Introduction
Neuroblastoma is an aggressive pediatric tumor that
accounts for ~15% of all cancer-related deaths in children. It
originates from neural crest cells and shows extreme heterogeneity
ranging from spontaneous regression to malignant progression.
Approximately 50% of neuroblastoma patients are stratified into a
high-risk group with the overall survival rate of <40% (1–3).
Over the years, two major advances have been incorporated into the
current therapy for high-risk patients (4). First, patients treated with the
differentiation agent 13-cis-retinoic acid (13-cis-RA) after
myeloablative consolidation therapy had a significantly decreased
rate of relapse (5). Second, the
combination of 13-cis-RA with anti-GD2 antibody and cytokines in
maintenance therapy further improved a relapse-free survival rate
(6). Despite these improvements,
50–60% of patients who complete these treatments still experience a
tumor relapse. As in other cancers, neuroblastoma relapse is
primarily driven by chemoresistant cancer stem cells (CSCs)
(7–9).
A number of studies have isolated neuroblastoma CSCs
as spheres grown in serum-free non-adherent culture used for neural
crest stem cell growth (10), side
population cells based on the efficient efflux of Hoechst 33342 dye
from stem cells used to isolate hematopoietic stem cells (11) and cell-surface marker-positive
cells based on the markers associated with stem cell populations in
other cancers (12–14). Although these studies provide an
important insight into the properties of neuroblastoma CSCs, their
definitive markers are still missing.
Aldehyde dehydrogenases (ALDHs) are a family of
NAD(P)+-dependent enzymes that catalyze the oxidation of
aldehydes to their corresponding carboxylic acids. They not only
serve to protect cells from the cytotoxic effects of xenobiotic and
intracellular aldehydes such as cyclophosphamide and ethanol, but
also generate important carboxylic acids in cellular physiology
such as retinoic acid (RA) and γ-aminobutyric acid (GABA) (15). High ALDH activity was first found
in hematopoietic stem cells and normal stem cells isolated from a
variety of tissues (16) and then
detected in CSCs of certain cancers (17,18).
Among all 19 ALDH isoforms identified in human cells, several
isoforms were proposed as CSC-markers; ALDH1A1 in lung cancer
(19), ALDH1B1 in colon cancer
(20) and ALDH7A1 in prostate
cancer (21). However, ALDH
remains elusive in neuroblastoma.
In the present study, we analyzed the ALDH activity
and expression of its 19 isoforms in spheres and parental cells of
different neuroblastoma cells and found that ALDH1A2 was involved
in the regulation of CSC properties in neuroblastoma.
Materials and methods
Neuroblastoma cells
BE(2)-C (CRL-2268) cells were obtained from the
American Type Culture Collection (Manassas, VA, USA). Human
neuroblastoma NBTT1, NBTT2D and NBTT3 cells were previously
described (22,23). Tumor tissue samples were obtained
from high-risk neuroblastoma patients with written informed
consent. The use of human tissues for this study was approved by
the Ethics Committee at Kobe University Graduate School of Medicine
and conducted in accordance with the Guidelines for the Clinical
Research of Kobe University Graduate School of Medicine.
Antibodies
The rabbit anti-PGK1 antibody was purchased from
Sigma (St. Louis, MO, USA), rabbit anti-NF-M antibody from
Millipore (Billerica, MA, USA), rabbit anti-ALDH1A2 antibody from
Atlas antibodies (Stockholm, Sweden) and rabbit anti-NANOG antibody
from ReproCELL (Tokyo, Japan).
Expression plasmids
The N-terminal 3xFLAG-tagged expression plasmid with
IRES-driven GFP and puromycin markers (pRS-3FLAG-IRES-GFP) was
constructed. Briefly, the U6 promoter expression unit of pRS vector
(Origene, Rockville, MD, USA) was first replaced with the
CMV-promoter expression unit of pCMV6-AC-IRES-GFP vector (Origene)
using In-Fusion HD Cloning kit (Takara, Otsu, Japan). The
3xFLAG-tag was then inserted into the SgfI site within multiple
cloning sites of the resulting plasmid. The full-length ALDH1A2
cDNA (NM_003888) was amplified by PCR using PrimeStar GXL DNA
polymerase (Takara), cloned into pRS-3FLAG-IRES-GFP and sequenced
using an ABI PRISM 3100 genetic analyzer (Applied Biosystems,
Foster City, CA, USA). Scramble and specific short hairpin RNA
(shRNA) expression plasmids (pGFP-V-RS-scramble shRNA,
pGFP-V-RS-ALDH1A2 shRNA, pGFP-V-RS-ALDH1L1 shRNA and
pGFP-V-RS-ALDH3B2 shRNA) were obtained from Origene and their
sequences are listed in Table
I.
 | Table IshRNA sequences. |
Table I
shRNA sequences.
| Gene name | Accession no. | Sequence
(5′-3′) |
|---|
| ALDH1A2 | NM_003888 | #1:
ccaataactcagactttggactcgtagca
#2: tgtgttcttcaatcaaggtcagtgctgca |
| ALDH1L1 | NM_012190 | #1:
gtggtcaccaaagcaggactcatcctctt
#2: catccagaccttccgctactttgctggct |
| ALDH3B2 | NM_000695 | #1:
cagtacctggaccagagctgctttgccgt
#2: tcatcaaccagaaacagttccagcggctg |
Real-time RT-PCR
Total RNA from neuroblastoma cells and spheres was
isolated with a TRIzol Plus RNA purification kit (Invitrogen,
Carlsbad, CA, USA) and reverse transcribed using a QuantiTect
Reverse Transcription kit (Qiagen, Valencia, CA, USA) according to
the manufacturer’s instructions. Real-time RT-PCR was performed as
described previously (22). Primer
sequences are listed in Table
II.
 | Table IIRT-PCR primer sequences. |
Table II
RT-PCR primer sequences.
| Gene name | Accession no. | Forward sequence
(5′-3′) | Reverse sequence
(5′-3′) |
|---|
| PGK1 | NM_000291 |
ggagaacctccgctttcat |
gctggctcggctttaacc |
| ALDH1A1 | NM_000689 |
tttggtggattcaagatgtctg |
cactgtgactgttttgacctctg |
| ALDH1A2 | NM_003888 |
tgcattcacagggtctactga |
tgcctccaagttccagagtt |
| ALDH1A3 | NM_000693 |
aacccctgcatcgtgtgt |
tggttgaagaacactccctga |
| ALDH1B1 | NM_000692 |
ttctcgagagaaccgtggag |
gtccagctcaaaggggttc |
| ALDH1L1 | NM_012190 |
gaccttccgctactttgctg |
ggtctggcctggttgatg |
| ALDH1L2 | NM_001034173 |
ttgacaaggctgtgcgaat |
cccagcagcaatacagttctc |
| ALDH2 | NM_000690 |
tggatttggacatggtcctc |
gatggttttcccgtggtactt |
| ALDH3A1 | NM_001135168 |
gatccaggagcaggagca |
tgtactcgatctcctctaggacgta |
| ALDH3A2 | NM_001031806 |
agcagagatgaacaccagatttc |
aggaggttgaacaggatcattc |
| ALDH3B1 | NM_000694 |
cgcatcatcaaccagaaaca |
tctcctgcacatccaccag |
| ALDH3B2 | NM_000695 |
ttcatcaaccggcaggag |
ctccagcatctggttcacaa |
| ALDH4A1 | NM_003748 |
agagcaaggaccctcagga |
cagacagtacaggcccgaag |
| ALDH5A1 | NM_170740 |
caacgtggaccaggctgta |
tgcaccaagaattggtttga |
| ALDH6A1 | NM_005589 |
gcccctgatggaacattaaa |
tccggatgatcgcaaataa |
| ALDH7A1 | NM_001182 |
gacctatcttgccttctgaaaga |
gattccaaccaggcctacg |
| ALDH8A1 | NM_022568 |
aaccgtcaggtccagcttt |
cactatagatgctcttctggacaaa |
| ALDH9A1 | NM_000696 |
ctccagcattagcctgtggt |
agccagtagcaatgcagaaac |
| ALDH16A1 | NM_153329 |
agacgtccaggccatgtg |
gaggcccactcgacaaact |
| ALDH18A1 | NM_002860 |
tctcgtcctgactgtctaccc |
taacaagccattgccacttg |
Cell culture and transfection
Parental cells and spheres of BE(2)-C, NBTT1, NBTT2D
and NBTT3 cells were cultured as described previously (24). BE(2)-C cells were transfected with
expression plasmids using Lipofectamine 2000 transfection reagent
(Invitrogen) according to the manufacturer’s instructions. Stably
transfected GFP-positive cells were selected by 2.0–3.0 μg/ml
puromycin (Invivogen, San Diego, CA, USA) and further isolated by
using MoFlo XDP (Beckman Coulter, Brea, CA, USA).
ALDH activity
ALDH activity was determined using the Aldefluor kit
(Stem Cell Technologies, Durham, NC, USA) according to the
manufacturer’s instructions. Briefly, neuroblastoma cells and
spheres were dissociated with Accumax (Innovative Cell
Technologies, San Diego, CA, USA) and suspended at
~1×106 cells/ml in Aldefluor assay buffer containing
BODIPY-aminoacetaldehyde (BAAA) in the presence or absence of 15 μM
diethylaminobenzaldehyde (DEAB), incubated at 37°C for 40 min and
then treated with 1 μg/ml propidium iodide (PI; Sigma). Flow
cytometric analysis was performed using MoFlo XDP. Specific ALDH
activity was based on the difference between the presence and
absence of DEAB.
Sphere and colony formation
Sphere formation was analyzed as described
previously (24). For colony
formation, BE(2)-C cells expressing the indicated shRNA or cDNA
were mixed in 0.325% SeaPlaque agarose (Lonza, Rockland, ME, USA)
in DMEM/Ham’s F12 (3:1) (Wako Pure Chemical, Osaka, Japan) with 10%
FBS and plated at 1,000 cells/well onto a solidified bottom layer
of 0.6% SeaPlaque agarose in DMEM/Ham’s F12 with 10% FBS in a
6-well plate. Cells were incubated at 37°C for 21 days, stained
with 0.5 mg/ml 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT; Sigma) and photographed. The total number of colonies
was counted manually.
Tumor formation
Four-week-old male athymic BALB/cAJcl nu/nu (nude)
mice were obtained from CLEA (Shizuoka, Japan). All procedures
involving animals were approved by the Animal Care and Use
Committee of Kobe University Graduate School of Medicine and
carried out in strict accordance with the Guidelines for the Care
and Use of Laboratory Animals of Kobe University Graduate School of
Medicine. BE(2)-C cells expressing the indicated shRNA or cDNA in
50% Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) were
injected subcutaneously into the flank of nude mice at a density of
1×105 cells per injection site. Tumor growth was
monitored 3 times per week by external caliper and tumor volume (V)
was calculated by the formula: V = 1/2 (L × W2), where L
and W were the greatest longitudinal and transverse diameters
(25). Mice were dissected when
the greatest diameter of tumor reached 20 mm. Xenograft tumors were
fixed in 20% buffered neutral formalin solution (Muto Pure
Chemicals, Tokyo, Japan) and embedded in paraffin. Tumor sample was
sectioned 4-μm thick, deparaffinized in xylene, rehydrated in
alcohol and stained with hematoxylin and eosin (H&E).
Immunohistochemistry
Immunohistochemical staining of neuroblastoma
xenografts was performed on the 4-μm thick sections of tumor
samples. After antigen retrieval using a conventional steamer with
citrate buffer (pH 6.0), the section was immunostained with a
primary antibody in REAL antibody diluent (Dako, Glostrup,
Denmark). The section was then blocked with peroxidase blocking
reagent (Dako) and incubated with EnVision labeled polymer
peroxidase (Dako). The immune complex was visualized using 3,
3′-diaminoben-zidine (DAB; Dako) as a chromogen and hematoxylin as
a counterstain. To quantify the average percentage of immunostained
cells, three fields containing ≥300 neuroblastoma cells were
randomly selected from each sample. Positive cells were identified
microscopically as brown cytoplasmic staining and manually
counted.
Other methods
Phase-contrast images were acquired using a BZ-9000E
fluorescence microscope (Keyence, Osaka, Japan). Western blotting
was performed as described previously (26).
Results
ALDH activity and ALDH isoforms
expression are consistently induced in spheres of different
neuroblastoma cells
To begin to characterize ALDH in neuroblastoma,
NBTT2D, NBTT1 and NBTT3 cells established from distinct high-risk
neuroblastoma patients were grown as spheres in a serum-free
non-adherent condition as described previously (24). The ALDH activities in spheres and
parental cells were then determined by Aldefluor assay. Higher ALDH
activity was constantly detected in spheres compared to parental
cells (Fig. 1A and B). Because the
ALDH isoform responsible for high ALDH activity measured by
Aldefluor assay is specific for each cancer type (18), we next analyzed the fold-change of
ALDH isoform expression in spheres compared to parental cells.
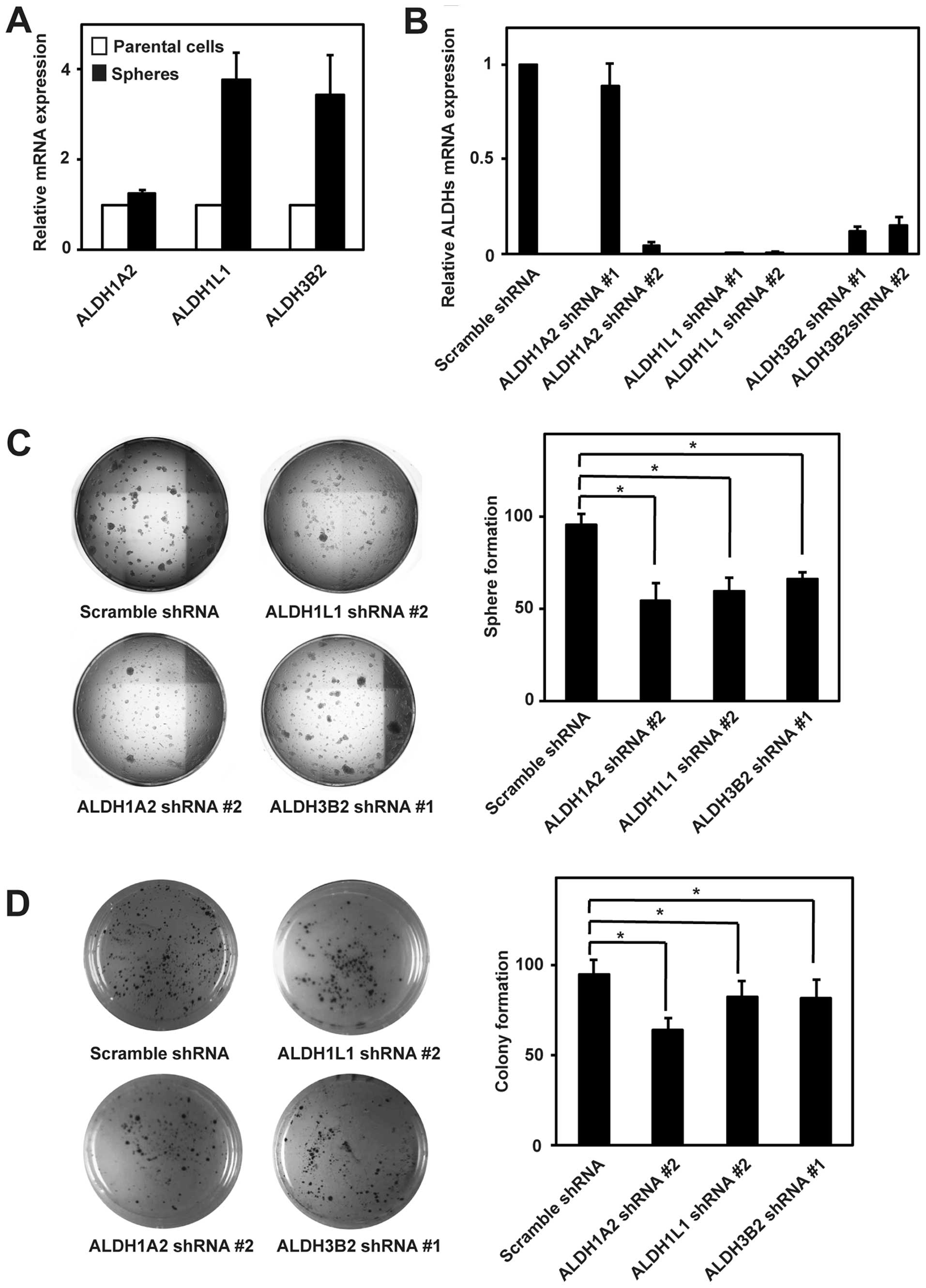
Among all 19 ALDH isoforms expressed in human cells, ALDH1A2,
ALDH1L1 and ALDH3B2 expression was consistently induced in spheres
compared to parental cells (Fig.
1C). While the induction of ALDH1A2 expression was not so high
in NBTT1 and NBTT3 cells, ALDH1A2 showed the best correlation
between mRNA induction and enzymatic activity induction among
ALDH1A2, ALDH1L1 and ALDH3B2 isoforms.
ALDH1A2, ALDH1L1 and ALDH3B2 isoforms are
associated with the sphere and colony formation in neuroblastoma
cells
To gain insight into the function of ALDH isoforms
induced in spheres, we first examined ALDH1A2, ALDH1L1 and ALDH3B2
mRNA expression in spheres and parental cells of neuroblastoma
BE(2)-C cells. Like NBTT2D, NBTT1 and NBTT3 cells, BE(2)-C cells
also showed the induction of expression of these ALDH isoforms in
spheres (Fig. 2A). We then
generated BE(2)-C cells stably expressing scramble, ALDH1A2,
ALDH1L1 and ALDH3B2 shRNA. ALDH1A2 shRNA #2, ALDH1L1 shRNA #2 and
ALDH3B2 shRNA #1 achieved effective knockdown and were used in the
present study (Fig. 2B). Next, we
performed the sphere and colony formation assays that are widely
used to examine the CSC properties in vitro. In the sphere
formation assay, cells are cultured in a serum-free non-adherent
condition so that only cells with ability to self-renew will form
spheres. Knockdown of ALDH1A2, ALDH1L1 and ALDH3B2 in BE(2)-C cells
significantly impaired the sphere formation (Fig. 2C). In the colony formation assay,
cells with a capacity of anchorage-independent growth can grow in
soft agar and form colonies. The colony formation was also
significantly impaired upon ALDH1A2, ALDH1L1 and ALDH3B2 knockdown
(Fig. 2D). Among these ALDH
isoforms, ALDH1A2 knockdown showed the most profound effect on both
the sphere and colony formation.
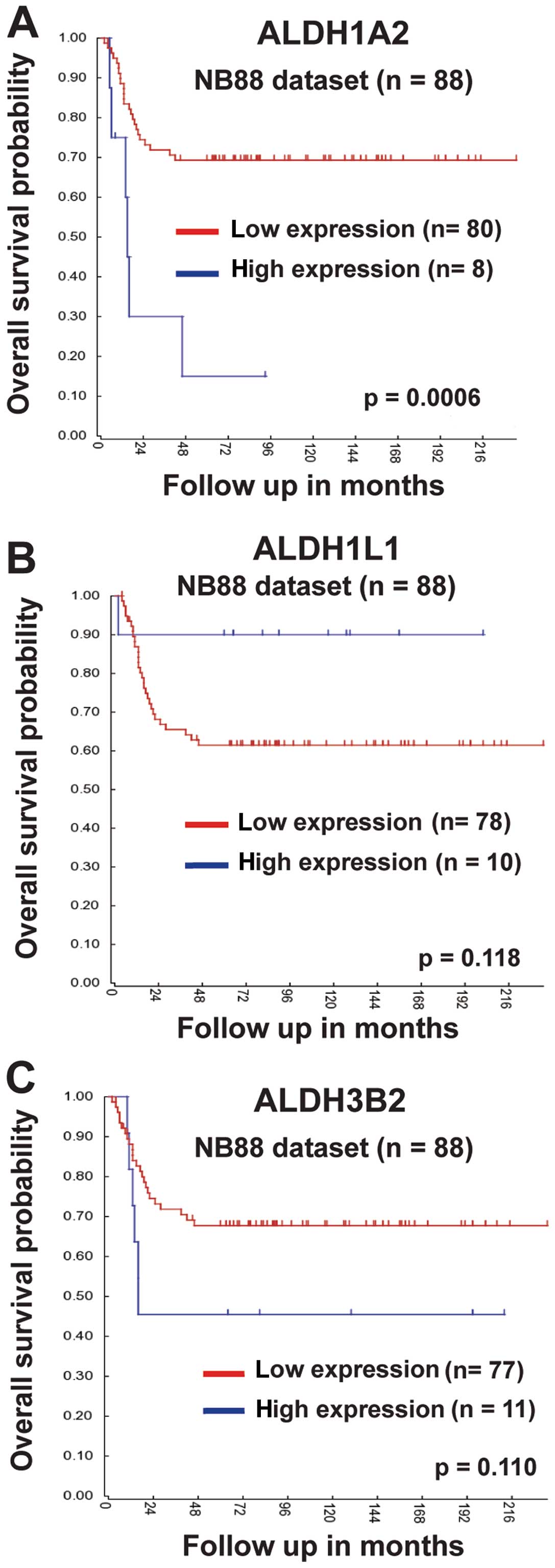
ALDH1A2 expression is correlated with the
prognosis of neuroblastoma patients
To further characterize the function of ALDH
isoforms induced in spheres, we next analyzed the correlation of
ALDH1A2, ALDH1L1 and ALDH3B2 expression with overall survival
probabilities of neuroblastoma patients. For this purpose, we used
the bioinformatics program R2 (http://r2.amc.nl)
and the NB88 dataset (Tumor
Neuroblastoma-Versteeg-88-MAS5.0-u133p2) that consisted of 88
primary neuroblastoma tumors of all stages. High expression of both
ALDH1A2 and ALDH3B2 was associated with low overall survival
probabilities (Fig. 3A and C). In
contrast, low ALDH1L1 expression tended to have low overall
survival probabilities (Fig. 3B).
Among ALDH1A2, ALDH1L1 and ALDH3B2 isoforms, only ALDH1A2
expression was significantly correlated with overall survival
probabilities.
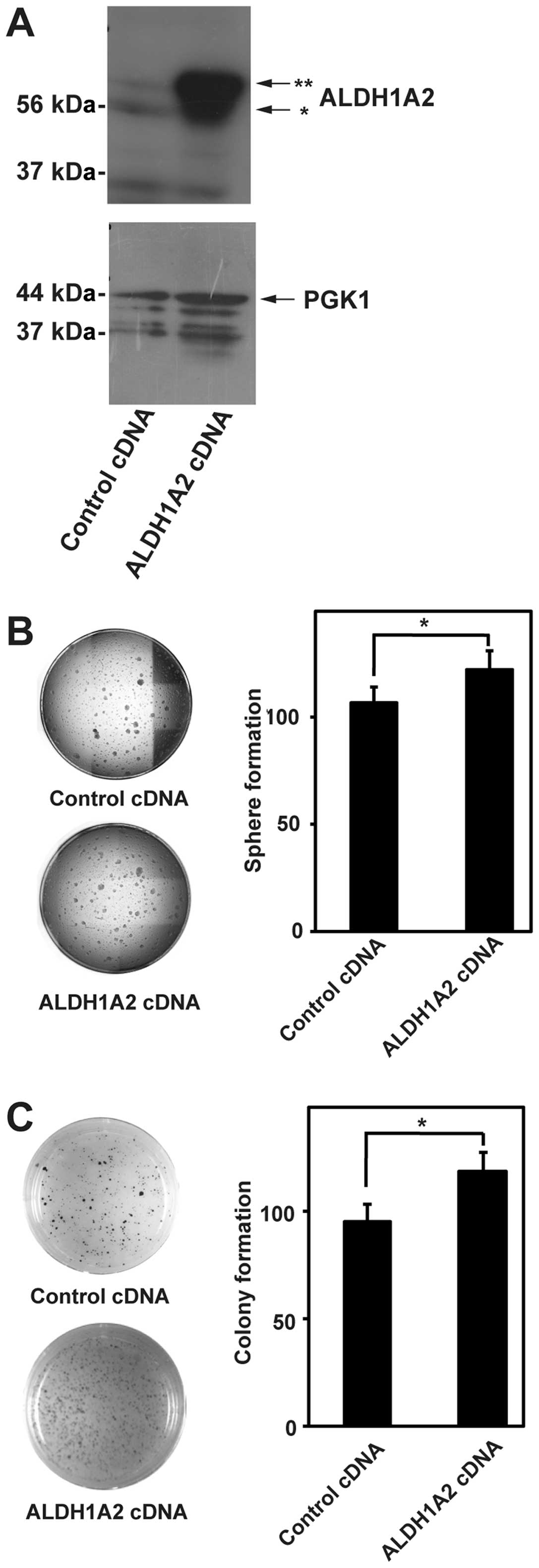
ALDH1A2 is involved in the sphere and
colony formation in neuroblastoma cells
Based on the above results, we focused on ALDH1A2
isoform in the subsequent study. If ALDH1A2 were involved in the
sphere and colony formation in neuroblastoma cells, ALDH1A2
overexpression would promote the sphere and colony formation. To
test this possibility, we generated BE(2)-C cells stably expressing
ALDH1A2 cDNA. ALDH1A2 overexpression was detected by western
blotting (Fig. 4A). The sphere and
colony formation were significantly promoted by ALDH1A2 cDNA
expression (Fig. 4B and C). These
results suggested that ALDH1A2 was involved in the sphere and
colony formation in neuroblastoma cells.
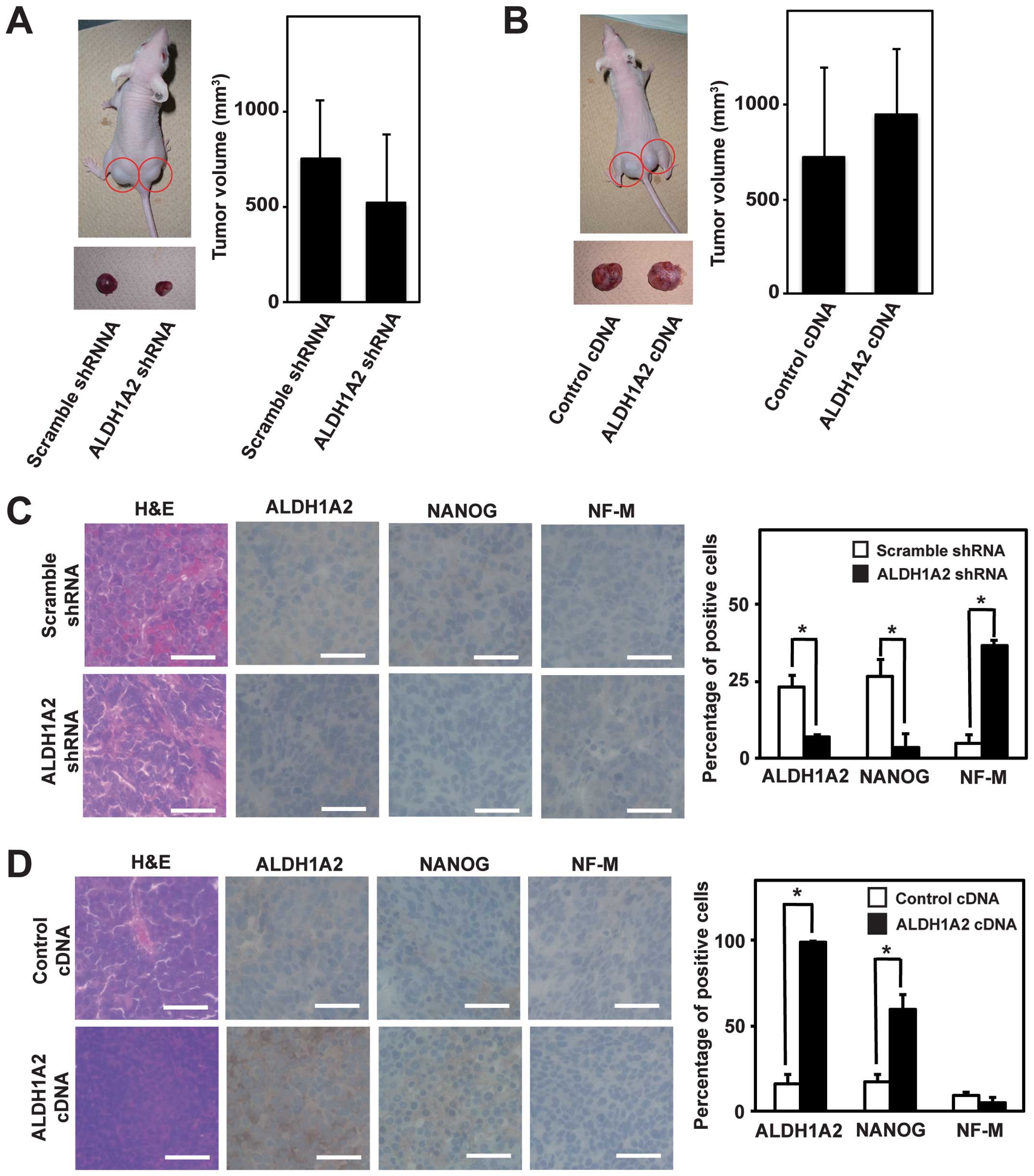
ALDH1A2 is involved in the growth and
undifferentiation of neuroblastoma xenografts
As CSC is defined as a subpopulation of cancer cells
that recapitulates their heterogeneous populations in xenograft
tumors, we next examined the function of ALDH1A2 in xenograft
tumors. To this end, we injected BE(2)-C cells stably expressing
scramble shRNA, ALDH1A2 shRNA, control cDNA and ALDH1A2 cDNA into
the flank of nude mice. All mice developed palpable tumors within
10–15 days and tumor volume was determined in 3–4 weeks. Tumor
volume tended to decrease upon ALDH1A2 knockdown and to increase
upon ALDH1A2 overexpression, albeit it was not statistically
significant (Fig. 5A and B). We
then performed the pathological examination with H&E staining.
Scramble shRNA and control cDNA tumors showed typical
characteristics of a small round cell tumor (Fig. 5C and D). Compared to scramble shRNA
tumors, ALDH1A2 shRNA tumors contained more stromal structures and
neuronal fibers (Fig. 5C,
H&E). In contrast, stromal structures were scarcer in
ALDH1A2 cDNA tumors than in control cDNA tumors (Fig. 5D, H&E). The tumors were further
examined by immunostaining with antibodies against ALDH1A2, NANOG
and NF-M. ALDH1A2-positive cells were reduced in ALDH1A2 shRNA
tumors compared to scramble shRNA tumors, whereas they were
increased in ALDH1A2 cDNA tumors compared to control cDNA tumors
(Fig. 5C and D, ALDH1A2). ALDH1A2
shRNA tumors showed more differentiated phenotypes with
NF-M-positive and NANOG-negative cells than scramble shRNA tumors,
while ALDH1A2 cDNA tumors had more undifferentiated phenotypes with
NANOG-positive and NF-M-negative cells than control cDNA tumors
(Fig. 5C and D, NANOG and NF-M).
These data suggested that ALDH1A2 was involved in the growth and
undifferentiation of neuroblastoma xenografts.
ALDH1A2 is involved in the resistance of
neuroblastoma cells to 13-cis-RA
Because CSC contributed to the chemoresistance in
addition to the sphere, colony and tumor formation, we finally
examined the function of ALDH1A2 in the resistance of neuroblastoma
cells to 13-cis-RA, which is currently used in the maintenance
therapy for high-risk neuroblastoma patients. As RA generally
induces the growth arrest and differentiation of neuroblastoma
cells, we first analyzed the growth arrest induced by 13-cis-RA
treatment for 72 h in BE(2)-C cells expressing scramble shRNA,
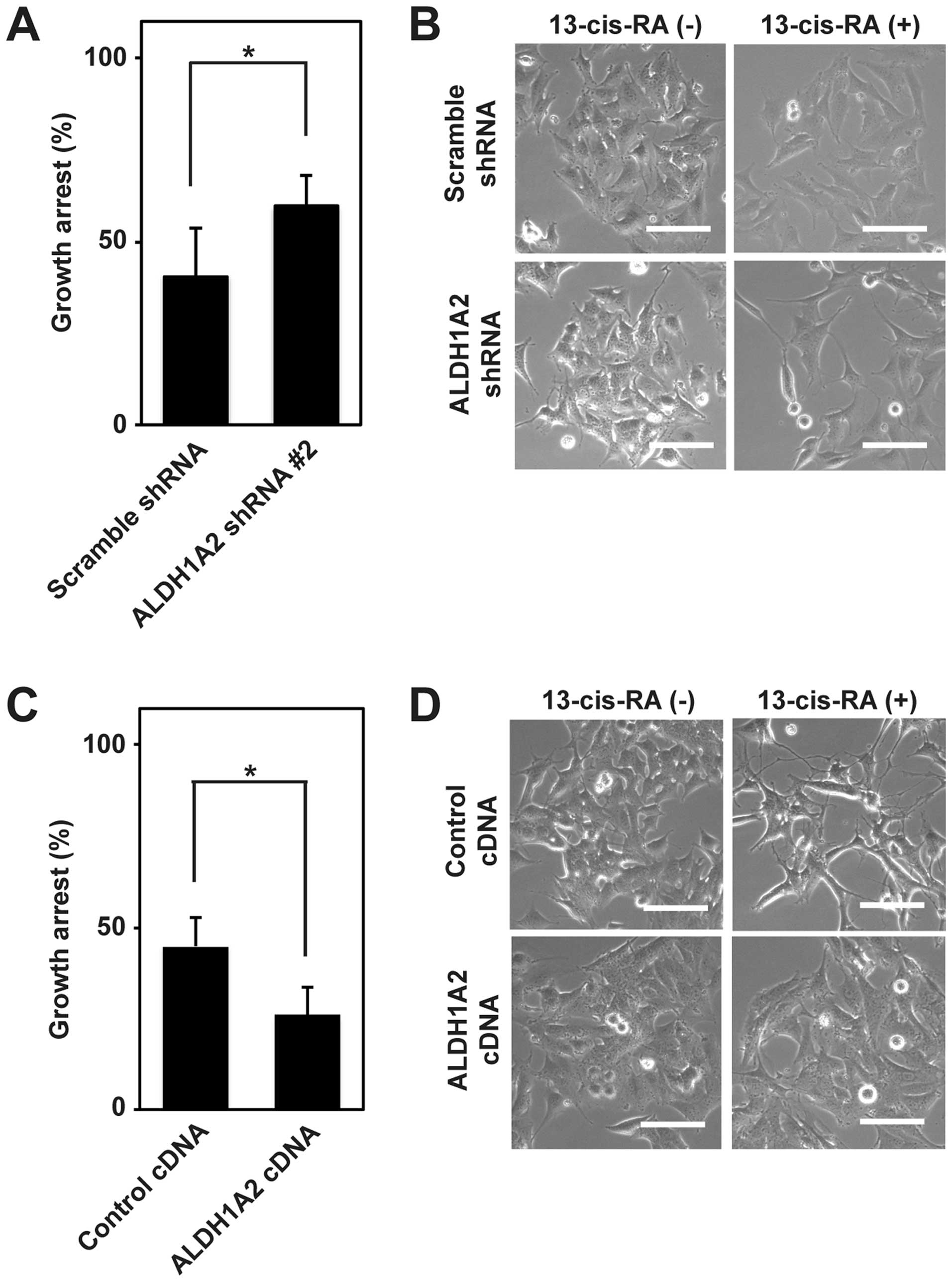
ALDH1A2 shRNA, control cDNA and ALDH1A2 cDNA. The 13-cis-RA-induced
growth arrest was significantly promoted by ALDH1A2 knockdown and
inhibited by ALDH1A2 overexpression (Fig. 6A and C). We then investigated their
differentiation by phase-contrast microscopy. In scramble shRNA and
control cDNA cells, the elongation of neurites started at ~48 h and
became evident at ~72 h after 13-cis-RA treatment. Compared to
scramble shRNA cells, ALDH1A2 shRNA cells showed more elongated
neurites at 48 h after 13-cis-RA treatment (Fig. 6B). In contrast, ALDH1A2 cDNA cells
did not elongate the neurites compared to control cDNA cells at 72
h after 13-cis-RA treatment (Fig.
6D). These results suggested that ALDH1A2 was involved in the
resistance of neuroblastoma cells to 13-cis-RA.
Discussion
More than half of high-risk neuroblastoma patients
experience tumor relapses and no curative salvage therapies for
recurrent neuroblastoma are currently known (4). In the present study, we analyzed ALDH
activity and expression of its 19 isoforms in spheres and parental
cells of different neuroblastoma cells. Consistent with our present
finding that ALDH1A2 was involved in the regulation of CSC
properties in neuroblastoma, ALDH1A2 was recently identified as the
highest upregulated gene along with marker genes of CD133, ABC
transporter, and WNT and NOTCH in neuroblastoma spheres (27).
While ALDH1A2 likely has diverse catalytic and
non-catalytic activities in addition to aldehyde metabolizing
activity, the physiological role of ALDH1A2 is best exemplified by
the association of genetic aberrations with disease phenotypes in
both mice and humans. ALDH1A2 knockout mice are embryonic lethal
(28), and mutations in ALDH1A2
gene are associated with spina bifida, congenital heart disease and
osteoarthritis of the hand (29–31).
As ALDH1A2 has the RA-biosynthesis activity by oxidizing retinal
aldehyde to RA, these phenotypes might be explained by the aberrant
RA-mediated cell signaling that has complex and pleiotropic
functions during development (32,33).
Among all 19 ALDH isoforms, other isoforms lacking
RA-biosynthesis activities were also upregulated in CSCs of several
cancer types (20,21,34).
Indeed, ALDH1L1 and ALDH3B2 expression was also consistently
induced in CSCs of neuroblastoma. The relation of ALDH1A2 to
ALDH1L1 and/or ALDH3B2 in neuroblastoma is currently under
investigation.
In addition to ALDH1A2, ALDH1A1, ALDH1A3 and ALDH8A1
can function in RA-signaling by their RA-biosynthesis activities
(18). Although these ALDH
isoforms were all expected to augment RA-signaling, their actual
roles were likely dependent on the cellular contexts of particular
cancer types. For instance, ALDH1A2 was downregulated and proposed
as a candidate tumor suppressor in prostate cancer (35,36),
whereas ALDH1A3 was upregulated and implicated in the maintenance
of CSCs in malignant high-grade gliomas (37).
In neuroblastoma, RA typically induces the
differentiation of tumor cells and 13-cis-RA is incorporated into
the maintenance therapy for high-risk patients with the purpose of
differentiating chemoresistant CSCs (3). However, the response to 13-cis-RA is
variable and still unpredictable in the clinic. Our present study
adds ALDH1A2 to a growing list of molecules responsible for
RA-resistance (38,39) and will provide a possible
therapeutic target.
In conclusion, we revealed that ALDH1A2 was involved
in the regulation of CSC properties in neuroblastoma. Inhibition of
ALDH1A2 deserves further evaluation as a new therapeutic approach
against high-risk neuroblastoma.
Acknowledgements
We thank the staff of animal facilities at Kobe
University Graduate School of Medicine and of Advanced Tissue
Staining Center at Kobe University Hospital for excellent technical
assistance. This study was supported in part by Grants-in-Aid for
Scientific Research from the Ministry of Education, Culture,
Sports, Science and Technology of Japan and grants from the
Children’s Cancer Association of Japan and Hyogo Science and
Technology Association.
References
|
1
|
Brodeur GM: Neuroblastoma: biological
insights into a clinical enigma. Nat Rev Cancer. 3:203–216. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Laverdière C, Liu Q, Yasui Y, et al:
Long-term outcomes in survivors of neuroblastoma: a report from the
Childhood Cancer Survivor Study. J Natl Cancer Inst. 101:1131–1140.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maris JM: Recent advances in
neuroblastoma. N Engl J Med. 362:2202–2211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cole KA and Maris JM: New strategies in
refractory and recurrent neuroblastoma: translational opportunities
to impact patient outcome. Clin Cancer Res. 18:2423–2428. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matthay KK, Villablanca JG, Seeger RC, et
al: Treatment of high-risk neuroblastoma with intensive
chemotherapy, radiotherapy, autologous bone marrow transplantation,
and 13-cis-retinoic acid. Children’s Cancer Group. N Engl J Med.
341:1165–1173. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu AL, Gilman AL, Ozkaynak MF, et al:
Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for
neuroblastoma. N Engl J Med. 363:1324–1334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vermeulen L, de Sousa e Melo F, Richel DJ
and Medema JP: The developing cancer stem-cell model: clinical
challenges and opportunities. Lancet Oncol. 13:e83–e89. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheung N-KV and Dyer MA: Neuroblastoma:
developmental biology, cancer genomics and immunotherapy. Nat Rev
Cancer. 13:397–411. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hansford LM, McKee AE, Zhang L, et al:
Neuroblastoma cells isolated from bone marrow metastases contain a
naturally enriched tumor-initiating cell. Cancer Res.
67:11234–11243. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hirschmann-Jax C, Foster AE, Wulf GG, et
al: A distinct ‘side population’ of cells with high drug efflux
capacity in human tumor cells. Proc Natl Acad Sci USA.
101:14228–14233. 2004. View Article : Google Scholar
|
|
12
|
Takenobu H, Shimozato O, Nakamura T, et
al: CD133 suppresses neuroblastoma cell differentiation via signal
pathway modification. Oncogene. 30:97–105. 2011. View Article : Google Scholar
|
|
13
|
Hsu DM, Agarwal S, Benham A, et al: G-CSF
receptor positive neuroblastoma subpopulations are enriched in
chemotherapy-resistant or relapsed tumors and are highly
tumorigenic. Cancer Res. 73:4134–4146. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sartelet H, Imbriglio T, Nyalendo C, et
al: CD133 expression is associated with poor outcome in
neuroblastoma via chemo-resistance mediated by the AKT pathway.
Histopathology. 60:1144–1155. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jackson B, Brocker C, Thompson DC, et al:
Update on the aldehyde dehydrogenase gene (ALDH) superfamily. Hum
Genomics. 5:283–303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kastan MB, Schlaffer E, Russo JE, Colvin
OM, Civin CI and Hilton J: Direct demonstration of elevated
aldehyde dehydrogenase in human hematopoietic progenitor cells.
Blood. 75:1947–1950. 1990.PubMed/NCBI
|
|
17
|
Ma I and Allan AL: The role of human
aldehyde dehydrogenase in normal and cancer stem cells. Stem Cell
Rev. 7:292–306. 2011. View Article : Google Scholar
|
|
18
|
Marcato P, Dean CA, Giacomantonio CA and
Lee PWK: Aldehyde dehydrogenase: its role as a cancer stem cell
marker comes down to the specific isoform. Cell Cycle.
10:1378–1384. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sullivan JP, Spinola M, Dodge M, et al:
Aldehyde dehydrogenase activity selects for lung adenocarcinoma
stem cells dependent on notch signaling. Cancer Res. 70:9937–9948.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen Y, Orlicky DJ, Matsumoto A, Singh S,
Thompson DC and Vasiliou V: Aldehyde dehydrogenase 1B1 (ALDH1B1) is
a potential biomarker for human colon cancer. Biochem Biophys Res
Commun. 405:173–179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
van den Hoogen C, van der Horst G, Cheung
H, et al: High aldehyde dehydrogenase activity identifies
tumor-initiating and metastasis-initiating cells in human prostate
cancer. Cancer Res. 70:5163–5173. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nishimura N, Pham TVH, Hartomo TB, et al:
Rab15 expression correlates with retinoic acid-induced
differentiation of neuroblastoma cells. Oncol Rep. 26:145–151.
2011.PubMed/NCBI
|
|
23
|
Pham TVH, Hartomo TB, Lee MJ, et al: Rab15
alternative splicing is altered in spheres of neuroblastoma cells.
Oncol Rep. 27:2045–2049. 2012.PubMed/NCBI
|
|
24
|
Nishimura N, Hartomo TB, Pham TVH, et al:
Epigallocatechin gallate inhibits sphere formation of neuroblastoma
BE(2)-C cells. Environ Health Prev Med. 17:246–251. 2012.
View Article : Google Scholar :
|
|
25
|
Tomayko MM and Reynolds CP: Determination
of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother
Pharmacol. 24:148–154. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kanda I, Nishimura N, Nakatsuji H,
Yamamura R, Nakanishi H and Sasaki T: Involvement of Rab13 and
JRAB/MICAL-L2 in epithelial cell scattering. Oncogene.
27:1687–1695. 2008. View Article : Google Scholar
|
|
27
|
Coulon A, Flahaut M, Mühlethaler-Mottet A,
et al: Functional sphere profiling reveals the complexity of
neuroblastoma tumor-initiating cell model. Neoplasia. 13:991–1004.
2011.PubMed/NCBI
|
|
28
|
Niederreither K, Subbarayan V, Dollé P and
Chambon P: Embryonic retinoic acid synthesis is essential for early
mouse post-implantation development. Nat Genet. 21:444–448. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Deak KL, Dickerson ME, Linney E, et al:
Analysis of ALDH1A2, CYP26A1, CYP26B1, CRABP1, and CRABP2 in human
neural tube defects suggests a possible association with alleles in
ALDH1A2. Birth Defects Res A Clin Mol Teratol. 73:868–875. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pavan M, Ruiz VF, Silva FA, et al: ALDH1A2
(RALDH2) genetic variation in human congenital heart disease. BMC
Med Genet. 10:1132009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Styrkarsdottir U, Thorleifsson G,
Helgadottir HT, et al: Severe osteoarthritis of the hand associates
with common variants within the ALDH1A2 gene and with rare variants
at 1p31. Nat Genet. 46:498–502. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Duester G: Retinoic acid synthesis and
signaling during early organogenesis. Cell. 134:921–931. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Niederreither K and Dollé P: Retinoic acid
in development: towards an integrated view. Nat Rev Genet.
9:541–553. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Patel M, Lu L, Zander DS, Sreerama L, Coco
D and Moreb JS: ALDH1A1 and ALDH3A1 expression in lung cancers:
correlation with histologic type and potential precursors. Lung
Cancer. 59:340–349. 2008. View Article : Google Scholar
|
|
35
|
Kim H, Lapointe J, Kaygusuz G, et al: The
retinoic acid synthesis gene ALDH1a2 is a candidate tumor
suppressor in prostate cancer. Cancer Res. 65:8118–8124. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Touma SE, Perner S, Rubin MA, Nanus DM and
Gudas LJ: Retinoid metabolism and ALDH1A2 (RALDH2) expression are
altered in the transgenic adenocarcinoma mouse prostate model.
Biochem Pharmacol. 78:1127–1138. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mao P, Joshi K, Li J, et al: Mesenchymal
glioma stem cells are maintained by activated glycolytic metabolism
involving aldehyde dehydrogenase 1A3. Proc Natl Acad Sci USA.
110:8644–8649. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang S, Laoukili J, Epping MT, et al:
ZNF423 is critically required for retinoic acid-induced
differentiation and is a marker of neuroblastoma outcome. Cancer
Cell. 15:328–340. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hölzel M, Huang S, Koster J, et al: NF1 is
a tumor suppressor in neuroblastoma that determines retinoic acid
response and disease outcome. Cell. 142:218–229. 2010. View Article : Google Scholar : PubMed/NCBI
|