Introduction
Ovarian cancer is in the fifth place in respect of
cancer-related death in woman (1).
Worldwide the annual number of new cases and deaths of ovarian
cancer is estimated to be around 0.22 and 0.14 million,
respectively (2). Combination of
surgery and chemotherapy has been used as a standard therapy for
the treatment of ovarian cancer patients, but overall 5-year
survival of the patients with stage III and IV still remains at
only 20 to 40%. Such poor prognosis of advanced stage ovarian
cancer is accounted for by the intrinsic and acquired
chemoresistance, since 30% of patients with advanced stages have
been reported not to respond to the first-line chemotherapy,
paclitaxel and cisplatin/carboplatin, and ~80% of the initial
responders eventually relapse and develop chemoresistance (3). However, the underlying molecular
mechanisms of chemoresistance in ovarian cancer are not fully
understood.
The signal transducers and activators of
transcription (STAT) family proteins have been reported to be
fairly upregulated and constitutively activated in many tumors
(4) and known to be resulted from
the upregulation of upstream signaling molecules such as
interleukin-6 (IL-6) (5). Of note,
in half of ovarian cancers, constitutive activation of STAT3 has
been observed and considered to play an important role for growth,
cell cycle progression and invasion of these cancer cells (4). Therefore, targeting IL-6/STAT3
signaling axis via inhibition of the IL-6–IL-6R interaction or
abrogation of STAT3 activation might be a clinically potential
therapy to treat cancers.
Among more than 90 protein tyrosine kinases
identified in the human genome, there are 58 receptor tyrosine
kinases (RTK), which are classified into 20 families (6). One of the subfamily of RTK is the TAM
family including three RTKs; Tyro3 (also called Sky), Axl (also
called Ark and Ufo) and Mer (7).
All the TAM RTKs have structural similarities, two
immunoglobin-like domains and two fibronectin type III repeats in
extracellular region and cytosolic kinase domain (8), and are recognized by growth
arrest-specific 6 (Gas 6) and protein S in common, which are
vitamin K-dependent proteins. In normal cells, intracellular
signaling via TAM RTKs has been reported to be responsible for a
various cellular functions such as survival, proliferation,
blockage of apoptosis, adhesion, morphology and motility (9,10).
However, in cancer cells, it plays a critical role in the
initiation as well as progression of cancers, since Axl, Mer and/or
Gas 6 have been demonstrated to be overexpressed in a variety of
cancer cell lines and patient samples including breast (11), colon (12), gastric (13), leukemias (14), melanoma (15), multiple myeloma (16), ovarian (17) and prostate cancer (18). Therefore, expression level of TAM
RTKs as well as their ligands and their changes seem to be good
prognostic marker and targeting these RTKs and their signaling
pathways might be a feasible strategy for the successful treatment
of many cancers.
Apigenin (4′,5,7,-tirhydoxyflavone), a dietary
flavone, is found in many fruits, vegetables and seasonings
(19–22) as a dimer. Anti-proliferative and
anti-angiogenic property of apigenin has been demonstrated in
various cancers including breast (23), cervical (23), lung (24), colon (25), hematologic (26), ovarian (27) and prostate cancer (28). Based on these unique effects of
apigenin on various cancers along with its low intrinsic toxicity,
apigenin has received great attention as a therapeutic as well as a
chemopreventive agent.
In the present study, we demonstrated that
inhibition of IL-6/STAT3 axis and targeting Axl and Tyro3 RTKs
resulted in the reduced proliferation of both parental SKOV3 cells
and taxol-resistant SKOV3/TR cells, suggesting a therapeutic
potential of these approaches to improve the overall outcome of
patients with chemoresistant ovarian cancer.
Materials and methods
Reagents and antibodies
Apigenin was from Sigma-Aldrich (St. Louis, MO,
USA). Primers for Axl, IL-6, IL-6 receptor, Mer, STAT3, Tyro3 and
GAPDH were synthesized by a domestic company, Bioneer Corp.
(Daejoun, Korea). TRI reagent was from Solgent (Daejoun, Korea).
AmpliTaq DNA polymerase was obtained from Roche Inc. (Indianapolis,
IN, USA). Enzyme-linked immunosorbent assay (ELISA) kit for
interleukin 6 (IL-6) was obtained from R&D Systems
(Minneapolis, MN, USA). For immunoblotting, specific antibodies
against Axl, STAT3, phosphoSTAT3, Tyro3 and GAPDH and secondary
antibodies were obtained from Santa Cruz Biotechnology Inc.
(Dallas, TX, USA).
Cell culture
SKOV3 cells were purchased from the American Type
Culture Collection (ATCC, Manassas, VA, USA). The cells were grown
in RPMI-1640 (Gibco-BRL, Grand Island, NY, USA) containing 10% FBS,
2 mM L-glutamine, 10 U/ml penicillin and 10 g/ml streptomycin at
37°C in 5% CO2 in a water-saturated atmosphere. The
taxol-resistant SKOV3/TR cells were established by stepwise
exposure of the parental SKOV3 cells to escalating concentrations
of taxol, ranging from 1.5 to 24 nM for more than 6 months.
RT-PCR
Cells (3×105) were seeded in 60-mm
culture dish and grown overnight at 37°C and then treated with the
indicated concentrations of apigenin for the 24 h. Total RNA was
extracted using TRI reagent and subjected to the cDNA synthesis and
PCR. The specific primers were as follows: Axl, sense
5′-AACCTTCAACTCCTGCCTTCTCG-3′ and antisense
5′-CAGCTTCTCCTTCAGCTCTTCAC-3′; Tyro3, sense
5′-GTGTGTGGCTGACTTCGGAC-3′ and antisense 5′-CAC
GTCCTCCATACACTCCG-3′; IL-6, sense 5′-ATGAACTCCT TCTCCACAAGCG-3′ and
antisense 5′-GAAGAGCCCTCA GGCTGGACT-3′; IL-6 receptor, sense
5′-CATTGCCATTGT TCTGAGGTTC-3′ and antisense 5′-AGTAGTCTGTATTG
CTGATGTC-3′; STAT3, sense 5′-TTCTCCTTCTGGGTCTG GCT-3′ and antisense
5′-CCACCCAAGTGAAAGTGACG-3′; GAPDH, sense 5′-GGAGCCAAAAGGGTCATCAT-3′
and antisense 5′-GTGATGGCATGGACTGTGGT-3′.
Western blot analysis
Cells were treated with the indicated concentration
of apigenin or stattic for 24 h. Total cell lysates were prepared
from those cells using lysis buffer [1% Triton X-100, 50 mM Tris
(pH 8.0), 150 mM NaCl, 1 mM PMSF, 1 mM
Na3VO4, and protease inhibitor cocktail].
Protein concentrations were determined using Bio-Rad protein
assays. Proteins from cell lysates (20–40 μg) were separated on 12%
SDS-PAGE, and electrotransferred to nitrocellulose membranes.
Membranes were blocked for 30 min at room temperature in
Tris-buffered saline-0.05% Tween-20 (TTBS) containing 5% non-fat
dry milk, and then incubated with TTBS containing a primary
antibody for 4 h at room temperature. After 3 × 10-min washes in
TTBS, membranes were incubated with peroxidase-conjugated secondary
antibody for 1 h. Following 3 additional 10-min washes with TTBS,
protein bands of interest were visualized using an enhanced
chemiluminescence detection system (Amersham).
siRNA transfection
RNA interference silencing was performed to inhibit
IL-6 production. SKVO3/TR cells (1×106) were seeded in
100-mm culture dish and grown overnight and then transfected with
50 nM siRNA against IL-6 (sc-39627; Santa Cruz Biotechnology,
Dallas, TX, USA), or control siRNA (sc-37007; Santa Cruz
Biotechnology). At 48 h post-transfection, cells were harvested and
the number of viable cells were counted and IL-6 level in
conditioned media were determined by ELISA. STAT3 and
phosphorylated STAT3 protein levels were determined by western blot
analysis using whole cell lysates.
Clonogenic assay
Cells were seeded in 35-mm culture dishes
(2×103 cells/dish) and allowed to grow for 7–10 days in
the presence of and/or absence of apigenin or stattic to form
colonies. Colonies of >50 cells were visualized by crystal
violet (in 60% methanol; Junsei Chemical Co., Ltd., Tokyo Japan)
staining and images were taken by RAS 3000 image analysis system
(Fuji Film, Tokyo, Japan).
Cell viability assay
The viability of cells was measured using Cell
Counting Kit-8 assay kit (Dojindo Laboratories, Kumamoto, Japan).
Cells (1–2×103 cells/well) were seeded in 96-well plates
and grown overnight at 37°C and then treated with the indicated
concentrations of stattic for the 24 h. At the end of the
treatment, 10 μl of CCK-8 solution was added and further incubated
for 4 h. The absorbance at 450 nm was measured using a microplate
reader (Model 680 microplate reader; Bio-Rad Laboratories). Values
are the mean ± SD for triplicate wells and normalized to that of
control group to determine the % of viability.
ELISA
The level of IL-6 in culture media was measured
using ELISA kit from R&D Systems according to the
manufacturer’s protocol. Cells were transfected with siRNAs, siCtrl
and siIL-6 or treated with apigenin for 24 h. Conditioned media
were harvested and assayed for IL-6. The data are representative of
at least three independent experiments.
Statistical analysis
Data are expressed as the mean ± SD of triplicate
samples or at least three independent experiments. For statistical
significance, Student’s t-test was used with a threshold of
P-values which is <0.05.
Results
IL-6, STAT3 and phosphorylated STAT3
levels are elevated in taxol-resistant ovarian cancer cells
To understand the molecular mechanisms underlying
taxol resistance in ovarian cancer cells, we established a
taxol-resistant subline, SKOV3/TR cells, by long-term and stepwise
exposure of taxol to parental SKOV3 cells. Since elevated
production of interleukin-6 (IL-6) and IL-6-mediated activation of
signal transducers and the activators of transcription 3 (STAT3)
have been reported to lead to chemoresistance to several
chemotherapeutic drugs in various cancers (29), we examined IL-6, IL-6 receptor,
STAT3, and phosphorylated STAT3 levels in both SKOV3 and SKOV3/TR
cells. RT-PCR result showed that IL-6 and IL-6 receptor mRNA levels
in SKOV3/TR cells were increased compared to those of SKOV3 cells,
respectively (Fig. 1A). In
addition, enzyme-linked immunosorbent assay (ELISA) result also
showed that the level of IL-6 in culture media of SKOV3/TR cells
was higher than that in culture media of parental cells, which is
consistent with the transcriptional upregulation of IL-6 in
SKOV3/TR cells (Fig. 1B).
Next, expression and phosphorylation status of STAT3
were examined. As shown in Fig.
1C, STAT3 mRNA level in SKOV3/TR cells was found to be
increased compared to that in parental cells. Western blot results
also showed that in SKOV3/TR cells, both STAT3 protein and
phosphoSTAT3 level were significantly elevated (Fig. 1D), indicating the induction of
STAT3 expression and its activation might be responsible for the
development of resistance to chemotherapy.
Silencing of IL-6 and inhibition of STAT3
reduce proliferation of taxol-resistant cells
The biological relevance of the increase of IL-6
production, STAT3 protein level, and its phosphorylation status in
SKOV3/TR cells was examined by silencing of IL-6 via siRNA and
inhibition of STAT3 using stattic, a small molecule inhibitor of
STAT3. SKOV3/TR cells were transfected with IL-6-specific siRNA,
siIL-6 or control siRNA, siCtrl and assessed IL-6 level in culture
media by ELISA. As shown in Fig.
2A, silencing of IL-6 via siIL-6 in SKOV3/TR cells resulted in
significant decrease of IL-6 production. Western blot results
further showed that phos-phorylation and expression of STAT3 was
also reduced in SKOV3/TR cells transfected with siIL-6 (Fig. 2B), confirming that STAT3 is a
downstream effector of IL-6-mediated signaling pathway.
Next, we examined the effect of siIL-6 on cell
proliferation. As shown in Fig.
2C, viability of SKOV3/TR cells transfected with siIL-6 was
fairly reduced compared to that transfected with siCtrl.
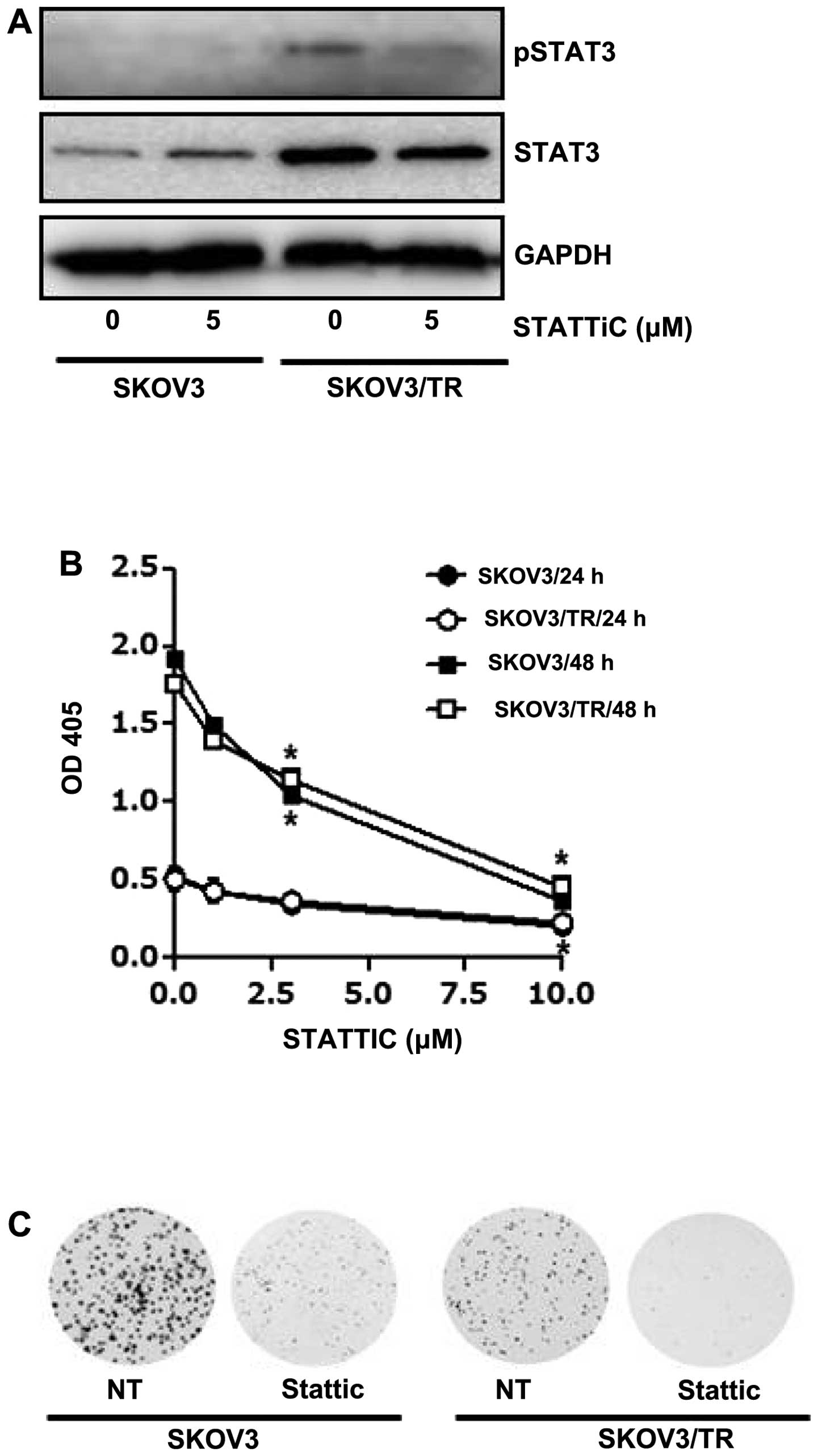
The effect of stattic, a small molecule inhibitor of
STAT3, on cell viability was also examined. We first found that
both expression and phosphorylation of STAT3 were decreased by
stattic treatment in SKOV3/TR cells (Fig. 3A). Then, both SKOV3 and SKOV3/TR
cells were treated with 1, 3 and 10 μM stattic for 24 h and CCK
assay results showed a dose-dependent decrease of the cell
viability (Fig. 3B). Consistent
with the CCK assay results, we also found that clonogenicity of
stattic-treated cells was considerably reduced (Fig. 3C), confirming its
anti-proliferative activity. Taken together, these results
demonstrated that upregulation of IL-6 and STAT3 expression as well
as the increased phosphorylation of STAT3 play a critical role in
proliferation of SKOV3/TR cells and are associated with taxol
resistance of SKOV3/TR cells.
Apigenin suppresses proliferation of both
parental and taxol- resistant cells
Since we previously reported that apigenin targets
Axl receptor tyrosine kinase (RTK), one of TAM family members,
which accounts for its anti-proliferative effects on non-small cell
lung carcinoma (NSCLC) cell lines, we asked whether apigenin was
cytotoxic in parental and taxol-resistant ovarian cancer cells,
which might result from downregulation of TAM expression. As shown
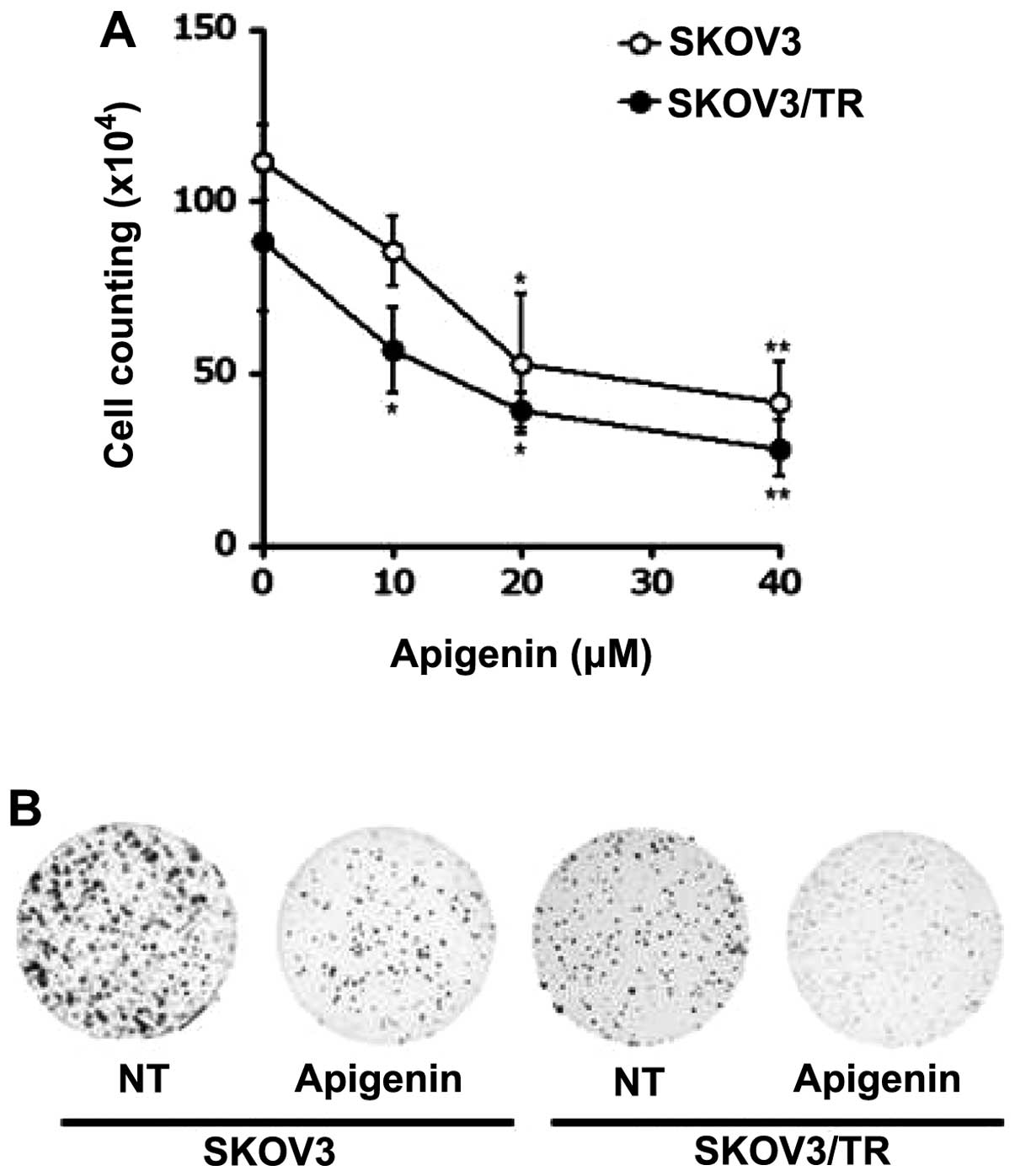
in Fig. 4A, apigenin treatment
decreased the viability of both SKOV3 and SKOV3/TR cells in a
dose-dependent manner. Of note, treatment with 40 μM apigenin for
24 h showed only 41.5% (SKOV3), and 28% (SKOV3/TR) survival of
these cells, respectively (Fig.
4A), indicating a more profound anti-proliferative effect of
apigenin on SKOV3/TR cells than parental SKOV3 cells.
Colony-forming assay further demonstrated cytotoxic activity of
apigenin on SKOV3 and SKOV3/TR cells. As shown in Fig. 4B, treatment of these cells with 40
μM apigenin was found to reduce not only the number of colonies but
also the size of each colony.
Anti-proliferative effect of apigenin is
mediated by the dysregulation of TAM RTKs and downstream effectors,
but not IL-6/STAT3 axis
Since TMA family of RTKs, Axl, Tyro3 and Mer is
known to be involved in cell survival, growth and proliferation, we
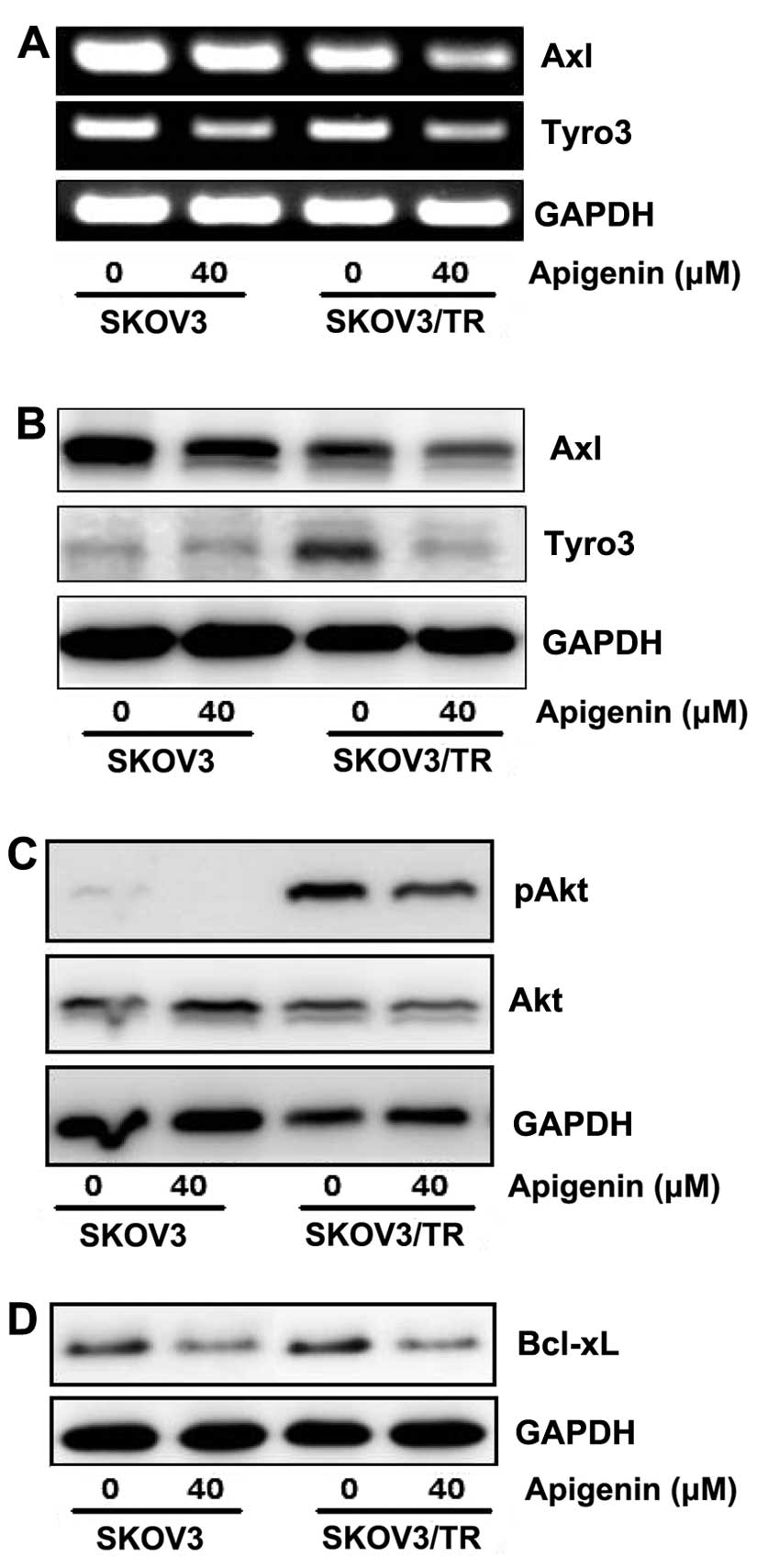
then examined the effect of apigenin on TAM RTKs expression.
Especially, in SKOV3/TR cells, Axl expression was found to be
slightly reduced, while Tyro3 expression was increased, compared to
those in parental SKOV3 cells, respectively. Both SKOV3 and
SKOV3/TR cells were treated with 40 μM apigenin for 24 h and then
expression of Axl and Tyro3 was examined at mRNA and protein level.
RT-PCR results showed that apigenin treatment led to significant
reduction of Axl and Tyro3 mRNA level in both parental and
taxol-resistant cells (Fig. 5A).
Downregulation of Axl and Tyro3 expression in apigenin-treated
cells was further confirmed by western blot analysis. As shown in
Fig. 5B, the protein levels of Axl
and Tyro3 were decreased by apigenin treatment, which is consistent
with RT-PCR results.
We next examined several downstream effectors which
might be affected after apigenin-mediated inhibition of Axl and
Tyro3 expression and subsequent reduction of cell proliferation.
Western blot results showed that apigenin treatment decreased the
level of phosphorylated Akt which transduces a strong signal for
cell cycle progression and is fairly increased in SKOV3/TR cells
(Fig. 5C). In addition, apigenin
was also found to reduce the level of B-cell lymphoma-extra large
(Bcl-xl, or BCL2-like 1 isoform 1) which is regulated by Akt and
inhibits apoptosis (Fig. 5D).
These data demonstrate that apigenin causes not only reduction of
Axl and Tyro3 expression but also the decrease of Akt
phosphorylation and Bcl-xl expression.
We further examined if apigenin affects IL-6/STAT3
axis, which is associated with cell viability. ELISA results showed
that total amount of IL-6 in culture media was slightly decreased
by apigenin treatment (Fig. 6A),
whereas IL-6 production per cell was increased, especially in
taxol-resistant SKOV3/TR cells (Fig.
6B). We also found that apigenin treatment had no effect on
STAT3 phosphorylation in these cells (Fig. 6C). Taken together, the results
indicate that apigenin has no effect on IL-6 production and
concomitant STAT3 phosphorylation and its anti-proliferative effect
does not result from suppression of IL-6/STAT3 axis.
Discussion
Cisplatin- and taxol-based chemotherapy is still the
first-line therapeutic choice for ovarian cancer. However, the
intrinsic and acquired resistance to these drugs have major
limitations leading to the failure of treatment (30–33).
Therefore, it is urgent to elucidate characteristics and underlying
molecular mechanisms of the resistance to improve final
outcomes.
We found that in taxol-resistant SKOV3/TR cells, the
levels of IL-6, IL-6 receptor, STAT3 and its phosphorylated form
were significantly increased compared to those in parental SKOV3
cells. Moreover, intervention of this IL-6/STAT3 signaling via
silencing of IL-6 and a STAT3 inhibitor, stattic, were found to
exert anti-proliferative effect on SKOV3/TR cells. These results
indicate that activation of IL-6/STAT3 axis resulted from the
long-term exposure of cells to taxol and need a strategy or a
compensation to survive under the pressure of taxol. Because of
dual function of STAT3 as a downstream effector of IL-6 and a
transcription factor to induce IL-6 expression, a positive feedback
loop between STAT3 and IL-6 is established, which results in
autocrine production of IL-6 and constitutive activation of STAT3.
Consistent with our data, the anti-apoptotic effect of IL-6 and the
involvement in drug resistance have been reported in various
cancers including myeloma (34),
prostate (35) and breast cancers
(36), which supports the idea
that combination of IL-6/STAT3 pathway inhibitor with
chemotherapeutic agents could be effective, in patients with
acquired chemoresistance.
A clinical significance of TAM receptor tyrosine
kinases (RTKs), Tyro3, Axl and Mer, as well as their ligands has
been demonstrated. For example, in 48.3% of ovarian adenocarcinoma
tissues, Axl protein level was elevated and reflected in disease
stage and lymph node metastasis. In lung cancer cases,
overexpression of Axl, Mer, and their ligands was also found in
more than half of non-small cell lung cancer (NSCLC) cell lines
(37,38) and RNA interference or monoclonal
antibodies against Axl have been reported to reduce NSCLC
proliferation, metastasis and xenograft tumor growth (39). In accordance with these studies, we
recently demonstrated that anti-proliferative effect on apigenin, a
dietary phytochemical derived from various fruits and vegetables
resulted from downregulation of Axl expression in NSCLC cells,
suggesting that Axl is a novel target of apigenin. Since the
initial report showed the inhibitory effect of apigenin on
mutagenesis and tumor promotion (40), many follow-up studies further
demonstrated its anti-oxidant, anti-inflammatory, anti-angiogenic
and anti-proliferative activities. Based on the above evidence,
apigenin has received considerable attention as a chemotherapeutic
and chemo-preventive agent. In the present study, apigenin was
further found to suppress the expression of Axl and Tyro3,
incurring decreased proliferation of both parental and
taxol-resistant SKOV3 cancer cells. Of note, Tyro3 induction
contrary to downregulation of Axl in SKOV3/TR cells seems to be a
compensation or another strategy for survival, which resulted from
long-term treatment of taxol. However, IL-6 and STAT3 expression
and STAT3 phosphorylation were not affected by apigenin, IL-6
production per cell was increased, suggesting that IL-6/STAT3
signaling pathway is not involved in the anti-proliferative effect
of apigenin.
In summary, our data demonstrated that silencing of
IL-6 and STAT3 inhibition intervened IL-6/STAT3 signaling pathway
and apigenin caused downregulation of expression in all TAM RTKs,
which eventually restricted in proliferation of taxol-resistant
ovarian cancer cells, suggesting that inhibition of IL-6/STAT3 axis
and targeting TAM RTKs might be feasible approaches to overcome
taxol resistance in ovarian cancer cells.
Acknowledgements
The present research was supported by the Basic
Science Research Program through the National Research Foundation
of Korea (NRF) funded by the Ministry of Education, Science and
Technology (grant no. 2006-2005303 and NRF-2014R1A1A2006192).
Abbreviations:
|
Bcl-xl
|
B-cell lymphoma-extra large
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
GAS 6
|
growth arrest-specific 6
|
|
IL-6
|
interleukin-6
|
|
RTK
|
receptor tyrosine kinase
|
|
STAT
|
signal transducers and activators of
transcription
|
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cohen JG, White M, Cruz A and
Farias-Eisner R: In 2014, can we do better than CA125 in the early
detection of ovarian cancer? World J Biol Chem. 5:286–300. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McGuire WP, Hoskins WJ, Brady MF, et al:
Cyclophosphamide and cisplatin compared with paclitaxel and
cisplatin in patients with stage III and stage IV ovarian cancer. N
Engl J Med. 334:1–6. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Levy DE and Darnell JE Jr: Stats:
transcriptional control and biological impact. Nat Rev Mol Cell
Biol. 3:651–662. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chang KT, Tsai CM, Chiou YC, Chiu CH, Jeng
KS and Huang CY: IL-6 induces neuroendocrine dedifferentiation and
cell proliferation in non-small cell lung cancer cells. Am J
Physiol Lung Cell Mol Physiol. 289:L446–L453. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Robinson DR, Wu YM and Lin SF: The protein
tyrosine kinase family of the human genome. Oncogene. 19:5548–5557.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ohashi K, Mizuno K, Kuma K, Miyata T and
Nakamura T: Cloning of the cDNA for a novel receptor tyrosine
kinase, Sky, predominantly expressed in brain. Oncogene. 9:699–705.
1994.PubMed/NCBI
|
|
8
|
Sasaki T, Knyazev PG, Clout NJ, et al:
Structural basis for Gas6-Axl signalling. EMBO J. 25:80–87. 2006.
View Article : Google Scholar
|
|
9
|
Stitt TN, Conn G, Gore M, et al: The
anticoagulation factor protein S and its relative, Gas6, are
ligands for the Tyro 3/Axl family of receptor tyrosine kinases.
Cell. 80:661–670. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hafizi S and Dahlback B: Gas6 and protein
S: Vitamin K-dependent ligands for the Axl receptor tyrosine kinase
subfamily. FEBS J. 273:5231–5244. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meric F, Lee WP, Sahin A, Zhang H, Kung HJ
and Hung MC: Expression profile of tyrosine kinases in breast
cancer. Clin Cancer Res. 8:361–367. 2002.PubMed/NCBI
|
|
12
|
Craven RJ, Xu LH, Weiner TM, et al:
Receptor tyrosine kinases expressed in metastatic colon cancer. Int
J Cancer. 60:791–797. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu CW, Li AF, Chi CW, et al: Clinical
significance of AXL kinase family in gastric cancer. Anticancer
Res. 22:1071–1078. 2002.PubMed/NCBI
|
|
14
|
Challier C, Uphoff CC, Janssen JW and
Drexler HG: Differential expression of the ufo/axl oncogene in
human leukemia-lymphoma cell lines. Leukemia. 10:781–787.
1996.PubMed/NCBI
|
|
15
|
Gyorffy B and Lage H: A Web-based data
warehouse on gene expression in human malignant melanoma. J Invest
Dermatol. 127:394–399. 2007. View Article : Google Scholar
|
|
16
|
De Vos J, Couderc G, Tarte K, et al:
Identifying intercellular signaling genes expressed in malignant
plasma cells by using complementary DNA arrays. Blood. 98:771–780.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Macleod K, Mullen P, Sewell J, et al:
Altered ErbB receptor signaling and gene expression in
cisplatin-resistant ovarian cancer. Cancer Res. 65:6789–6800. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sainaghi PP, Castello L, Bergamasco L,
Galletti M, Bellosta P and Avanzi GC: Gas6 induces proliferation in
prostate carcinoma cell lines expressing the Axl receptor. J Cell
Physiol. 204:36–44. 2005. View Article : Google Scholar
|
|
19
|
Birt DF, Hendrich S and Wang W: Dietary
agents in cancer prevention: flavonoids and isoflavonoids.
Pharmacol Ther. 90:157–177. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Surh YJ: Cancer chemoprevention with
dietary phytochemicals. Nat Rev Cancer. 3:768–780. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Manach C, Scalbert A, Morand C, Remesy C
and Jimenez L: Polyphenols: food sources and bioavailability. Am J
Clin Nutr. 79:727–747. 2004.PubMed/NCBI
|
|
22
|
Yang CS, Landau JM, Huang MT and Newmark
HL: Inhibition of carcinogenesis by dietary polyphenolic compounds.
Annu Rev Nutr. 21:381–406. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zheng PW, Chiang LC and Lin CC: Apigenin
induced apoptosis through p53-dependent pathway in human cervical
carcinoma cells. Life Sci. 76:1367–1379. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu HF, Chie YJ, Yang MS, et al: Apigenin
induces apoptosis in human lung cancer H460 cells through caspase-
and mitochondria-dependent pathways. Hum Exp Toxicol. 30:1053–1061.
2011. View Article : Google Scholar
|
|
25
|
Zhong Y, Krisanapun C, Lee SH, et al:
Molecular targets of apigenin in colorectal cancer cells:
involvement of p21, NAG-1 and p53. Eur J Cancer. 46:3365–3374.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ruela-de-Sousa RR, Fuhler GM, Blom N,
Ferreira CV, Aoyama H and Peppelenbosch MP: Cytotoxicity of
apigenin on leukemia cell lines: implications for prevention and
therapy. Cell Death Dis. 1:e192010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li ZD, Hu XW, Wang YT and Fang J: Apigenin
inhibits proliferation of ovarian cancer A2780 cells through Id1.
FEBS Lett. 583:1999–2003. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mirzoeva S, Kim ND, Chiu K, Franzen CA,
Bergan RC and Pelling JC: Inhibition of HIF-1 alpha and VEGF
expression by the chemopreventive bioflavonoid apigenin is
accompanied by Akt inhibition in human prostate carcinoma PC3-M
cells. Mol Carcinog. 47:686–700. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Barre B, Vigneron A, Perkins N, Roninson
IB, Gamelin E and Coqueret O: The STAT3 oncogene as a predictive
marker of drug resistance. Trends Mol Med. 13:4–11. 2007.
View Article : Google Scholar
|
|
30
|
McGuire WP III: Current status of taxane
and platinum-based chemotherapy in ovarian cancer. J Clin Oncol.
21(Suppl 10): 133–135. 2003. View Article : Google Scholar
|
|
31
|
Cannistra SA: Cancer of the ovary. N Engl
J Med. 351:2519–2529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ozols RF: Systemic therapy for ovarian
cancer: current status and new treatments. Semin Oncol. 33:S3–S11.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tian C, Ambrosone CB, Darcy KM, et al:
Common variants in ABCB1, ABCC2 and ABCG2 genes and clinical
outcomes among women with advanced stage ovarian cancer treated
with platinum and taxane-based chemotherapy: a Gynecologic Oncology
Group study. Gynecol Oncol. 124:575–581. 2012. View Article : Google Scholar :
|
|
34
|
Catlett-Falcone R, Landowski TH, Oshiro
MM, et al: Constitutive activation of Stat3 signaling confers
resistance to apoptosis in human U266 myeloma cells. Immunity.
10:105–115. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pu YS, Hour TC, Chuang SE, Cheng AL, Lai
MK and Kuo ML: Interleukin-6 is responsible for drug resistance and
anti-apoptotic effects in prostatic cancer cells. Prostate.
60:120–129. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Conze D, Weiss L, Regen PS, et al:
Autocrine production of interleukin 6 causes multidrug resistance
in breast cancer cells. Cancer Res. 61:8851–8858. 2001.PubMed/NCBI
|
|
37
|
Wimmel A, Glitz D, Kraus A, Roeder J and
Schuermann M: Axl receptor tyrosine kinase expression in human lung
cancer cell lines correlates with cellular adhesion. Eur J Cancer.
37:2264–2274. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Linger RM, Keating AK, Earp HS and Graham
DK: Taking aim at Mer and Axl receptor tyrosine kinases as novel
therapeutic targets in solid tumors. Expert Opin Ther Targets.
14:1073–1090. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ye X, Li Y, Stawicki S, et al: An anti-Axl
monoclonal antibody attenuates xenograft tumor growth and enhances
the effect of multiple anticancer therapies. Oncogene.
29:5254–5264. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Birt DF, Walker B, Tibbels MG and Bresnick
E: Anti-mutagenesis and anti-promotion by apigenin, robinetin and
indole-3-carbinol. Carcinogenesis. 7:959–963. 1986. View Article : Google Scholar : PubMed/NCBI
|