Introduction
Kojic acid
(5-hydroxy-2-hydroxymethyl-4H-pyran-4-one) is a biologically
important natural product and used widely as an additive in the
food industry and bleaching agent in cosmetic preparations
(1). Kojic acid and its
derivatives also possess other various bioactivities such as
antimicrobial (2,3), antidiabetic (4) and antitumor activity (5). Moreover, it can chelate many metal
ions as bidentate ligand, such copper (6) and its skin lightening ability is
stemmed from its copper-chelating property. Copper chelation is
currently being investigated as an antiangiogenic and
antineoplastic agent in patients diagnosed with esophageal
carcinoma, hormone-refractory prostate cancer, colorectal cancer,
and breast cancer (7). In light of
its copper-chelating property with tyrosinase, kojic acid appears
to be a potential candidate to develop novel classes of
metal-chelating antiproliferative agents (8), and many of its derivatives of it have
been synthesized to improve their biological activity (9,10).
The fluoroquinolones belong to the group of
synthetic antibiotics that exert broad-spectrum antimicrobial
activities. Ciprofloxacin is one of the fluoroquinolones and widely
used in clinic as antimicrobial agent. In addition to antimicrobial
activity, antitumor activity against colon cancer, bladder cancer,
leukemia and hepatic cell lines have been found and linked to
topoisomerase II (Top II) inhibitory (11–13).
The structure-activity relationship (SAR) and the functions of
different positions on the quinolone core have been identified
(14). The SAR studies indicated
that the group at position 7 affects its antibacterial spectrum
(15). The substituted
7-piperazinyl derivatives of ciprofloxacin, such as azo (11), Mannich base (16,17),
N-alkylated (18) and N-acylated
ciprofloxacin derivatives (19)
have been synthesized and evaluated for antibacterial activity.
Combined multifunctional units into a molecule is a
strategy of fragment-based drug discovery to find lead compounds
for different research aims, the Mannich reaction is one of ways
used to perform this task. In addition, the Mannich bases
themselves have good biological activity, so many of these
compounds were synthesized and investigated for antimicrobial and
antitumor activities. In our previous investigation (13), we found that the structural
modification on ciprofloxacin may improve its antitumor activity,
as extended study, the compound of 7-piperazinyl ciprofloxacin with
kojic acid and its copper complex were synthesized in order to
investigate whether there is potent enhancement in antitumor
activities. In this study, the potent molecular mechanism of action
of the Mannich base and its copper complex was explored via RT-PCR,
cell cycle analysis, topoisomerase inhibition and molecular
docking, revealing a new action mechanism for the copper
complex.
Materials and methods
Materials
All chemical reagents were analytical reagent grade.
MTT, rhodamine 123, propidium iodide, ethidium bromide and SDS were
purchased from Sigma-Aldrich, Inc. (Shanghai, China). Ciprofloxacin
hydrochloride was obtained from Sangon Biotech (Shanghai, China).
Kojic acid was purchased from Aladdin Industrial Corp. (Shanghai,
China).
Preparation of Mannich base of kojic acid
with ciprofloxacin
Mannich bases were prepared by the reaction of
ciprofloxacin (0.01 mol) and kojic acid (0.01 mol) in methanol with
37% formalin (0.012 mol). The mixture was stirred vigorously for 4
h. The resulting precipitate was collected by filtration and washed
with cold methanol. The crude product was recrystallized from
methanol. The Mannich base was characterized by 1H NMR
(400 MHz, Bruker, Switzerland) spectrum and Mass spectrum:
1H NMR (d6-DMSO) 1H NMR d (DMSO-d6, 400 MHz)
δ (ppm): 2.61 (4H; t; piperazine), 3.35 (4H; s;
piperazine), 3.58 (2H; s; -CH2-), 4.34 (2H; s;
-HOCH2-), 5.77 (1H; t;
CH2-OH),
6.38 (1H; s; H5-pyran), 9.45 ppm (1H; s; 3-OH pyran), 1.18 – 1.32
(m, 4H, -CH2CH2-), 3.16 – 3.24 (m, 8H,
piperazine-H), 3.35 (m, 1H, CH), 7.58 (d, 1H, J = 7.2 Hz,
H8 of cip), 7.89 (d, J = 13.5 Hz, 1H, H5 of
cip), 8.66 (s, 1H, H2 of cip), 15.16 (s, 1H, -COO H); MS
(VG Autospec-3000 spectrometer, Micromass, UK): m/e 485.46. The
structure of the Mannich base and its copper complex are shown in
Fig. 1.
Cell culture and cytotoxicity assay
HCT-116 and HepG2 human hepatoma cell lines (Y-S
Biotechnology Inc., Shanghai, China) were propagated continuously
in RPMI-1640 medium supplemented with 10% freshly inactivated fetal
calf serum and antibiotics (100 U/ml penicillin G, and 100 μg/ml
streptomycin) in a humidified atmosphere of 5% CO2 and
95% air at 37°C. The HCT-116 and HepG2 cells in exponential-phase
were detached by 0.25% trypsin, followed by centrifugation, PBS
washing, and re-suspended in RPMI-1640, then the equal cells were
seeded (5×103/well) into a 96-well plate. When the cells
attached to the Mannich base the copper complex was added at the
final concentrations at 200, 100, 50, 25, 12.5, 6, 3 and 1.5 μM
which varied with the drug and cell line. After incubation for 48
h, 10 μl MTT solution (1 mg/ml) was added to each well, the plate
was further incubated for 4 h. The cell culture was removed by
aspiration and 100 μl DMSO was added into each well to dissolve the
formazan crystals. The measurement of absorbance at 570 nm was
performed on an ELISA spectrophotometer (MK3, Thermo Scientific,
Shanghai, China). Percent growth inhibition was defined as percent
absorbance inhibition within appropriate absorbance in each cell
line. The same assay was done in triplet.
RT-PCR
Total RNA was extracted from the HepG2 cells using
TRIzol reagent (Shangong Biological, Shanghai, China) according to
the manufacturer’s protocol. Three micrograms of total RNA were
used for reverse transcription in a total volume of 20 μl following
the manufacturer’s recommendation (Lifefeng, Shanghai, China).
Aliquots of 2 μl cDNA were subsequently amplified in a total volume
of 25 μl. The sense and antisense primer for β-actin were
5′-ACACTGTGCC CATCTACGAGG-3′ and 5′-CGGACTCGTCATACTCC TGCT-3′ (615
bp) that were used as an internal control; the sense and antisense
primers for bcl2 were 5′-TTACCAAGCAG CCGAAGA-3′ and
5′-TCCCTCCTTTACATTCACAA-3′ (309 bp, NM_ 138621); the sense and
antisense primers for bax, 5′-TTTTGCTTCAGGGTTTCATC-3′ and 5′-GGCCTT
GAGCACCAGTTT-3′ (299 bp, BC014175); the sense and antisense primers
for p53, 5′-GTCTACCTCCCGCCATAA-3′, 5′-CATCTCCCAAACATCCCT-3′ (316
bp, NM_ 001126114.2); the sense and antisense primers for cyclin A,
5′-TTAGGGAAA TGGAGGTTA-3′ and 5′-CAGAAAGTATTGGGTAAGAA-3′ (404 bp,
NM_001237.3); the sense and antisense primers for cyclin B,
5′-TCTGCTGGGTGTAGGTCC-3′ and 5′-AATA GGCTCAGGCGAAAG-3′ (444 bp,
NM_0319 66.3); the sense and antisense primers for cyclin D1,
5′-CTGGATGCTGGAGG TCTGCGAGGA-3′ and 5′-TGAACTTCACATCTGTGGCAC AGA-3′
(400 bp, M 73554), respectively. The cycling conditions: 94°C for 5
min, followed by 28 cycles of 94°C for 30 sec, 58°C for 30 sec and
72°C for 1 min, and a final extension of 72°C for 10 min. PCR
products were separated on the 1.5% agarose gel viewed by ethidium
bromide staining. These data were acquired with Tocan 360 gel
imager (version 3.2.1 software, Shanghai Tiancheng Technology Inc.,
Shanghai, China). The RT-PCR experiments were done in
duplicates.
Cell cycle analysis
HepG2 cells (1×105) were seeded in a
6-well plate. After 24-h incubation at 37°C (5% CO2),
the medium was changed with fresh, supplemented or not (control)
with the Mannich base (50 and 100 μM). After 24-h incubation, cells
were harvested with trypsin, washed by PBS, fixed in 70% ethanol
and stored at −20°C for 1 h. The cellular nuclear DNA was stained
by propidium iodide (PI) as described (13), briefly, followed by removing the
ethanol, washed with PBS, the cells were suspended in 0.5 ml PBS
containing 50 μg/ml PI and 100 μg/ml RNase and incubated at 37°C
for 30 min. Flow cytometry was performed in duplicate with a
FACScalibur flow cytometer (Becton-Dickinson, USA). From each
sample 10,000 events were collected and fluorescent signal
intensity was recorded and analyzed by CellQuest and Modifit
(Becton-Dickinson).
Flow cytometry analysis of mitochondrial
membrane potential
Following similar procedure as mentioned in cell
cycle analysis, the HepG2 cells were treated with the Mannich base
(50 and 100 μM) or its copper complex (6 and 12 μM) for 24 h, the
cells were washed with PBS twice after removing the cell medium and
digested with trypsin. Treated cells were collected and resuspended
at a concentration of 1×105/ml in PBS containing 1
μmol/l rhodamine 123 and then incubated at 37°C for 30 min for
direct use in flow cytometry. Samples were analyzed by FACSCalibur
flow cytometer (Becton-Dickinson) with an excitation wavelength of
488 nm and an emission wavelength of 525 nm. Data collection and
analysis were as described above.
DNA Top activity assay
The nuclear extract from HepG2 cells was prepared as
described (13). Briefly, cells
were detached using trypsin followed by centrifugation and washing
twice with cold nucleus buffer (NB) (150 mM sodium chloride, 1 mM
potassium dihydrogen phosphate, 5 mM magnesium chloride, 1 mM EDTA,
2 mM dithiotreitol and 1 mM PMSF, pH 6.4) at 4°C. The supernatant
was discarded and the cell pellet was re-suspended in 300 μl NB
with 0.3% Triton X-100 and placed on ice for 10 min, then
transferred into a glass Dounce homogenizer with ten up-and-down
strokes using a loose-fitting pestle. The suspension solution was
centrifuged at 150 × g for 10 min at 4°C, the pellet washed with
Triton X-l00-free cold NB. Cold NB solution (150 μl) containing
0.35 M NaCl was added to the pellet and allowed to stand 30 min at
4°C in order to extract the nuclear proteins. The supernatant was
obtained by centrifugation at 10,000 × g for 10 min at 4°C. The
protein concentration was determined using the Bradford method.
Nuclear extract (0.4 μg) was added to the Top reaction mixture
containing 10 mM Tris-HCl (pH 7.5), 1 mM EDTA, 150 mM NaCl, 0.1%
BSA (bovine serum albumin), 0.1 mM spermidine, 5% glycerol and 0.4
μg pUC18 and 3 μl (or 2, or 1 μl) test compound (1 or 0.3 mM) at a
final volume of 10 μl. Following incubation at 37°C for 30 min, the
reaction was terminated by adding 5 μl of stopping buffer (10% SDS,
0.025% bromophenol blue and 5% glycerol). The reaction products
were analyzed by electrophoresis on 1% agarose gel using a TBE
buffer with 0.1% SDS (89 mM Tris-HCl, 89 mM boric acid and 62 mM
EDTA) at 45 V for 3 h, stained by ethidium bromide (0.5 μg/ml) and
photographed using a short wavelength UV lamp on Tocan 360 gel
scanner (Shanghai Tiancheng Technology Inc.). The assay was
conducted in duplicate.
Molecular docking studies
The structure of human type I DNA Top with DNA and
Topotecan (1K4T) and human type II Top (3QX3) were obtained from
RCSB Protein Data Bank (20). The
Mannich base was generated from Chemdraw (Chemdraw Ultra 8.0,
CambridgeSoft, USA). Similarly structure of its copper complex was
proposed based on copper (II) ion coordination geometry in general.
The energy minimization was conducted by Chem3D (Ultra 8.0,
CambridgeSoft) (21). The
resulting models were displayed in PyMol (The PyMOL Molecular
Graphics System, Version 1.4.1, Schrödinger, LLC, USA).
Molecular docking studies were performed by AutoDock
Vina and AutoDock Tools based on the recommended procedure
(22). Grid boxes were set to the
center of ciprofloxacin (or topotecan) model, and the grid box size
for Mannich base models was set to 22, 24, and 28 for x-, y- and
z-axis, respectively. The Mannich base was set as a flexible ligand
by using the default parameters of the AutoDock Tools. The optimal
conformation of the ligand was generated by AutoDock Vina.
Results
Antitumor activity
It has been demonstrated that ciprofloxacin and
kojic acid exhibit their cytotoxicity against some tumor cell
lines. The Mannich base containing ciprofloxacin structural unit
might have similar property. To determine their potential antitumor
activity, the proliferation inhibition of the Mannich base against
HepG2 and HCT-116 cell lines was investigated by MTT assay
(Fig. 2). For both cell lines, the
Mannich base was shown to be more toxic than ciprofloxacin, with
IC50 = 103.3±5.1 μM for HepG2, and 87.9±8.0 μM for
HCT-116, respectively (Fig. 2).
Due to copper chelator ability of kojic acid, the copper complex of
the Mannich base was prepared and evaluated. It is notable that the
copper complex exhibited excellent biological activities and
selectivity, indicating there is a synergistic effect when the
copper ion was chelated by the Mannich base. The IC50 of
the copper complex was 11.5±3.0 μM for HepG2 cell, a ~10-fold
decrease in IC50 compared to that of the Mannich base.
Similar trend against HCT-116 cell line was also observed, except
the 2-fold dropped IC50 (87 vs 44 μM in
IC50). The copper complex seemed to exhibit to some
extent selectivity for HepG2 cell.
The regulation of the Mannich base and
its copper complex on apoptotic gene expression
The Mannich base and its copper complex showed good
antitumor activities, it was necessary to determine their action
mechanism. Ciprofloxacin induces apoptosis via regulation of
pro-apoptotic Bax, anti-apoptotic Bcl-2 genes to exert its
cytotoxicity, so RT-PCR was employed to evaluate the gene
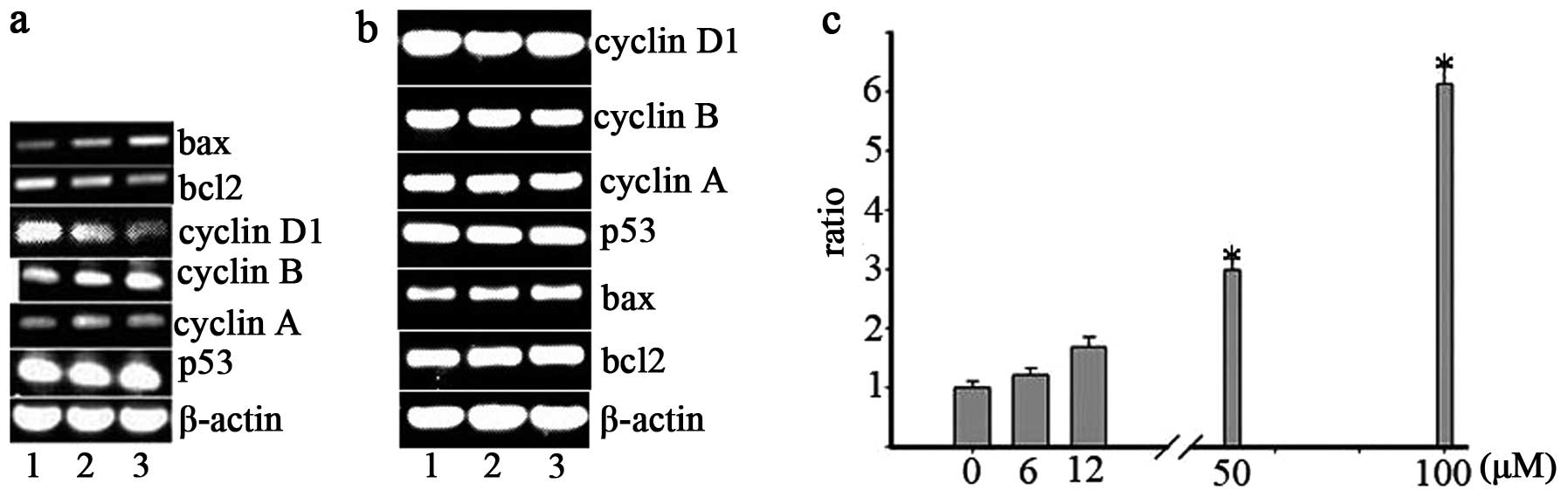
regulation of the Mannich base and its copper complex. As shown in
Fig. 3a, Bax increased
dose-dependently after 24 h of incubation of the Mannich base,
while Bcl-2 was decreased. For easy comparison, the ratio of
Bax/Bcl-2 was set at 1.0 in untreated cells, and the changes are
shown in Fig. 3c (narrow bar). It
was clear that the Bax/Bcl-2 ratio in the Mannich base treated
cells was significantly increased in a concentration-dependent
manner, indicating that the cytotoxicity of the investigated
compounds might be involved in apoptosis. The cell cycle factor was
also checked, it is of interest that the cyclin D1 was
downregulated, which was correlated to G1 arrest. However, other
cell cycle factors and p53 were not clearly changed (Fig. 3a). Similar procedure was applied on
the gene regulation of the copper complex (Fig. 3b), the investigated gene expression
was not significantly changed except the Bax slight increased,
showing that a different way occurred compared to the Mannich
base.
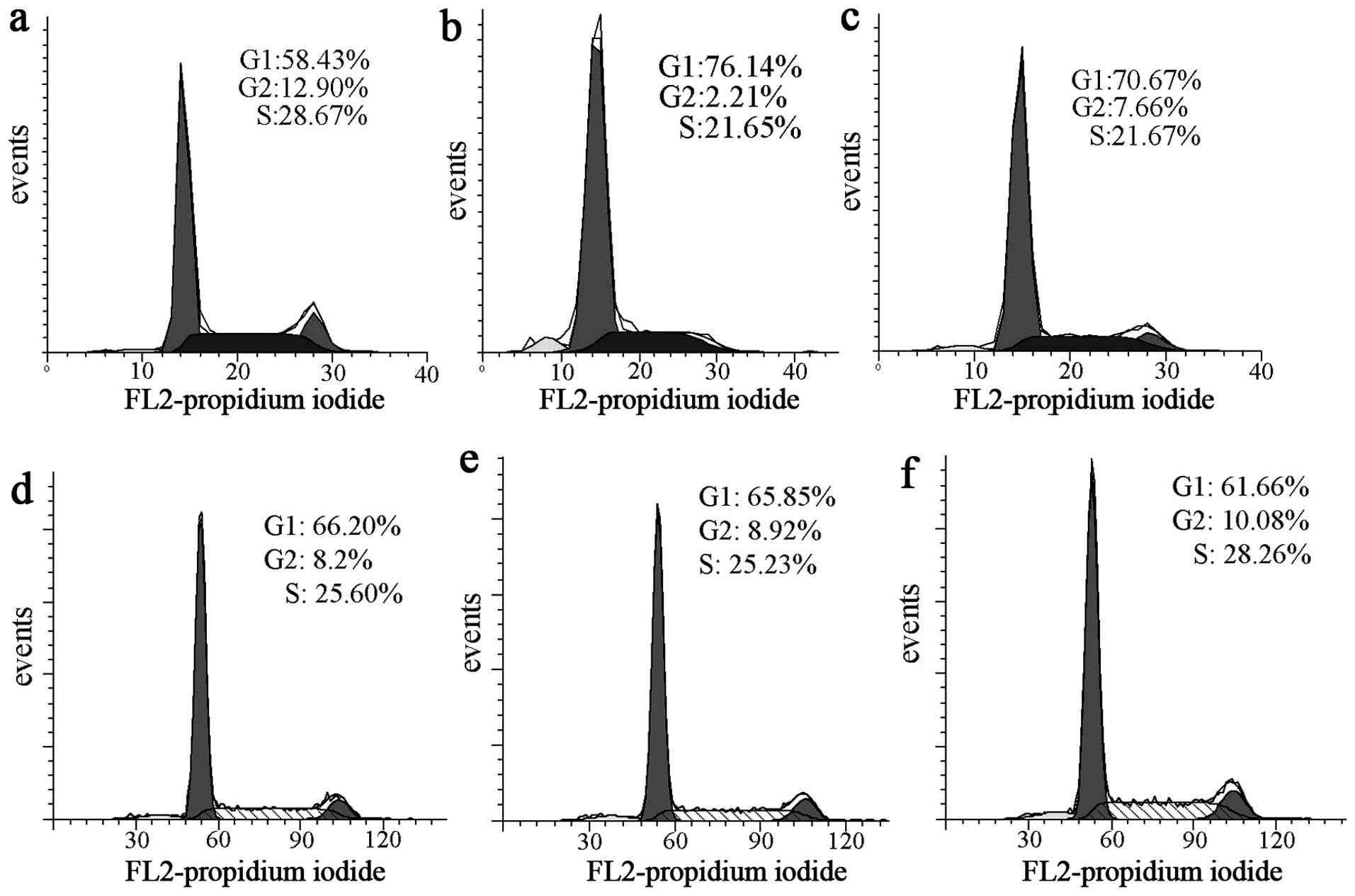
Cell cycle arrest
The proliferation inhibition may stem from
disturbing the cell cycle, to test the hypothesis, cell cycle
analyses were conducted after 24-h exposure of the drugs to HepG2
cells under IC50. As shown in Fig. 4a–c, the Mannich base caused an
accumulation of cells in the G1-phase. The percentage of cells at
the G1-phase significantly increased from 58.43 to 76.14 and 70.67%
after treatment with 50 and 100 μM Mannich base, respectively. The
result indicated that the anti-proliferative effect of the Mannich
base could be partly derived from inducing cell cycle arrest. When
the cells were treated with the copper complex, the G1 arrest was
not observed (Fig. 4d–f),
indicating that the cytotoxicity of the copper complex was not due
to the disturbance of cell cycle.
Mitochondrial membrane potential
Ciprofloxacin can depolarize the mitochondrial
membrane and inhibit mtDNA synthesis, so it was necessary to
determine whether the Mannich base and its copper complex has
similar function. The depolarization of mitochondrial membrane was
evaluated via rhodamine 123 staining on BD flow cytometry. The
results are shown in Fig. 5.
Compared to control, the distribution of cells with different
fluorescence was significantly altered both for the Mannich base
and its copper complex treated groups. Almost all the cells
(87–95%) were in M2 gate after the drugs treatments, a ~38–46%
increase could be seen (Fig. 5).
These observations indicated that both the Mannich base and its
copper complex could depolarize the mitochondrial membrane,
decreasing its electrochemical potential.
Inhibition of DNA human Top
Ciprofloxacin as a type II DNA Top inhibitor is well
documented. To determine whether the Mannich base and its copper
complex recapitulate such activity, plasmid DNA was incubated with
nucleic extract in the presence of a varied concentration of the
investigated compounds, and reaction products were analyzed by gel
electrophoresis. As shown in Fig.
6a, the Mannich base exhibited inhibition of human Top II (at
300 μM), but the Top I inhibition was not observed. The copper
complex exhibited dual Top inhibition both for type I and II at
much lower concentration (Fig.
6b). To further reveal the action mode of the copper complex in
Top inhibition, the reaction mixtures were subjected to
electrophoresis on EtB containing agarose gel. As shown in the gel
(Fig. 6c), the topology was
dominated by the intercalative dye, all closed circular forms of
DNA would be positively supercoiled and migrate with similar rate.
The Top II cleavage complex could be identified by comparison with
the positive control of etoposide. From Fig. 6c, the Top II-DNA cleavage complex
was seen and the amount of cleavage DNA was increased with
increasing of the concentration of the copper complex, indicating
that the potent cytotoxicity of the copper complex may contribute
to stabilize the Top II-DNA cleavage complex.
 | Figure 6The topoisomerase inhibition of
Mannich base and its copper complex. I, supercoiled; II, cleaved
DNA; and III, relaxed DNA. (a) 1 and 6, pUC18 only; 2, pUC18 +
nucleic extract; 3–5, pUC18 + nucleic extract in the presence of
200, 400 and 600 μM Mannich base without ATP; 7, pUC18 + nucleic
extract with ATP; 8–10, pUC18 + nucleic extract in the presence of
200, 400 and 600 μM Mannich base with ATP. (b) 1 and 6, pUC18 only;
2–4, pUC18 + nucleic extract in the presence of 30, 60 and 90 μM
Mannich base copper complex without ATP; 7, pUC18 + nucleic extract
with ATP; 8–10, pUC18 + nucleic extract in the presence of 30, 60
and 90 μM Mannich base copper complex with ATP; 11, pUC18 + nucleic
extract in the presence of 300 μM ciprofloxacin. (c) 1, pUC18; 2,
pUC18 + nucleic extract; 3–5, pUC18 + nucleic extract in the
presence of 30, 60 and 90 μM Mannich base copper complex with ATP;
6, pUC18 + nucleic extract + 3 mM etoposide; arrow, potent
DNA-topoisomerase complex. |
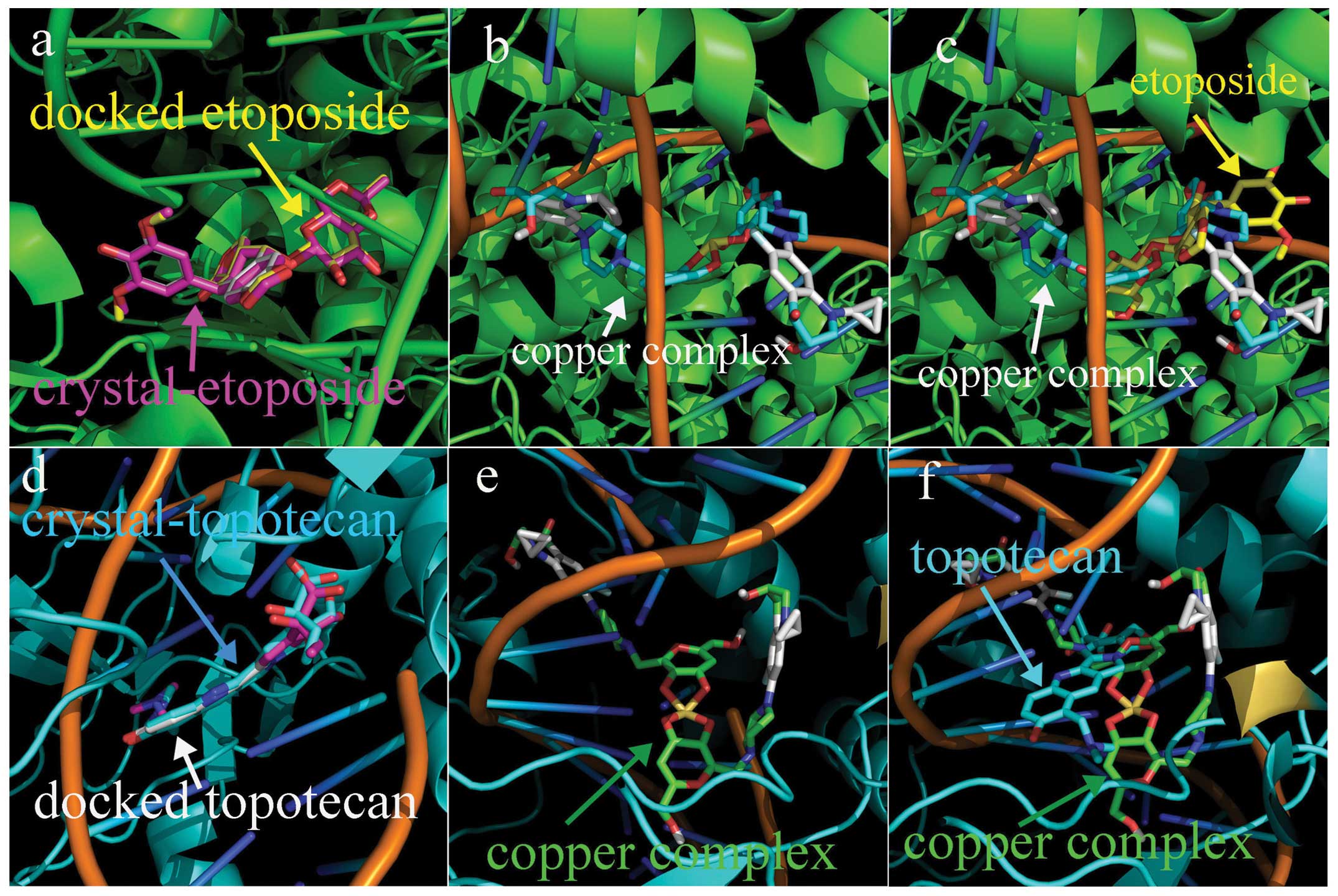
Molecular docking analysis
Top inhibition exerted by the copper complex in
vitro made us look for potent structural basis, the molecular
docking was conducted. The human type I and II Top crystal
structures were from RCSB Protein Data Bank. In order to evaluate
the accuracy of our docking protocol, etoposide and topotecan were
re-docked into each Top-DNA complex based on their crystal
structures. The human type I (PDB ID:1K4T) and II Top (PDB ID:3QX3)
were chosen as receptors, and the copper complex was used as
ligand. The input pbdqt files were generated by Autodocktool and
the optimal conformations of ligands were calculated on AutoDock
Vina. The Top-inhibitor complex derived from docking simulation
showed that the docked etoposide and topotecan were almost fully
superimposed on the native co-crystallized ones (Fig. 7a and d). Following the same
protocol the copper complex was individually docked into the Top I
and II, the simulating affinity energy was −11.5 and −10.9
kcal/mol, respectively. Compared to that of redocked etoposide,
−14.6 and topotecan, −11.2 kcal/mol, the affinity energy of the
copper complex with the Top was quite close to the clinically used
drugs. The orientation of the copper complex in the Top I and II is
shown in Fig. 7b and e,
respectively. In addition, the superimposition of the copper
complex on the co-crystallized etoposide and topotecan are
presented in Fig. 7c and f.
Discussion
Ciprofloxacin has a certain degree of antitumor
activity (23), so does the
ciprofloxacin containing Mannich base. The proliferation inhibition
of the Mannich base clearly showed that the structural modification
on 7-piperdinyl ciprofloxacin with kojic acid unit leads to
enhancement in antitumor activity. However, its antimicrobial
activities was decreased from that reported recently (16). It should be realized that
ciprofloxacin and kojic acid both have potential chelating ability,
and the derivatives of kojic acid have been synthesized as a copper
chelator, corresponding metal complexes of the derivatives have
also been investigated (24,25).
The excellent proliferation inhibition of the copper complex
encouraged us to probe the potent molecular mechanism. A previous
study showed that the copper chloride has no detectable
cytotoxicity at 50 μM (data not shown), so the enhancement of the
copper complex of the Mannich base in proliferation inhibition
could not be simply explained as synergistic effect because
synchronous exposure of the copper chloride and the Mannich base to
the investigated cell lines did not induce higher cytotoxicity
(data not shown). However, after adjusting the pH of Mannich base
with base, a copper chloride addition leads to a suspension with
color change, indicating the copper complex of the Mannich base was
formed (26). Thus additional
studies are required to probe potent molecular mechanism, which may
provide some meaningful information to explain the phenomenon. It
has been well demonstrated that many chemotherapeutic agents exert
their cytotoxicity via apoptotic path. Bax and Bcl-2 are
apoptosis-related genes, the Bax upregulation and Bcl-2
downregulation are indicative of apoptosis involved. In our study
the RT-PCR data clearly show that the Bax/Bcl-2 ratio in the
Mannich base treated HepG2 cells was significantly increased,
indicating that the Mannich base induced apoptosis via the same
molecular path as ciprofloxacin. However, this situation for its
copper complex was not observed, indicating the cytotoxicity of the
copper complex might not be involved in apoptotic pathway.
It has been demonstrated that quinolone derivatives
induce cell death through causing double-stranded DNA breakage,
inhibition of Top function (27,28),
so the ciprofloxacin containing Mannich base in this study might
share a similar action mechanism. To test the hypothesis Top
inhibition assay was carried out. Unsurprisingly, type II
topoisomerase inhibition by the Mannich base was observed at higher
concentration, but the type I Top inhibition was not evident.
Beyond our expectations, the copper complex exhibited dual Top
inhibition, this situation has been only found in a few metal
complexes (29–32), so we speculated that the excellent
antitumor activity may be closely related to its dual Top
inhibition. However, due to lack of evidence from crystal
structure, how this kind of metal complex interacts with Top, via
competitive ATP binding or poisoning DNA complex or allosteric
effect to disturb unwinding DNA helix are largely unknown. In our
experiment, the DNA-Top complex poisoning function of the copper
complex could be seen, stabilizing the intermediate and causing
more DNA cleavage in a concentration-dependent manner. As mentioned
above due to lack of detailed structural information, in
silico study to simulate the potent interaction between the
copper complex and the DNA-Top complex may give some helpful
information. Molecular docking is a well established computational
technique to predict the interaction between enzyme and inhibitor
(33,34). In addition, AutoDock software is
widely used to predict the binding information of test compounds
(35,36). In general, divalence copper ion
(II) can be chelated by many compounds contained heteroatom, such
as kojic acid, forming square planar molecules in geometry
(37). So 1:2 molar ratio of
copper ion to the Mannich base may be speculated, and a square
planar geometry for the copper complex was tentatively proposed for
the docking study. In addition, in this case, the copper complex
may have non-electrolyte property and thus can easily cross the
cellular membrane. It is interesting that the docked copper complex
has affinity energy with the Top I or II quite close to that of
etoposide or topotecan used in clinic, indicating the copper
complex might have similar manner as poison to stabilize the
cleavage DNA-Top complex and cause DNA fragmentation. The data from
molecular docking was supportive of the Top inhibition experiment
in vitro and corelated with assessment of the cytotoxicity
of the complex.
Top IIα inhibition or poisoning leads G2/M-phase
arrest in general (38,39), to look for further support, the
cell cycle was assessed in the presence of the investigated
compounds. Surprisingly, both the Mannich base and its copper
complex did not lead to G2/M-phase arrest. It unambiguously
demonstrated that the Mannich base caused a G1-phase arrest. Cyclin
D1 plays an important role in regulating cell cycle progression and
cyclin D1 degradation is sufficient to induce G1 cell cycle arrest
(38). The cyclin D1
downregulation at mRNA level in the treatment of Mannich base might
be also correlated with the G1 arrest. This situation was also
found in Top inhibitors, for the cell cycle arrest dose-dependently
in some cell lines (40,41). Unlike the Mannich base, its copper
complex was not able to disturb the cell cycle, indicating that the
mechanisms between them were different.
Mitochondria are producing energy plants, and the
ATP was produced by utilizing the proton electrochemical gradient
potential between inner and outer mitochondrial membrane. The
depolarization of mitochondrial membrane will result in decreases
of the potential and proton gradient, consequently leading to less
efficient ATP production (42).
Our observations also support that both the Mannich base and its
copper complex have functions of depolarization of mitochondrial
membrane compared to the untreated group from the fluorescence
intensity of mitochondria stained by rhodamine 123 (Fig. 6), which cause ATP shortage,
consequently slowing down cell growth. So it is reasonable to
speculate that the disturbance of mitochondrial respiration and ATP
synthesis may contribute to the cytotoxic effect of the Mannich or
its copper complex (43).
In conclusion, the Mannich base derived from
ciprofloxacin is more toxic than its parent compound in antitumor
activity. Its copper complex showed excellent antitumor activity.
Mechanistic studies revealed preliminarily that the Mannich base
exerted cytotoxicity via regulation of a pro-apoptotic gene,
causing cell cycle arrest, disturbing ATP production and inhibiting
Top II. Unlike the Mannich base, the cytotoxicity of its copper
complex mainly stem from its ability to stabilize the intermediate
of cleavage DNA-Top complex as well as disturbance of the
mitochondrial respiration chain. However, detailed mechanism of the
copper complex, especially in Top inhibition in vivo is
largely unknown and require more study in the future.
Acknowledgements
This study was supported by grants CP1204 from
Xinxiang Scientific and Technology Division; ZD2011-06 from
Xinxiang Medical University and 2109901 from plan of Health
Scientific and Technological Innovation Talents of Henan Province
for Shaoshan Li.
References
|
1
|
Bentley R: From miso, sake and shoyu to
cosmetics: a century of science for kojic acid. Nat Prod Rep.
23:1046–1062. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Noh JM, Kwak SY, Kim DH, et al: Kojic
acid-tripeptide amide as a new tyrosinase inhibitor. Biopolymers.
88:300–307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aytemir MD and Özçelik B: A study of
cytotoxicity of novel chlorokojic acid derivatives with their
antimicrobial and antiviral activities. Eur J Med Chem.
45:4089–4095. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xiong X and Pirrung MC: Modular synthesis
of candidate indole-based insulin mimics by Claisen rearrangement.
Org Lett. 10:1151–1154. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kasser JH, Kandioller W, Hartinger CG, et
al: Mannich products of kojic acid and N-heterocycles and their
Ru(II) - arene complexes: synthesis, characterization and
stability. J Organomet Chem. 695:875–881. 2010. View Article : Google Scholar
|
|
6
|
Toy AD and Smith TD: Electron spin
resonance evidence for the existence of a dimeric form of the
copper (II) chelate of kojic acid. J Am Chem Soc. 93:3049–3050.
1971. View Article : Google Scholar
|
|
7
|
Yoo JY, Pradarelli J, Haseley A, et al:
Copper chelation enhances antitumor efficacy and systemic delivery
of oncolytic HSV. Clin Cancer Res. 18:4931–4941. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen YH, Lu PJ, Hulme C, et al: Synthesis
of kojic acid-derived copper-chelating apoptosis inducing agents.
Med Chem Res. 22:995–1003. 2013. View Article : Google Scholar
|
|
9
|
Fickova M, Pravodova E, Rondhal L, et al:
In vitro antiproliferative and cytotoxic activities of novel kojic
acid derivatives: 5-benzyloxy-2-selenocyanatomethyl- and
5-methoxy-2-selenocyanatomethyl-4-pyranone. J Appl Toxicol.
28:554–559. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoo DS, Lee J, Choi SS, et al: A
modulatory effect of novel kojic acid derivatives on cancer cell
proliferation and macrophage activation. Pharmazie. 65:261–266.
2010.PubMed/NCBI
|
|
11
|
Hawtin RE, Stockett DE, Byl JAW, et al:
Voreloxin is an anticancer quinolone derivative that intercalates
DNA and poisons topoisomerase II. PLoS One. 5:e101862010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Seo KW, Holt R, Jung YS, et al:
Fluoroquinolone-mediated inhibition of cell growth, S-G2/M cell
cycle arrest, and apoptosis in canine osteosarcoma cell lines. PLoS
One. 7:e429602012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fu Y, Zhang Y, Zhou SF, et al: The effects
of substitution of carboxyl with hydrazide group on position 3 of
ciprofloxacin on its antimicrobial and antitumor activity. Int J
Pharmacol. 9:416–429. 2013. View Article : Google Scholar
|
|
14
|
Ahmed A and Daneshtalab M: Nonclassical
biological activities of quinolone derivatives. J Pharm Pharmaceut
Sci. 15:52–72. 2012.PubMed/NCBI
|
|
15
|
Yadav P and Joshi YC: Syntheses and
spectral studies of novel ciprofloxacin derivatives. Bull Chem Soc
Ethiop. 22:459–464. 2008.
|
|
16
|
Emami S, Ghafouri E, Faramarzi MA, et al:
Mannich bases of 7-piperazinylquinolones and kojic acid
derivatives: synthesis, in vitro antibacterial activity and in
silico study. Eur J Med Chem. 68:185–191. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jubie S, Sikdar P, Kalirajan R, et al:
Synthesis and antimicrobial activity of some novel ciprofloxacin
analogues. J Pharm Res. 3:511–513. 2010.
|
|
18
|
Alipour E, Mohammadhosseini N, Panah F, et
al: Synthesis and in vitro cytotoxic activity of
N-2-(2-Furyl)-2-(chlorobenzyloxyimino) ethyl ciprofloxacin
derivatives. E-J Chem. 8:1226–1231. 2011. View Article : Google Scholar
|
|
19
|
Cormiera R, Burdab WN, Harringtonb L, et
al: Studies on the antimicrobial properties of N-acylated
ciprofloxacins. Bioorg Med Chem Lett. 22:6513–6520. 2012.
View Article : Google Scholar
|
|
20
|
Bax BD, Chan PF, Eggleston DS, et al: Type
IIa topoisomerase inhibition by a new class of antibacterial
agents. Nature. 466:935–940. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ok K, Jung YW, Jee JG, et al: Facile
docking and scoring studies of carborane ligands with estrogen
receptor. Bull Korean Chem Soc. 34:1051–1054. 2013. View Article : Google Scholar
|
|
22
|
Trott O and Olson AJ: AutoDock Vina:
improving the speed and accuracy of docking with a new scoring
function, efficient optimization and multithreading. J Comput Chem.
31:455–461. 2010.PubMed/NCBI
|
|
23
|
Bourikas LA, Kolios G, Valatas V, et al:
Ciprofloxacin decreases survival in HT-29 cells via the induction
of TGF-β1 secretion and enhances the anti-proliferative effect of
5-fluorouracil. Br J Pharmacol. 157:362–370. 2009.PubMed/NCBI
|
|
24
|
Kwak SY, Choi HR, Park KC, et al: Kojic
acid-amino acid amide metal complexes and their melanogenesis
inhibitory activities. J Pept Sci. 17:791–797. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kwak SY, Noh JM, Park SH, et al: Enhanced
cell permeability of kojic acid-phenylalanine amide with metal
complex. Bioorg Med Chem Lett. 20:738–741. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kenyon GD, Chen Di, Shirley O, et al:
Clioquinol and pyrrolidine dithiocarbamate complex with copper to
form proteasome inhibitors and apoptosis inducers in human breast
cancer cells. Breast Cancer Res. 7:R897–R908. 2005. View Article : Google Scholar
|
|
27
|
Drlica K, Malik M, Kerns RJ and Zhao X:
Quinolone-mediated bacterial death. Antimicrob Agents Chemother.
52:385–392. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun JP, Shi ZY, Liu SM, et al:
Trimethoxy-benzaldehyde levofloxacin hydrazone inducing the growth
arrest and apoptosis of human hepatocarcinoma cells. Cancer Cell
Int. 13:672013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ahmad M, Afzal M, Tabassum S, et al:
Synthesis and structure elucidation of a cobalt(II) complex as
topoisomerase I inhibitor: in vitro DNA binding, nuclease and RBC
hemolysis. Eur J Med Chem. 74:683–693. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kou JF, Qian C, Wang JQ, et al: Chiral
ruthenium(II) anthraquinone complexes as dual inhibitors of
topoisomerases I and II. J Biol Inorg Chem. 17:81–96. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Patel MN, Bhatt BS and Dosi PA:
Topoisomerase inhibition, nucleolytic and electrolytic contribution
on DNA binding activity exerted by biological active analogue of
coordination compounds. Appl Biochem Biotechnol. 166:1949–1968.
2012. View Article : Google Scholar
|
|
32
|
Castelli S, Vassallo O, Katkar P, et al:
Inhibition of human DNA topoisomerase IB by a cyclometalated gold
III compound: analysis on the different steps of the enzyme
catalytic cycle. Arch Biochem Biophys. 516:108–112. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang B: MetaPocket: a meta approach to
improve protein ligand binding site prediction. Omics. 13:325–330.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fukunishi Y and Nakamura H: Prediction of
ligand-binding sites of proteins by molecular docking calculation
for a random ligand library. Protein Sci. 20:95–106. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nikaido H and Pagès JM: Broad specificity
efflux pumps and their role in multidrug resistance of Gram
negative bacteria. FE MS Microbiol Rev. 36:340–363. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Takatsuka Y, Chen C and Nikaido H:
Mechanism of recognition of compounds of diverse structures by the
multidrug efflux pump AcrB of Escherichia coli. Proc Natl
Acad Sci USA. 107:6559–6565. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Singh S, Singh J, Gulia S, et al: Metal
ion selectivity of kojate complexes: a theoretical study. J Theor
Chem. 2013:3427832013. View Article : Google Scholar
|
|
38
|
Guo L, Liu XJ, Nishikawa K, et al:
Inhibition of topoisomerase IIA and G2 cell cycle arrest by NK314,
a novel benzo[c] phenanthridine currently in clinical trials. Mol
Cancer Ther. 6:1501–1508. 2007.PubMed/NCBI
|
|
39
|
Masamha CP and Benbrook DM: Cyclin D1
degradation is sufficient to induce G1 cell cycle arrest despite
constitutive expression of cyclin E2 in ovarian cancer cells.
Cancer Res. 69:6565–6572. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li TM, Chen GW, Su CC, et al: Ellagic acid
induced p53/p21 expression, G1 arrest and apoptosis in human
bladder cancer T24 cells. Anticancer Res. 25:971–980.
2005.PubMed/NCBI
|
|
41
|
Stravopodis DJ, Karkoulis PK, Konstantakou
EG, et al: Grade-dependent effects on cell cycle progression and
apoptosis in response to doxorubicin in human bladder cancer cell
lines. Int J Oncol. 34:137–160. 2009.PubMed/NCBI
|
|
42
|
Perry SW, Norman JP, Barbieri J, et al:
Mitochondrial membrane potential probes and the proton gradient: a
practical usage guide. Biotechniques. 50:98–115. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rawi SM, Mourad IM, Arafa NMS, et al:
Effect of ciprofloxacin and levofloxacin on some oxidative stress
parameters in brain regions of male albino rats. Afr J Pharm
Pharmacol. 5:1888–1897. 2011.
|