Introduction
Colorectal cancer (CRC) is one of the most common
cancers worldwide (1). The high
morbidity and mortality associated with CRC is frequently caused by
development of metastasis, i.e., liver metastasis (2). This liver metastasis determines the
survival rates of CRC patients. The development of liver metastasis
comprises many steps: proliferation of the primary tumor, transfer
to the circulating blood, adhesion to the liver sinusoids, and
extravasation and proliferation into the liver (3). Adhesion of the circulating tumor
cells (TCs) to the liver sinusoids is the most important step, and
thus, suppression of this TC adhesion is crucial for the control of
liver metastasis. Several authors have focused on the various steps
of liver metastasis in animal models, and Kupffer cells (KCs) have
been revealed to play an important role in the metastasis (4–6). KCs
had a promotive effect on liver metastasis; for example, the
release of inflammatory cytokines and matrix metalloproteinase by
KCs caused facilitation of adhesion, extravasation, and
proliferation of TCs (7–11). On the other hand, a suppressive
effect of KCs on liver metastasis was demonstrated by their
capacity to kill TCs (12–16). Therefore, the role of KCs in liver
metastasis remains controversial. The previous studies analyzed
data obtained from fixed specimens; i.e., they did not observe TCs
in real time. However, with intravital microscopy (IVM), the
interaction of KCs and TCs in the same location can be
consecutively observed in real time. We sought, therefore, to
analyze in real time the relationship between KCs and TCs in liver
metastasis by means of IVM.
Materials and methods
Animals
Male Fischer 344 (F344) rats, weighing 200–250 g,
were obtained from CLEA Japan, Inc. (Tokyo, Japan). The animal
experiments were carried out in a humane manner after approval was
received from the Animal Experiment Committee of the University of
Tsukuba.
Cell lines
RCN-H4 cells (17,18),
derived from liver metastasis of an F344 rat colon adenocarcinoma
cell line, were provided by the Riken Cell Bank (Ibaraki, Japan).
The cells were maintained in RPMI-1640 medium supplemented with 10%
heat-inactivated FBS, 100 U/ml penicillin G, and 100 g/ml
streptomycin in humidified 95% air-5% CO2 at 37°C.
KC elimination
KCs were eliminated by use of Cl2MDP
liposomes, as reported by van Rooijen et al (19). The liposomes were injected into the
rats via the tail vein (1 ml/rat). Twenty-four hours after the
injection, no KCs were found. Repopulation of the KCs started at
day 3 after the injection and was completed at day 8 (20).
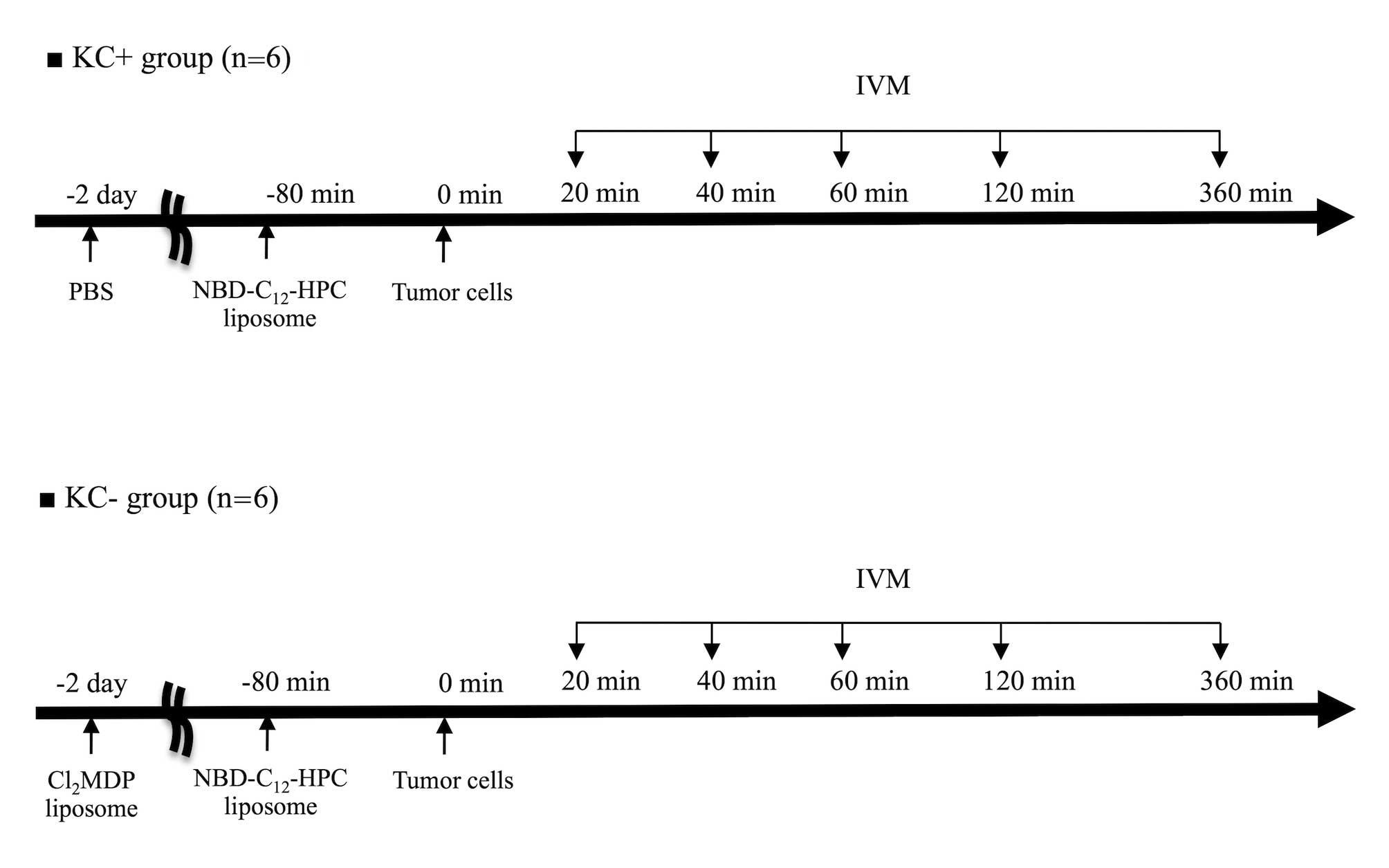
IVM model
The rats were divided into two groups: the control
group (KC+ group; n=6) and the KC elimination group (KC- group;
n=6). In the KC+ group, calcium- and magnesium-free PBS (CMF-PBS)
was injected via the tail vein (1 ml/rat) 48 h before the
operation. In the KC- group, Cl2MDP liposomes were
injected intravenously via the tail vein (1 ml/rat) 48 h before the
operation.
Fluorescence labeling for IVM (labeling
of KCs)
As previously reported, KCs were labeled with
fluorescence dye by means of the liposome entrapment method
(21). Fluorescently labeled
phosphatidylcholine was incorporated into the liposomes according
to the method of Watanabe et al (22). The fluorescent pigment used was
2-(12-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)
amino)dodecanoyl-1-hexadecanoyl-sn-glycero-3-phosphocholine
(NBD-C12-HPC) (Molecular Probes, Eugene, OR, USA).
Eighty minutes before the TC injection, the liposomes (4 ml/kg)
were administered via a carotid artery catheter. After that, the
KCs in the rat livers were stained and were clearly delineated in
the fluorescent IVM image.
Labeling of TCs
After trypsinization, the TCs were washed with
CMF-PBS and kept in serum-free medium (RPMI-1640) for 60 min for
reconstitution of the cell surfaces. During reconstitution, the
cells were incubated with rhodamine 6G (10-7 M; Sigma-Aldrich
Japan, Tokyo, Japan) to label them for the fluorescence microscopy.
Subsequent to rhodamine-6G staining, the percentage of living cells
exceeded 90%, as assessed by the trypan blue exclusion method.
After a wash with CMF-PBS, the cells were resuspended as a
single-cell solution in CMF-PBS at a final concentration of
5×106 cells/ml. No detrimental influence of the
preparation on the cells’ migratory or adhesive activity was
observed.
IVM
IVM were performed as previously reported (21). The rats were tracheotomized under
isoflurane-induced anesthesia. After a transverse laparotomy, the
left hepatic lobe was exteriorized and placed on a plate specially
designed to minimize movements caused by respiration and then
covered with a gridded coverslip (Olympus Corporation, Tokyo,
Japan) (23). Using the gridded
coverslips, selected regions of interest could be relocated at
later time points. The TCs (5×106 cells) were
intra-arterially injected for 60 sec (24). TC injection via portal vein caused
portal tumor embolism, therefore we took a conscious dicision to TC
injection via intra-artery, as reported by other authors (24,25).
This route of cell application was reported not to influence the
adhesive behavior of the cells within the liver sinusoids (26). The circulating TCs in the sinusoids
were observed at 30 sec after the end of the TC injection. IVM was
performed using a modified microscope (BX30 FLA-SP; Olympus
Corporation). The hepatic microcirculation was recorded by means of
a CCD camera (DVC-0; DVC, Austin, TX, USA) and a digital video
recorder (GV-HD700/1; Sony, Tokyo, Japan) for offline analysis.
Using objective lenses (10× 0.3 – 20× 0.7; Olympus Corporation), a
final magnification of ×325–×650 was achieved on the video screen.
In each rat, analysis of the liver microcirculatory para meters was
performed in 10 randomly selected acini at 20, 40, 60, 120, and 360
min after the TC injection (Fig.
1). The observation time for each field was 30 sec. The grid
number was recorded, so that the same acini could be observed at
each time point. At the end of the observation, the rats were
euthanized by exsanguination. The microcirculatory parameters were
quantitatively assessed using WinROOF imaging software (version
5.0; Mitani Shoji Co., Ltd, Tokyo, Japan).
Microcirculatory analysis of KCs and
TCs
The following parameters were analyzed: ) the number
of adherent TCs, i.e., the TCs firmly adherent to the sinusoids for
longer than 20 sec [The number of adherent TCs in the scanned acini
was counted and the results were expressed as the number of
adherent TCs per field (1 field = ~0.2 mm2)]; ) the
number of TCs adherent to KCs, i.e., the TCs detected at the same
locations as the KCs; and ) the number of KCs.
Biochemical analysis
As markers of KC activation, tumor necrosis factor
(TNF)-α and interleukin (IL)-1β levels in liver tissues were
measured 6 h after the TC injection using a commercial
enzyme-linked immunosorbent assay kit (R&D Systems,
Minneapolis, MN, USA).
Transmission electron microscopy
KCs after the TC injection were assessed by
transmission electron microscopy. Two hours after the injection,
the livers were quickly resected. Tissue samples from the right
lobe were cut into 1-mm3 cubes and stored in 2.5%
glutaraldehyde. The specimens were post-fixed with osmium
tetroxide, dehydrated through a graded alcohol series, and embedded
in Epon mixture. Ultrathin sections were prepared using an Ultracut
S microtome (Leica Aktiengesellschaft, Vienna, Austria) and mounted
on copper grids. The sections were treated with uranyl acetate and
lead citrate to enhance the contrast. Specimens were examined using
a Hitachi H-7000 transmission electron microscope (Hitachi, Tokyo,
Japan).
KC elimination model before and after TC
injection
To evaluate which timing of the KC elimination and
TC injection was involved in liver metastasis, the rats were
divided into five groups: the KC+ group without the
Cl2MDP liposome injection (n=6); the KC- group with the
Cl2MDP liposome injection 2 days before the TC injection
(n=6); and the KC- group with the Cl2MDP liposome
injection 1, 3, and 7 days after the TC injection (KC 1D- group, KC
3D- group, and KC 7D- group, respectively; n=6 each; Fig. 2). In all groups, 5×108
RCN-H4 cells were injected via the left carotid artery. The number
of metastatic nodules in the liver was evaluated by hematoxylin and
eosin (H&E) staining of the liver sections obtained 2 weeks
after the TC injection.
Histologic analysis
Liver tissues were obtained from each rat, fixed
with 10% formaldehyde, and embedded in paraffin. The left and
medial lobes were evenly cut into 10 slices. Thin sections (4 μm)
were prepared and stained with H&E. The number of the
metastatic nodules was assessed using WinROOF software.
Statistical analysis
All data were expressed as the means ± standard
errors of the mean (SEMs). The Mann-Whitney test and analysis of
variance were used, followed by the Scheffé’s test. P<0.05 was
considered significant.
Results
IVM model
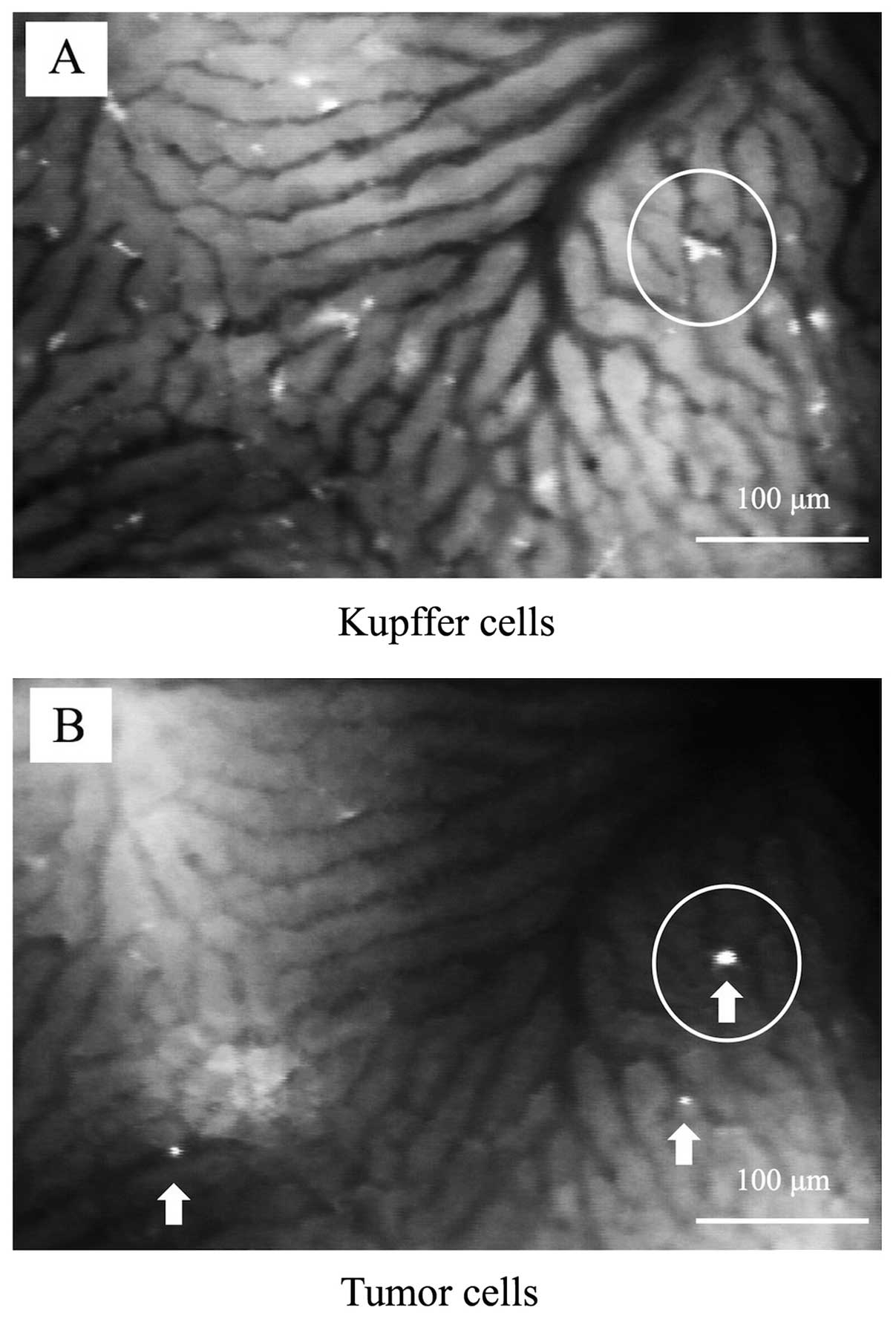
The circulating TCs rapidly adhered to the
sinusoids, and some adherent TCs were detected in the same
locations as the KCs (Fig. 3A and
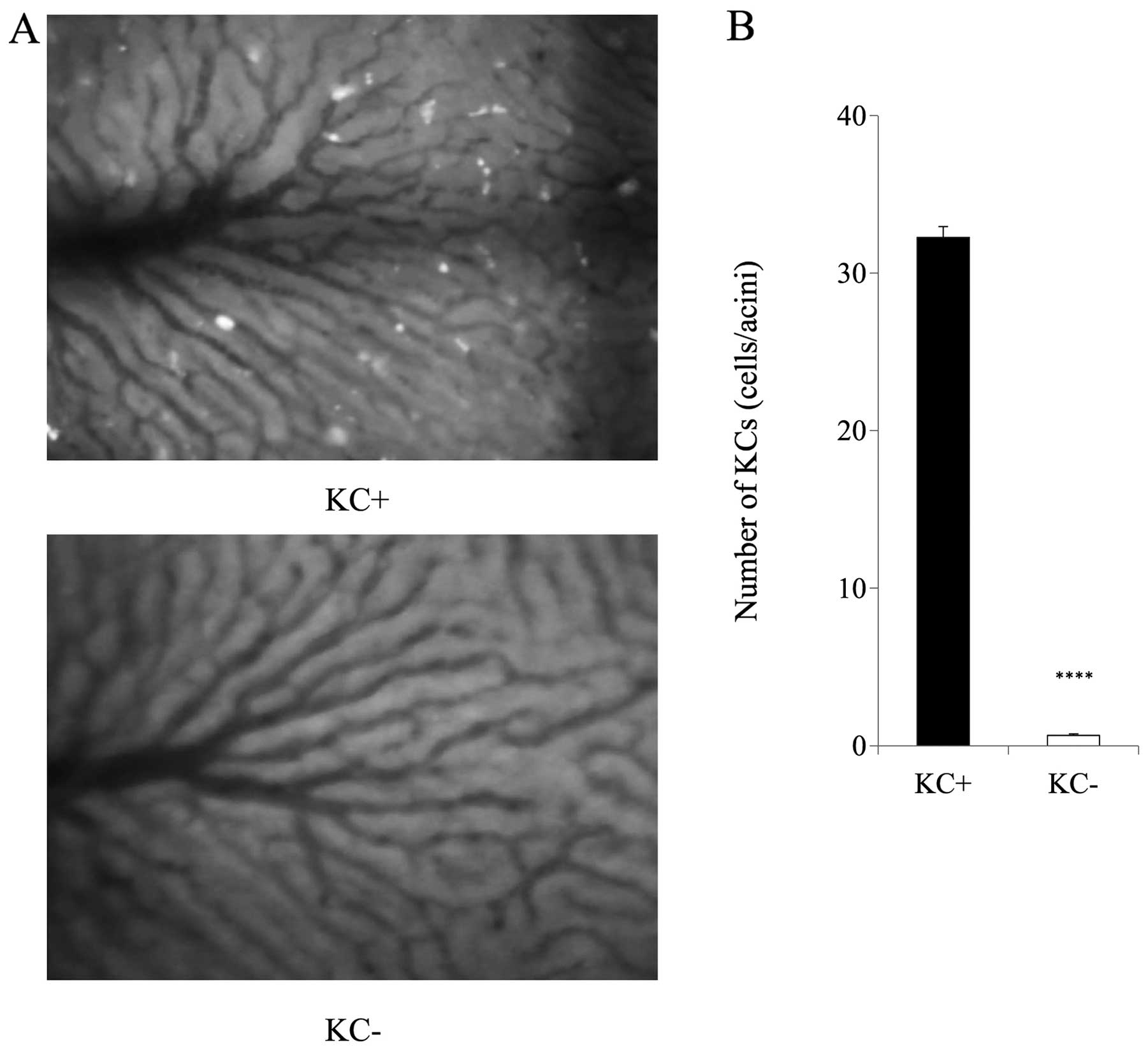
B). The number of KCs in the sinusoids was significantly
decreased in the KC- group when compared with the KC+ group before
the TC injection (Fig. 4A and B).
Cl2MDP successfully eliminated KCs from the liver
tissue. No side-effects of Cl2MDP administration, such
as liver injury, were observed.
Number of adherent TCs in the
sinusoids
The total number of adherent TCs in the sinusoids
peaked at 20 min after the TC injection and then decreased in both
groups (Fig. 5A). Regardless of
the presence of KCs, the total number of adherent TCs was almost
the same in both groups until 2 h after the injection. However, the
total number of adherent TCs at 6 h after the injection in the KC+
group was less than that in the KC- group. The adherent TCs in the
same location as the KCs comprised 19% of the total number of
adherent TCs at 20 min and increased over time, i.e., by 74% at 6 h
(Fig. 5B).
TNF-α and IL-1β in liver tissue
The concentrations of TNF-α and IL-1β in the liver
tissue were significantly elevated at 6 h after the TC injection in
the KC+ group (Fig. 6A and B).
Transmission electron microscopy
In the transmission electron microscopy findings,
some TCs were adherent to the KCs in the liver sinusoids. All of
the TCs were phagocytosed by the KCs (Fig. 7A and B).
KC elimination model before and after TC
injection
In the KC- group, the number of liver metastatic
nodules was remarkably increased when compared with that in the KC+
group (Fig. 8A). A large number of
metastatic nodules were observed in the portal area. The morphology
of the metastatic nodules in both groups was the same (Fig. 8B). In the KC+ group, the number of
metastatic nodules was 0.15±0.04 per slice for the left and middle
liver lobes. In the KC- group, in which Cl2MDP liposomes
had been administered 2 days before the TC injection, the
metastases were markedly increased (57.55±4.41; Fig. 9). In the other three KC- groups, in
which the Cl2MDP liposome injection had been
administered 1–7 days after the TC injection, there were no
significant differences in the number of metastases as compared
with the KC+ group (Fig. 9). The
numbers of metastatic nodules were 3.91±0.43, 0.19±0.04, and
0.19±0.05 in the KC 1D- group, KC 3D- group, and KC 7D- group,
respectively.
Discussion
Proliferation of TCs in the liver after migration
from the primary lesion causes CRC metastases to the liver. Some
patients with CRC or CRC liver metastasis had TCs in the
circulating blood (27–30). KCs play an important role in liver
metastasis; however, whether the role of KCs in the metastasis is
promotive or suppressive remains unclear (6–16,31,32).
Previous studies consisted mostly of histologic evaluations of
fixed specimens (31,33,34),
and the relationship between KCs and TCs in the same individual was
not observed in real time. To clarify the role of KCs, especially
in the early stage of liver metastasis, we used IVM to observe
adhesion of TCs to the liver sinusoids. Our results showed that the
total number of adherent TCs was significantly increased by
elimination of KCs. Moreover, in the metastasis formation model,
the number of liver metastases was increased by elimination of KCs.
These results suggested that KCs suppressed liver metastasis.
Furthermore, we found that the number of adherent TCs in the
sinusoids in the early stage of liver metastasis was strongly
related with the formation of the liver metastatic nodules
thereafter.
IVM allows for consecutive observation of the same
region, and we could observe the adhesion of the TCs to the
sinusoids for up to 6 h after the TC injection. We found that the
total number of adherent TCs in the KC+ group decreased
time-dependently, and at 6 h after the TC injection, it was
significantly smaller than that in the KC- group. Moreover, in the
KC+ group, the percentage of adherent TCs detected at the same
locations as the KCs increased time-dependently. These results
strongly suggested that the KCs interacted with the TCs, and to
elucidate this fact, we used transmission electron microscopy to
demonstrate that the KCs phagocytosed the TCs. The result clearly
indicated that the TCs adherent to the KCs were caught by the
pseudopodia of the KCs and that the KCs phagocytosed the TCs,
resulting in the decreased number of adherent TCs after the TC
injection. Previous studies observed that a coculture of KCs and
TCs led to the formation of pseudopodia in KCs and that the KCs
attached to the TCs in vitro (35). Moreover, the TCs that were strongly
caught by the pseudopodia of the KCs were phagocytosed and
destroyed by the KCs (36).
Timmers et al observed that KCs interacted with most
injected TCs and phagocytosed most of them in vivo (16). Therefore, it was strongly suggested
that KCs phagocytosed TCs in the early stage of liver metastasis
and suppressed formation of liver metastasis.
KC phagocytosis of TCs needs activation of the KCs
(36). To evaluate the activation
of KCs, we investigated the cytokines involved in KCs, i.e., TNF-α
and IL-1β (37–39), in liver tissue. In the KC+ group,
both cytokines were elevated 6 h after the TC injection. TCs have
been reported to activate KCs; i.e., TCs led to secretion of TNF-α
involved in KCs in vitro (33) and to increase of peroxidase
activity in KCs in vivo (40). Moreover, KC activation, which was
induced by OK432, an immunopotentiator prepared from streptococci,
led to a decrease in liver metastasis in a rat model (6). It was postulated that this effect
resulted from the augmentation of the tumoricidal activity and
antigen-presenting activity of the KCs (6). Mise et al reported that OK432
increased the TNF production and tumoricidal activity of KCs in
vitro (32). Therefore, the
augmentation of KC tumoricidal activity might be a strategy to
prevent liver metastases (6).
Bayon et al demonstrated tumoricidal activity of KCs in
vitro using rat colon adenocarcinoma CC531 cells, and reported
that TC injection after the elimination of KCs led to increased
liver metastasis in vivo study (31). From these reports and our results,
we concluded that the activation of KCs was strongly associated
with the TC injection.
After clarifying our results that KCs are necessary
to suppress liver metastasis, we next sought to elucidate when the
presence of KCs is necessary for suppression of liver metastasis.
In the groups in which KCs were eliminated 1–7 days after the TC
injection, the number of metastatic nodules was significantly
decreased when compared with the group in which KCs were eliminated
before the TC injection. It has been reported that circulating TCs
were increased by surgical manipulation. Nishizaki et al
reported that liver metastasis progressed after surgical
manipulation in a VX-2 carcinoma cell line in a rabbit model
(41). In clinical studies of
patients with resectable CRC, circulating TCs were increased by
surgical manipulation, and the presence of the circulating TCs
negatively affected the prognosis after surgery (42). In another clinical study,
circulating TCs were detected in 20–30% of patients with liver
metastases from CRC before surgery, and the rate was increased to
50% during liver resection (27,43).
Koch et al postulated that intraoperative tumor
manipulation, which resulted in an enhanced release of TCs, caused
tumor recurrence, at least in patients undergoing liver resection
for colorectal metastases (27).
Some studies demonstrated that the presence of circulating TCs
within 24 h of CRC resection significantly increased the rate of
recurrence (27,29,30).
The circulating TCs, which were increased by surgical manipulation,
were considered to contribute to the recurrence of liver metastasis
following curative surgical resection. These findings suggested
that the augmentation of KC phagocytosis of TCs had the potential
to suppress liver metastasis after CRC surgical resection.
To our knowledge, this is the first study to show
adhesion between KCs and TCs in real time by using IVM. The TCs
adhered to the KCs, and the KCs were then activated and
phagocytosed the TCs. We also demonstrated that KCs played a
suppressive role in liver metastasis. In this study, the role of
KCs in the early stage of liver metastasis was revealed. Future
study of the detailed mechanism of KC phagocytosis of TCs may
provide new therapies for the prevention of liver metastasis after
curative resection of CRC.
Acknowledgements
This study was supported in part by the Japanese
Ministry of Education, Culture, Sports, Science, and Technology of
Japan (MEXT) (Kakenhi no. 25462078). We thank F. Miyamasu for
grammatical revision.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2
|
van der Bij GJ, Bögels M, Otten MA, et al:
Experimentally induced liver metastases from colorectal cancer can
be prevented by mononuclear phagocyte-mediated monoclonal antibody
therapy. J Hepatol. 53:677–685. 2010.
|
|
3
|
Gout S and Huot J: Role of cancer
microenvironment in metastasis: focus on colon cancer. Cancer
Microenviron. 1:69–83. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Williams DL, Sherwood ER, McNamee RB,
Jones EL and Di Luzio NR: Therapeutic efficacy of glucan in a
murine model of hepatic metastatic disease. Hepatology. 5:198–206.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heuff G, Oldenburg HS, Boutkan H, et al:
Enhanced tumour growth in the rat liver after selective elimination
of Kupffer cells. Cancer Immunol Immunother. 37:125–130. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang W, Arii S, Sasaoki T, et al: The
role of Kupffer cells in the surveillance of tumor growth in the
liver. J Surg Res. 55:140–146. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thomas P, Hayashi H, Zimmer R and Forse
RA: Regulation of cytokine production in carcinoembryonic antigen
stimulated Kupffer cells by beta-2 adrenergic receptors:
implications for hepatic metastasis. Cancer Lett. 209:251–257.
2004. View Article : Google Scholar
|
|
8
|
Gangopadhyay A, Lazure DA and Thomas P:
Carcinoembryonic antigen induces signal transduction in Kupffer
cells. Cancer Lett. 118:1–6. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gangopadhyay A, Lazure DA and Thomas P:
Adhesion of colorectal carcinoma cells to the endothelium is
mediated by cytokines from CEA stimulated Kupffer cells. Clin Exp
Metastasis. 16:703–712. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Auguste P, Fallavollita L, Wang N, Burnier
J, Bikfalvi A and Brodt P: The host inflammatory response promotes
liver metastasis by increasing tumor cell arrest and extravasation.
Am J Pathol. 170:1781–1792. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Christophi C, Harun N and Fifis T: Liver
regeneration and tumor stimulation - a review of cytokine and
angiogenic factors. J Gastrointest Surg. 12:966–980. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roh MS, Wang L, Oyedeji C, et al: Human
Kupffer cells are cytotoxic against human colon adenocarcinoma.
Surgery. 108:400–405. 1990.PubMed/NCBI
|
|
13
|
Curley SA, Roh MS, Feig B, Oyedeji C,
Kleinerman ES and Klostergaard J: Mechanisms of Kupffer cell
cytotoxicity in vitro against the syngeneic murine colon
adenocarcinoma line MCA26. J Leukoc Biol. 53:715–721.
1993.PubMed/NCBI
|
|
14
|
Thomas C, Nijenhuis AM, Dontje B, Daemen T
and Scherphof GL: Tumoricidal response of liver macrophages
isolated from rats bearing liver metastases of colon
adenocarcinoma. J Leukoc Biol. 57:617–623. 1995.PubMed/NCBI
|
|
15
|
Heuff G, Steenbergen JJ, Van de Loosdrecht
AA, et al: Isolation of cytotoxic Kupffer cells by a modified
enzymatic assay: a methodological study. J Immunol Methods.
159:115–123. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Timmers M, Vekemans K, Vermijlen D, et al:
Interactions between rat colon carcinoma cells and Kupffer cells
during the onset of hepatic metastasis. Int J Cancer. 112:793–802.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Inoue Y, Kashima Y, Aizawa K and
Hatakeyama K: A new rat colon cancer cell line metastasizes
spontaneously: biologic cha racteristics and chemotherapeutic
response. Jpn J Cancer Res. 82:90–97. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Okuno K, Hirai N, Lee YS, Kawai I,
Shigeoka H and Yasutomi M: Involvement of liver-associated immunity
in hepatic metastasis formation. J Surg Res. 75:148–152. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van Rooijen N and Kors N: Effects of
intracellular diphosphonates on cells of the mononuclear phagocyte
system: in vivo effects of liposome-encapsulated diphosphonates on
different macrophage subpopulations in the spleen. Calcif Tissue
Int. 45:153–156. 1989.
|
|
20
|
Bogers WM, Stad RK, Janssen DJ, et al:
Kupffer cell depletion in vivo results in clearance of large-sized
IgA aggregates in rats by liver endothelial cells. Clin Exp
Immunol. 85:128–136. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tamura T, Kondo T, Pak S, et al:
Interaction between Kupffer cells and platelets in the early period
of hepatic ischemia-reperfusion injury - an in vivo study. J Surg
Res. 178:443–451. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Watanabe R, Munemasa T, Matsumura M and
Fujimaki M: Fluorescent liposomes for intravital staining of
Kupffer cells to aid in vivo microscopy in rats. Methods Find Exp
Clin Pharmacol. 29:321–327. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kondo T, Okamoto S, Todoroki T, Hirano T,
Schildberg FW and Messmer K: Application of a novel method for
subsequent evaluation of sinusoids and postsinusoidal venules after
ischemia-reperfusion injury of rat liver. Eur Surg Res. 30:252–258.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Enns A, Korb T, Schlüter K, et al:
Alphavbeta5-integrins mediate early steps of metastasis formation.
Eur J Cancer. 41:1065–1072. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schlüter K, Gassmann P, Enns A, et al:
Organ-specific metastatic tumor cell adhesion and extravasation of
colon carcinoma cells with different metastatic potential. Am J
Pathol. 169:1064–1073. 2006.PubMed/NCBI
|
|
26
|
Haier J, Korb T, Hotz B, Spiegel HU and
Senninger N: An intravital model to monitor steps of metastatic
tumor cell adhesion within the hepatic microcirculation. J
Gastrointest Surg. 7:507–514. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Koch M, Kienle P, Hinz U, et al: Detection
of hematogenous tumor cell dissemination predicts tumor relapse in
patients undergoing surgical resection of colorectal liver
metastases. Ann Surg. 241:199–205. 2005. View Article : Google Scholar
|
|
28
|
Yamaguchi K, Takagi Y, Aoki S, Futamura M
and Saji S: Significant detection of circulating cancer cells in
the blood by reverse transcriptase-polymerase chain reaction during
colorectal cancer resection. Ann Surg. 232:58–65. 2000. View Article : Google Scholar
|
|
29
|
Allen-Mersh TG, McCullough TK, Patel H,
Wharton RQ, Glover C and Jonas SK: Role of circulating tumour cells
in predicting recurrence after excision of primary colorectal
carcinoma. Br J Surg. 94:96–105. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Patel H, Le Marer N, Wharton RQ, et al:
Clearance of circulating tumor cells after excision of primary
colorectal cancer. Ann Surg. 235:226–231. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bayón LG, Izquierdo MA, Sirovich I, van
Rooijen N, Beelen RH and Meijer S: Role of Kupffer cells in
arresting circulating tumor cells and controlling metastatic growth
in the liver. Hepatology. 23:1224–1231. 1996.PubMed/NCBI
|
|
32
|
Mise M, Arii S, Higashitsuji H, et al:
Augmented local immunity in the liver by a streptococcal
preparation, OK432, related to antitumor activity of hepatic
macrophages. Immunopharmacology. 27:31–41. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Khatib AM, Auguste P, Fallavollita L, et
al: Characterization of the host proinflammatory response to tumor
cells during the initial stages of liver metastasis. Am J Pathol.
167:749–759. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Higashi N, Ishii H, Fujiwara T,
Morimoto-Tomita M and Irimura T: Redistribution of fibroblasts and
macrophages as micrometastases develop into established liver
metastases. Clin Exp Metastasis. 19:631–638. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yonei Y, Kurose I, Fukumura D, et al:
Evidence of direct interaction between Kupffer cells and colon
cancer cells: an ultrastructural study of the co-culture. Liver.
14:37–44. 1994.PubMed/NCBI
|
|
36
|
Gardner CR, Wasserman AJ and Laskin DL:
Liver macrophage-mediated cytotoxicity toward mastocytoma cells
involves phagocytosis of tumor targets. Hepatology. 14:318–324.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Decker K: Biologically active products of
stimulated liver macrophages (Kupffer cells). Eur J Biochem.
192:245–261. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Laskin DL, Weinberger B and Laskin JD:
Functional heterogeneity in liver and lung macrophages. J Leukoc
Biol. 70:163–170. 2001.PubMed/NCBI
|
|
39
|
Roberts RA, Ganey PE, Ju C, Kamendulis LM,
Rusyn I and Klaunig JE: Role of the Kupffer cell in mediating
hepatic toxicity and carcinogenesis. Toxicol Sci. 96:2–15. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kruskal JB, Azouz A, Korideck H, et al:
Hepatic colorectal cancer metastases: imaging initial steps of
formation in mice. Radiology. 243:703–711. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nishizaki T, Matsumata T, Kanematsu T,
Yasunaga C and Sugimachi K: Surgical manipulation of VX2 carcinoma
in the rabbit liver evokes enhancement of metastasis. J Surg Res.
49:92–97. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ito S, Nakanishi H, Hirai T, et al:
Quantitative detection of CEA expressing free tumor cells in the
peripheral blood of colorectal cancer patients during surgery with
real-time RT-PCR on a LightCycler. Cancer Lett. 183:195–203. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Weitz J, Koch M, Kienle P, et al:
Detection of hematogenic tumor cell dissemination in patients
undergoing resection of liver metastases of colorectal cancer. Ann
Surg. 232:66–72. 2000. View Article : Google Scholar : PubMed/NCBI
|