Introduction
Colorectal cancer (CRC) is one of the most common
cancers and a leading cause of cancer-related deaths (1,2). To
date, the mainstay of anti-CRC treatment includes surgery,
chemotherapy and radiotherapy. However, due to tumor recurrence and
metastasis the long-term survival and prognosis of patients remains
quite poor (3,4). Tumor metastasis is a complex process
involving the spread of malignant tumor cells from a primary tumor
site to a distant organ, which is a major cause of failure of
cancer treatment (5–7). Epithelial-mesenchymal transition
(EMT) is a critical step for the initiation of cancer metastasis
(8,9). The processes of EMT and metastasis
are highly regulated by multiple mechanisms, including TGF-β1/ZEB
pathways and miRNA 200 family (10–14).
TGF-β1 is the prototypic member of transforming
growth factor β superfamily. The activation of TGF-β signaling
pathway is initiated by the binding of ligands to a type II
receptor, resulting in the phosphorylation/activation of a type I
receptor. The activated type I receptor then phosphorylates SMAD2/3
that in turn bind to SMAD4. The SMAD complex translocates to the
nucleus to regulate the expression of target genes, including the
ZEB (zinc finger E-box-binding homeobox) transcription factor
family (15,16). Upon activation, ZEB transcription
factors suppress epithelial marker gene expression and upregulate
mesenchymal gene expression, leading to the processes of EMT and
cancer metastasis (17,18). MicroRNAs (miRNA) are a class of
endogenous short non-coding RNAs (19–24 nucleotides), which
function primarily to negatively regulate target gene expression by
specifically binding to the 3′-untranslational region (3′-UTR) of
target mRNAs (19–21). It has been shown that miRNAs
function more likely as oncogenes or tumor suppressors to modulate
multiple oncogenic cellular processes, such as cell proliferation,
apoptosis and metastasis (22–24).
The miR-200 family members, including miR-200a, miR-200b and
miR-200c, have been proposed to act as tumor suppressors that
inhibit EMT by downregulating the expression of ZEB1 and ZEB2
(11,14,25–27).
However, the expression of miR-200 family is negatively regulated
by TGF-β signaling, probably via TGF-β-induced DNA methylation of
the miR-200 loci (12). Thus,
TGF-β/ZEB/miR-200 signaling network creates a double-negative
feedback loop that plays an essential role in the initiation of EMT
and cancer metastasis; which therefore becomes a promising target
for cancer chemotherapy (13,14).
Recently, traditional Chinese medicines (TCM) have
received great interest in the field of anticancer treatment since
they have fewer adverse effects as compared to modern
chemotherapeutics and have been used in China for thousands of
years as important alternative remedies for various diseases
including cancer (28,29). Pien Tze Huang (PZH) is a well-known
TCM formula that was first prescribed >450 years ago in the Ming
Dynasty. The main ingredients of PZH include Moschus, Calculus
Bovis, Snake Gall and Radix Notoginseng. These products
together confer PZH properties of heat clearing, detoxification,
dissipation of hard mass, detumescence and analgesia (30). Traditionally, PZH has been used to
clinically treat traumatic injuries and a variety of inflammatory
diseases, particularly hepatitis (30–32).
More importantly, PZH has also been used in China and Southeast
Asia for centuries as a folk remedy for treatment of various types
of human cancer. We recently reported that PZH can inhibit colon
cancer growth through the promotion of cancer cell apoptosis, the
inhibition of cell proliferation and tumor angiogenesis, which is
probably mediated by modulation of multiple signaling pathways
(33–40). To further elucidate the mode of
action of PZH, in the present study we evaluated its effects on the
metastatic capacities of human colorectal carcinoma HCT-8 cells and
investigated the underlying molecular mechanisms.
Materials and methods
Materials and reagents
Roswell Park Memorial Institute (RPMI)-1640 medium,
fetal bovine serum (FBS), penicillin-streptomycin, were obtained
from Life Technologies Corp. (Grand Island, NY, USA). N-cadherin
and E-cadherin antibodies were purchased from Abcam (HK) Ltd. (Hong
Kong, China). TGF-β1, SMAD2/3, SMAD4, ZEB1, ZEB2 and β-actin
antibodies, horseradish peroxidase (HRP)-conjugated secondary
antibodies were provided by Cell Signaling Technology (Beverly, MA,
USA). Transwell chambers were obtained from Corning Life Sciences
(Tewksbury, MA, USA). BD BioCoat Matrigel Invasion Chamber was
purchased from BD Bioscience (San Jose, CA, USA). PrimeScript RT
reagent kit, RNAiso for Small RNA kit and SYBR Premix Ex Taq II kit
were provided by Dalian Takara Biotechnology Co., Ltd. (Dalian,
Liaoning, China). All the other chemicals, unless otherwise stated,
were obtained from Sigma Chemicals (St. Louis, MO, USA).
Preparations of PZH
PZH was obtained from, and authenticated by the sole
manufacturer Zhangzhou Pien Tze Huang Pharmaceutical Co. Ltd.,
China (Chinese FDA approval no. Z35020242). Stock solutions of PZH
were prepared just before use by dissolving the PZH powder in PBS
(phosphate-buffered saline) to a concentration of 20 mg/ml. The
working concentrations of PZH were made by diluting the stock
solution in the culture medium.
Cell culture
Human colorectal carcinoma HCT-8 cells were obtained
from Nanjing KeyGen Biotech. Co. Ltd. (Nanjing, Jiangsu, China).
Cells were grown in RPMI-1640 medium containing 10% (v/v) FBS, 100
U/ml penicillin and 100 μg/ml streptomycin in a 37°C humidified
incubator with 5% CO2. The cells were subcultured at
80–90% confluency.
Evaluation of cell migration by
wound-healing assay
Migration of HCT-8 cells was examined by
wound-healing assay. Cells were seeded into 6-well plate at a
density of 1×106 cells/well in 2 ml medium. After 24 h
of incubation, cells were scraped away vertically in each well by
using a P100 pipette tip. Three randomly selected views along the
scraped line were photographed on each well using a phase-contrast
inverted microscope (Leica, Germany) at a magnification of ×100.
Cells were then treated with indicated concentrations of PZH for 24
h, and another set of images were taken using the same method. A
reduction in the scraped area indicates a sign of migration.
Measurement of cell migration and
invasion by transwell assay
Migration assay was performed using transwell cell
culture chambers with 8-μm pore filters (Corning Life Sciences,
USA). After treatment with various concentrations of PZH for 24 h,
HCT-8 cells were trypsinized and resuspended in serum-free
RPMI-1640. A total of 5×104 cells in 200 μl of
serum-free RPMI-1640 were plated in the upper chambers. RPMI-1640
media containing 10% (v/v) FBS was used in the lower chambers as a
chemoattractant. Cells were allowed to migrate for 12 h, and the
non-migrated cells were removed from the upper surface of transwell
membranes by a cotton swab. Membranes were then stained with
crystal violet. For quantification, the average number of migrating
cells per field was assessed by counting 3 random fields under a
phase-contrast microscope (Leica) at a magnification of ×200. For
cell invasion assay, the procedure was the same as that of
above-described migration analysis, except that the upper chambers
were coated with Matrigel Matrix (BD Biosciences, USA).
Western blot analysis
HCT-8 cells were seeded into 25 cm2
flasks at a density of 1.5×106 cells/flask in 5 ml
medium. After incubation for 24 h, the cells were treated with the
indicated concentrations of PZH for 24 h. The treated cells were
lysed with mammalian cell lysis buffer containing protease and
phosphatase inhibitor cocktails. Total protein concentrations were
determined by BCA assay. Equal amounts of total proteins were
resolved in 12% SDS-PAGE gels and electroblotted. The PVDF
membranes were blocked with 5% skimmed milk and probed with primary
antibodies N-cadherin, E-cadherin, TGF-β1, SMAD2/3, SMAD4, ZEB1,
ZEB2 and β-actin overnight at 4°C and subsequently with the
appropriate HRP-conjugated secondary antibody followed by enhanced
chemiluminescence detection.
Q-PCR analysis
Total small RNA from HCT-8 cells was isolated with
RNAiso for Small RNA kit. Total small RNA (500 ng) was
reverse-transcribed with SYBR PrimeScript miRNA RT-PCR kit
according to the manufacturer’s instructions. The obtained cDNA was
used to determine the miRNA amount of miR-200a, miR-200b and
miR-200c, U6 was used as an internal control. The primers of
miR-200a (DHM0178), miR-200b (DHM0179), miR-200c (DHM0180) and U6
(D356-03) were obtained from Dalian Takara Biotechnology Co., Ltd.
Quantitative PCR was performed using SYBR Premix Ex Taq II in an
ABI 7500 Fast instrument. Q-PCR reactions were carried out
following the manufacturer’s protocol. miRNA expression values were
determined as ΔCt=Ct (sample)−Ct (U6) and relative quantities
between different samples were determined as ΔΔCt=ΔCt (sample
1)−ΔCt (sample 2), the values were expressed as 2−ΔΔCt.
All Q-PCR reactions were conducted in triplicate.
Statistical analysis
The data are presented as the means of three
determinations and was analyzed using the SPSS package for Windows
(Version 18.0). Statistical analysis of the data was performed with
Student’s t-test and ANOVA. Differences with P<0.05 were
considered statistically significant.
Results
PZH inhibits migration and invasion of
HCT-8 cells
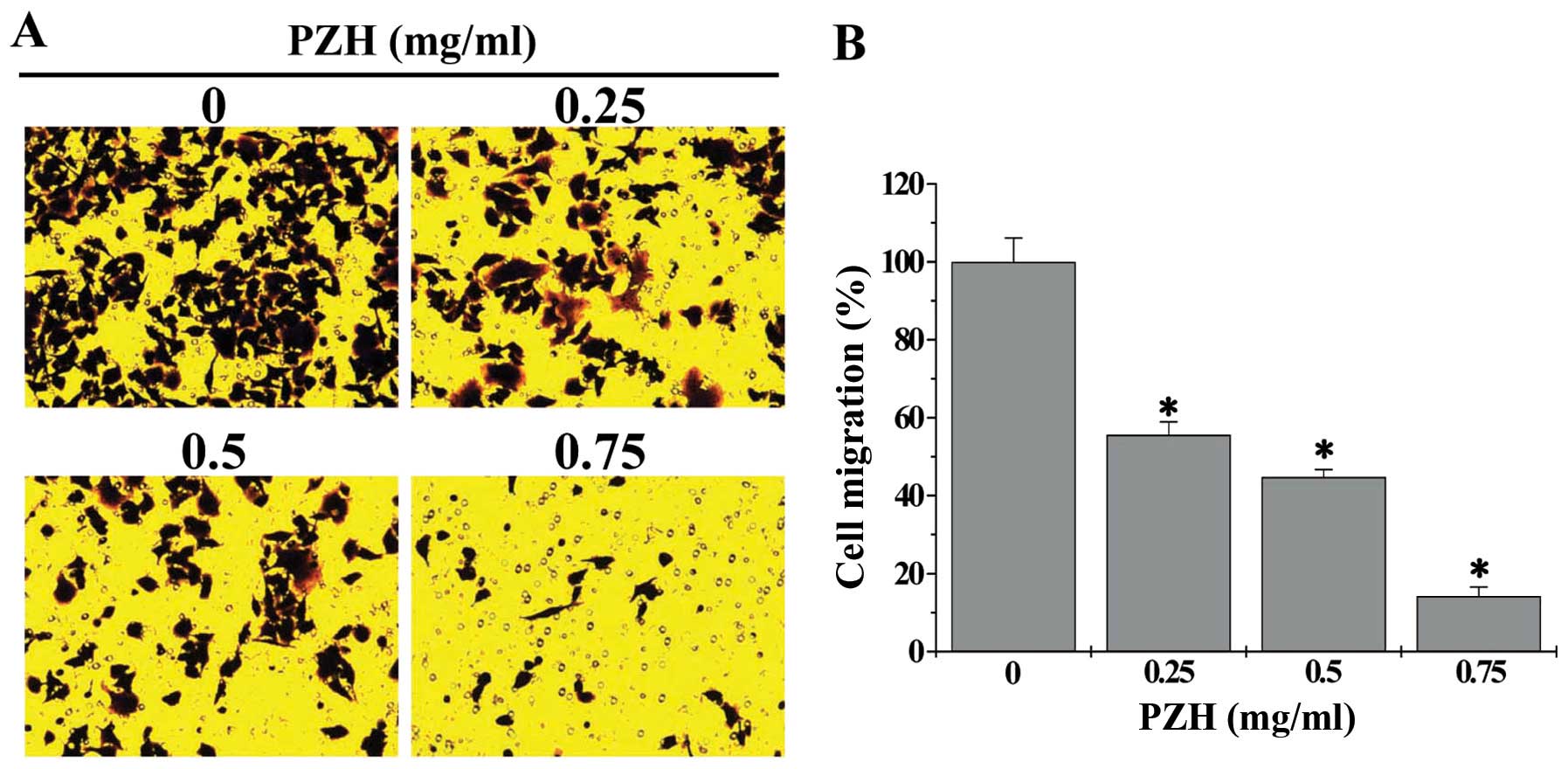
We first performed a wound-healing assay to evaluate
the effect of PZH on the migration of HCT-8 cells. As shown in
Fig. 1, after post-wounding for 24
h, untreated HCT-8 cells migrated into the clear area, whereas PZH
treatment dose-dependently inhibited HCT-8 cell migration. We
further verified these results using transwell assay; and the data
showed that treatment with 0.25–0.75 mg/ml of PZH for 24 h
dose-dependently reduced cell migratory rate of HCT-8 cells by
44.4–85.8%, as compared to untreated cells (Fig. 2, P<0.05). We next determined the
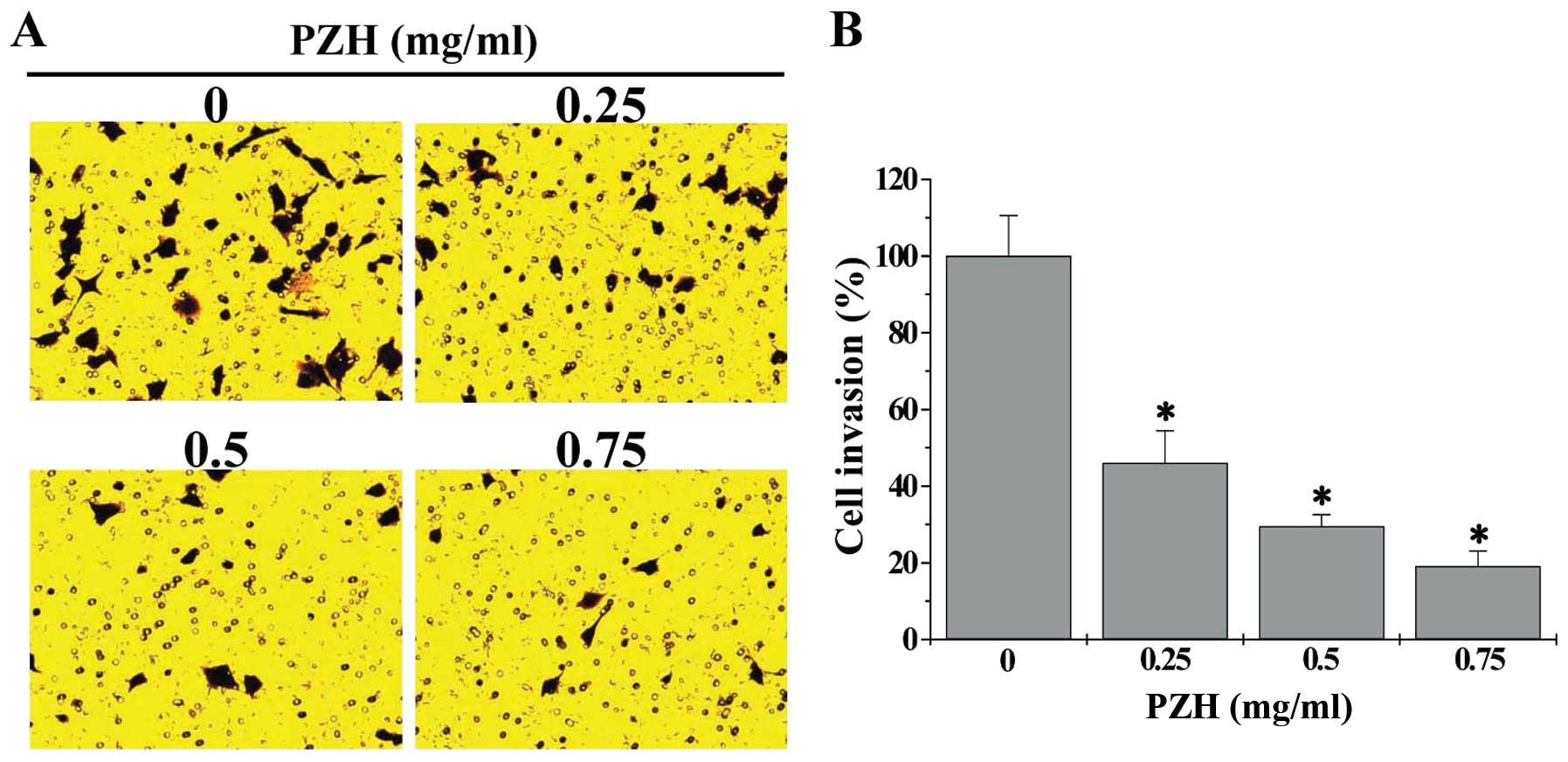
effect of PZH on the invasion capacity of HCT-8 cells using the
transwell assay. As shown in Fig.
3, compared with untreated cells (100%), the invasion rate of
HCT-8 cells following treatment with 0.25, 0.5 or 0.75 mg/ml of PZH
was 46.0±8.4, 29.6±3.0 or 19.1±4.0%, respectively (P<0.05).
Taken together, these data suggest that PZH can inhibit metastasis
of human colorectal cancer cells.
PZH modulates the activation of TGF-β1
pathway and the expression of EMT-regulatory genes in HCT-8
cells
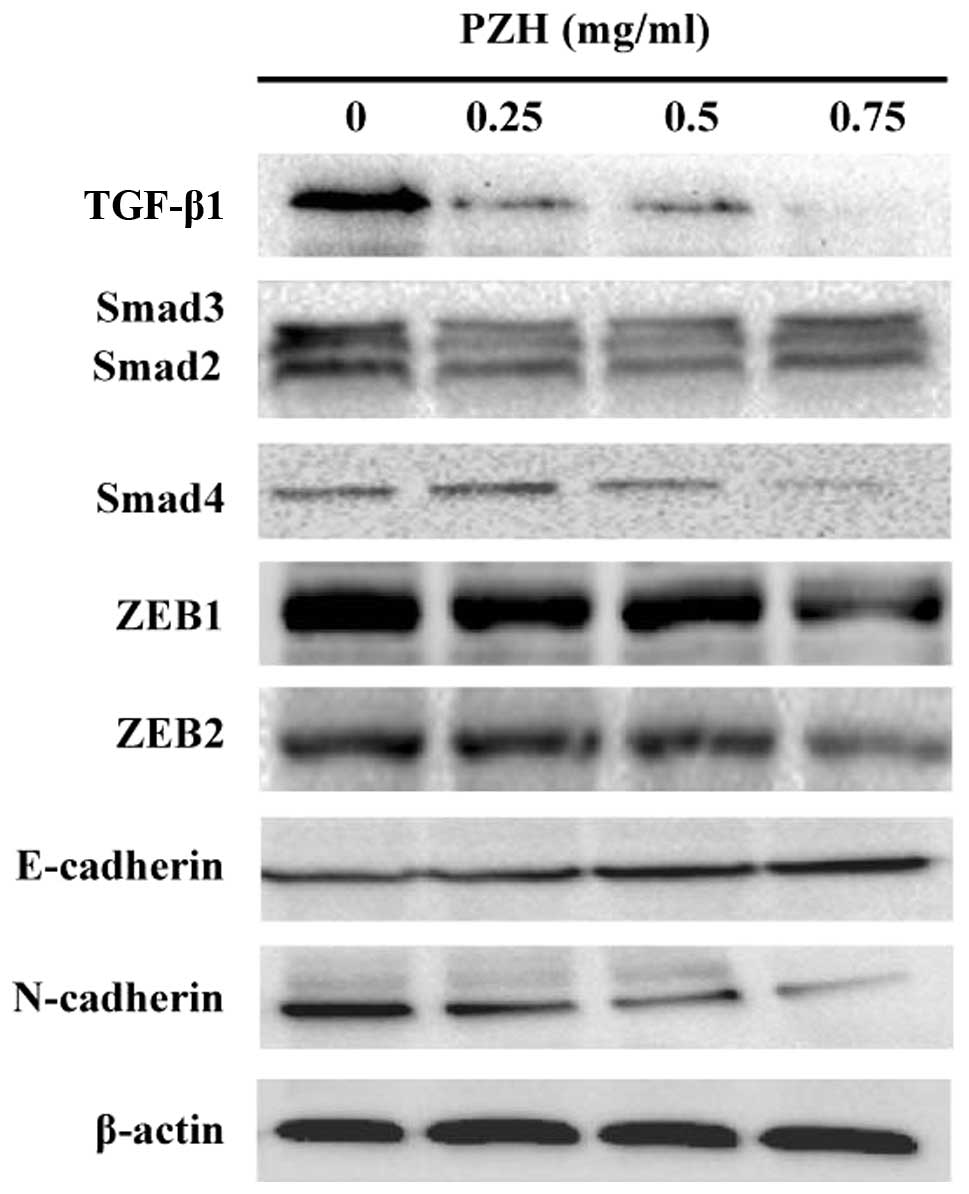
To determine the PZH effect on the activation of
TGF-β1 signaling, we examined the protein expression of several key
mediators of this pathway using western blot analysis. As shown in
Fig. 4, the protein expression
levels of TGF-β1, Smad2/3 and Smad4 were downregulated by PZH
treatment in a dose-dependent manner. Moreover, PZH treatment
suppressed the expression of TGF-β1 target genes ZEB1 and ZEB2,
leading to the downregulation of expression of mesenchymal marker
N-cadherin as well as an increase in the expression of epithelial
marker E-cadherin (Fig. 4).
Therefore, the inhibitory effect of PZH on cancer cell metastasis
might be mediated by the suppression of TGF-β1 pathway and the
process of EMT.
PZH upregulates the expression of
miR-200a, miR-200b and miR-200c in HCT-8 cells
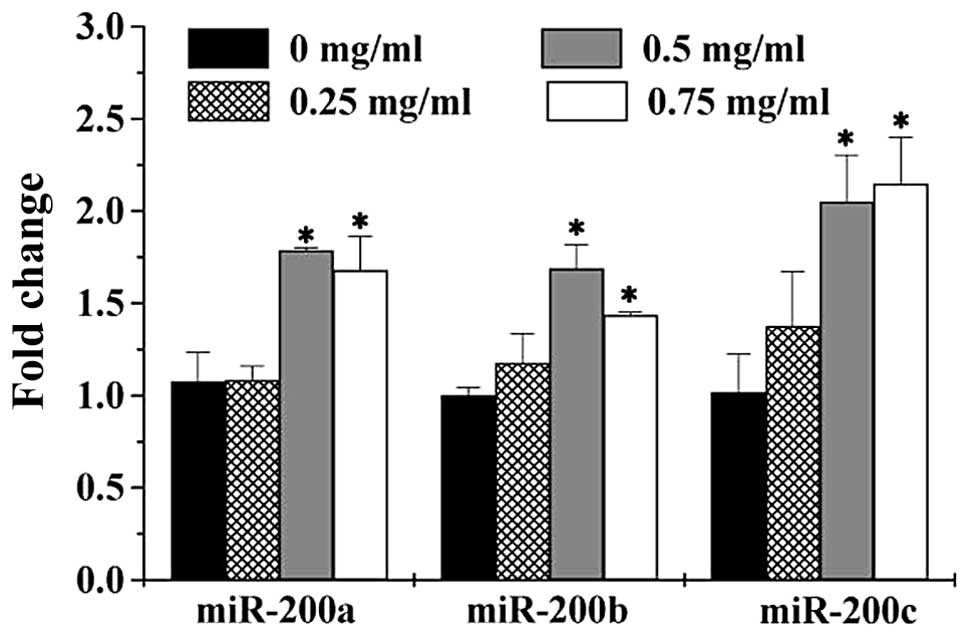
To further explore the mechanism of anti-metastasis
activities of PZH, we determined the expression of miR-200 family
in HCT-8 cells using Q-PCR assay. As shown in Fig. 5, PZH treatment significantly and
dose-dependently increased the expression of miR-200a, miR-200b and
miR-200c, consistent with the observations that PZH inhibited the
TGF-β1 pathway and expression of ZEB transcription factors
(Fig. 4).
Discussion
Drug resistance and intrinsic cytotoxicity against
normal cells profoundly limit the long-term use of currently-used
chemotherapeutic regimens and thereby their therapeutic
effectiveness (41,42), emphasizing the need for the
development of novel antitumor drugs. Due to the relatively higher
safety and the long history of pharmacological applications,
traditional Chinese medicines (TCM) have attracted great interest
in the field of cancer treatment (28,29).
TCM formula is a complex combination of many natural products, each
of which contains numerous chemical compounds. Therefore, TCM
formulas are considered to be multi-component and multi-target
agents exerting their therapeutic function in a more holistic way;
and discovering naturally-occurring agents could be a promising
approach of cancer treatment. Pien Tze Huang (PZH) is a well-known
TCM formula that has been used in China and Southeast Asia for
centuries as a folk remedy for various types of cancer. We recently
reported that PZH can inhibit colon cancer growth through the
promotion of cancer cell apoptosis, the inhibition of cell
proliferation and tumor angiogenesis, which is probably mediated by
modulation of multiple signaling pathways (33–40).
These data demonstrate that PZH possesses a broad range of
anticancer activities due to its ability to affect multiple
intracellular targets, suggesting that PZH could be a novel
multi-target anticancer agent.
Tumor metastasis is a complex process involving the
spread of malignant tumor cells from a primary tumor site to a
distant organ, which is a major cause of failure of clinical cancer
chemotherapy and therefore has become an important focus for
anticancer therapies (5–7,13,14).
To further elucidate the mode of action of PZH, in the present
study we evaluated its effects on cancer metastasis. Using wound
healing and transwell assays we found that PZH treatment
significantly inhibited the migration and invasion of human
colorectal carcinoma HCT-8 cells in a dose-dependent manner,
demonstrating the inhibitory activity of PZH on the metastatic
capacities of colorectal cancer cells. Epithelial-mesenchymal
transition (EMT) is a biological process in which epithelial cells
lose their polarity and cell-cell adhesion, and acquire migratory
and invasive properties of mesenchymal cells (8,9,14,15).
After acquiring a mesenchymal phenotype through the process of EMT,
carcinoma cells invade adjacent tissues, break through the basement
membrane, and eventually enter the bloodstream leading to cancer
metastasis (8,9,14–17).
Therefore, EMT is an essential step for the initiation of cancer
metastasis. Using western blot analysis we found that PZH treatment
reduced the protein expression of mesenchymal marker N-cadherin but
increased that of epithelial marker E-cadherin, indicating that the
anti-metastasis activity of PZH was associated with its inhibitory
effect on EMT. The processes of EMT and metastasis are highly
regulated by multiple mechanisms, including TGF-β1/SMAD pathways
and miRNA 200 family (10–14). The activation of TGF-β1 signaling
enhances the expression of ZEB transcription factors, which in turn
modulates the expression of EMT-regulatory genes resulting in the
initiation of EMT (15,16). Interestingly, the expression of ZEB
transcription factors can be downregulated by miR-200 family
members (11,14,25–27);
but miR-200 family expression is negatively regulated by TGF-β1
signaling (12), forming a
double-negative feedback loop to control the processes of EMT and
metastasis (13,14). Data from western blot and Q-PCR
analyses indicated that PZH suppressed the activation of TGF-β1
pathway and the expression of ZEB1 and ZEB2, whereas the expression
of miR-200a, miR-200b and miR-200c was upregulated after PZH
treatment.
In conclusion, here we report that PZH can inhibit
the metastatic capacity of human colorectal carcinoma cells via
modulating TGF-β1/ZEB/miR-200 signaling network, which might be one
of the mechanisms whereby PZH exerts its anticancer function.
Acknowledgements
This study was supported by the National Natural
Science Foundations of China (81373819 and 81202790) and the China
Postdoctoral Science Foundation (2013T60636).
Abbreviations:
|
CRC
|
colorectal cancer
|
|
PZH
|
Pien Tze Huang
|
|
TCM
|
traditional Chinese medicine
|
|
TGF-β
|
transforming growth factor-β
|
|
EMT
|
epithelial-to-mesenchymal
transition
|
|
ZEB
|
zinc finger E-box-binding homeobox
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Markowitz SD and Bertagnolli MM: Molecular
basis of colorectal cancer. N Engl J Med. 361:2449–2460. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koyanagi K, Bilchik AJ, Saha S, Turner RR,
Wiese D, McCarter M, Shen P, Deacon L, Elashoff D and Hoon DS:
Prognostic relevance of occult nodal micrometastases and
circulating tumor cells in colorectal cancer in a prospective
multicenter trial. Clin Cancer Res. 14:7391–7396. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bilchik AJ, Hoon DS, Saha S, Turner RR,
Wiese D, DiNome M, Koyanagi K, McCarter M, Shen P, Iddings D, Chen
SL, Gonzalez M, Elashoff D and Morton DL: Prognostic impact of
micrometastases in colon cancer: interim results of a prospective
multicenter trial. Ann Surg. 246:568–575. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Johnson SM, Gulhati P, Rampy BA, Han Y,
Rychahou PG, Doan HQ, Weiss HL and Evers BM: Novel expression
patterns of PI3K/Akt/mTOR signaling pathway components in
colorectal cancer. J Am Coll Surg. 210:767–778. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Christofori G: New signals from the
invasive front. Nature. 441:444–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stein U and Schlag PM: Clinical,
biological, and molecular aspects of metastasis in colorectal
cancer. Recent Results Cancer Res. 176:61–80. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pecina-Slaus N, Cicvara-Pecina T and Kafka
A: Epithelial-to-mesenchymal transition: possible role in
meningiomas. Front Biosci (Elite Ed). 13:889–896. 2012. View Article : Google Scholar
|
|
9
|
Guarino M, Rubino B and Ballabio G: The
role of epithelial-mesenchymal transition in cancer pathology.
Pathology. 13:305–318. 2007. View Article : Google Scholar
|
|
10
|
Xu Y and Pasche B: TGF-beta signaling
alterations and susceptibility to colorectal cancer. Hum Mol Genet.
16:R14–R20. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eades G, Yao Y, Yang M, Zhang Y, Chumsri S
and Zhou Q: miR-200a regulates SIRT1 expression and epithelial to
mesenchymal transition (EMT)-like transformation in mammary
epithelial cells. J Biol Chem. 286:25992–26002. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gregory PA, Bracken CP, Smith E, Bert AG,
Wright JA, Roslan S, Morris M, Wyatt L, Farshid G, Lim YY, Lindeman
GJ, Shannon MF, Drew PA, Khew-Goodall Y and Goodall GJ: An
autocrine TGF-beta/ZEB/miR-200 signaling network regulates
establishment and maintenance of epithelial-mesenchymal transition.
Mol Biol Cell. 22:1686–1698. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiong M, Jiang L, Zhou Y, Qiu W, Fang L,
Tan R, Wen P and Yang J: The miR-200 family regulates
TGF-β1-induced renal tubular epithelial to mesenchymal transition
through Smad pathway by targeting ZEB1 and ZEB2 expression. Am J
Physiol Renal Physiol. 302:F369–F379. 2012. View Article : Google Scholar
|
|
14
|
Hur K, Toiyama Y, Takahashi M, Balaguer F,
Nagasaka T, Koike J, Hemmi H, Koi M, Boland CR and Goel A:
MicroRNA-200c modulates epithelial-to-mesenchymal transition (EMT)
in human colorectal cancer metastasis. Gut. 62:1315–1326. 2013.
View Article : Google Scholar :
|
|
15
|
Moustakas A and Heldin CH: Signaling
networks guiding epithelial-mesenchymal transitions during
embryogenesis and cancer progression. Cancer Sci. 98:1512–1520.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu Y, Sato F, Yamada T, Bhawal UK,
Kawamoto T, Fujimoto K, Noshiro M, Seino H, Morohashi S, Hakamada
K, Abiko Y, Kato Y and Kijima H: The BHLH transcription factor DEC1
plays an important role in the epithelial-mesenchymal transition of
pancreatic cancer. Int J Oncol. 41:1337–1346. 2012.PubMed/NCBI
|
|
17
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: an alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Comijn J, Berx G, Vermassen P, Verschueren
K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D and van Roy
F: The two-handed E box binding zinc finger protein SIP1
downregulates E-cadherin and induces invasion. Mol Cell.
7:1267–1278. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Valencia-Sachez MA, Liu J, Hannon GJ and
Parker R: Control of translation and mRNA degradation by miRNAs and
siRNAs. Genes Dev. 20:515–524. 2006. View Article : Google Scholar
|
|
20
|
Bagga S and Pasquinelli AE: Identification
and analysis of microRNAs. Genet Eng. 27:1–20. 2006. View Article : Google Scholar
|
|
21
|
Bar N and Dikstein R: miR-22 forms a
regulatory loop in PTEN/AKT pathway and modulates signaling
kinetics. PLoS One. 5:e108592010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Leskelä S, Leandro-García LJ, Mendiola M,
Barriuso J, Inglada-Pérez L, Muñoz I, Martínez-Delgado B, Redondo
A, de Santiago J and Robledo M: The miR-200 family controls
β-tubulin iii expression and is associated with paclitaxel-based
treatment response and progression-free survival in ovarian cancer
patients. Endocr Relat Cancer. 18:85–95. 2011. View Article : Google Scholar
|
|
23
|
Uhlmann S, Zhang J, Schwäger A,
Mannsperger H, Riazalhosseini Y, Burmester S, Ward A, Korf U,
Wiemann S and Sahin Ö: MiR-200bc/429 cluster targets plcγ1 and
differentially regulates proliferation and egf-driven invasion than
miR-200a/141 in breast cancer. Oncogene. 29:4297–4306. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li H, Tang J, Lei H, Cai P, Zhu H, Li B,
Xu X, Xia Y and Tang W: Decreased MiR-200a/141 suppress cell
migration and proliferation by targeting PTEN in Hirschsprung’s
disease. Cell Physiol Biochem. 34:543–553. 2014. View Article : Google Scholar
|
|
25
|
Gregory PA, Bert AG, Paterson EL, Barry
SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y and Goodall GJ:
The miR-200 family and miR-205 regulate epithelial to mesenchymal
transition by targeting ZEB1 and SIP1. Nat Cell Biol. 10:593–601.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park SM, Gaur AB, Lengyel E and Peter ME:
The miR-200 family determines the epithelial phenotype of cancer
cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes
Dev. 22:894–907. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang CH, Chen CL, More SV, Hsiao PW, Hung
WC and Li WS: The tetraindole SK228 reverses the
epithelial-to-mesenchymal transition of breast cancer cells by
up-regulating members of the miR-200 family. PLoS One.
9:e1010882014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gordaliza M: Natural products as leads to
anticancer drugs. Clin Transl Oncol. 9:767–776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ji HF, Li XJ and Zhang HY: Natural
products and drug discovery. EMBO Rep. 10:194–200. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chinese Pharmacopoeia Commission.
Pharmacopoeia of the Peoples Republic of China. 1. Chinese Medical
Science and Technology Press; Beijing: pp. 573–575. 2010
|
|
31
|
Lee KK, Kwong WH, Chau FT, Yew DT and Chan
WY: Pien Tze Huang protects the liver against carbon
tetrachloride-induced damage. Pharmacol Toxicol. 91:185–192. 2002.
View Article : Google Scholar
|
|
32
|
Chan WY, Chau FT, Lee KK, Kwong WH and Yew
DT: Substitution for natural musk in Pien Tze Huang does not affect
its hepatoprotective activities. Hum Exp Toxicol. 23:35–47. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin JM, Wei LH, Chen YQ, Liu XX, Hong ZF,
Sferra TJ and Peng J: Pien Tze Huang-induced apoptosis in human
colon cancer HT-29 cells is associated with regulation of the Bcl-2
family and activation of caspase 3. Chin J Integr Med. 17:685–690.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhuang QC, Hong F, Shen AL, Zheng LP, Zeng
JW, Lin W, Chen YQ, Sferra TJ, Hong ZF and Peng J: Pien Tze Huang
inhibits tumor cell proliferation and promotes apoptosis via
suppressing the STAT3 pathway in colorectal cancer mouse. Int J
Oncol. 26:1569–1574. 2012.
|
|
35
|
Shen AL, Hong F, Liu LY, Lin JM, Zhuang
QC, Hong ZF and Peng J: Effects of Pien Tze Huang on angiogenesis
in vivo and in vitro. Chin J Integr Med. 18:431–436. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shen AL, Hong F, Liu LY, Lin JM, Wei LH,
Cai QY, Hong ZF and Peng J: Pien Tze Huang inhibits the
proliferation of human colon carcinoma cells by arresting G1/S cell
cycle progression. Oncol Lett. 4:767–770. 2012.PubMed/NCBI
|
|
37
|
Shen AL, Chen YQ, Hong F, Lin JM, Wei LH,
Hong ZF, Sferra TJ and Peng J: Pien Tze Huang suppresses
IL-6-inducible STAT3 activation in human colon carcinoma cells
through induction of SOCS3. Oncol Rep. 28:2125–2130.
2012.PubMed/NCBI
|
|
38
|
Shen A, Lin J, Chen Y, Lin W, Liu L, Hong
Z, Sferra TJ and Peng J: Pien Tze Huang inhibits tumor angiogenesis
in a mouse model of colorectal cancer via suppression of multiple
cellular pathways. Oncol Rep. 30:1701–1706. 2013.PubMed/NCBI
|
|
39
|
Chen HW, Shen AL, Zhang YC, Chen YQ, Lin
JM, Lin W, Sferra TJ and Peng J: Pien Tze Huang inhibits
hypoxia-induced epithelial-mesenchymal transition in human colon
carcinoma cells via suppression of HIF-1 pathway. Exp Ther Med.
7:1237–1242. 2014.PubMed/NCBI
|
|
40
|
Wei LH, Chen PY, Chen YQ, Shen AL, Chen
HW, Lin W, Hong ZF, Sferra T and Peng J: Pien Tze Huang suppresses
the stem-like side population in colorectal cancer cells. Mol Med
Rep. 9:261–266. 2014.
|
|
41
|
Van Cutsem E and Costa F: Progress in the
adjuvant treatment of colon cancer: Has it influenced clinical
practice? JAMA. 294:2758–2760. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lippman SM: The dilemma and promise of
cancer chemoprevention. Nat Clin Pract Oncol. 10:5232006.
View Article : Google Scholar
|