Introduction
Notch signaling is involved in the tumorigenesis and
progression of nearly all solid tumors (1), which makes this cascade a highly
desirable therapeutic target. In particular, Notch signaling has
been shown to be involved in the tumorigenesis and proliferation of
hepatocellular carcinoma (HCC). However, the reports on this topic
have been contradictory (2–6), and
the differential expression patterns of Notch cascade members are
correlated with this variability in results. In the liver, Notch
acts in a developmental stage- and dose-dependent manner to
coordinate biliary fate and morphogenesis (7), and similar activities have also been
detected in HCC. However, temporal and concentration differences in
the Notch cascade cannot completely explain the discrepancies in
the Notch-related findings in HCC, which suggests the existence of
other factors that affect Notch activation in HCC.
Notch signaling is controlled at multiple levels,
including through gene dosage sensitivity (8) and through cis and trans
interactions between the ligand and receptor on neighboring cells
or on the same cell membrane (9).
Unlike most major signaling pathways, which rely on enzymatically
amplified signals, Notch signaling is mediated by stoichiometric
interactions between the elements of the pathway. The
stoichiometric difference between receptor and ligand expression on
the membrane is an important factor that affects the signaling
between two interacting cells (10). Additionally, post-translational
modifications introduced in the cytoplasm, such as ubiquitylation,
glycosylation and phosphorylation, have emerged as key regulators
of Notch signaling (11–13). Therefore, the localization of
Jagged1 (JAG1) and Notch1 to the membrane or the cytoplasm directly
correlates with the activity of the Notch cascade. Thus, we
directed our attention to the effects of the spatial distribution
of Notch signaling factors on HCC progression.
Similar to its role in organ development (14), Notch signaling impacts tumor
metastasis by regulating epithelial-mesenchymal transition
(EMT)-related genes (15). Breast
cancer and prostate tumor models have indicated that JAG1 mediates
EMT through the up-regulation of Slug and AKT signaling (16,17).
Recent data have further indicated that osteopontin (OPN), another
EMT-related gene, is dramatically up-regulated as a result of Notch
activation in HCC, suggesting that OPN is one of the main target
genes of Notch signaling in HCC (18). Our previous research demonstrated
the crucial role of OPN in HCC metastasis (19) and suggested that the
JAG1-Notch1-OPN axis may play a pivotal role in HCC metastasis.
In this study, we used immunohistochemical analyses
of tissue microarrays (TMAs) to evaluate whether the localization
patterns of the Notch1 cascade members could affect HCC metastasis.
The predictive value of the localization of JAG1-Notch1-OPN cascade
members in HCC metastasis was also evaluated.
Materials and methods
Cell line
Human HCC cell lines with different metastatic
potential, or MHCC97H (100% lung metatsis) and MHCC97L were
established in Liver Cancer Institute of Fudan University
(Shanghai, China). HCC cell line SMMC-7721 was established at
Second Military Medical University (Shanghai, China); Hep3B, and
HepG2 were obtained from American Type Culture Collection
(Manassas, VA, USA). L02, an immortalized human liver cell line,
and HCC cell lines Huh7 and HCC-7402 was obtained from Chinese
Scientific Academy. The cell line was cultured in high glucose DMEM
(Gibco-BRL, Grand Island, NY, USA) supplemented with 10% fetal
bovine serum (Hyclone Laboratories, Inc., Logan, UT, USA).
Patients and follow-up
One hundred twelve patients were sampled from a
prospectively designed database. Ethical approval was obtained from
the Zhongshan Hospital Research Ethics Committee, and informed
consent was obtained from each patient. The included patients
underwent hepatectomy by the same surgical team between January,
2000 and May, 2004. All of the patients had pathologically
diagnosed HCC and were classified as Child-Pugh A. Routine
follow-up procedures at our clinic were performed as previously
described (20). Through May 2009,
56 patients were found to have lung metastasis. Ten patients with a
resectable lung metastasis received a partial pneumonectomy.
TMA and immunohistochemistry
First, hematoxylin and eosin-stained slides were
screened for optimal tumor content and at least 2 cm of tissue
adjacent to the tumor (TAT). Then, the TMA was constructed
according to standard procedures (21). Rabbit anti-human JAG1 (Santa Cruz
Biotechnology, Santa Cruz, CA, USA) at 1:50, rabbit anti-human
Notch1 (Epitomics, Burlingame, CA, USA) at 1:100 and rabbit
antihuman OPN (Santa Cruz Biotechnology) at 1:200 were used as
primary antibodies for detection. Detection without a primary
antibody was used as a negative control.
Categories of JAG1 and Notch1
expression
Two categories for JAG1 expression were established
based on the distribution of immunostaining patterns:
JAG1Mem which has clear membrane expression no matter
whether expression level in cytoplasm; and JAG1Cyto
which only can be detected the cytoplasm expression). Similarly,
Notch1 expression was categorized as Notch1Mem and
Notch1Cyto based on the spatial staining pattern. Two
pathologists reviewed the results independently. In addition,
because OPN was only evaluated as a target of the JAG1-Notch1
cascade, we categorized OPN expression as negative or positive
based on the staining intensity.
Western blot analysis
Membrane protein was extracted according to the
instructions of Mem-PER Plus Membrane Protein Extraction Kit
(Thermo Fisher Scientific, Rockford, IL, USA). Western blot
analysis was performed according to the protocol of Bio-Rad wet
transfer using the Bio-Rad Transfer Cell System (Bio-Rad,
Mississauga, ON, Canada). Analyses of protein expression were
performed according to the manufacturer’s instructions. Rabbit
anti-human JAG1 (Santa Cruz Biotechnology) 1:200, rabbit anti-human
Notch1 (Epitomics) at 1:1,000, rabbit anti-human OPN (Abcam,
Cambridge, MA, USA) at 1:1,000 and rabbit anti-human β-actin mAb
(Epitomics) 1:1,000 were used as primary antibodies in
detection.
RNAi
Small interference RNAs (siRNAs) to target
expression of JAG1 and Notch1 were synthesized by GenePharma Corp.
(Shanghai, China) The coding sequences were as follows:
siJAG1-1845, 5′-GGGUCAGAAUUGUGACAUATT-3′; siJAG1-5396,
5′-GGAGUAUUCUAAUAAGCUATT-3′; siNotch1-3918,
5′-CGUCAUCAAUGGCUGCAAATT-3′; siNotch1-8240,
5′-GGAUUAAUUUGCAUCUGAATT-3′; negative control siRNA,
5′-UUCUCCGAACGUGUCACG UTT-3′. siRNA transfection of HCCLM3 cells
was performed according to the Lipofectamine 2000 protocol.
Statistical analysis
Pearson’s χ2 test was used to compare
qualitative variables in the clinical pathology analysis. When the
expected sample numbers were below 5, Fisher’s exact test was used.
Spearman’s rank test was used to detect the correlation between
variables. The survival analysis included time-to-lung metastasis,
overall survival (OS) and time-to-progression (TTP). The
time-to-lung metastasis was calculated from the date of hepatectomy
to the date of lung metastasis with a definite clinical diagnosis.
The Kaplan-Meier method was used to generate the survival curves,
and the log-rank test was used to compare the survival
distributions between the groups. The log-rank test was also used
to screen for prognostic factors in the univariate analysis. A Cox
regression model was used to identify the prognostic factors
related to the time-to-lung metastasis. A receiver operation curve
(ROC) was used to confirm the predictive accuracy of the prognostic
factors. All of the p-values were 2-tailed, and statistical
significance was set at 0.05. The statistical analyses were
completed using SPSS 18.0 software (SPSS Inc., Chicago, IL,
USA).
Results
Tumor cells with JAG1Mem are
more likely to undergo metastasis
The 112 tumors evaluated demonstrated JAG1
expression; 39 exhibited membrane expression, and 73 exhibited
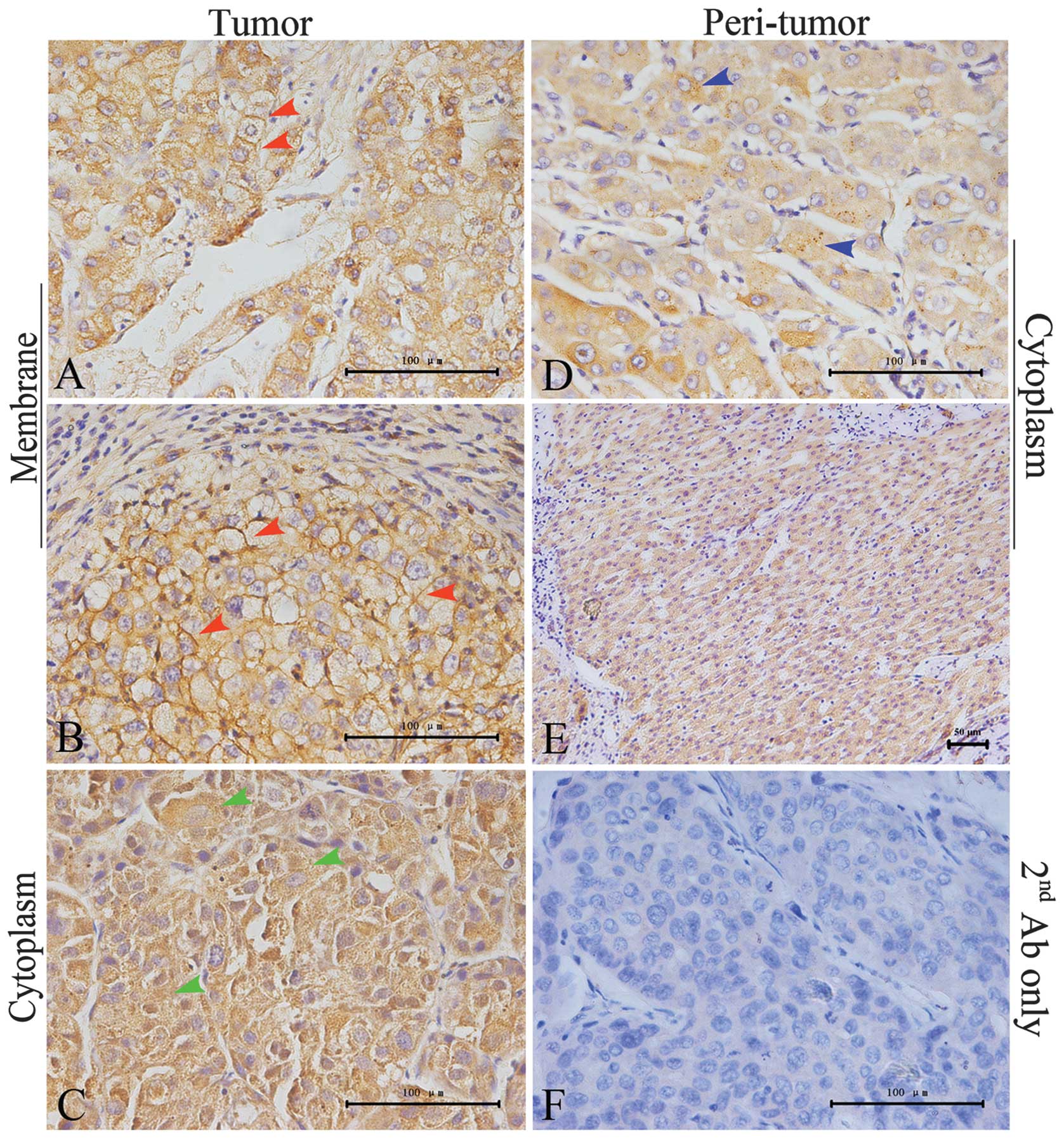
cytoplasmic expression. Fig. 1
shows a representative image of strong JAG1 staining in the
membranes of neighboring tumor cells.
In patients with lung metastasis (n=56), 54% (n=30)
of the samples demonstrated JAG1Mem expression, whereas
most of the samples (84%) from patients without lung metastasis
(n=56) demonstrated JAG1Cyto expression. The tumor cells
with JAG1Mem frequently underwent extra-hepatic
metastasis [hazard ratio (HR), 2.160; 95% confidence interval (CI),
1.517–3.074; p<0.001], and JAG1Mem correlated
positively with metastasis (Spearman’s rho =0.394; p=0.000). In
addition, there were no statistically significant differences in
any clinicopathological variables between JAG1Mem and
JAG1Cyto samples, with the exception of the tumor
capsule (Table I); tumors with
JAG1Mem had a more poorly encapsulated membrane
(Spearman’s rho =0.232; p=0.014).
 | Table ICorrelation between the localized
expression of JAG1 and the clinical characteristics of 112 patients
with hepatocellular carcinoma. |
Table I
Correlation between the localized
expression of JAG1 and the clinical characteristics of 112 patients
with hepatocellular carcinoma.
| No. of
patients | |
|---|
|
| |
|---|
| Variables | JAG1Mem
(n=39) | JAG1Cyto
(n=73) | P-value |
|---|
| Age, years | | | 0.067 |
| ≤60 | 36 | 57 | |
| >60 | 3 | 16 | |
| Gendera | | | 0.765 |
| Male | 34 | 65 | |
| Female | 5 | 8 | |
| HBsAg | | | 0.801 |
| Negative | 8 | 13 | |
| Positive | 31 | 60 | |
| Cirrhosis | | | 1.000 |
| Absent | 31 | 57 | |
| Present | 8 | 16 | |
| AFP (μg/l) | | | 0.528 |
| ≤20 | 11 | 26 | |
| >20 | 28 | 47 | |
| Tumor size
(cm) | | | 0.539 |
| ≤5 | 16 | 25 | |
| >5 | 23 | 48 | |
| No. of tumor
nodules | | | 0.343 |
| Single | 33 | 56 | |
| Multiple | 6 | 17 | |
| Tumor
encapsulation | | | 0.016 |
| Well
encapsulated | 18 | 51 | |
| Poorly
encapsulated | 21 | 22 | |
| Microvascular
invasion | | | 0.404 |
| Negative | 34 | 51 | |
| Positive | 15 | 22 | |
| Edmondson
grade | | | 0.539 |
| Grade (1/2) | 23 | 48 | |
| Grade (3/4) | 16 | 25 | |
| Portal lymphatic
involvementa | | | 0.446 |
| No | 35 | 69 | |
| Yes | 4 | 4 | |
Patients with JAG1Mem tumors were also
more likely to experience lung metastasis within a shorter period
of time compared to those with JAG1Cyto tumors. In the
JAG1Cyto group, the cumulative 1-, 3- and 5-year rates
of lung metastasis-free were 73, 66 and 64%, respectively, whereas
in the JAG1Mem group, the cumulative 1-, 3- and 5-year
rates were 51, 31 and 22%, respectively. The time-to-lung
metastasis for the JAG1Mem group was significantly
shorter than that for the JAG1Cyto group (p<0.001,
log-rank test). The curve for time-to-lung metastasis is shown in
Fig. 2A. In addition, there were
significant differences in the OS and TTP between the
JAG1Mem and JAG1Cyto groups (log-rank test,
p=0.024 and p=0.002, respectively) (Fig. 3A and C).
Membrane expression of Notch1 in tumors
correlates closely with metastasis
Notch1 staining was not detectable in 23 samples. In
the 89 tumors with detectable Notch1 staining, 44 were
Notch1Mem, and 45 were Notch1Cyto. The
typical membrane staining for Notch1 could also be observed in
neighboring tumor cells (Fig.
4).
Overall, 63% (n=26) of the samples from patients
with lung metastases (n=41) were classified as
Notch1Mem, whereas in the patients without lung
metastasis (n=48), only 18 samples were Notch1Mem.
Furthermore, tumors with Notch1Mem expression were
highly likely to undergo extra-hepatic metastasis (HR, 1.773, 95%
CI, 1.096–2.867; p=0.015), and Notch1Mem was positively
correlated with metastasis (Spearman’s rho =0.258; p=0.015). In
addition, there were no statistically significant differences in
any clinicopathological variables between JAG1Mem and
JAG1Cyto (data not shown).
Patients with Notch1Mem tumors were more
likely to develop lung metastases in a shorter period of time. The
time-to-lung metastasis for the Notch1Mem group was also
significantly shorter than that in the Notch1Cyto group
(log-rank test; p=0.05). The time-to-lung metastasis curves are
shown in Fig. 2B. In addition,
there were potential differences in OS and TTP between the
Notch1Mem and Notch1Cyto groups (log-rank
test, p=0.066 and p=0.065, respectively) (Fig. 3B and D).
JAG1Mem-Notch1Mem
cascade member expression is predictive of time-to-lung
metastasis
To evaluate one of the most important
ligand-receptor pairs in Notch signaling, we next studied the
prognostic value of the localization of JAG1-Notch1 expression. The
results demonstrated that 76% (n=26) of JAG1Mem tumors
exhibited Notch1Mem expression, whereas only 8 of 44
Notch1Cyto tumors exhibited JAG1Mem
expression. JAG1Mem expression also demonstrated a
strong correlation with Notch1Mem in HCC (HR, 6.5; 95%
CI, 2.455–17.210; p<0.001), and JAG1Mem positively
correlated with Notch1Mem (Spearman’s rho =0.420;
p<0.001).
Univariate analyses indicated that AFP (p=0.013),
microvascular invasion (p<0.001), portal lymphatic involvement
(p=0.003), Edmondson grade (p=0.05), JAG1Mem
(p<0.001) and Notch1Mem (p=0.05) were potential
prognostic factors for lung metastasis. Furthermore, Cox analysis
showed that JAG1Mem (HR, 0.467; 95% CI, 0.271–0.806;
p=0.006), microvascular invasion (HR, 2.597; 95% CI, 1.472–4.582;
p=0.001) and Edmondson grade (HR, 1.791; 95% CI, 1.045–3.070;
p=0.034) served as independent prognostic factors for HCC lung
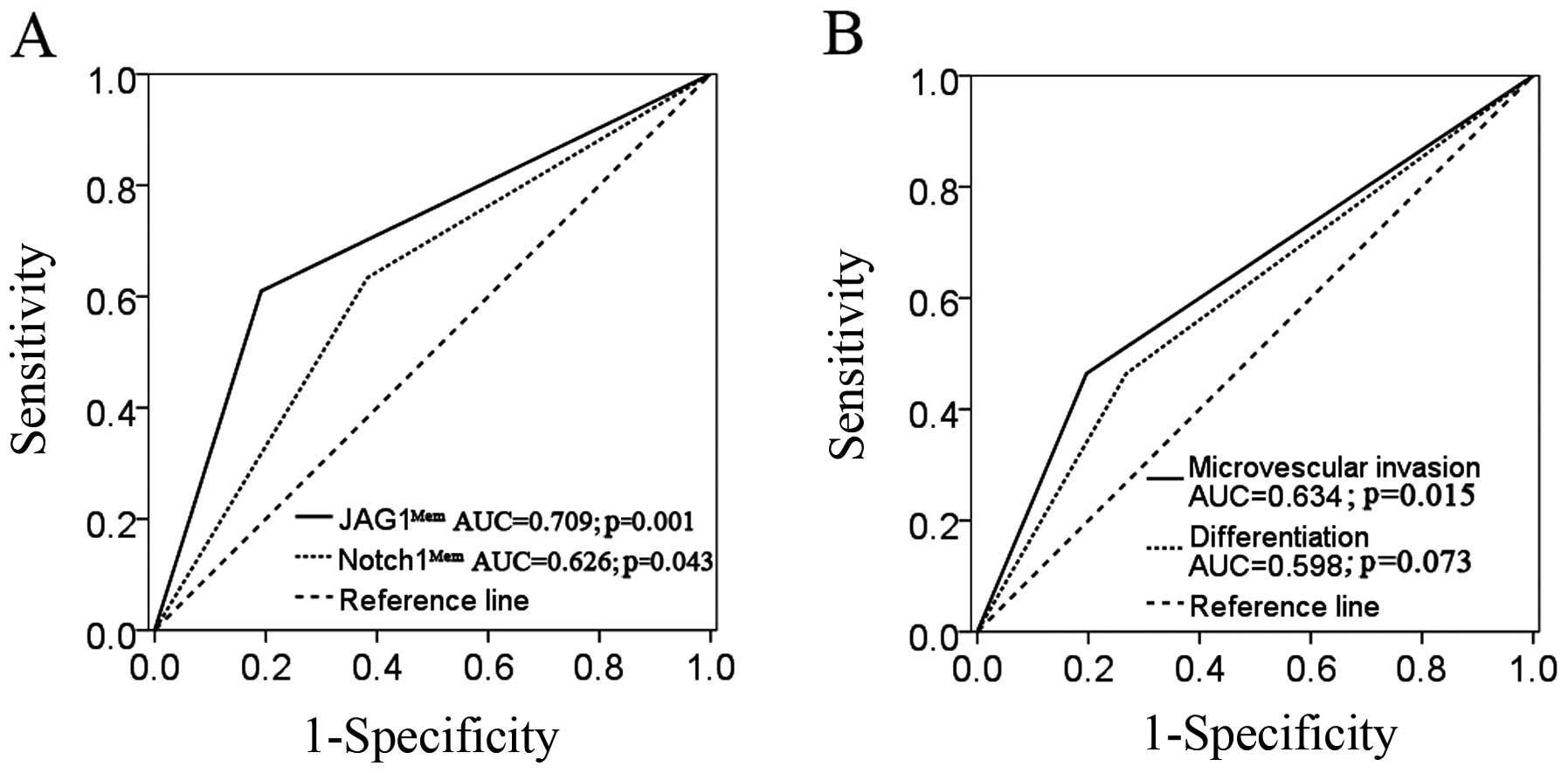
metastasis. In the ROC analysis, JAG1Mem showed better
specificity and sensitivity, with an AUC of 0.709 (95% CI,
0.598–0.820; p=0.001), than Notch1Mem (AUC 0.626; 95%
CI, 0.508–0.743; p=0.043) (Fig.
5A), microvascular invasion (AUC, 0.634; 95% CI, 0.530–0.737;
p=0.015) or Edmondson grade (AUC, 0.598; 95% CI, 0.493–0.704; p=
0.073) (Fig. 5B).
Cytoplasmic JAG1 and Notch1 expression is
observed mainly in peri-tumor tissues
To evaluate the roles of JAG1-Notch1 signaling in
tumors and the tumor microenvironment, we compared the spatial
distributions of JAG1 and Notch1 expression in TATs, including 23
peri-tumor tissues from patients with metastasis and another 23
peri-tumor tissues from patients without metastasis. Generally, the
immuno-staining for JAG1 and Notch1 in the peri-tumor tissue was
weaker than that in the tumors; only one of the 46 TATs evaluated
was of the JAG1Mem type. The distribution of
JAG1Cyto in most of the peri-tumor samples was
homogenous. Small JAG1-positive granules were found in the
cytoplasm (Fig. 1D). Similar to
JAG1, Notch1 expression was localized to the cytoplasm in most of
the peri-tumor samples (41/46). In addition, in the
Notch1Cyto samples, the Notch1 protein typically
aggregated in the cytoplasm in a granule-type pattern, which was
either well distributed or regional (Fig. 4E–F).
High OPN expression correlates
significantly with JAG1Mem
Emerging reports have strongly suggested that the
activation of the Notch pathway can upregulate OPN, and OPN has
been shown to play critical roles in HCC metastasis. Thus, we
examined the relationship between the expression of JAG1-Notch1 and
that of its target gene OPN. In total, 45% (n=50) of the samples
with positive JAG1 had positive OPN expression, including 19
samples with strong OPN staining. Further, 56% (n=22) of the
samples in the JAG1Mem group (n=39) had strong OPN
staining, whereas only 38% of the samples in the
JAG1Cyto group (n=72) had detectable OPN expression. HCC
cells with JAG1Mem expression also had a strong tendency
to express OPN (HR, 0.434; 95% CI, 0.193–0.975; p=0.041), and
JAG1Mem positively correlated with OPN expression
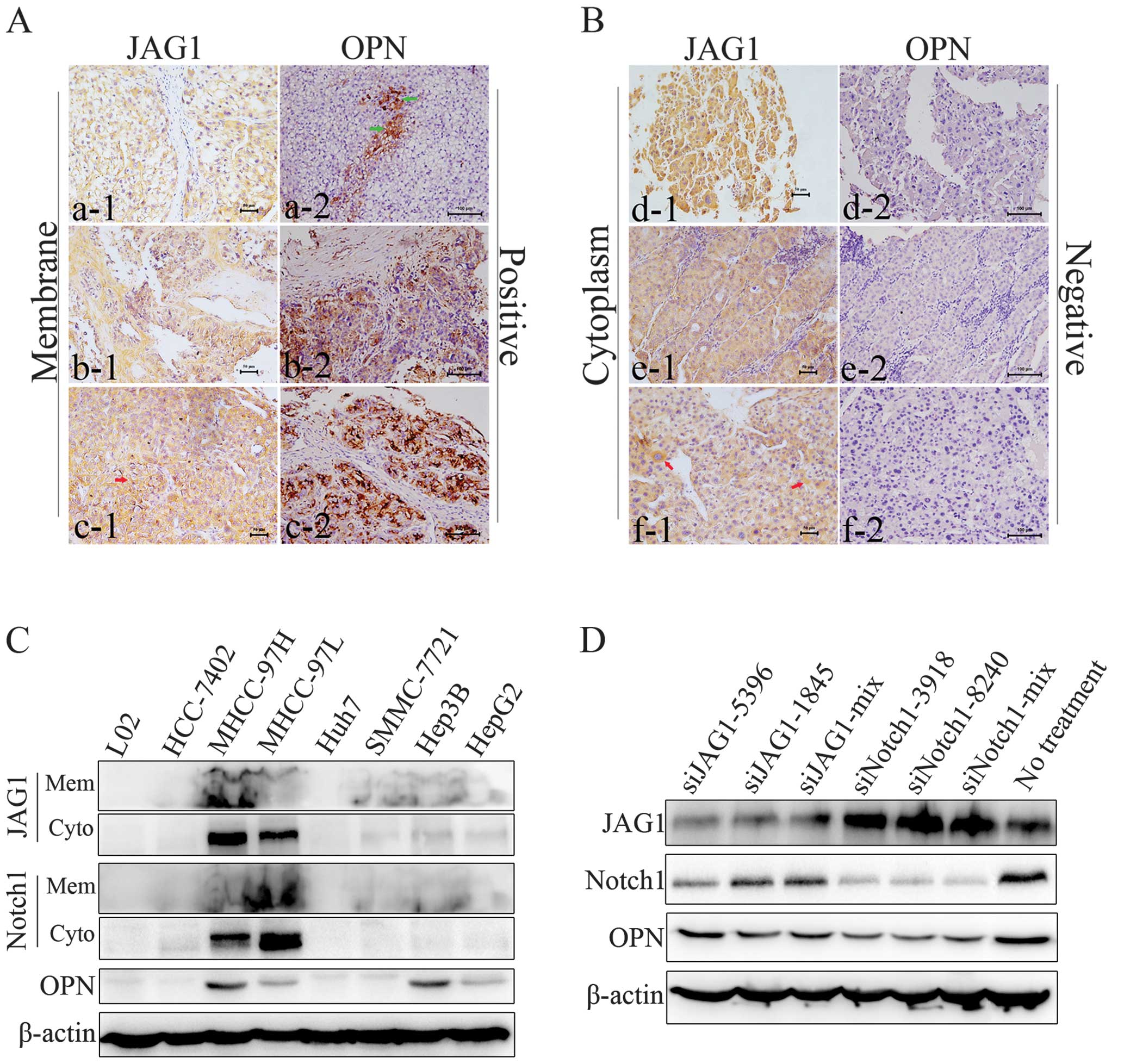
(Spearman’s rho =0.195; p=0.042). In tumor cells in which JAG1 was
clearly stained on the membrane, OPN was expressed either in
aggregates or uniformly (Fig. 6A),
whereas in JAG1Cyto tumor cells, OPN was expressed
relative lowly (Fig. 6B).
Knockdown of JAG1-Notch1 downregulated
the OPN level
The membrane expression of JAG1-Notch1 and the
coincidence of expressions between JAG1-Notch1 and OPN were
detected in HCC cell lines. As shown in Fig. 6C, the most of examined HCC cells
had upregulated membrane expressions of both JAG1 and Notch1 when
compared to normal liver cell line L02. Particularly, highly
metastatic MHCC97H had strong membrane expression and cytoplasm
expression of JAG1, with the consistently high expression of OPN.
Further, downregulation of JAG1 expression in MHCC97H decreased OPN
level. Similarly, knockdown of Notch1 induced the downregulation of
OPN expression in HCC cells (Fig.
6D).
Discussion
Although a developmental stage- and dose-dependent
mechanism of action for the Notch cascade was previously observed
in HCC and liver tissues, the reports concerning Notch-driven
carcinogenesis and HCC progression are controversial (1,22,23)
and suggest another possible mechanism of Notch activation. To our
knowledge, this study is the first to show that the spatial
distributions of Notch signaling proteins affect their roles in the
development of HCC.
Notch signaling has been shown to have unique
features, including gene dosage sensitivity and both cis and
trans regulation mechanisms. Our findings suggest that
enhanced membrane localization and expression may lead to an
increased opportunity for signal communication and activation
between neighboring tumor cells. In addition, strong
JAG1Mem and Notch1Mem expression likely leads
to increased trans interactions between cells and cis interactions
between ligands and receptors on the same cell membrane.
Additionally, because Notch activity is highly dependent on
contextual cues, JAG1Mem and Notch1Mem
expression may facilitate communication between tumor cells and the
tumor microenvironment. Cytoplasmic JAG1 or Notch1 may be degraded
or subject to post-translational modifications. In addition, recent
evidence supports the hypothesis that the Notch-DSL pathway is
bidirectional (24), suggesting
that the membrane localization of JAG1-Notch1 has a unique role in
Notch signaling.
In this study, the close correlation between
membrane-localized JAG1-Notch1 and extra-hepatic metastasis
supplied further evidence for Notch signaling in promoting HCC
progression. Consistent with our findings, Notch signaling was
previously shown to be involved in metastasis through an EMT
mechanism in tumors and was correlated with the upregulation of
E-cadherin expression in HCC (2,25).
Furthermore, the inhibition of Notch signaling using a DAPT
inhibitor was able to decrease the invasive potential of HCC
(26).
To confirm the role of the JAG1-Notch1 cascade in
HCC metastasis, we further evaluated the expression of the target
gene OPN and the relationship between OPN and JAG1. As expected,
strong OPN staining correlated closely with JAG1Mem
expression in the TMA analysis. Also, OPN was expressed highly in
HCC cell line with strong membrane expression of JAG1. Further,
knockdown of JAG1 or knockdown of Notch1 induced the downregulation
of OPN expression in HCC cells. The high expression of OPN in the
JAG1Mem-Notch1Mem tumors further suggests the
crucial role of the active JAG1-Notch1-OPN cascade in extra-hepatic
metastasis. Furthermore, OPN was previously identified as a pivotal
metastasis-related gene in the genomic profiling of multiple HCC
pairs, suggesting that OPN is tumor specific (27). Additionally, the critical role of
OPN in metastatic HCC has been well demonstrated (28). Therefore, the JAG1-Notch1-OPN
cascade in HCC metastasis has tumor-specific features.
In conclusion, the spatial distribution of Notch
signaling components represents another crucial factor regulating
the Notch cascade in HCC. The tumor-specific JAG1-Notch1-OPN
cascade was determined to be predictive of extra-hepatic HCC
metastasis and poor prognosis, which suggests that this pathway may
represent a therapeutic target for HCC.
Acknowledgements
This study was supported by State Key Project on
Infectious Diseases of China (no. 2012ZX10002-016) and the Youth
Backbone Fund from Fudan University (B-233).
References
|
1
|
Ranganathan P, Weaver KL and Capobianco
AJ: Notch signalling in solid tumours: a little bit of everything
but not all the time. Nat Rev Cancer. 11:338–351. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou L, Wang DS, Li QJ, Sun W, Zhang Y and
Dou KF: The down-regulation of Notch1 inhibits the invasion and
migration of hepatocellular carcinoma cells by inactivating the
cyclo-oxygenase-2/Snail/E-cadherin pathway in vitro. Dig Dis Sci.
58:1016–1025. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lim SO, Park YM, Kim HS, et al: Notch1
differentially regulates oncogenesis by wildtype p53 overexpression
and p53 mutation in grade III hepatocellular carcinoma. Hepatology.
53:1352–1362. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu M, Lee DF, Chen CT, et al: IKKalpha
activation of NOTCH links tumorigenesis via FOXA2 suppression. Mol
Cell. 45:171–184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qi R, An H, Yu Y, et al: Notch1 signaling
inhibits growth of human hepatocellular carcinoma through induction
of cell cycle arrest and apoptosis. Cancer Res. 63:8323–8329.
2003.PubMed/NCBI
|
|
6
|
Viatour P, Ehmer U, Saddic LA, et al:
Notch signaling inhibits hepatocellular carcinoma following
inactivation of the RB pathway. J Exp Med. 208:1963–1976. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zong Y, Panikkar A, Xu J, et al: Notch
signaling controls liver development by regulating biliary
differentiation. Development. 136:1727–1739. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mazzone M, Selfors LM, Albeck J, et al:
Dose-dependent induction of distinct phenotypic responses to Notch
pathway activation in mammary epithelial cells. Proc Natl Acad Sci
USA. 107:5012–5017. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
D’Souza B, Meloty-Kapella L and Weinmaster
G: Canonical and non-canonical Notch ligands. Curr Top Dev Biol.
92:73–129. 2010.
|
|
10
|
Artavanis-Tsakonas S and Muskavitch MA:
Notch: the past, the present, and the future. Curr Top Dev Biol.
92:1–29. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kovall RA and Blacklow SC: Mechanistic
insights into Notch receptor signaling from structural and
biochemical studies. Curr Top Dev Biol. 92:31–71. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Le Bras S, Loyer N and Le Borgne R: The
multiple facets of ubiquitination in the regulation of notch
signaling pathway. Traffic. 12:149–161. 2011.PubMed/NCBI
|
|
13
|
Fortini ME: Notch signaling: the core
pathway and its posttranslational regulation. Dev Cell. 16:633–647.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
De la Pompa JL and Epstein JA:
Coordinating tissue interactions: Notch signaling in cardiac
development and disease. Dev Cell. 22:244–254. 2012.PubMed/NCBI
|
|
15
|
Wang Z, Li Y, Kong D and Sarkar FH: The
role of Notch signaling pathway in epithelial-mesenchymal
transition (EMT) during development and tumor aggressiveness. Curr
Drug Targets. 11:745–751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Leong KG, Niessen K, Kulic I, et al:
Jagged1-mediated Notch activation induces epithelial-to-mesenchymal
transition through Slug-induced repression of E-cadherin. J Exp
Med. 204:2935–2948. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Santagata S, Demichelis F, Riva A, et al:
JAGGED1 expression is associated with prostate cancer metastasis
and recurrence. Cancer Res. 64:6854–6857. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Villanueva A, Alsinet C, Yanger K, et al:
Notch signaling is activated in human hepatocellular carcinoma and
induces tumor formation in mice. Gastroenterology.
143:1660–1669.e7. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen RX, Xia YH, Xue TC, Zhang H and Ye
SL: Downregulation of osteopontin inhibits metastasis of
hepatocellular carcinoma cells via a mechanism involving MMP-2 and
uPA. Oncol Rep. 25:803–808. 2011.PubMed/NCBI
|
|
20
|
Xue TC, Han D, Chen RX, et al: High
expression of CXCR7 combined with alpha fetoprotein in
hepatocellular carcinoma correlates with extra-hepatic metastasis
to lung after hepatectomy. Asian Pac J Cancer Prev. 12:657–663.
2011.PubMed/NCBI
|
|
21
|
Simon R, Mirlacher M and Sauter G:
Immunohistochemical analysis of tissue microarrays. Methods Mol
Biol. 664:113–126. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang M, Xue L, Cao Q, et al: Expression of
Notch1, Jagged1 and beta-catenin and their clinicopathological
significance in hepatocellular carcinoma. Neoplasma. 56:533–541.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lim SO, Kim HS, Quan X, et al: Notch1
binds and induces degradation of Snail in hepatocellular carcinoma.
BMC Biol. 9:832011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Andersen P, Uosaki H, Shenje LT and Kwon
C: Non-canonical Notch signaling: emerging role and mechanism.
Trends Cell Biol. 22:257–265. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang XQ, Zhang W, Lui EL, et al:
Notch1-Snail1-E-cadherin pathway in metastatic hepatocellular
carcinoma. Int J Cancer. 131:E163–E172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou L, Wang DS, Li QJ, Sun W, Zhang Y and
Dou KF: Downregulation of the Notch signaling pathway inhibits
hepatocellular carcinoma cell invasion by inactivation of matrix
metalloproteinase-2 and -9 and vascular endothelial growth factor.
Oncol Rep. 28:874–882. 2012.
|
|
27
|
Ye QH, Qin LX, Forgues M, et al:
Predicting hepatitis B virus-positive metastatic hepatocellular
carcinomas using gene expression profiling and supervised machine
learning. Nat Med. 9:416–423. 2003. View
Article : Google Scholar
|
|
28
|
Dong QZ, Zhang XF, Zhao Y, et al:
Osteopontin promoter polymorphisms at locus −443 significantly
affect the metastasis and prognosis of human hepatocellular
carcinoma. Hepatology. 57:1024–1034. 2012.
|