Introduction
Breast cancer is the most frequently diagnosed
cancer and is therefore the leading cause of cancer death in women
worldwide (1). While marked
progress has been made in the treatment of breast cancer, such as
hormonal therapy for estrogen receptor and/or progesterone
receptor-positive tumors, other targeted therapies for selected
subgroups of patients, such as HER2-positive cancers, have also
been successfully developed. Despite these advances, the mortality
rate is still high in breast cancer patients, mainly due to
metastasis spread (2); therefore
the development of metastasis-targeted therapy for breast cancer is
clinically important.
Phytochemicals from natural products are a promising
source for the development of novel cancer therapeutics. Because of
their potential effectiveness and low toxicity profiles (3), many phytochemicals have been
successful in clinical development of many diseases (4,5).
Nuclear factor-κB (NF-κB), defined as a multi-functional
transcription factor, is widely involved in a variety of
physiological and pathological processes (6). It has been widely recognized that
NF-κB plays a critical role in the initiation, promotion and
progression of certain types of cancers through its ability to
upregulate genes responsible for cell survival, invasion,
angiogenesis and metastasis (7–12).
Consequently, the NF-κB pathway is regarded as a potential new drug
target in cancer metastasis and progression (13–15).
In this study, we screened 56 phytochemical
compounds for their inhibitory activity in NF-κB. Hirsutine was
found to be a prominent NF-κB inhibitor and significantly inhibited
the metastatic potential of murine 4T1 breast cancer cells both
in vitro and in vivo. Since Hirsutine further reduced
the metastatic potential of human breast cancer cells, it is an
attractive lead compound targeting metastasis and the progression
of breast cancer.
Materials and methods
Reagents
All the compounds (in DMSO) shown in Table I were provided by the Cooperative
Research Project I of the Institute of Natural Medicine, University
of Toyama, Japan. Antibody against MMP-9 or β-actin was obtained
from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Mouse TNF-α
was purchased from Jena Bioscience GmbH (Jena, Germany). Hirsutine
was purchased from Wako Pure Chemical Industries, Ltd. (Osaka,
Japan). pGL4.50 [luc2P/CMV-RE/Hygro] and pGL4.32
[luc2P/NF-κB-RE/Hygro] vector and D-Luciferin were obtained from
Promega (Sunnyvale, CA, USA). Lipofectamine 2000 was purchased from
Invitrogen (Carlsbad, CA, USA). Hygromycin B was obtained from
Nacalai Tesque (Kyoto, Japan).
 | Table INatural compounds. |
Table I
Natural compounds.
| No. | Compound |
|---|
| 1 | Galangin |
| 2 |
Galangin-7-glucoside |
| 3 |
6-Hydroxygalangin-7-glucoside |
| 4 | Scutellarin |
| 5 | Resveratrol |
| 6 | Artemisine |
| 7 | Acoinitine |
| 8 | Albiflorin |
| 9 | Alisol A |
| 10 | Alisol B |
| 11 | Alkannin |
| 12 | Amygdalin |
| 13 | Arbutin |
| 14 | Astragaloside
IV |
| 15 | Atractylenolide
III |
| 16 | Aucubin |
| 17 | Baicalein |
| 18 | Baicalin |
| 19 | Barbaloin |
| 20 | Bergenin |
| 21 | Catalpol |
| 22 | E-Cinnamic
acid |
| 23 | Cinobufagin |
| 24 | Cinobufotalin |
| 25 | Corydaline |
| 26 | Curcumin |
| 27 |
Dehydrocostuslactone |
| 28 |
Dimethylesculetin |
| 29 |
Eleutheroside-B |
| 30 | Epihesperidin |
| 31 | Ergosterol |
| 32 | β-Eudesmol |
| 33 | E-Ferulic acid |
| 34 | Geniposide |
| 35 | Geniposidic
acid |
| 36 |
Gentiopicroside |
| 37 | Ginsenoside Rc |
| 38 | Ginsenoside Rd |
| 39 | Glabridin |
| 40 | Glycyrrhizic
acid |
| 41 | Hirsutine |
| 42 | Icariin |
| 43 | Isofraxidine |
| 44 | Ligustilide |
| 45 | Loganin |
| 46 | Magnolol |
| 47 | Mesaconitine |
| 48 | Naringin |
| 49 | Paeonol |
| 50 | Palmatine
chloride |
| 51 |
S-Perilladelhyde |
| 52 | Puerarin |
| 53 |
Rhynchophylline |
| 54 | Sinomenine |
| 55 | Swertiamarin |
| 56 | Wogonin |
Cells
Mouse mammary carcinoma 4T1 cells were maintained in
RPMI-1640 medium (Nissui, Tokyo, Japan) supplemented with 10%
bovine serum. MDA-MB-231 and MDA-MB-468 human breast cancer cell
lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM;
Nissui) supplemented with 10% bovine serum. To establish the
luciferase gene expressing 4T1 cells (4T1-luc or 4T1-NFκB-luc), and
4T1 cells (5×105/well) were seeded in a 6-well plate and
pGL4.50 or pGL4.32 vector was transfected using Lipofectamine 2000.
The cells were selected with Hygromycin B (100 μg/ml) and cloned by
limiting dilution.
NF-κB luciferase reporter assay
For the detection of luciferase activity, cells were
stimulated with or without TNF-α (10 ng/ml) in the screening
experiments. Briefly, for screening experiments without
stimulation, 4T1-NFκB-luc cells in exponential growth were placed
at a final concentration of 2×104 cells/well in a
96-well plate. After 3-h incubation, the cells were treated with
compounds or with the vehicle (vehicle control, 0.5% DMSO) for 24
h. Then 20 μl of luciferin (900 μg/ml) was added, and the plates
were incubated for another 30 min. Luciferase activity was measured
by the Glomax multi detection system (Promega). For the experiments
with TNF-α (10 ng/ml) stimulation, 4T1-NFκB-luc cells
(2×104 cells/well) were placed in a 96-well plate for 12
h. After incubation, the cells were treated with compounds and
TNF-α (10 ng/ml) for 12 h. Then luciferin was added and luciferase
activity was measured.
Cell viability assay
Viability of cells was assessed using a WST-1 Cell
Counting kit (Wako Pure Chemical Industries). The experimental
conditions for cell viability were similar to the previous
luciferase reporter assay. Twenty-four hours after treatment with
compounds, 10 μl WST-1 reagent was added and incubated for another
2 h (37°C, 5% CO2). The absorbance at 450 nm was
measured using a microplate reader.
In vitro wound healing assay
4T1-NFκB-luc cells were plated in a 24-well plate at
a concentration of (1×105 cells/well) and allowed to
form a confluent monolayer for 24 h. The monolayer was then
scratched with a sterile pipette tip (1,000 μl), washed with medium
to remove floated and detached cells, and photographed (time 0 h).
Cells were successively treated in medium in the presence of
different concentrations of Hirsutine (12.5 and 25 μM) along with
the vehicle DMSO for 24 h. Scratched areas were photographed
(magnification, ×40) at 0 h and then again 24 h later to assess the
degree of wound healing. The percentage of wound closure was
estimated by the following equation: wound closure % = 1 − (wound
area at t24/wound area at t0) × 100%, where
t24 is the time after wounding and t0 is the
time immediately after wounding.
Haptotaxis and hapto-invasion assay
The filters of a Transwell cell culture insert (8 μm
pore size; Whatman Japan KK, Tokyo, Japan) were pre-coated with
fibronectin (Iwaki, Tokyo, Japan, 1.25 μg/filter) on the lower
surfaces. For the hapto-invasion assay, the upper surface of the
filters was coated with Matrigel (Becton-Dickinson, Bedford, MA, 1
μg/filter). Cells were pre-incubated with or without Hirsutine (25
μM) for 24 h. After trypsinization, cells in 0.1% (v/v) BSA medium
(1.5×104) were placed in the upper chamber of
Transwells. After the subsequent incubation at 37°C, the residual
cells were removed from the upper surface of the membrane and the
migrated cells on the lower surface of the membrane were fixed in
100% methanol and stained with hematoxylin and eosin. Migration was
determined by counting the cells with a microscope at ×100
magnification. Five visual fields were chosen randomly and the
average number of migrating cells in the five fields was taken for
each group.
Gelatin zymography
Subconfluent monolayers of 4T1-NFκB-luc cells
(5×104/well) pretreated for 24 h with Hirsutine (12.5
and 25 μM) were cultured for another 24 h in serum-free RPMI-1640.
After incubation, cell-free supernatants were collected and mixed
with sample buffer containing 2% SDS (without 2-mercaptoethalnol)
and incubated at 37°C for 20 min. Comparative gelatin zymography
was performed on 10% SDS-PAGE with 0.1% gelatin. Samples were
electrophoresed at 10 mA for 4–5 h at 4°C. Gels were washed with
buffer containing 2.5% Triton X-100 and 0.01 M Tris-HCl for 2 h at
4°C and then washed with 0.01 M Tris-HCl for 40 min at room
temperature. Gels were incubated in buffer containing 0.05 M
Tris-HCl, 0.5 mM CaCl2 and 1 μM ZnCl2 for 48
h at 37°C. After incubation, gels were stained with Coomassie
Brilliant Blue for 6 h and destained with 5% acetic acid and 10%
methanol. The bands were scanned by an image scanner and quantified
by Image J software.
Western blot analysis
4T1-NFκB-luc cells were treated with Hirsutine for
24 h. Treated cells were collected, washed with phosphate-buffered
saline (PBS), and lysed in lysis buffer [25 mM HEPES (pH 7.7), 0.3
M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.1% Triton X-100, 20
mM b-glycerophosphate, 0.1 mM sodium orthovanadate, 0.5 mM
phenylemethylsulfonyl fluoride (PMSF), 1 mM dithiothreitol, 10
mg/ml aprotinin and 10 mg/ml leupeptin]. The cell lysates were
separated by 10% SDS-PAGE and transferred to PVDF membranes using a
glycine transfer buffer [192 mM glycine, 25 mM Tris-HCl (pH 8.8)
and 20% (v/v) methanol]. After blocking with Block Ace for 4 h at
room temperature, the membrane was incubated overnight with primary
antibodies, and then for 60 min with secondary antibodies. Primary
antibodies were used at a dilution of 1:1,000. The secondary
antibodies (horseradish peroxidase-conjugated goat anti-mouse IgG)
were used at a dilution of 1:2,000 and visualized with an enhanced
chemiluminescence system (Amersham Biosciences).
Experimental lung metastasis model
Female BALB/c mice were purchased from Japan SLC,
Inc. (Hamamatsu, Japan) and maintained in a temperature-controlled,
pathogen-free room. All animals were handled according to the
approved protocols and guidelines of the Animal Committee of Toyama
University (A2012INM-6). For the experimental metastasis model,
4T1-luc cells were inoculated intravenously (i.v.,
5×105) with or without pre-treatment with Magnolol or
Hirsutine (24 h, 25 μM). For lung metastasis imaging, mice were
injected with D-luciferin 7 days after tumor inoculation, then the
lungs were removed to measure luminescence using in vivo
imaging system (IVIS Lumina II; Caliper Life Sciences, MA,
USA).
Statistical analysis
All the data are expressed as the mean ± SD of at
least two or three independent experiments unless otherwise stated.
Statistical significance was analyzed using Student’s t-test.
P<0.05 was considered significant.
Results
Hirsutine inhibits NF-κB activation in
metastatic 4T1 breast cancer cells
In order to identify novel anti-metastatic drug
candidates targeting inflammatory signals in cancer cells, we first
screened our library of natural product-derived compounds using
murine 4T1 breast cancer cells stably expressing NF-κB luciferase
reporter (4T1-NFκB-luc cells). 4T1-NFκB-luc cells were incubated
with a series of natural product-derived compounds (Table I) for 24 h and luminescence was
measured to determine NF-κB activity. To discriminate whether the
inhibition of NF-κB activity is related to the reduction of cell
viability, all tested compounds were measured for their direct
cytotoxicity on 4T1-NFκB-luc cells in parallel with the luciferase
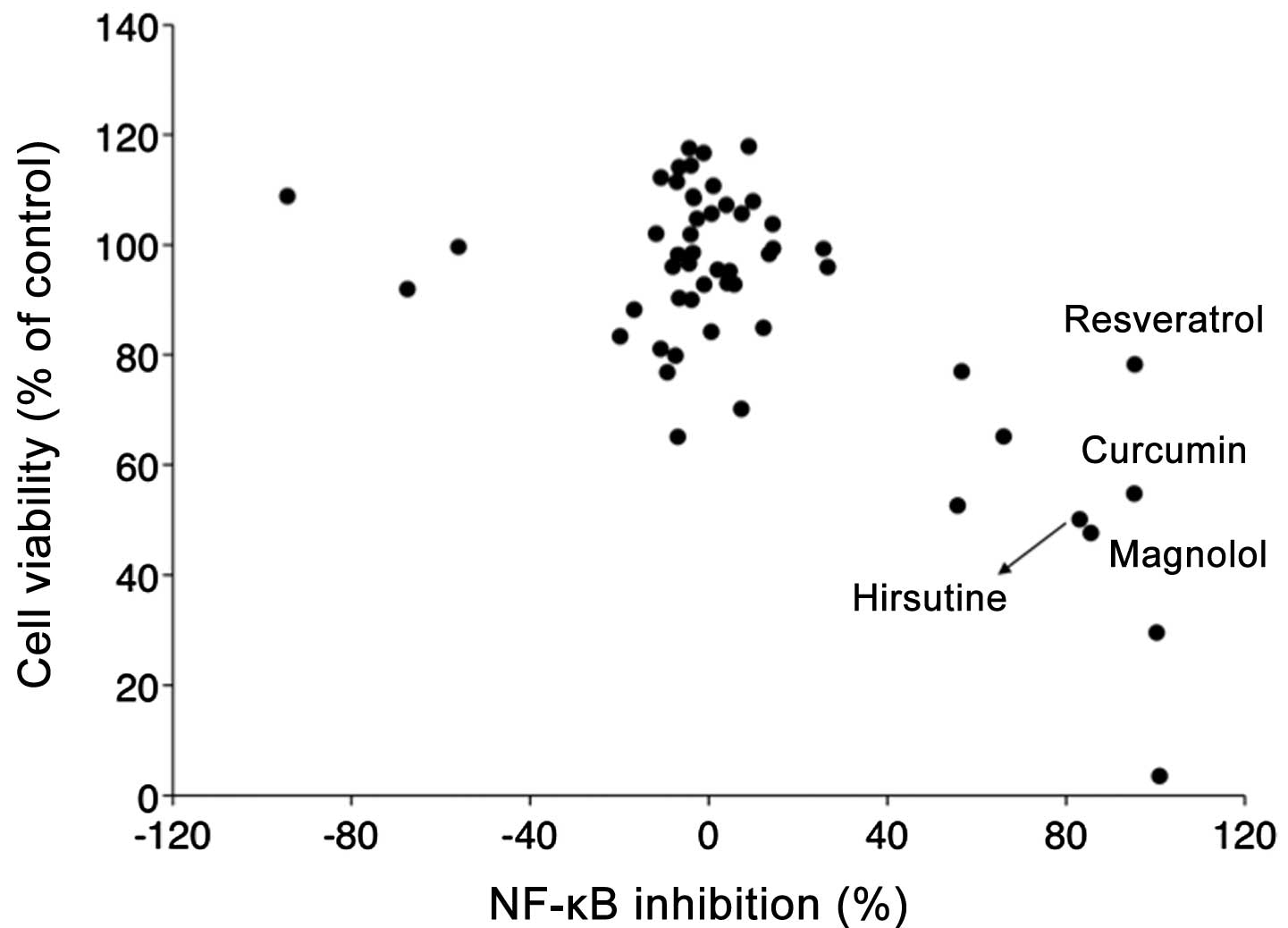
reporter assays. As summarized in Fig.
1, 4 out of 56 compounds
(resveratrol, curcumin, magnolol, Hirsutine) significantly
suppressed NF-κB activity in 4T1-NFκB-luc cells (>80%) with
relatively little effect on cell viability (<50%). While the
pharmacological effect of resveratrol (16–18),
curcumin (19,20) and magnolol (21–31),
in NF-κB inhibition has been previously recognized, there is almost
no information of Hirsutine on its effect on NF-κB inhibition in
cancer cells; therefore, we decided to further explore the
potential of Hirsutine as novel anti-metastatic drug candidate
targeting inflammatory signals in cancer cells.
To further determine the specificity of Hirsutine in
its inhibition of NF-κB activation, 4T1-NFκB-luc cells were treated
with different doses of Hirsutine to evaluate their effect on NF-κB
reporter activity and cell viability. Hirsutine specifically
inhibited NF-κB activation of 4T1 cells at a concentration that did
not largely affect cell viability (Fig. 2A and C) and further inhibited NF-κB
activation even in the presence of TNF-α stimulation in 4T1 cells
(Fig. 2B and C). Collectively, the
presented results strongly support that Hirsutine is a potent
inhibitor of NF-κB in metastatic 4T1 breast cancer cells.
Hirsutine reduces metastatic potential of
4T1 cells
We next examined the effect of Hirsutine on the
metastatic potential of 4T1 cells accordaning to the inhibition of
NF-κB. As shown in Fig. 3,
Hirsutine showed dose-dependent inhibition of the cell migration of
4T1 cells, as determined by the wound closure assay. In addition,
pre-treatment with Hirsutine inhibited 4T1 cell haptotaxis
(Fig. 4A) towards fibronectin in
the Transwell chamber assay. Furthermore, Hirsutine pre-treatment
showed significant inhibition of the invasion activity of 4T1 cells
(Fig. 4B). In order to determine
whether the inhibitory effect of Hirsutine on cellular invasion was
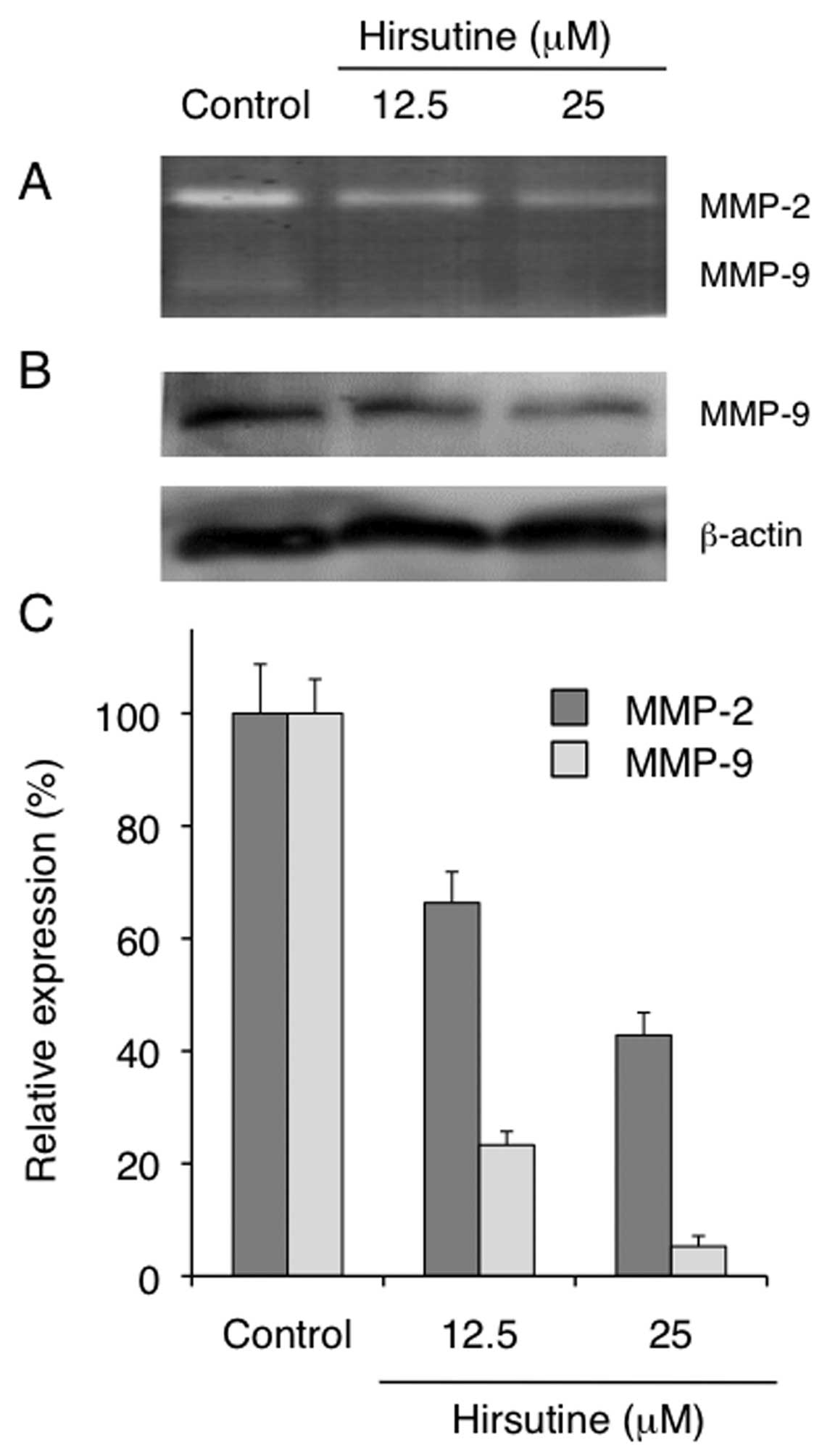
relative to the proteolytic activity of 4T1 cells, we employed the
gelatin zymography assay to examine the effect of Hirsutine
treatment on the production of MMP-2 and MMP-9 in 4T1 cells. As
shown in Fig. 5, Hirsutine-treated
4T1 cells showed a reduction in the activity of both MMP-2 and
MMP-9 (Fig. 5A and C). We further
confirmed that the cytoplasmic expression of MMP-9 was also reduced
in Hirsutine-treated 4T1 cells as evaluated by western blotting
(Fig. 5B). Collectively, these
results clearly indicate that Hirsutine reduces the metastatic
potential of 4T1 cells in vitro by regulating cellular
migration or invasion in accordance with the inhibitory activity of
NF-κB activation. We then tested the therapeutic potential of
Hirsutine in breast cancer using an in vivo animal model. In
an experimental lung metastasis model of 4T1 breast cancer, we
found that pre-treatment with Hirsutine inhibited the metastatic
lung colonization of 4T1 cells (Fig.
6).
Hirsutine reduces the migration of human
breast cancer cells in vitro
To further explore the clinical relevance of our
findings, we tested the effect of Hirsutine on human breast cancer
cell lines MDA-MB-231 and MDA-MB-468. Similar to our observation in
murine 4T1 cells, both Magnolol and Hirsutine inhibited the
haptotaxis of MDA-MB-231 or MDA-MB-468 cells (Fig. 7) at a non-cytotoxic dose (data not
shown) implying the efficacy of the compounds in human breast
cancer cells in vitro.
Discussion
Activation of NF-κB has been observed in many
cancers, including breast cancer, melanoma, lung cancer and various
types of other cancers (32–34).
It is known that NF-κB activation has not only been implicated in
carcinogenesis (35,36) but also in cancer cell invasion and
the metastatic process (37–40);
therefore, targeting the NF-κB-mediated inflammatory signaling
pathway is an attractive strategy for controlling metastasis.
Amongst 56 chemically defined phytochemicals derived from natural
products, we identified a new compound, Hirsutine, that strongly
suppressed NF-κB activity in 4T1 breast cancer cells. In accordance
with the NF-κB inhibition, Hirsutine reduced the metastatic
potential of 4T1 cells, as seen in their inhibition of the
migration and invasion of 4T1 cells. Importantly, Hirsutine showed
anti-metastasis activity against 4T1 breast cancer cells in
vivo and therefore could be useful as a lead compound for
cancer therapy.
Hirsutine is one of the major alkaloids in
Uncaria species and its cardioprotective (41), antihypertensive and anti-arrhythmic
activity has been reported (42).
The presented activity of Hirsutine in the inhibition of NF-κB
activation and the reduction of the metastatic potential of cancer
cells is unexplored. While the underlying mechanisms that account
for the multifaceted and differential role of NF-κB in cancer
metastasis are presently unknown, the NF-κB-mediated inflammatory
signaling pathway is an attractive clinical target for controlling
metastasis in humans. Importantly, our present observation is
applicable clinically because we observed the inhibitory effect of
Hirsutine on NF-κB activation in human breast cancer cell lines as
seen in the inhibition of p-65 phosphorylation (data not shown) and
further in the inhibition of their migration (Fig. 7). Further studies are still
required to determine the exact mechanisms of action of Hirsutine
in its anti-metastatic activity by clarifying the potential
involvement of other signaling pathways and/or transcriptional
factors. Considering that the effective doses of Hirsutine shown in
this study were relatively high, we also need to consider either
chemical or biological modification of Hirsutine to achieve higher
potency. Nevertheless, our current study clearly indicates that
Hirsutine could be an attractive lead compound for reducing the
metastasis potential of cancer cells by regulating NF-κB
tumor-promoting activity.
Acknowledgements
We are grateful to Satoru J. Yokoyama and Hiroaki
Sakurai for discussions and colleagues in Saiki Laboratory for
generous support. This study was partly supported by Yokoyama
Foundation and a Grant-in-aid for the 2012 and 2013 Cooperative
Research Project I from the Institute of Natural Medicine,
University of Toyama. Chenghua Lou is a graduate student supported
by the Campus Asian Program of the University of Toyama.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Mann J: Natural products in cancer
chemotherapy: past, present and future. Nat Rev Cancer. 2:143–148.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Crowell JA: The chemopreventive agent
development research program in the Division of Cancer Prevention
of the US National Cancer Institute: an overview. Eur J Cancer.
41:1889–1910. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cragg GM, Newman DJ and Snader KM: Natural
products in drug discovery and development. J Nat Prod. 60:52–60.
1997. View Article : Google Scholar
|
|
5
|
Harvey AL: Natural products in drug
discovery. Drug Discov Today. 13:894–901. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sha WC: Regulation of immune responses by
NF-kappa B/Rel transcription factor. J Exp Med. 187:143–146. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xiao G and Fu J: NF-κB and cancer: a
paradigm of Yin-Yang. Am J Cancer Res. 1:192–221. 2011.
|
|
8
|
Basseres DS and Baldwin AS: Nuclear
factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic
initiation and progression. Oncogene. 25:6817–6830. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bours V, Bonizzi G, Bentires-Alj M, et al:
NF-kappaB activation in response to toxical and therapeutical
agents: role in inflammation and cancer treatment. Toxicology.
153:27–38. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang CY, Fong YC, Lee CY, et al: CCL5
increases lung cancer migration via PI3K, Akt and NF-kappaB
pathways. Biochem Pharmacol. 77:794–803. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nottingham LK, Yan CH, Yang X, et al:
Aberrant IKKα and IKKβ cooperatively activate NF-κB and induce
EGFR/AP1 signaling to promote survival and migration of head and
neck cancer. Oncogene. 33:1135–1147. 2014.
|
|
12
|
Zhang W, Tan W, Wu X, et al: A NIK-IKKα
module expands ErbB2-induced tumor-initiating cells by stimulating
nuclear export of p27/Kip1. Cancer Cell. 23:647–659. 2013.
|
|
13
|
Shaffer AL, Rosenwald A and Staudt LM:
Lymphoid malignancies: the dark side of B-cell differentiation. Nat
Rev Immunol. 2:920–932. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Panwalkar A, Verstovsek S and Giles F:
Nuclear factor-kappaB modulation as a therapeutic approach in
hematologic malignancies. Cancer. 100:1578–1589. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Orlowski RZ and Baldwin AS Jr: NF-kappaB
as a therapeutic target in cancer. Trends Mol Med. 8:385–389. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Singh NP, Singh UP, Hegde VL, et al:
Resveratrol (trans-3,5,4′-trihydroxystilbene) suppresses EL4 tumor
growth by induction of apoptosis involving reciprocal regulation of
SIRT1 and NF-κB. Mol Nutr Food Res. 55:1207–1218. 2011.
|
|
17
|
Kumar A and Sharma SS: NF-kappaB
inhibitory action of resveratrol: a probable mechanism of
neuroprotection in experimental diabetic neuropathy. Biochem
Biophys Res Commun. 394:360–365. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Youn J, Lee JS, Na HK, Kundu JK and Surh
YJ: Resveratrol and piceatannol inhibit iNOS expression and
NF-kappaB activation in dextran sulfate sodium-induced mouse
colitis. Nutr Cancer. 61:847–854. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chhunchha B, Fatma N, Kubo E, Rai P, Singh
SP and Singh DP: Curcumin abates hypoxia-induced oxidative stress
based-ER stress-mediated cell death in mouse hippocampal cells
(HT22) by controlling Prdx6 and NF-κB regulation. Am J Physiol Cell
Physiol. 304:C636–C655. 2013.PubMed/NCBI
|
|
20
|
Leclercq IA, Farrell GC, Sempoux C, dela
Pena A and Horsmans Y: Curcumin inhibits NF-kappaB activation and
reduces the severity of experimental steatohepatitis in mice. J
Hepatol. 41:926–934. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin SY, Chang YT, Liu JD, et al: Molecular
mechanisms of apoptosis induced by magnolol in colon and liver
cancer cells. Mol Carcinog. 32:73–83. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ikeda K, Sakai Y and Nagase H: Inhibitory
effect of magnolol on tumour metastasis in mice. Phytother Res.
17:933–937. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang SE, Hsieh MT, Tsai TH and Hsu SL:
Effector mechanism of magnolol-induced apoptosis in human lung
squamous carcinoma CH27 cells. Br J Pharmacol. 138:193–201. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hsu YF, Lee TS, Lin SY, et al: Involvement
of Ras/Raf-1/ERK actions in the magnolol-induced upregulation of
p21 and cellcycle arrest in colon cancer cells. Mol Carcinog.
46:275–283. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang SH, Chen Y, Tung PY, et al:
Mechanisms for the magnolol-induced cell death of CGTH W-2 thyroid
carcinoma cells. J Cell Biochem. 101:1011–1022. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee SJ, Cho YH, Park K, et al: Magnolol
elicits activation of the extracellular signal-regulated kinase
pathway by inducing p27KIP1-mediated G2/M-phase cell cycle arrest
in human urinary bladder cancer 5637 cells. Biochem Pharmacol.
75:2289–2300. 2008. View Article : Google Scholar
|
|
27
|
Tsai JR, Chong IW, Chen YH, et al:
Magnolol induces apoptosis via caspase-independent pathways in
non-small cell lung cancer cells. Arch Pharm Res. 37:548–557. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen MC, Lee CF, Huang WH and Chou TC:
Magnolol suppresses hypoxia-induced angiogenesis via inhibition of
HIF-1α/VEGF signaling pathway in human bladder cancer cells.
Biochem Pharmacol. 85:1278–1287. 2013.PubMed/NCBI
|
|
29
|
Rasul A, Yu B, Khan M, et al: Magnolol, a
natural compound, induces apoptosis of SGC-7901 human gastric
adenocarcinoma cells via the mitochondrial and PI3K/Akt signaling
pathways. Int J Oncol. 40:1153–1161. 2012.PubMed/NCBI
|
|
30
|
Lee J, Jung E, Park J, et al:
Anti-inflammatory effects of magnolol and honokiol are mediated
through inhibition of the downstream pathway of MEKK-1 in NF-kappaB
activation signaling. Planta Med. 71:338–343. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tse AK, Wan CK, Zhu GY, et al: Magnolol
suppresses NF-kappaB activation and NF-kappaB regulated gene
expression through inhibition of IkappaB kinase activation. Mol
Immunol. 44:2647–2658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chua HL, Bhat-Nakshatri P, Clare SE,
Morimiya A, Badve S and Nakshatri H: NF-kappaB represses E-cadherin
expression and enhances epithelial to mesenchymal transition of
mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2.
Oncogene. 26:711–724. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang J, Pan WH, Clawson GA and Richmond A:
Systemic targeting inhibitor of kappaB kinase inhibits melanoma
tumor growth. Cancer Res. 67:3127–3134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Scartozzi M, Bearzi I, Pierantoni C, et
al: Nuclear factor-κB tumor expression predicts response and
survival in irinotecan-refractory metastatic colorectal cancer
treated with cetuximab-irinotecan therapy. J Clin Oncol.
25:3930–3935. 2007.
|
|
35
|
Keats JJ, Fonseca R, Chesi M, et al:
Promiscuous mutations activate the noncanonical NF-kappaB pathway
in multiple myeloma. Cancer Cell. 12:131–144. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Annunziata CM, Davis RE, Demchenko Y, et
al: Frequent engagement of the classical and alternative NF-kappaB
pathways by diverse genetic abnormalities in multiple myeloma.
Cancer Cell. 12:115–130. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yeh CB, Hsieh MJ, Hsieh YH, Chien MH,
Chiou HL and Yang SF: Antimetastatic effects of norcantharidin on
hepatocellular carcinoma by transcriptional inhibition of MMP-9
through modulation of NF-κB activity. PLoS One.
7:e310552012.PubMed/NCBI
|
|
38
|
Lin TH, Tan TW, Tsai TH, et al: D-pinitol
inhibits prostate cancer metastasis through inhibition of αVβ3
integrin by modulating FAK, c-Src and NF-κB pathways. Int J Mol
Sci. 14:9790–9802. 2013.PubMed/NCBI
|
|
39
|
Chen YY, Lu HF, Hsu SC, et al: Bufalin
inhibits migration and invasion in human hepatocellular carcinoma
SK-Hep1 cells through the inhibitions of NF-κB and matrix
metalloproteinase-2/-9-signaling pathways. Environ Toxicol. Aug
16–2013.(Epub ahead of print).
|
|
40
|
Yang XC, Wang X, Luo L, et al: RNA
interference suppression of A100A4 reduces the growth and
metastatic phenotype of human renal cancer cells via
NF-κB-dependent MMP-2 and bcl-2 pathway. Eur Rev Med Pharmacol Sci.
17:1669–1680. 2013.PubMed/NCBI
|
|
41
|
Wu LX, Gu XF, Zhu YC and Zhu YZ:
Protective effects of novel single compound, Hirsutine on hypoxic
neonatal rat cardiomyocytes. Eur J Pharmacol. 650:290–297. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Horie S, Yano S, Aimi N, Sakai S and
Watanabe K: Effects of hirsutine, an antihypertensive indole
alkaloid from Uncaria rhynchophylla, on intracellular
calcium in rat thoracic aorta. Life Sci. 50:491–498. 1992.
View Article : Google Scholar : PubMed/NCBI
|