tanabe KK, Ellis LM and Saya H: Expression
of CD44R1 adhesion molecule in colon carcinomas and metastases.
Lancet. 341:725–726. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abdullah LN and Chow EK: Mechanisms of
chemoresistance in cancer stem cells. Clin Transl Med. 2:32013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dick JE: Stem cell concepts renew cancer
research. Blood. 112:4793–4807. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Prat A, Parker JS, Karginova O, et al:
Phenotypic and molecular characterization of the claudin-low
intrinsic subtype of breast cancer. Breast Cancer Res. 12:R682010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mani SA, Guo W, Liao MJ, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morel AP, Lievre M, Thomas C, Hinkal G,
Ansieau S and Puisieux A: Generation of breast cancer stem cells
through epithelial-mesenchymal transition. PLoS One. 3:e28882008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mor G, Yin G, Chefetz I, Yang Y and Alvero
A: Ovarian cancer stem cells and inflammation. Cancer Biol Ther.
11:708–713. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chiou SH, Yu CC, Huang CY, et al: Positive
correlations of Oct-4 and Nanog in oral cancer stem-like cells and
high-grade oral squamous cell carcinoma. Clin Cancer Res.
14:4085–4095. 2008. View Article : Google Scholar
|
|
28
|
Lee J, Kotliarova S, Kotliarov Y, et al:
Tumor stem cells derived from glioblastomas cultured in bFGF and
EGF more closely mirror the phenotype and genotype of primary
tumors than do serum-cultured cell lines. Cancer Cell. 9:391–403.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hueng DY, Sytwu HK, Huang SM, Chang C and
Ma HI: Isolation and characterization of tumor stem-like cells from
human meningiomas. J Neurooncol. 104:45–53. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhong Y, Guan K, Guo S, et al: Spheres
derived from the human SK-RC-42 renal cell carcinoma cell line are
enriched in cancer stem cells. Cancer Lett. 299:150–160. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pollard SM, Yoshikawa K, Clarke ID, et al:
Glioma stem cell lines expanded in adherent culture have
tumor-specific phenotypes and are suitable for chemical and genetic
screens. Cell Stem Cell. 4:568–580. 2009. View Article : Google Scholar
|
|
32
|
Ziober AF, Falls EM and Ziober BL: The
extracellular matrix in oral squamous cell carcinoma: friend or
foe? Head Neck. 28:740–749. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Timpl R, Rohde H, Robey PG, Rennard SI,
Foidart JM and Martin GR: Laminin - a glycoprotein from basement
membranes. J Biol Chem. 254:9933–9937. 1979.PubMed/NCBI
|
|
34
|
Calabrese C, Poppleton H, Kocak M, et al:
A perivascular niche for brain tumor stem cells. Cancer Cell.
11:69–82. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Takeishi S, Matsumoto A, Onoyama I, Naka
K, Hirao A and Nakayama KI: Ablation of Fbxw7 eliminates
leukemia-initiating cells by preventing quiescence. Cancer Cell.
23:347–361. 2013. View Article : Google Scholar : PubMed/NCBI
|