Introduction
Oral squamous cell carcinoma (OSCC) is the 8th most
common cancer in humans, which accounts for ~2% of all carcinomas
in women and 4% in men worldwide (1,2).
Over 300,000 new cases of OSCC are diagnosed annually with ~11,000
new cases in Japan (3). Surgery,
chemotherapy and radiotherapy are the standards for the treatment
of head and neck squamous cell carcinomas (HNSCCs) including OSCC.
However, despite recent advances in cancer diagnosis, surgery,
chemotherapy, radiotherapy and other treatment methods, the overall
survival rate of OSCC is ~50% in the advanced stage of the disease
(4,5). Almost 60% of HNSCC patients are
diagnosed with locally advanced disease at presentation (6). Therefore, it is important to
establish more promising therapeutic strategies.
Cetuximab (Erbitux®; formerly IMC-C225)
is a chimeric (mouse/human) IgG1 monoclonal antibody that targets
the extracellular ligand-binding domain of EGFR with high affinity
and inhibits tumor growth, invasion, angiogenesis and metastasis
(7,8). Cetuximab causes G1 phase cell cycle
arrest by decreasing cyclin-dependent kinase 2 (CDK2) and
increasing p27Kip1 levels in tumor cell lines (9). Paclitaxel (PTX) is a diterpenoid
isolated from the bark of the Pacific yew, Taxus brevifolia
(10). PTX induces mitotic arrest
and cell death by binding to microtubules, promoting microtubule
assembly, and stabilizing tubulin polymers against depolymerization
affecting cells in the G2/M-phase (11,12).
Most of the available reports on HNSCC demonstrated
the effect of cetuximab in combination with
cisplatin/platinum-based drugs or radiotherapy (7,13,14).
However, cetuximab with paclitaxel combination therapy has been
proven to be effective in HNSCC including several other cancers
(15–19). For last few years the number HNSCC
patients treated with PTX and cetuximab combined therapy has been
increased gradually. This combination therapy could be a promising
regimen for patients with advanced head and neck cancer after
failure of platinum-based therapy (15).
The transcription factor NF-κB plays an important
role in regulating various genes involved in inflammatory and
immune responses as well as in cell survival (20–24).
A major form of NF-κB is composed of a dimer of p50 and p65
subunits, and this complex is retained in the cytoplasm by
inhibitory molecules (IκBs) (25).
When the IκB-α is phosphorylated through the stimulation of
radiation or anticancer agents, it becomes degraded through the
ubiquitin-proteasome pathway which leads to the nuclear
translocation of NF-κB to stimulate the expression of its target
genes (21). The high constitutive
levels of NF-κB activity is seen in Hodgkin’s disease tumor cells,
in breast cancer cells, and in head and neck cancer cells, and may
contribute to the abnormal survival of these cells (22,23,26).
Briefly, cancer cells can manage to defend themselves from
radiation or anticancer agents by the activation of NF-κB (21–23).
Therefore, suppression of NF-κB activity in cancer cells may be
crucial to induce marked cell death by chemotherapeutic agents.
However, it is reported that some anticancer drugs, including
vinblastine, vincristine, daunomycin, doxorubicin, etoposide, and
PTX can upregulate the expression of NF-κB (27–30).
Hence the efficacy of PTX-cetuximab combination therapy might be
dependent upon the ability of cetuximab to control the PTX
influenced upregulation of NF-κB.
In this study, we investigated the antitumor
efficacy of PTX in combination with cetuximab against OSCC both
in vitro and in vivo. We also examined whether
cetuximab can regulate the expression of p65 NF-κB induced by PTX
in OSCC cells.
Materials and methods
Cell lines and nude mice
Human lung fibroblast cell line (Wi-38) and Human
tongue squamous cell carcinoma (HSC2, HSC3 and HSC4) cell lines
were purchased from Cell Bank, RIKEN BioResource Center (Ibaraki,
Japan) respectively. These cell lines were cultured in 100-mm
culture dish (Becton-Dickinson Labware, Franklin Lakes, NJ, USA)
and maintained in Dulbecco’s modified Eagle’s medium (DMEM,
Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal
bovine serum (FBS, Thermo Fisher Scientific Inc., Waltham, MA,
USA), 5% of antibiotic-antimycotic solution (Thermo Fisher
Scientific). In all experiments, cells were incubated at 37°C in a
humidified atmosphere containing 5% CO2.
Four-week-old female Balb/c nu/nu athymic nude mice
(average weight 15.0 g) were purchased from CLEA Japan, Co., Ltd.
(Tokyo, Japan). The mice were provided with sterile water and food
ad libitum, and maintained under pathogen-free conditions in
accordance with the Guidelines for Animal Experimentation of
Yamaguchi University.
Agents
Cetuximab was purchased from Merck Serono
(Dermstadt, Germany) and PTX was purchased from Wako Pure Chemical
Industries (Osaka, Japan).
Western blot analysis
Untreated Wi-38, HSC2, HSC3 and HSC4 cells were used
for the detection of EGFR expression. PTX and/or cetuximab treated
(0, 3, 6, 12, 24 and 48 h) HSC2, HSC3 and HSC4 cells were used for
the analysis of p65 NF-κB expression. Whole cell lysates from
control and treated cells were prepared with RIPA buffer (Thermo
Fisher Scientific) and the amount of protein in the cell lysates
were quantified with NanoDrop 1000 (Thermo Fisher Scientific). Cell
lysates containing 50 μg protein/sample were subjected to
electrophoresis on 10% SDS-polyacrylamide gels, and then
transferred to a PVDF membrane. The membranes were blocked and
treated with the anti-EGFR rabbit polyclonal antibody (Santa Cruz
Biotechnology, Santa Cruz, CA, USA) or anti-p65 NF-κB rabbit
polyclonal antibody (Santa Cruz). All antibodies were detected
using Western Breeze chromogenic immunodetection system (Thermo
Fisher Scientific) according to the manufacturer’s instructions.
Anti-actin monoclonal antibody (Santa Cruz) was used for
normalization of western blot analysis.
In vitro cell growth inhibition
assay
Wi-38, HSC2, HSC3 and HSC4 cells (5×103
cells/well) were seeded on 96-well plates (Becton-Dickinson
Labware) in DMEM supplemented with 10% FBS. Twenty-four hours
later, the cells were treated with different concentrations of
cetuximab (0, 0.1, 1 and 10 μg/ml) or PTX (0, 0.01, 0.02 and 0.05
μg/ml) alone or in combination to determine the suitable
concentration of these drugs in cell growth inhibition. After 48 h,
3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide
(MTT, 25 μl/well) was added to the 96-well plate and incubated for
4 h. The blue dye taken up by cells was dissolved in dimethyl
sulfoxide (100 μl/well), and the absorbance was measured with a
spectrophotometer (Bio-Rad Laboratories, Hercules, CA, USA) at 490
nm. Growth inhibitory effects were compared among the groups. All
assays were run in triplicate.
Hoechst staining
Cells (5×105 cells/well) were cultured on
cover glasses in 6-well plates (Becton-Dickinson Labware) in DMEM
with 10% FBS alone or with PTX and/or cetuximab. Forty-eight hours
later, cells were washed with phosphate-buffered saline (PBS),
fixed with 4% paraformaldehyde and stained with 2 μg/ml Hoechst
33258 (Dojindo Laboratories, Kumamoto, Japan), a fluorescent DNA
binding dye, for 30 min at 37°C. Fluorescence of Hoechst 33258 was
observed under a fluorescent microscope (FLoid® Cell
Imaging Station, Thermo Fisher Scientific). Apoptotic cells were
identified by their typical morphological appearance, including
chromatin condensation, nuclear fragmentation, formation of
membrane blebs and apoptotic bodies. The mean percentages of
apoptotic cells were estimated by counting 500 cells/area from at
least three different areas/sample.
In vivo tumor growth inhibition
assay
HSC2 cells (1×106) were suspended in 0.1
ml of serum-free medium and injected into the subcutaneous tissue
of 5-week old nude mice using a 27-gauge needle. When the estimated
tumor volume (0.5 × length × width2) reached 100–150
mm3, the tumor-bearing mice were allocated randomly into
control and treatment groups (5 mice/group). HSC2 tumors were
treated for 3 weeks with PTX (20 mg/kg, twice/week) and/or
cetuximab (20 mg/kg, twice/week) dissolved in 0.5% hydroxypropyl
methylcellulose (HPMC). Control group received HPMC (0.5%) only.
Tumor size and body weight were monitored and measured every three
days. Antitumor effects and body weight changes were compared among
the groups. All mice were sacrificed at the end of 3 weeks/21
days.
Immunohistochemical staining
HSC2 tumors harvested at autopsy were embedded in
paraffin blocks. Four-micrometer-thick sections were prepared from
the blocks and mounted on slides. These sections were processed for
immunostaining using the anti-p65 NF-κB rabbit polyclonal antibody
(Santa Cruz), and appropriate peroxidase conjugated anti-rabbit IgG
second antibody. Negative controls were done using PBS instead of
the primary antibody. The blocking and immunostaining were
performed using Dako Envision kit/HRP (Dako, Glostrup, Denmark).
Slides were counterstained with hematoxylin. The slides were then
examined under a bright-field microscope. A positive reaction was
detected as reddish-brown precipitates.
TUNEL (terminal deoxynucleotidyl
transferase (Tdt)-mediated nick end labeling) assay
To detect apoptotic cells in mouse tumor tissues,
TUNEL assay using the Apoptosis In Situ Detection kit (Wako)
was performed, labeling 3′-OH DNA ends generated by DNA
fragmentation. Four-micrometer-thick paraffin sections of tumor
were deparaffinized in xylene and rehydrated in decreasing
concentrations of ethanol. Tissue sections were incubated in 20
μg/ml proteinase K (Dako) for 15 min. After sections were rinsed in
distilled water, endogenous peroxidase was blocked by incubating
the slides in 3% hydrogen peroxide in PBS for 5 min. After washing
with PBS, the sections were incubated with equilibration buffer and
then TdT enzyme in a humidified chamber at 37°C for 60 min. They
were subsequently put into prewarmed stop wash buffer for 10 min.
After rinsing in PBS, the sections were incubated with
antidigoxigenin-peroxidase conjugate for 30 min. Peroxidase
activity in each section was demonstrated by the application of
diaminobenzidine (Peroxidase Substrate kit; Vector Laboratories,
Burlingame, CA, USA). Hematoxylin was used as a counterstain. At
least 1,000 cells were counted under a microscope in several random
fields of each section. The number of apoptotic cells was divided
by the total number of cells counted and the result was expressed
as a percentage.
Statistical analyses
All data are expressed as mean ± SD. The
significance of the experiment results was determined by the
Mann-Whitney’s U test or one-way ANOVA. The differences were
considered statistically significant when P<0.05.
Results
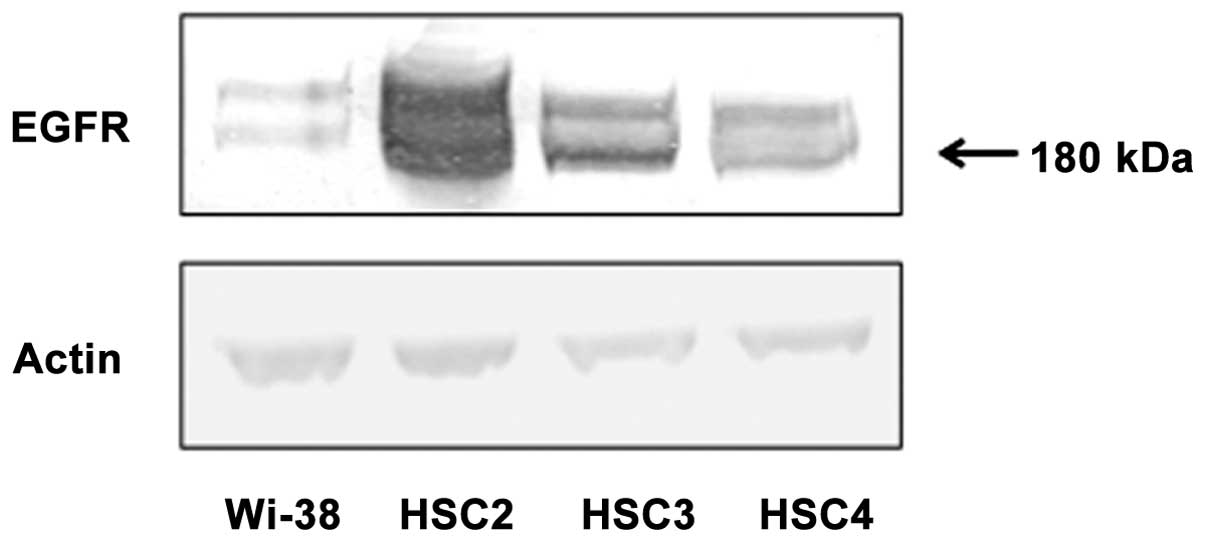
Analysis of EGFR expression in cells
To evaluate the expression pattern of EGFR in Wi-38,
HSC2, HSC3 and HSC4 cells, western blotting was performed. Wi-38
cells showed relatively weak expression of EGFR compared to the
other three cell lines. HSC2 cells showed high EGFR expression
compared to HSC3 and HSC4 cells (Fig.
1).
Effect of PTX and cetuximab on cell
growth inhibition of oral squamous cell carcinoma cell lines in
vitro
The growth inhibitory effect of PTX and cetuximab on
Wi-38, HSC2, HSC3 and HSC4 cells was analyzed by the MTT assay.
Cells were treated with different concentrations of PTX (0.01, 0.02
and 0.05 μg/ml) or cetuximab (0.1, 1 and 10 μg/ml) alone for 48 h.
PTX inhibited the growth of Wi-38, HSC2, HSC3 and HSC4 cells in a
dose-dependent manner (Fig. 2B).
Similar result was observed after cetuximab treatment in all cells
except Wi-38 cell (Fig. 2A). As
combination treatment, we selected the concentrations of 0.02 μg/ml
PTX and 1 μg/ml cetuximab as these concentrations significantly
inhibited the growth of all cell lines (Fig. 2A and B). As shown in Fig. 2C, 48-h treatment with PTX and
cetuximab combination significantly inhibited the growth of HSC2,
HSC3 and HSC4 cells compared to PTX or cetuximab alone, or the
untreated control.
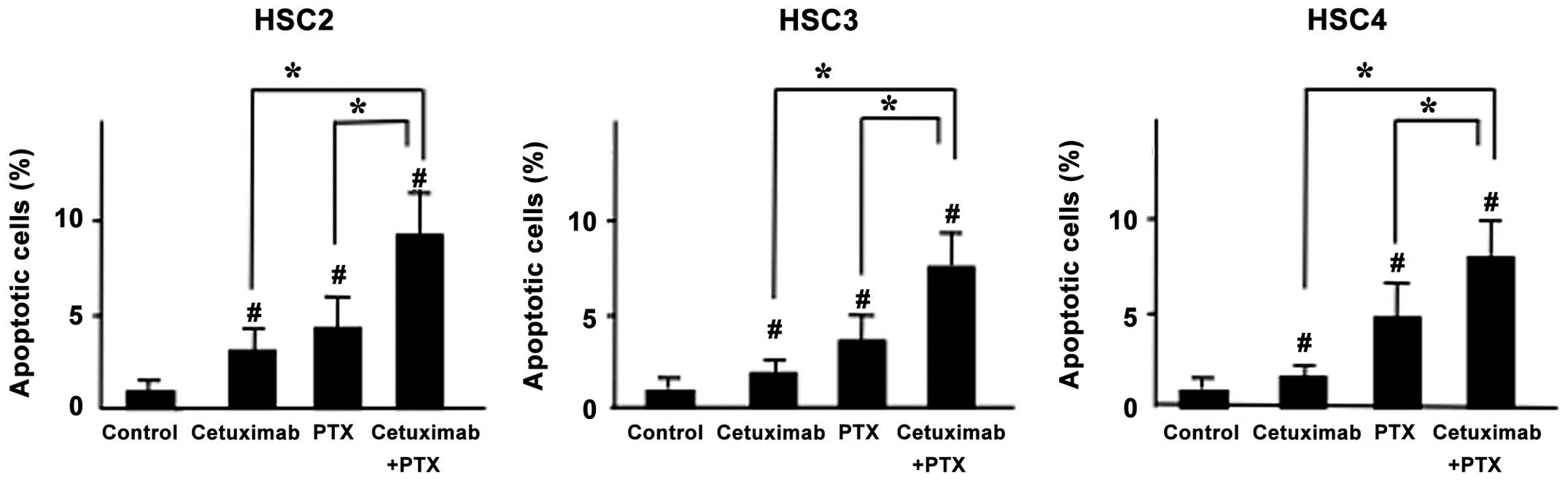
Effect of PTX and cetuximab on induction
of apoptosis in vitro
To understand whether the enhanced cell growth
inhibitory effect of PTX and cetuximab combined treatment was due
to apoptosis, we performed Hoechst staining to detect DNA
fragmentation and chromatin condensation in treated cells. The
numbers of apoptotic cells were significantly increased after PTX
and cetuximab combined treatment compared to treatment with either
agent alone (Fig. 3).
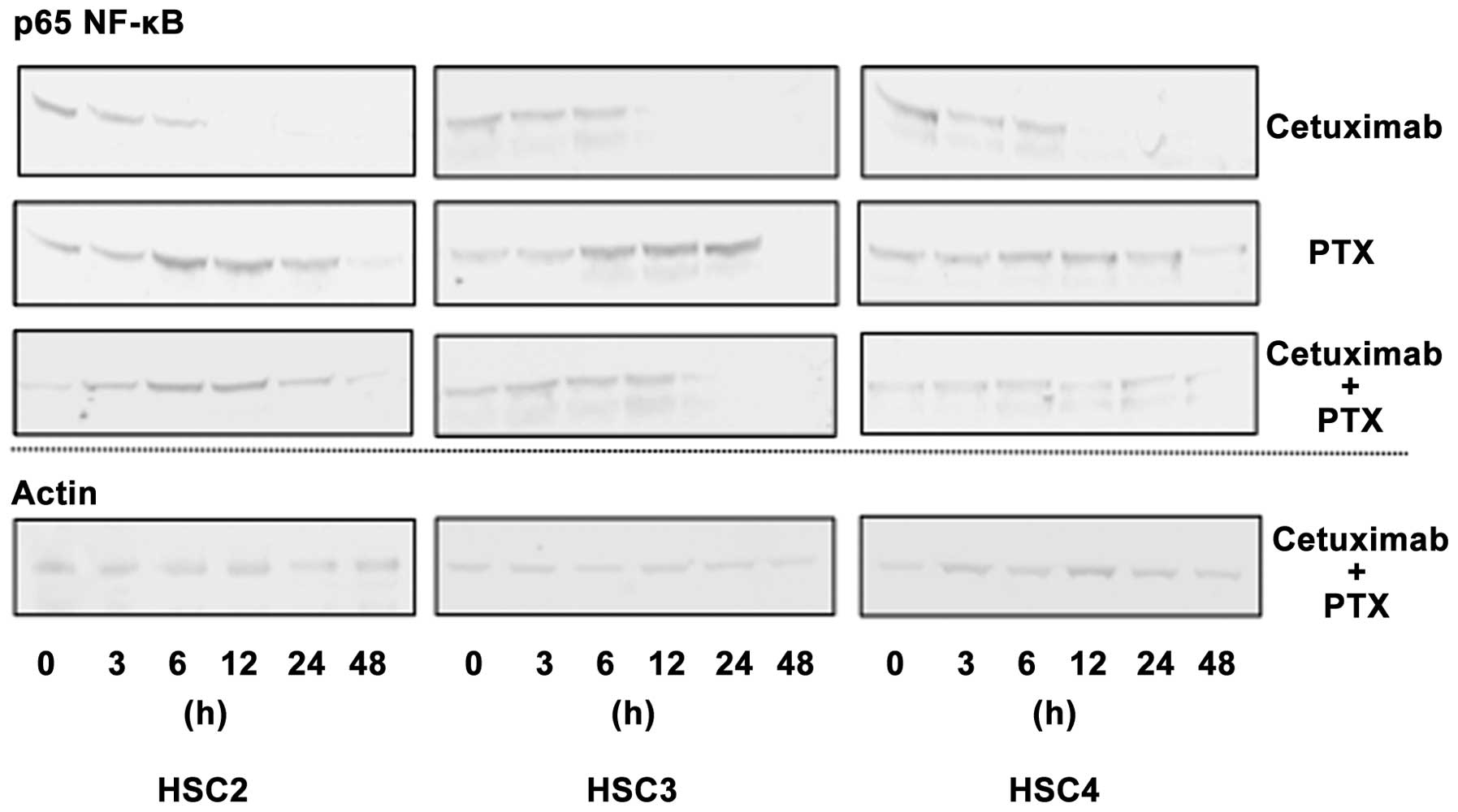
Effect of PTX and cetuximab on the
expression of p65 in vitro
To clarify the mechanisms behind the apoptosis
inducing activities of cetuximab and PTX combination treatment, we
examined the expression of p65 NF-κB in cells by western blotting.
Cetuximab reduced the expression of p65 in a time-dependent manner,
while the highest reduction of p65 expression was observed after 12
h of treatment. On the other hand, PTX treatment induced the
expression of p65 after 6 h of treatment. Combined treatment with
PTX and cetuximab induced p65 expression slightly, and reduced it
after 24 h of treatment (Fig.
4).
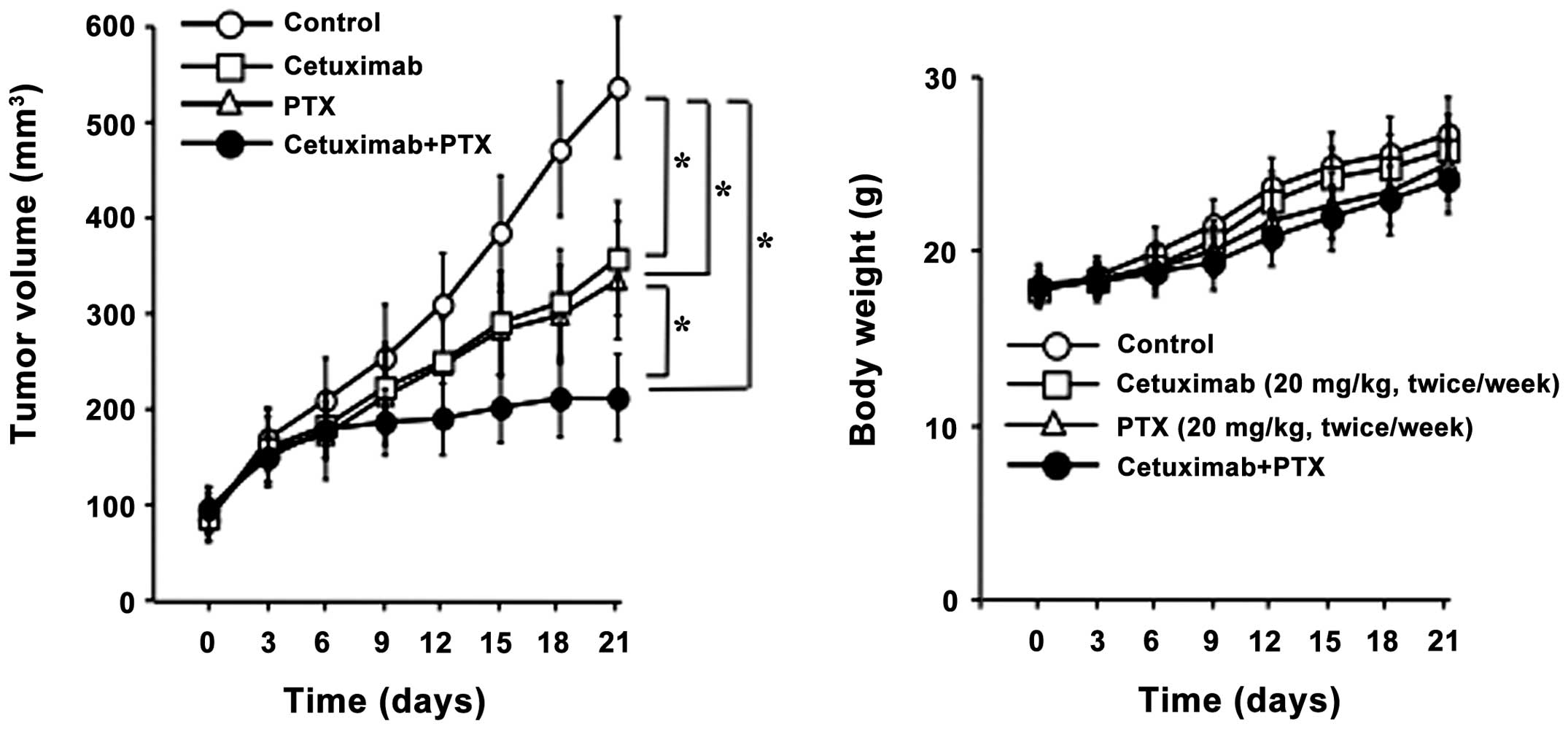
Effect of PTX and cetuximab on tumor
growth inhibition in vivo
Nude mice with HSC2 tumor xenografts were used to
examine the antitumor activity of PTX and cetuximab
single/combination treatment. Control group received 0.5% HPMC
only, while treatment groups were treated with either PTX (20
mg/kg/day, twice/week) or cetuximab (20 mg/kg/day, twice/week)
alone, or in combination for 3 weeks. Fig. 5 shows the result of the in
vivo experiment. All the treatment groups significantly
inhibited tumor growth compared to the untreated control. Antitumor
effect of cetuximab alone was in the same range as PTX alone.
However, the maximum reduction of tumor growth was observed with
PTX and cetuximab combination treatment, which is significantly
different than treatment with either agent alone. Compared to the
control, mice in all treatment groups showed no toxicity or
significant weight loss during the treatment.
Effect of PTX and cetuximab on the
expression of p65 in vivo
We examined the expression of p65 in mouse tumors by
immunohistochemistry. The expression of p65 was detected in both
the nucleus and the cytoplasm of untreated HSC2 tumor cells.
However, p65 expression was mainly detected in cytoplasm of
cetuximab treated tumors, while it was detected in the nucleus of
PTX treated tumor cells. Moreover, the expression of p65 was
decreased and was mainly detected in the cytoplasm of tumors that
received PTX and cetuximab combined treatment (Fig. 6).
Effect of PTX and cetuximab on induction
of apoptosis in vivo
To detect the degree of apoptosis induced by PTX
and/or cetuximab in vivo, tumors were removed from mice
after treatment and the number of apoptotic cells was quantified by
the TUNEL assay. Although treatment with PTX or cetuximab alone
moderately induced apoptosis in mice tumors compared to the
untreated control, PTX and cetuximab combined treatment
significantly upregulated the expressions of TUNEL-positive cells
in mice tumors than all other treatment groups or control (Fig. 7).
Discussion
In this study, we have shown the efficacy of PTX in
combination with cetuximab on OSCC both in vitro and in
vivo. In addition, the results suggest that cetuximab may
enhance the effect of PTX on OSCC through the downregulation of p65
NF-κB.
Cetuximab has attracted attention over the years
because it is thus far the most effective drug shown to improve
survival when combined with cisplatin and 5-fluorouracil (5-FU) in
recurrent and/or metastatic squamous cell carcinoma of the head and
neck (R/M-SCCHN) (31). Briefly,
adding cetuximab to platinum/5-FU as first-line treatment of
R/M-SCCHN significantly improved median overall survival (OS) by
2.7 months versus chemotherapy alone, without adversely impacting
patients’ quality of life (31,32).
Based on these reports, we consider that the current first-line
standard treatment approach for R/M-SCCHN must be the combination
of platinum/5-FU and cetuximab. However, it has been reported
recently that the weekly PTX-cetuximab combination might be an
effective first-line treatment option for patients in a poor state
of health and for patients with R/M-SCCHN, particularly where
platinum-based chemotherapy is contraindicated and there are few
treatment options (33,34). However, the mechanism of antitumor
effect of PTX in combination with cetuximab is still unclear.
First of all, we examined whether EGFR protein was
consistently expressed in all OSCC cell lines studied. Our results
revealed that expression of EGFR proteins varied among different
OSCC cell lines. HSC2 cells had the highest levels of EGFR
expression, while HSC3 cells expressed moderate levels of EGFR
protein and HSC4 contained the lowest level of EGFR protein. This
variable EGFR expression in different cell lines might be related
with the characteristics of primary tumor tissues. Moreover, we
detected very low EGFR proteins expression in Wi-38 (Fig. 1).
In accordance with our expectation, cetuximab
exerted the strongest growth inhibitory effect in HSC2 cells, while
it exhibited the lowest growth inhibitory effect in HSC4. In
addition, cetuximab did not suppress the cell growth of Wi-38
(Fig. 2A). The activity of
cetuximab may be related to the level of EGFR protein expression in
OSCC cells. On the other hand, PTX exerted the strongest growth
inhibitory effect in HSC4 cell, and it also suppressed the cell
growth of Wi-38 (Fig. 2B).
Interestingly, PTX in combination with cetuximab showed strong
growth inhibitory effect in all three OSCC cell lines. Therefore,
it appears that the level of EGFR expression alone cannot be
considered sufficient as a predictive marker of PTX-cetuximab
combined activity. Janmaat et al reported similar
observations in non-small cell lung cancer cells, where the EGFR
expression level showed no correlation with sensitivity to
gefitinib and cetuximab (34).
Our Hoechst staining data showed that, apoptosis was
induced almost equally in all three OSCC cell lines after
PTX-cetuximab combination treatment (Fig. 3). Therefore, we assumed that the
growth inhibitory effect of PTX and cetuximab combined treatment
was due to apoptosis. Cetuximab might be acting as a enhancer of
the PTX-induced apoptosis in PTX-cetuximab combination treatment,
as cetuximab alone could not exert strong apoptosis inducing
activity on OSCC cells (Fig. 3).
PTX is reported to exert antitumor effects on cancer cells by
inhibiting cell division, however, it can also upregulate NF-κB
activity, which might lead to defend cancer cells from
chemotherapeutic agents (30). We
observed similar results in case of PTX single treatment on OSCC
(Fig. 4). Therefore, we examined
whether cetuximab can regulate the expression of p65 NF-κB induced
by PTX. As we expected, the expression of p65 NF-κB was reduced
after PTX-cetuximab combination treatment both in vitro and
in vivo, which suggests that cetuximab can regulate
PTX-induced p65 NF-κB activity in OSCC cells (Figs. 4 and 6). The precise mechanism responsible for
the antitumor effects of PTX and cetuximab combined treatment in
OSCC is still unclear and will require further study. Nevertheless,
the combination of PTX and cetuximab could be a promising
therapeutic strategy for OSCC patients with poor prognosis. Future
studies should aim at defining the most appropriate dose and
schedule of administration of this combination treatment.
Acknowledgements
This study was supported in part by a Grant-in-Aid
from the Japanese Ministry of Education, Science and Culture.
References
|
1
|
Exarchos KP, Goletsis Y and Fotiadis DI: A
multiscale and multiparametric approach for modeling the
progression of oral cancer. BMC Med Inform Decis Mak. 12:1362012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global Cancer Statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
3
|
Tanaka T, Tanaka M and Tanaka T: Oral
carcinogenesis and oral cancer chemoprevention (review). Patholog
Res Int. 2011:4312462011.PubMed/NCBI
|
|
4
|
Inagi K, Takahashi H, Okamoto M, Nakayama
M, Makoshi T and Nagai H: Treatment effects in patients with
squamous cell carcinoma of the oral cavity. Acta Otolaryngol
(Suppl). 547:25–29. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shingaki S, Takada M, Sasai K, Bibi R,
Kobayashi T, Nomura T and Saito C: Impact of lymph node metastasis
on the pattern of failure and survival in oral carcinomas. Am J
Surg. 185:278–284. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wise-Draper TM, Draper DJ, Gutkind JS,
Molinolo AA, Wikenheiser-Brokamp KA and Wells SI: Future directions
and treatment strategies for head and neck squamous cell
carcinomas. Transl Res. 160:167–177. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Russell JS and Colevas AD: The use of
epidermal growth factor receptor monoclonal antibodies in squamous
cell carcinoma of the head and neck. Chemother Res Pract.
2012:7615182012.PubMed/NCBI
|
|
8
|
Vincenzi B, Zoccoli A, Pantano F, Venditti
O and Galluzzo S: Cetuximab: from bench to bedside. Curr Cancer
Drug Targets. 10:80–95. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peng D, Fan Z, Lu Y, DeBlasio T, Scher H
and Mendelsohn J: Anti-epidermal growth factor receptor monoclonal
antibody 225 upregulates p27KIP1 and induces G1 arrest
in prostatic cancer cell line DU145. Cancer Res. 56:3666–3669.
1996.PubMed/NCBI
|
|
10
|
Wani MC, Taylor HL, Wall ME, Coggon P and
McPhail AT: Plant antitumor agents. VI The isolation and structure
of taxol, a novel antileukemic and antitumor agent from Taxus
brevifolia. J Am Chem Soc. 93:2325–2327. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rowinsky EK, Donehower RC, Jones RJ and
Tucker RW: Microtubule changes and cytotoxicity in leukemic cell
lines treated with taxol. Cancer Res. 48:4093–4100. 1988.PubMed/NCBI
|
|
12
|
Schiff PB, Fant J and Horwitz SB:
Promotion of microtubule assembly in vitro by taxol. Nature.
277:665–667. 1979. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Langer CJ, Lee JW, Patel UA, Shin DM,
Argiris AE, Quon H, Ridge JA and Forastiere AA: Concurrent
radiation (RT), cisplatin (DDP) and cetuximab (C) in unresectable,
locally advanced (LA) squamous cell carcinoma of the head and neck
(SCCHN). J Clin Oncol. 26:20(abst 6006). 2008.PubMed/NCBI
|
|
14
|
Bonner JA, Harari PM, Giralt J, Cohen RB,
Jones CU, Sur RK, Raben D, Baselga J, Spencer SA, Zhu J,
Youssoufian H, Rowinsky EK and Ang KK: Radiotherapy plus cetuximab
for locoregionally advanced head and neck cancer: 5-year survival
data from a phase 3 randomised trial, and relation between
cetuximab-induced rash and survival. Lancet Oncol. 11:21–28.
2010.PubMed/NCBI
|
|
15
|
Sosa AE, Grau JJ, Feliz L, Pereira V,
Alcaraz D, Muñoz-García C and Caballero M: Outcome of patients
treated with palliative weekly paclitaxel plus cetuximab in
recurrent head and neck cancer after failure of platinum-based
therapy. Eur Arch Otorhinolaryngol. 271:373–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiménez B, Trigo JM, Pajares BI, Sáez MI,
Quero C, Navarro V, Llácer C, Medina L, Rueda A and Alba E:
Efficacy and safety of weekly paclitaxel combined with cetuximab in
the treatment of pretreated recurrent/metastatic head and neck
cancer patients. Oral Oncol. 49:182–185. 2013.PubMed/NCBI
|
|
17
|
Hitt R, Irigoyen A, Cortes-Funes H, Grau
JJ, García-Sáenz JA and Cruz-Hernandez JJ; Spanish Head and Neck
Cancer Cooperative Group (TTCC). Phase II study of the combination
of cetuximab and weekly paclitaxel in the first-line treatment of
patients with recurrent and/or metastatic squamous cell carcinoma
of head and neck. Ann Oncol. 23:1016–1022. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wong YN, Litwin S, Vaughn D, Cohen S,
Plimack ER, Lee J, Song W, Dabrow M, Brody M, Tuttle H and Hudes G:
Phase II trial of cetuximab with or without paclitaxel in patients
with advanced urothelial tract carcinoma. J Clin Oncol.
30:3545–3551. 2012. View Article : Google Scholar
|
|
19
|
Mecca C, Ponzetti A, Caliendo V, Ciuffreda
L and Lista P: Complete response of metastatic cutaneous squamous
cell carcinoma to cetuximab plus paclitaxel. Eur J Dermatol.
22:758–761. 2012.PubMed/NCBI
|
|
20
|
Beg AA, Sha WC, Bronson RT, Ghosh S and
Baltimore D: Embryonic lethality and liver degeneration in mice
lacking the RelA component of NF-κB. Nature. 376:167–170.
1995.PubMed/NCBI
|
|
21
|
Cai Z, Korner M, Tarantino N and Chouaib
S: IκB-α overexpression in human breast carcinoma MCF7 cells
inhibits nuclear factor-κB activation but not tumor necrosis
factor-α-induced apoptosis. J Biol Chem. 272:96–101. 1997.
|
|
22
|
Bargou RC, Emmerich F, Krappmann D,
Bommert K, Mapara MY, Arnold W, Royer HD, Grinstein E, Greiner A,
Scheidereit C and Dorken B: Constitutive nuclear factor-kappa
B-RelA activation is required for proliferation and survival of
Hodgkin’s disease tumor cells. J Clin Invest. 100:2961–2969.
1997.PubMed/NCBI
|
|
23
|
Sovak MA, Bellas RE, Kim DW, Zanieski GJ,
Rogers AE, Traish AM and Sonenshein GE: Aberrant nuclear
factor-κB/Rel expression and the pathogenesis of breast cancer. J
Clin Invest. 100:2952–2960. 1997.
|
|
24
|
Shen HM and Tergaonkar V: NFkappaB
signaling in carcinogenesis and as a potential molecular target for
cancer therapy (review). Apoptosis. 14:348–363. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Beg AA and Baldwin AS Jr: The IκB
proteins: multifunctional regulators of Rel/NF-κB transcription
factors. Genes Dev. 7:2064–2070. 1993.
|
|
26
|
Duffey D, Chen Z, Dong G, Ondrey FG, Wolf
JF, Brown K, Siebenlist U and Van Waes C: Expression of a
dominant-negative mutant inhibitor-κBα of nuclear factor-κB in
human head and neck squamous cell carcinoma inhibits survival,
proinflammatory cytokine expression, and tumor growth in vivo.
Cancer Res. 59:3468–3474. 1999.
|
|
27
|
Wang CY, Mayo MW and Baldwin AS Jr: TNF-
and cancer therapy-induced apoptosis: Potentiation by inhibition of
NF-κB. Science. 274:784–787. 1996.PubMed/NCBI
|
|
28
|
Wang CY, Mayo MW, Korneluk RG, Goeddel DV
and Baldwin AS Jr: NF-κB antiapoptosis: Induction of TRAF1 and
TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation.
Science. 281:1680–1683. 1998.
|
|
29
|
Das KC and White CW: Activation of NF-κB
by antineoplastic agents. J Biol Chem. 272:14914–14920. 1997.
|
|
30
|
Spencer W, Kwon H, Crépieux P, Leclerc N,
Lin R and Hiscott J: Taxol selectively blocks microtubule dependent
NF-κB activation by phorbol ester via inhibition of IκB-α
phosphorylation and degradation. Oncogene. 18:495–505.
1999.PubMed/NCBI
|
|
31
|
Vermorken JB, Mesia R, Rivera F, Remenar
E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol
D, Peyrade F, Benasso M, Vynnychenko I, De Raucourt D, Bokemeyer C,
Schueler A, Amellal N and Hitt R: Platinum-based chemotherapy plus
cetuximab in head and neck cancer. N Engl J Med. 359:1116–1127.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mesia R, Rivera F, Kawecki A, Rottey S,
Hitt R, Kienzer H, Cupissol D, De Raucourt D, Benasso M, Koralewski
P, Delord JP, Bokemeyer C, Curran D, Gross A and Vermorken JB:
Quality of life of patients receiving platinum-based chemotherapy
plus cetuximab first line for recurrent and/or metastatic squamous
cell carcinoma of the head and neck. Ann Oncol. 21:1967–1973. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Leon X, Hitt R, Constenla M, Rocca A,
Stupp R, Kovács AF, Amellal N, Bessa EH and Bourhis J: A
retrospective analysis of the outcome of patients with recurrent
and/or metastatic squamous cell carcinoma of the head and neck
refractory to a platinum-based chemotherapy. Clin Oncol (R Coll
Radiol). 17:418–424. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Janmaat ML, Kruyt FA, Rodriguez JA and
Giaccone G: Response to epidermal growth factor receptor inhibitors
in nonsmall cell lung cancer cells: limited antiproliferative
effects and absence of apoptosis associated with persistent
activity of extracellular signal-regulated kinase or Akt kinase
pathways. Clin Cancer Res. 9:2316–2326. 2003.
|