Introduction
Squamous cell carcinoma of head and neck (SCCHN), a
malignant tumor of epithelial origin, represents >90% of all
head and neck cancers. The 5-year survival rate is only 30–40% in
SCCHN, the main reasons are invasion and metastasis, especially
metastasis to lymph nodes (1). The
mechanisms leading to SCCHN metastasis are incompletely
understood.
Chemokines are a group of small,
structurally-related molecules that constitute a superfamily of
inducible, secreted, proinflammatory proteins that are involved in
a variety of immune responses (2–5).
Chemokines are classified into four major groups based on the
number and spacing of conserved cysteines: CXC, CC, C and CX3C. The
CC chemokine receptor 7 (CCR7) has two ligands: CCL19 and CCL21.
The interaction between CCR7 and its ligands promotes the
migration, invasion and chemotaxis of T cells, B cells, natural
killer cells (NK cells), mature dendritic cells (DC) and some
tumors (6–9). The tumors affected by this
interaction include: esophageal squamous cell carcinoma, colorectal
carcinoma, non-small cell lung cancer, hepatocellular cancer,
breast cancer, and melanoma (10–15).
We have reported that CCR7 regulates cell migration and adhesion in
metastatic SCCHN by activating integrin αvβ3, integrin β1 and
PI3K/cdc42 (16–24). However, when these downstream
molecules are inhibited, the role of CCR7 could not be blocked
completely. Thus, we hypothesize there may be other molecules in
the CCR7 signal pathway.
Generally, chemokine receptors relay intracellular
signals that regulate chemotaxis through the Gi subfamily of G
proteins (25). These
intracellular signaling molecules include mitogen-activated protein
kinase (MAPK) family members (26–28).
MAPKs comprise a family of protein-serine/threonine kinases, which
are highly conserved in protein structures from unicellular
eukaryotic organisms to multicellular organisms, including mammals
(29). Mammalian cells contain
three major classes of MAPKs: ERK1/2, JNK, and p38. These molecules
are important regulators of chemotaxis and/or random motility in a
variety of cell types (28,30–32).
We hypothesized that MAPK members may be downstream
molecules of the CCR7 pathway induced by CCL19 in SCCHN. The goals
of this study were to determine whether MAPK members are activated
by CCR7, the role and the molecular mechanisms of MAPK in
CCR7-regulating SCCHN metastasis.
Materials and methods
Human tumor samples and cell lines
All clinical investigations were conducted according
to the principles expressed in the Declaration of Helsinki. The
study protocol was granted approval from the Ethics Committee of
the China Medical University, and all participants provided their
written informed consent to participate in this study.
SCCHN tissue specimens were obtained from 78
patients by biopsy prior to chemotherapy or radiotherapy at the
Department of Oral and Maxillofacial Surgery, School and Hospital
of Stomatology, China Medical University. The term ‘metastatic’ in
this study refers to patients with positive lymph nodes that were
recognized either at initial presentation or later based on the
histopathological diagnosis after neck dissection. The
classification of SCCHN, including primary tumors (T), regional
lymph nodes (N), distant metastasis (M) and stage grouping, was
determined according to the rules of the Union for International
Cancer Control (UICC) for head and neck cancer (Tumor node
metastasis, TNM classification, 1997). Ten samples of normal
tissues adjacent to the benign tumor were chosen as controls.
PCI-4B and PCI-37B, which are well-characterized
SCCHN cell lines that are derived from the metastatic lymph node of
SCCHN patients, were kindly donated by the University of Pittsburgh
Cancer Institute (33,34). The cells were cultured in DMEM
medium (Invitrogen Life Technologies, Carlsbad, CA, USA) containing
10% fetal bovine serum (Gibco, Carlsbad, CA, USA), 100 U/ml
penicillin G and 100 U/ml streptomycin. When inhibitors were used,
we ensured that the dosage used did not affect the viability or
expression of CCR7 of the cells.
Reagents and antibodies
CCL19, CCR7 specific monoclonal antibody (mouse
anti-human CCR7 antibody) were purchased from R&D Systems
(Minneapolis, MN, USA), PD98059 (ERK inhibitor) was purchased from
Promega Corporation (Madison, WI, USA), SP600125 (JNK inhibitor)
was purchased from Biomol GmbH (Hamburg, Germany). The
anti-phospho-JNK, anti-JNK, anti-phospho-ERK, anti-ERK,
anti-phospho-p38 MAPK, anti-p38 MAPK, anti-E-cadherin and
anti-Vimentin were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA).
Immunohistochemical staining and
evaluation
Sections were deparaffinized in xylene for 10 min
and were then rehydrated through graded alcohols. To inhibit
endogenous peroxide activity, sections were immersed in 100%
methanol containing 0.3% hydrogen peroxide for 40 min. Following
immersion, sections were put in a microwave oven in a jar filled
with 10 mM sodium citrate buffer (pH 6.0) for 10 min and cooled at
room temperature. Sections were incubated with normal goat serum
for 20 min and were then incubated with the primary antibody for 1
h. After the incubation period, sections were washed three times
with PBS and were then incubated with the linking reagent
(biotinylated anti-immunoglobulin; Zymed Laboratories, Inc., South
San Francisco, CA, USA) at room temperature for 1 h. After being
washed three times with PBS, the sections were incubated with a
complex of Avidin DH and biotinlylated enzyme (Zymed Laboratories,
Inc.) for 30 min. The sections were again washed three times with
PBS and incubated with a medium consisting of an equal volume of
0.02% hydrogen peroxide and diaminobenzidine tetrahydrochloride
(Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing,
China) for 1 min in the dark. After chromogen development, sections
were washed in water and counterstained with hematoxylin. The
stained slides were investigated independently by two pathologists
who had no knowledge of the clinical parameters and outcomes. All
these cells were scored as negative (−) (<10% or no staining),
weak positive (+) (11–50%), positive (++) (51–75%), or strongly
positive (+++) (>75%).
Western blotting
Cells were harvested in a lysis buffer (10 mM
tris(hydroxymethyl)aminomethane (Tris) HCl, pH 7.6, 50 mM
Na4P2O7, 50 mM NaF, 1 mM
NaV3O4, 1% Triton X-100 and 1X protease
inhibitor of protein tyrosine phosphatases). Lysates were sonicated
for 3 sec and centrifuged at 4°C, 14,000 rpm for 30 min. The
supernatant was collected for protein quantification using the
Bio-Rad Protein Assay dye reagent (Bio-Rad Laboratories, Richmond,
CA, USA). Protein (50 μg) was size-fractionated through a 10%
SDS-PAGE gel and transferred onto nitrocellulose filters. The
filters were blocked (1% non-fat dry milk, 0.1% Triton X-100, 150
mM NaCl, 50 mM Tris (pH 7.5) and incubated with the primary
antibody, which was diluted to a ratio of 1:1,000. Nitrocellulose
filters were incubated with horseradish peroxidase-conjugated
secondary antibodies. Bands were visualized using the enhanced
chemiluminescence system (Amersham Pharmacia Biotech, Piscataway,
NJ, USA) and quantified by scanning densitometry using FlourChem
V2.0 software.
Immunostaining and fluorescence
microscopy
Cells were fixed in 4% paraformaldehyde in PBS (10
min at room temperature) and permeabilized with 0.2% Triton X-100
(10 min at room temperature). Cells were then incubated
individually in anti-E-cadherin or anti-Vimentin (1 h at room
temperature) and FITC-conjugated immunoglobulins (1 h at room
temperature). Cell nuclei were stained by DAPI. Representative
fields of cells were photographed by fluorescence microscopy.
Migration assay
Disposable 24-well Transwell inserts with 8 μm pore
size were run in triplicate in DMEM with 0.5% (w/v) BSA. Aliquots
of the chemokine CCL19 were added to the lower chamber at a
concentration of 500 ng/ml. The inhibitors-pre-treated PCI-4B and
PCI-37B cell suspensions (2×105) were placed in the top
of inserts. After 24 h of incubation, the cells on the upper
surface of inserts were removed with a cell harvester, and the
membrane was washed with medium. Cells that penetrated the membrane
were fixed with ice-cold methanol, stained with 0.5% crystal
violet, photographed, and counted under the microscope. Mean ±
standard deviation (SD) was recorded for each condition and
migration index was calculated based on the control, random
migration.
Matrigel invasion assay
Cell invasion was quantified in vitro using
Matrigel-coated semipermeable, modified inserts with a pore size of
8 μm. The analysis of Matrigel invasion assay was performed as
described in the migration assay incubated with CCL19 for 36 h.
Mean ± SD was recorded for each condition and invasion index was
calculated based on the control, random invasion.
Wound-healing assay
SCCHN cells were plated in a 24-well plate at
initial density of 1.5×105 cells/cm2. A
uniform monolayer formed in 2–3 days. The wounding assays were
performed in a serum-free medium. A micropipette tip was used to
create a wound in the monolayer by scrapping. The relative cell
free area was calculated based on the control group.
Statistical analysis
Data were expressed as the mean ± SD of repeated
assays. The correlation was analyzed using the Spearman’s test and
χ2 test. Statistical differences between the two groups
were evaluated using an unpaired Student’s t-test. P<0.05 were
considered to be significant. All statistical analyses were
performed with SPSS 11.0 software.
Results
CCR7 stimulates the phosphorylation of
ERK1/2 and JNK
MAPK family members (ERK1/2, p38, and JNK) have been
implicated in regulating chemotaxis in some systems and random
motility in others (28,30,31).
Our previous results demonstrated that CCR7 mediated SCCHN cell
migration and invasion (17–19).
Therefore, we analyzed whether CCR7 induced activation of MAPKs in
SCCHN cells. PCI-4B and PCI-37B cells were stimulated with CCL19
for various time periods, then lysed, and the lysates were analyzed
by western blotting using antibodies specific for the
phosphorylated/active forms and total protein of the three MAPKs.
The results showed that stimulation with CCL19 resulted in a
transient and potent phosphorylation of ERK1/2 and JNK, but no
effect on p38. Phosphorylation of ERK1/2 and JNK reached a maximum
after 15–30 min, and returned to levels close to baseline by 60
min. Total ERK1/2, JNK, p38 and the phosphorylation of p38 had no
change under the inducation (Fig.
1).
To further determine whether CCR7 regulates the
activation of MAPKs, PCI-4B and PCI-37B cells were pre-treated with
CCR7 mAb, the antibody can neutralize the bioactivity of CCR7.
Control and CCR7 mAb-treated PCI-4B and PCI-37B cells were
stimulated with CCL19, and activation of ERK1/2 and JNK was
analyzed. Treatment with CCR7 mAb completely abrogated the
CCL19-dependent activation of ERK1/2 and JNK (Fig. 2), indicated that its CCR7
activation stimulates the phosphorylation of ERK1/2 and JNK in
SCCHN cells.
To analyze the possible relationship between ERK1/2
and JNK after stimulation of CCR7, we used pharmacological agents.
PCI-4B and PCI-37B cells were pre-treated with ERK1/2 inhibitor
(PD98059) and JNK inhibitor (SP600125) respectively. The results
showed, PD98059 could blunt the increase of the phosphorylation of
ERK1/2 induced by stimulation with CCL19, without affecting JNK,
and SP600125 could blunt the increase of the phosphorylation of
JNK, without affecting ERK1/2, indicating that ERK1/2 and JNK
activated independently (Fig.
2).
ERK1/2 and JNK regulate CCR7-dependent
migration and invasion
Our previous results have shown that CCL19 induces
PCI-4B and PCI-37B cell migration and invasion, and this can be
blocked by CCR7 mAb (17,19) (Figs.
3 and 4). In this study, we
examined whether ERK1/2 and JNK were involved in regulating the
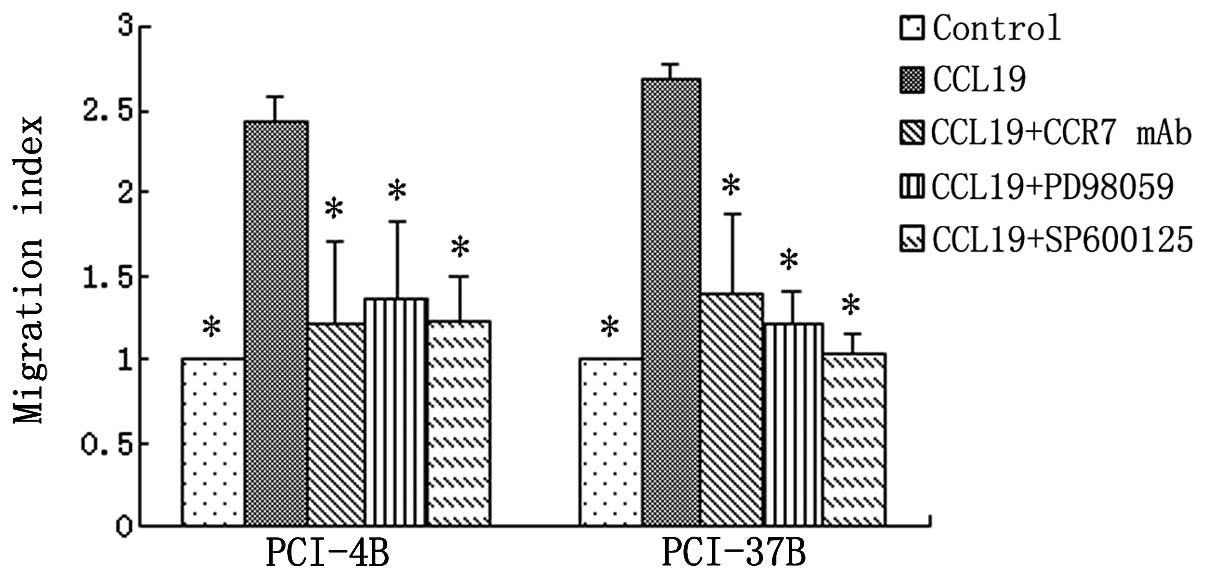
migratory and invasive speed induced by CCR7 activation. The
results showed, the cell invasion index induced by CCL19 was almost
three times that of the control group, and the inhibition of ERK1/2
and JNK significantly blocked the effect of CCL19 in both cell
lines, leading to decreased cell invasion to almost the baseline,
as well as CCR7 mAb (Fig. 3). The
migration index was the same as the invasion index. The CCL19
induced migration was significantly blocked by the ERK1/2 and JNK
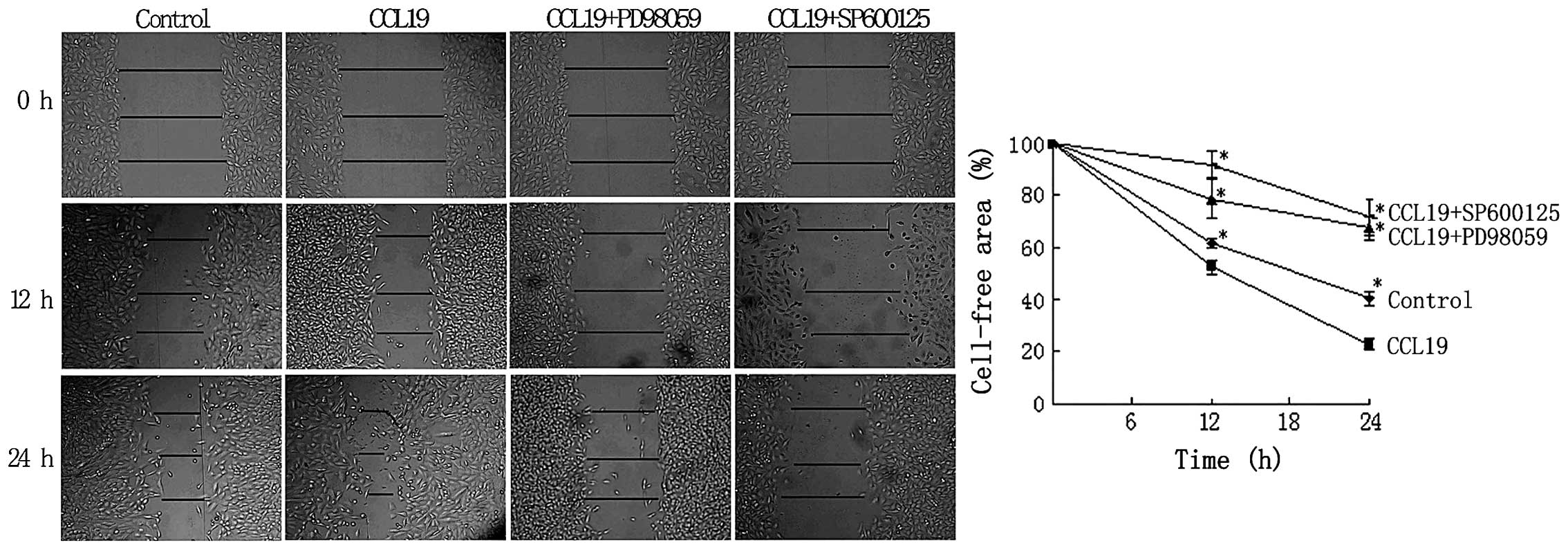
inhibitors (Fig. 4). We also used
the wound-healing assay that requires both migration and
proliferation of cells. The defined lesions were generated in
subconfluent layers of cells and the repopulation of denuded areas
was studied. After 12 and 24 h, the gap started to close slowly in
the control group, and in the CCL19 group the gap closure was
accelerated, and almost merged. After the inhibitor of ERK1/2 and
JNK were pre-treated, the cell migration and proliferation
decreased significantly, free area was even larger than the control
group (Fig. 5). The results
indicated that ERK1/2 and JNK regulate CCR7-depedent migration and
invasion.
ERK1/2 and JNK mediate the expression
levels of E-cadherin and Vimentin induced by CCR7
CCL19 can induce SCCHN cell migration and invasion.
E-cadherin and Vimentin are known to associate with
epithelial-mesenchymal transition (EMT), a key point in tumor
progress, and to generally participate in migration and invasion.
Our results showed that CCL19-treated PCI-4B and PCI-37B cells led
to a significant increase in the level of Vimentin protein and a
significant decrease in the level of E-cadherin, which can be
reversed by CCR7 mAb, implying that CCR7-induced cell migration and
invasion may be through E-cadherin and Vimentin expression. After
the inhibitor of ERK1/2 and JNK were pre-treated, CCL19-induced
high expression of Vimentin was decreased and low expression of
E-cadherin was increased (Fig. 6).
Combined with previous results, we think ERK1/2 and JNK mediate the
expression levels of E-cadherin and Vimentin induced by CCR7, and
this pathway may play a key role in SCCHN metastasis.
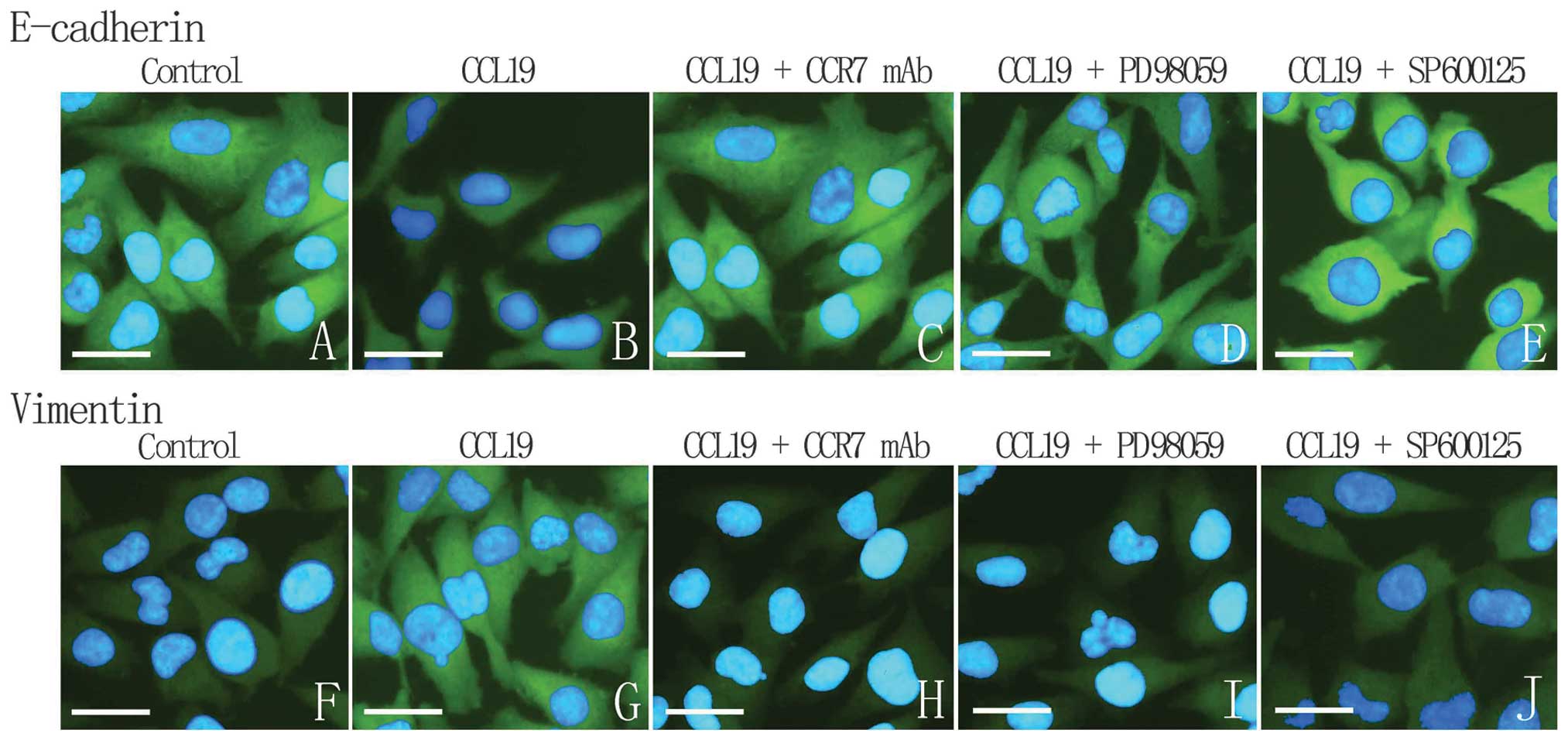
To demonstrate this conclusions further, we designed
an immunofluorescence assay. As Fig.
7 shows, the control cells contacted each other and the
immunostaining of E-cadherin is very strong. CCL19 group presents
no cell-cell contact, and the E-cadherin expression is very low and
diffused. When pre-treated with CCR7 mAb, the inhibitors of ERK1/2
or JNK, the E-cadherin expression becomes stronger, although CCL19
induced. On the contrary, control group exhibited diffuse and low
cytoplasmic Vimentin staining. In response to CCL19, a network of
Vimentin filaments spanning the cell and establishing cell-to-cell
contacts was observed. Application of CCR7, ERK1/2 or JNK inhibitor
blocked CCL19-induced Vimentin fiber formation and restored the
cellular distribution pattern to that under control conditions.
CCR7 and phosphorylation of ERK1/2 and
JNK expressed by immunohistochemical staining had significant
positive correlation in tumor tissues
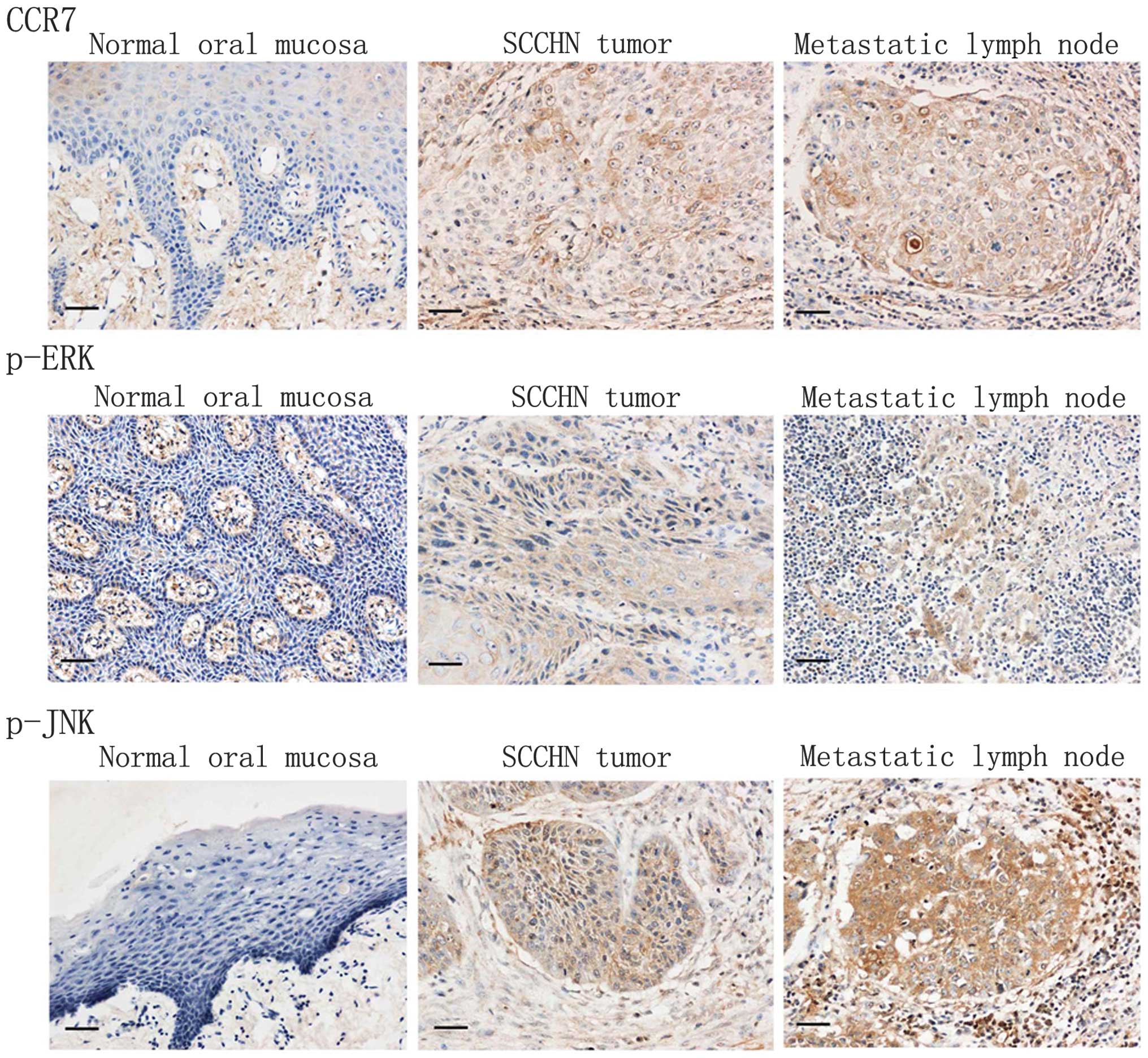
Firstly, we studied the expression of CCR7 and
phosphorylation of ERK1/2 and JNK in specimens from SCCHN patients
by immunohistochemical staining. As Fig. 8 shows, in normal mucosa, CCR7 and
phosphorylation of ERK1/2 and JNK were almost not stained. However,
CCR7 was strongly immunolocalized in the membrane and the cytoplasm
of cancer cells, and phosphorylation of ERK and JNK were detected
in the nucleus and the cytoplasm. In metastatic lymph nodes, they
were all highly expressed in tumor cells. Of the 78 patients, 48
cases were positive for CCR7 (48/78), and 46 cases were positive
for phosphorylation of ERK1/2 (46/78), 41 cases were positive for
phosphorylation of JNK (41/78). However, in ten control cases,
CCR7, ERK1/2 and JNK phosphorylation was noted only in one case
(1/10). SCCHN and normal tissues have significant difference in the
expression of CCR7, phosphorylation of ERK1/2 and phosphorylation
of JNK (P<0.05). Table I
summarizes the relationship between CCR7 expression,
phosphorylation of ERK1/2 and JNK, and the clinicopathological
factors of the 78 SCCHN patients. The expression of CCR7,
phosphorylation of ERK1/2 and JNK were all significantly correlated
with cervical lymph node metastasis and clinical stage (P<0.05),
and had no significant difference with age or gender (P>0.05).
Furthermore, our data also suggested that the phosphorylation of
ERK1/2 and JNK was correlated with CCR7 expression, respectively
(P<0.05) (Table II).
 | Table ICorrelations between CCR7, ERK1/2
phosphorylation, JNK phosphorylation and the clinicopathological
factors of SCCHN. |
Table I
Correlations between CCR7, ERK1/2
phosphorylation, JNK phosphorylation and the clinicopathological
factors of SCCHN.
| Clinicopathological
characteristics | No. of cases | CCR7 | Statistical
analysis χ2 | ERK1/2
phosphorylation | Statistical
analysis χ2 | JNK
phosphorylation | Statistical
analysis χ2 |
|---|
|
|
|
|---|
| + ~ +++ | − | + ~ +++ | − | + ~ +++ | − |
|---|
| Age |
| ≥60 | 40 | 25 | 15 | 0.032 | 21 | 19 | 1.422 | 21 | 19 | 0.000 |
| <60 | 38 | 23 | 15 | | 25 | 13 | | 20 | 18 | |
| Gender |
| Male | 50 | 32 | 18 | 0.357 | 32 | 18 | 1.454 | 23 | 27 | 2.407 |
| Female | 28 | 16 | 12 | | 14 | 14 | | 18 | 10 | |
| Tumor size |
| T1, T2 | 65 | 37 | 28 | 3.510 | 37 | 28 | 0.678 | 29 | 36 | 9.882a |
| T3, T4 | 13 | 11 | 2 | | 9 | 4 | | 12 | 1 | |
| Clinical stage |
| I, II | 37 | 15 | 22 | 13.113a | 17 | 20 | 4.938a | 13 | 24 | 8.575a |
| III, IV | 41 | 33 | 8 | | 29 | 12 | | 28 | 13 | |
| Nodal
metastasis |
| Yes | 37 | 29 | 8 | 8.434a | 28 | 9 | 8.115a | 24 | 13 | 4.271a |
| No | 41 | 19 | 22 | | 18 | 23 | | 17 | 24 | |
 | Table IICorrelations between CCR7 expression,
ERK1/2 phosphorylation and JNK phosphorylation in SCCHN primary
tumor. |
Table II
Correlations between CCR7 expression,
ERK1/2 phosphorylation and JNK phosphorylation in SCCHN primary
tumor.
| ERK1/2
phosphorylation | JNK
phosphorylation |
|---|
|
|
|
|---|
| + ~ +++ | − | + ~ +++ | − |
|---|
| CCR7 | | | | |
| + ~ +++ | 32 | 16 | 31 | 17 |
| − | 14 | 16 | 10 | 20 |
Discussion
Metastasis involves the separation from the primary
tumor, migration into the extracellular matrix, blood vessel
invasion, adhesion to endothelium and extravasation and growth in a
secondary organ (35). Therefore,
these steps will regulate cancer cells metastasis. CCL19 is
expressed constitutively within lymphoid tissues (36). The interaction of CCR7 and CCL19
promotes cell migration and adhesion in metastatic SCCHN (17–19).
However, the mechanisms of adhesion and migration and the signaling
pathway involved remains poorly understood.
MAPKs regulate many physiological processes in
response to diverse stimuli including cytokines, growth factors,
antigens, toxins, drugs, cell shape, adherence to extracellular
matrix, and cell-cell interactions. The activation of MAPKs in
response to these diverse stimuli contributes to the control of
transcription, proliferation, development, cell death, motility,
and many other important regulatory responses in cells. To control
such diverse biological responses, MAPKs are activated and
inactivated with spatial and temporal accuracy within the cell
(37). Riol-Blanco et al
investigated the intracellular pathways that regulate
CCR7-dependent chemotaxis and migratory speed in DCs, and found
that CCR7 induced a G(i)-dependent activation of MAPK members
ERK1/2, JNK, and p38, with ERK1/2 and p38 controlling JNK (38). Recently, Shannon et al
reported that the CCR7 signaling pathway leading to T-lymphocyte
migration on fibronectin is a β1 integrin-dependent pathway
involving ERK1/2 phosphorylation (39). Our results also showed that, in
solid tumor (SCCHN), stimulation of CCL19 and the activation of
CCR7 can induce ERK1/2 and JNK phosphorylation, while has no effect
on p38, suggesting ERK1/2 and JNK may be the downstream signaling
pathway of CCR7 in SCCHN. Furthermore, the phosphorylation of
ERK1/2 or JNK did not influence each other, suggesting the two
molecules participate in the signaling pathway independently. In
DC, ERK1/2, JNK, and p38 only regulated chemotaxis, but not the
migratory speed (38), and in
B-cell chronic lymphocytic leukemia, ERK1/2 participates in
CCL21-dependent migration and invasion simultaneously (40). In our results, ERK1/2 and JNK not
only mediated CCR7-induced cell migration, but also mediated the
speed of invasion.
MAPKs are members of a three-kinase phosphorylate
system composed of the MAPK, MAPK kinase (MKK) and MAPK kinase
kinase (MKKK). MKKKs phosphorylate and activate MKKs, which in turn
phosphorylate and activate MAPKs. Scaffolding proteins organize
MKK-MAPK complexes for activation by specific MKKKs and do so in
specific locations in the cell. It is the MKKK associated with the
scaffolded complex that provides selectivity for activation by
upstream stimuli including GTPases, additional kinases and
receptors (41).
The activation of MKK-MAPK complexes regulate the
physiological processes by many target proteins. E-cadherin is a
transmembrane glycoprotein associated with the cytoskeleton via
cytoplasmic proteins. Normal squamous epithelium of the esophagus
showed strong E-cadherin/β-catenin expression especially on
cell-cell boundaries except in the superficial layer (42). EMT plays an important role in tumor
prognosis, known to dismantle cadherin-medicated cell-cell
junctions (43). Thus, disruption
of E-cadherin-mediated adhesion is considered as a key step in the
progression toward the malignant phase of carcinoma (44). In ovarian cancer, it has been shown
that E-cadherin is downregulated by epidermal growth factor (EGF)
receptor (EGFR) activation via p38 MAPK, and that cells with low
E-cadherin expression are particularly invasive (45). In human oral squamous cancer CAL-27
cells, treatment with the ERK inhibitor could also upregulate the
expression of the E-cadherin molecule. Vimentin, the major
intermediate filament (IF) protein of mesenchymal cells, is also
associated with EMT. In lung cancer, transforming growth
factor-β1-induced EMT was reversed by ERK inhibitor by attenuating
the expression of Vimentin (46).
In our study, the CCR7-induced ERK and JNK activation downregulated
E-cadherin and upregulated Vimentin expression simultaneously, and
then affected cell-cell contact and expansion. Therefore, we
presumed that E-cadherin and Vimentin are ERK and JNK downstream
target molecules in CCR7 regulating SCCHN cell migration and
invasion, and this MAPK pathway may generally participate in tumor
cells separated from the primary tumor, migrating into
extracellular matrix, invading blood vessels and adhering to the
endothelium. The immunohistochemistry results not only showed that
CCR7 was correlated with the phosphorylation of ERK1/2 and JNK in
SCCHN, but also showed that these molecules are all associated with
lymph node metastasis. This confirmed the results in
vitro.
Taken together, our study supports a hypothesis that
CCR7 regulate SCCHN metastasis via MAPK members (ERK1/2 and JNK).
However, signaling pathways controlling directional cell migration
are not linear, but integrate signals from a plethora of upstream
switches into a molecular matrix, resulting in complex cellular
responses. Further study is required to elucidate the sequence of
events leading to the CCR7-mediated metastatic phenotype, which
will enable the development of therapeutic strategies aiming at
blocking these carcinogenic and metastatic effects.
Acknowledgements
This research was supported by grants from the
National Natural Science Foundation of China (No. 81372877), the
National Young Scholars Science Foundation of China (No. 81102058),
the Foundation of Education Bureau of Liaoning Province (No.
2009A755, No. L2014317), the Public Welfare Fund Project for
Science of Liaoning Province (No. 2011002001), Natural Science
Foundation of Liaoning Province (No. 2014021096), and Excellent
Talent Fund Project of Higher Education of Liaoning Province
(LJQ2014087).
References
|
1
|
Greenlee RT, Hill-Harmon MB, Murray T and
Thun M: Cancer statistics, 2001. CA Cancer J Clin. 51:15–36. 2001.
View Article : Google Scholar
|
|
2
|
Butcher EC, Williams M, Youngman K, Rott L
and Briskin M: Lymphocyte trafficking and regional immunity. Adv
Immunol. 72:209–253. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Campbell JJ and Butcher EC: Chemokines in
tissue-specific and microenvironment-specific lymphocyte homing.
Curr Opin Immunol. 12:336–341. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Morales J, Homey B, Vicari AP, et al:
CTACK, a skin-associated chemokine that preferentially attracts
skin-homing memory T cells. Proc Natl Acad Sci USA. 96:14470–14475.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zlotnik A and Yoshie O: Chemokines: a new
classification system and their role in immunity. Immunity.
12:121–127. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mburu YK, Abe K, Ferris LK, Sarkar SN and
Ferris RL: Human β-defensin 3 promotes NF-κB-mediated CCR7
expression and anti-apoptotic signals in squamous cell carcinoma of
the head and neck. Carcinogenesis. 32:168–174. 2011.
|
|
7
|
Nagira M, Imai T, Yoshida R, et al: A
lymphocyte-specific CC chemokine, secondary lymphoid tissue
chemokine (SLC), is a highly efficient chemoattractant for B cells
and activated T cells. Eur J Immunol. 28:1516–1523. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iijima N, Yanagawa Y, Clingan JM and Onoé
K: CCR7-mediated c-Jun N-terminal kinase activation regulates cell
migration in mature dendritic cells. Int Immunol. 17:1201–1212.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Emmett MS, Lanati S, Dunn DB, Stone OA and
Bates DO: CCR7 mediates directed growth of melanomas towards
lymphatics. Microcirculation. 18:172–182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ding Y, Shimada Y, Maeda M, et al:
Association of CC chemokine receptor 7 with lymph node metastasis
of esophageal squamous cell carcinoma. Clin Cancer Res.
9:3406–3412. 2003.PubMed/NCBI
|
|
11
|
Takanami I: Overexpression of CCR7 mRNA in
nonsmall cell lung cancer: correlation with lymph node metastasis.
Int J Cancer. 105:186–189. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Murakami T, Cardones AR and Hwang ST:
Chemokine receptors and melanoma metastasis. J Dermatol Sci.
36:71–78. 2004. View Article : Google Scholar
|
|
13
|
Gunther K, Leier J, Henning G, et al:
Prediction of lymph node metastasis in colorectal carcinoma by
expression of chemokine receptor CCR7. Int J Cancer. 116:726–733.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cabioglu N, Yazici MS, Arun B, et al: CCR7
and CXCR4 as novel biomarkers predicting axillary lymph node
metastasis in T1 breast cancer. Clin Cancer Res. 11:5686–5693.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schimanski CC, Bahre R, Gockel I, et al:
Chemokine receptor CCR7 enhances intrahepatic and lymphatic
dissemination of human hepatocellular cancer. Oncol Rep.
16:109–113. 2006.PubMed/NCBI
|
|
16
|
Zhao ZJ, Li P, Liu FY, Sun LY and Sun CF:
PKCα take part in CCR7/NF-κB autocrine signaling loop in
CCR7-positive squamous cell carcinoma of head and neck. Mol Cell
Biochem. 357:181–187. 2011.
|
|
17
|
Zhao ZJ, Liu FY, Li P, Ding X, Zong ZH and
Sun CF: CCL19-induced chemokine receptor 7 activates the
phosphoinositide-3 kinase-mediated invasive pathway through Cdc42
in metastatic squamous cell carcinoma of the head and neck. Oncol
Rep. 25:729–737. 2011.PubMed/NCBI
|
|
18
|
Li P, Zhao ZJ, Liu FY, et al: The
chemokine receptor 7 regulates cell adhesion and migration via
beta1 integrin in metastatic squamous cell carcinoma of the head
and neck. Oncol Rep. 24:989–995. 2010.PubMed/NCBI
|
|
19
|
Li P, Liu F, Sun L, et al: Chemokine
receptor 7 promotes cell migration and adhesion in metastatic
squamous cell carcinoma of the head and neck by activating integrin
αvβ3. Int J Mol Med. 27:679–687. 2011.PubMed/NCBI
|
|
20
|
Liu FY, Zhao ZJ, Li P, Ding X, Zong ZH and
Sun CF: Mammalian target of rapamycin (mTOR) is involved in the
survival of cells mediated by chemokine receptor 7 through PI3K/Akt
in metastatic squamous cell carcinoma of the head and neck. Br J
Oral Maxillofac Surg. 48:291–296. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu FY, Zhao ZJ, Li P, Ding X and Sun CF:
Effect of rapamycin combined with cisplatin on head and neck
squamous cancer cells regulated by CCL19. Zhonghua Kou Qiang Yi Xue
Za Zhi. 46:197–200. 2011.(In Chinese).
|
|
22
|
Liu FY, Zhao ZJ, Li P, Ding X and Sun CF:
The effect of CCL19 on the viability of head and neck squamous
cancer cells. Shanghai Kou Qiang Yi Xue. 19:158–161. 2010.(In
Chinese).
|
|
23
|
Liu FY, Zhao ZJ, Li P, et al: NF-κB
participates in chemokine receptor 7-mediated cell survival in
metastatic squamous cell carcinoma of the head and neck. Oncol Rep.
25:383–391. 2011.
|
|
24
|
Liu FY, Zhao ZJ, Huang SH and Sun CF: Role
of PDTC in CCL19 regulating the activity of human head and neck
squamous cancer cells. J Chin Med Univ. 37:847–849. 2008.(In
Chinese).
|
|
25
|
Thelen M: Dancing to the tune of
chemokines. Nat Immunol. 2:129–134. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ganju RK, Brubaker SA, Meyer J, et al: The
alpha-chemokine, stromal cell-derived factor-1alpha, binds to the
transmembrane G-protein-coupled CXCR-4 receptor and activates
multiple signal transduction pathways. J Biol Chem.
273:23169–23175. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ganju RK, Dutt P, Wu L, et al:
Beta-chemokine receptor CCR5 signals via the novel tyrosine kinase
RAFTK. Blood. 91:791–797. 1998.PubMed/NCBI
|
|
28
|
Wong MM and Fish EN: Chemokines:
attractive mediators of the immune response. Semin Immunol.
15:5–14. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang P, Han J and Hui L: MAPK signaling
in inflammation-associated cancer development. Protein Cell.
1:218–226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Klemke RL, Cai S, Giannini AL, Gallagher
PJ, de Lanerolle P and Cheresh DA: Regulation of cell motility by
mitogen-activated protein kinase. J Cell Biol. 137:481–492. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang C, Rajfur Z, Borchers C, Schaller MD
and Jacobson K: JNK phosphorylates paxillin and regulates cell
migration. Nature. 424:219–223. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang H, Wu C, Wan S, Zhang H, Zhou S and
Liu G: Shikonin attenuates lung cancer cell adhesion to
extracellular matrix and metastasis by inhibiting integrin β1
expression and the ERK1/2 signaling pathway. Toxicology.
308:104–112. 2013.PubMed/NCBI
|
|
33
|
Wang J, Zhang X, Thomas SM, et al:
Chemokine receptor 7 activates phosphoinositide-3 kinase-mediated
invasive and prosurvival pathways in head and neck cancer cells
independent of EGFR. Oncogene. 24:5897–5904. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mburu YK, Wang J, Wood MA, Walker WH and
Ferris RL: CCR7 mediates inflammation-associated tumor progression.
Immunol Res. 36:61–72. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liotta LA, Steeg PS and Stetler-Stevenson
WG: Cancer metastasis and angiogenesis: an imbalance of positive
and negative regulation. Cell. 64:327–336. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ngo VN, Tang HL and Cyster JG:
Epstein-Barr virus-induced molecule 1 ligand chemokine is expressed
by dendritic cells in lymphoid tissues and strongly attracts naive
T cells and activated B cells. J Exp Med. 188:181–191. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Johnson GL: Defining MAPK interactomes.
ACS Chem Biol. 6:18–20. 2011. View Article : Google Scholar
|
|
38
|
Riol-Blanco L, Sánchez-Sánchez N, Torres
A, et al: The chemokine receptor CCR7 activates in dendritic cells
two signaling modules that independently regulate chemotaxis and
migratory speed. J Immunol. 174:4070–4080. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shannon LA, Calloway PA, Welch TP and
Vines CM: CCR7/CCL21 migration on fibronectin is mediated by
phospholipase Cgamma1 and ERK1/2 in primary T lymphocytes. J Biol
Chem. 285:38781–38787. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Redondo-Muñoz J, José Terol M,
García-Marco JA and García-Pardo A: Matrix metalloproteinase-9 is
up-regulated by CCL21/CCR7 interaction via extracellular
signa-lregulated kinase-1/2 signaling and is involved in
CCL21-driven B-cell chronic lymphocytic leukemia cell invasion and
migration. Blood. 111:383–386. 2008.
|
|
41
|
Johnson GL, Dohlman HG and Graves LM: MAPK
kinase kinases (MKKKs) as a target class for small-molecule
inhibition to modulate signaling networks and gene expression. Curr
Opin Chem Biol. 9:325–331. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Takayama T, Shiozaki H, Shibamoto S, et
al: Beta-catenin expression in human cancers. Am J Pathol.
148:39–46. 1996.
|
|
43
|
Savagner P: Leaving the neighborhood:
molecular mechanisms involved during epithelial-mesenchymal
transition. Bioessays. 23:912–923. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Behrens J: Cadherins and catenins: role in
signal transduction and tumor progression. Cancer Metastasis Rev.
18:15–30. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cheng JC, Klausen C and Leung PC: Hydrogen
peroxide mediates EGF-induced down-regulation of E-cadherin
expression via p38 MAPK and snail in human ovarian cancer cells.
Mol Endocrinol. 24:1569–1580. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen XF, Zhang HJ, Wang HB, et al:
Transforming growth factor-β1 induces epithelial-to-mesenchymal
transition in human lung cancer cells via PI3K/Akt and MEK/Erk1/2
signaling pathways. Mol Biol Rep. 39:3549–3556. 2011.
|