Introduction
The lifetime risk to develop colorectal carcinoma
(CRC) is >5% and because current therapy is not curative in all
cases this disease is one of the leading causes of death worldwide
(1). All curative resected CRC
patients (30–50%) will develop local recurrence or distant
metastatic disease (2,3). Adjuvant chemotherapy is used in high
risk patients, often defined as patients above stage IIB according
to the TNM classification, angio-invasive growth, tumor perforation
or obstruction and <10 detectable lymph nodes. Adjuvant
chemotherapy results in a relative risk reduction of ~30% in
disease recurrence (3,4). Because of a lack of 100% sensitivity
and specificity of the known risk factors for disease recurrence,
numerous patients receive adjuvant therapy without having presumed
micro-metastasis. On the other hand, a subgroup of patients
classified as having a low risk for disease recurrence, thus, not
receiving adjuvant treatment will develop disease recurrence.
Better tools are necessary to discriminate between these patient
groups.
The presence of tumor cells in blood of cancer
patients may help to discriminate between these patient groups. The
introduction of a validated system for the enumeration of
circulating tumor cells (CTC) (5)
enabled prospective clinical studies in both the metastatic and
non-metastatic setting. Data from the multicenter prospective
studies of CTC in metastatic breast (6), prostate (7) and colorectal (8) and a single center prospective study
at diagnosis of breast cancer (9,10)
were reported earlier. These studies demonstrated that CTC are an
independent predicting factor for disease-free survival and overall
survival and these findings were confirmed by other studies
(11–16). In the present study, we report on a
single center prospective study in newly diagnosed CRC that was
initiated at the same time as the original study in the metastatic
colorectal cancer. The frequency of CTC in patients with metastatic
colorectal cancer is extremely low, no CTC were detected with the
CellSearch system in 52% of these patients using the FDA cleared
protocol for 7.5 ml of blood (8).
In this study we investigate CTC in newly diagnosed patients
without overt metastasis, the incidence of detectable CTC is
expected to be lower and therefore, larger blood volumes will need
to be analyzed to detect significant amount of CTC. To explore
whether or not the presence of CTC in newly diagnosed patients
could predict recurrence, 30 ml of blood was analyzed for the
presence of CTC before surgery, after surgery and at several
time-points during a four-year follow-up.
Patients and methods
Study design and patients
In this double blind single center cohort study 216
patients with colorectal malignancy and 58 patients with benign
colorectal disease were enrolled at Medisch Spectrum Twente (MST),
Enschede, the Netherlands. The ethics board of Medisch Spectrum
Twente, approved the study protocol and all patients provided
written informed consent. Patients were included between September
2003 and November 2008. Inclusion criteria were defined as patients
aged ≥18 years, newly diagnosed colorectal cancer without
metastases and scheduled for surgery, ECOG performance state 0–1.
The main exclusion criterion was the presence of malignancy in the
5 years before inclusion in the medical history (excluding
non-melanoma skin carcinoma or cervix carcinoma in situ).
Thirty-three patients were excluded from analyses because of the
following reasons: 15 patients were diagnosed with distant
metastasis perioperative, 15 patients had a malignancy in their
medical history, and 3 patients did not have CTC data at the
inclusion time-point. This resulted in final cohort of 183
patients. The control group consisted of 58 patients undergoing
colonoscopy or abdominal surgery in which no malignancy was
detected. These patients were included throughout the study period
to prevent bias in the staff performing the laboratory CTC
analysis.
CTC enumeration during follow-up was done coinciding
with a routine follow-up visit according to the Dutch guidelines
for treatment of colorectal cancer (17). Treatment intention of all included
patients with colorectal cancer was curative surgery.
Peri-operative findings and pathologic outcome would define
adjuvant therapy. Presence of CTC was blinded and did not influence
adjuvant therapy. Patient records were reviewed in June of 2013 to
record whether or not disease recurrence had occurred and if so
when and whether or not the patient died and if this was related to
colon cancer.
The primary end point of the study was to determine
a correlation between the presence of CTC prior to surgery and
recurrence-free survival (RFS). Secondary end points were defined
as a correlation of CTC prior to surgery with colon cancer related
death (CCRD) and correlation of blood draws during follow-up after
surgery with RFS and CCRD.
Blood collection and CTC detection
Four peripheral blood samples were drawn by
venipuncture into a 10-ml CellSave Preservative tube (Veridex LLC,
Raritan, NJ, USA). Time-points of blood draw were before surgery or
colonoscopy (Draw A), after surgery/before adjuvant therapy (Draw
B), after adjuvant therapy (Draw C), after 1 year (Draw D), after 2
years (Draw E), after 3 years (Draw F) and after 4 years (Draw G).
Four aliquots of 7.5 ml were examined for the presence of CTC with
the CellSearch system (Veridex). The CTC number was the total of
the number found in the four aliquots. Analysis took place within
72 h after the blood draw. The CellSearch system enriched CTC using
antibodies directed against the epithelial cell adhesion antigen
(EpCAM) coupled to ferro-fluids. The enriched cells were
fluorescently labeled with the nucleic acid dye
4,6-diaminodino-2-phenylindole (DAPI) and phycoerythrin (PE)
labeled monoclonal antibodies against cytokeratin 8, 18 and 19 and
allophycocyanin (APC) labeled antibodies directed against CD45.
Images of CTC candidates were captured by the CellTracks Analyzer
II and presented to experienced operators for classification and
assigned as CTC when the objects were >4 μm, stained with DAPI
and cytokeratin, lacked CD45 and had morphological features
consistent with that of a cell (5). The operators were blinded to the
clinical status of the patient.
Statistical analysis
All patient data were collected in an Access
database including demographic parameters such as age and gender
and pathological findings including histological grade and TNM
staging. Follow-up findings included recurrence date, adjuvant
therapy and last outpatient control visit. The patient data was
merged with the CTC enumeration at the moment of final analysis.
Statistical analysis was performed using SPSS version 20.0 and R
version 3.0.2 (18). A P-value
<0.05 was considered to indicate a significant difference. All
tests were two-sided. When dividing patients into a favorable and
unfavorable group using CTC counts, unfavorable was considered one
or more CTC. Kaplan Meier curves for RFS and CCRD were compared
using the log-rank test. Between-group differences in categorical
variables were tested by the Pearson’s Chi-square test. The
following significant univariate prognostic factors were included
in a multivariate Cox proportional regression model: T stage, N
stage and CTC status. Due to the low numbers of patients with T1,
T1 and T2 were grouped together in the multivariate model. The
proportional hazard assumption was checked for all factors included
in the model. Factors were removed from the multivariate model
using stepwise elimination, using P>0.10 as criteria.
Results
Patient characteristics and univariate
analysis
The follow-up ranged from 1 to 109 months with a
mean of 60 months and a median of 61 months. Recurrence of disease
was observed in 48 of 183 (26%) patients; 36 (20%) patients died of
causes related to colorectal cancer and 23 (12%) patients died of
other causes. The median follow-up of the patients alive at the end
of the follow-up was 66 months. Patient characteristics and their
relation with CTC, RFS and CCRD is shown in Table I. Univariate analysis showed a
significant relationship between RFS and CCRD with T-stage, N stage
and adjuvant therapy. The other variables: histological grade,
tumor size and gender were not significant. T-stage (P=0.016)
showed a significant difference in coincidence with unfavorable CTC
counts.
 | Table ICharacteristics of the 183 patients
and their relation to recurrence-free survival (RFS) and colon
cancer related death (CCRD) using a log-rank test. |
Table I
Characteristics of the 183 patients
and their relation to recurrence-free survival (RFS) and colon
cancer related death (CCRD) using a log-rank test.
| N | % | % CTC ≥1 | RFS P-value | CCRD P-value |
|---|
| T stage | | | | 0.008 | 0.001 |
| T4 | 19 | 10 | 37a | | |
| T3 | 108 | 59 | 28a | | |
| T2 | 39 | 21 | 8a | | |
| T1 | 13 | 7 | 8a | | |
| Unkown | 4 | 2 | | | |
| N stage | | | | 0.025 | 0.006 |
| N0 | 112 | 61 | 19 | | |
| N1 | 41 | 22 | 34 | | |
| N2 | 26 | 14 | 27 | | |
| Unkown | 4 | 2 | | | |
| M stage |
| M0 | 183 | 100 | 24 | | |
| M1 | 0 | | | | |
| Histology grade | | | | 0.115 | 0.125 |
| Poor | 11 | 7 | 36 | | |
| Moderate | 133 | 73 | 25 | | |
| Good | 13 | 6 | 8 | | |
| Unkown | 26 | 14 | | | |
| Adjuvant therapy | | | | <0.001 | <0.001 |
| Yes | 54 | 30 | 23 | | |
| No | 129 | 70 | 28 | | |
| Gender | | | | 0.510 | 0.537 |
| Male | 116 | 63 | 28 | | |
| Female | 67 | 37 | 18 | | |
| Continues | Mean | Min-max | | | |
| Age | 66 | 37–85 | | | |
| Follow-up | 60 | 1–109 | | | |
Frequency of circulating tumor cells
In 44 out of the 183 patients (24%) CTC were
detected before surgery. This decreased to 29 (19.9%) in the sample
drawn weeks after surgery (and before the initiation of the
adjuvant therapy when indicated). Ten of the 29 patients with CTC
after surgery had CTC before surgery. The number of patients with
one or more CTC found at the different time-points are provided in
Table II.
 | Table IIPrevalence of circulating tumor cells
before colon cancer surgery and at several time-points after
surgery. |
Table II
Prevalence of circulating tumor cells
before colon cancer surgery and at several time-points after
surgery.
| CTCs | Benign
disease
N=58
n (%) | (A) Before
surgery
n=183
n (%) | (B) After
surgery
n=146
n (%) | (C) After adjuvant
therapy
n=42
n (%) | (D) One year after
surgery
n=116
n (%) | (E) Two years after
surgery
n=82
n (%) | (F) Three years
after surgery
n=47
n (%) | (G) Four years
after surgery
n=16
n (%) |
|---|
| 0 | 50 (86) | 139 (76) | 117 (80) | 32 (76) | 96 (83) | 74 (90) | 42 (89) | 13 (81) |
| ≥1 | 8 (14) | 44 (24) | 29 (20) | 10 (24) | 20 (17) | 8 (10) | 5 (11) | 3 (19) |
| 1 | 7 (12) | 28 (15) | 17 (12) | 6 (14) | 11 (9) | 3 (4) | 3 (6) | 2 (13) |
| 2 | 1 (2) | 11 (6) | 5 (3) | 3 (7) | 3 (3) | 1 (1) | 0 (0) | 0 (0) |
| 3 | 0 (0) | 0 (0) | 2 (1) | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 0 (0) |
| 4 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 1 (2) | 0 (0) |
| >4 | 0 (0) | 5 (3) | 5 (3) | 1 (2) | 5 (4) | 3 (4) | 1 (2) | 1 (6) |
Relation between circulating tumor cells
and recurrence-free survival or colon cancer related death
Patients were divided into those with favorable (0
CTC) and unfavorable (≥1 CTC) CTC for the different time-points.
Table III shows the result of
the log-rank test for each of the different blood draws for RFS and
CCRD. The number of patients participating in the follow-up CTC
measurements decreased and to verify potential bias, the before
surgery results of the same patients are provided in italics below
each draw. When comparing unfavorable CTC to favorable CTC counts
the risk of recurrence (Hazard ratio, 2.07, P=0.016) and CCRD
(Hazard ratio, 2.74, P=0.003) was significantly increased before
surgery. The associated Kaplan-Meier curves for RFS and CCRD are
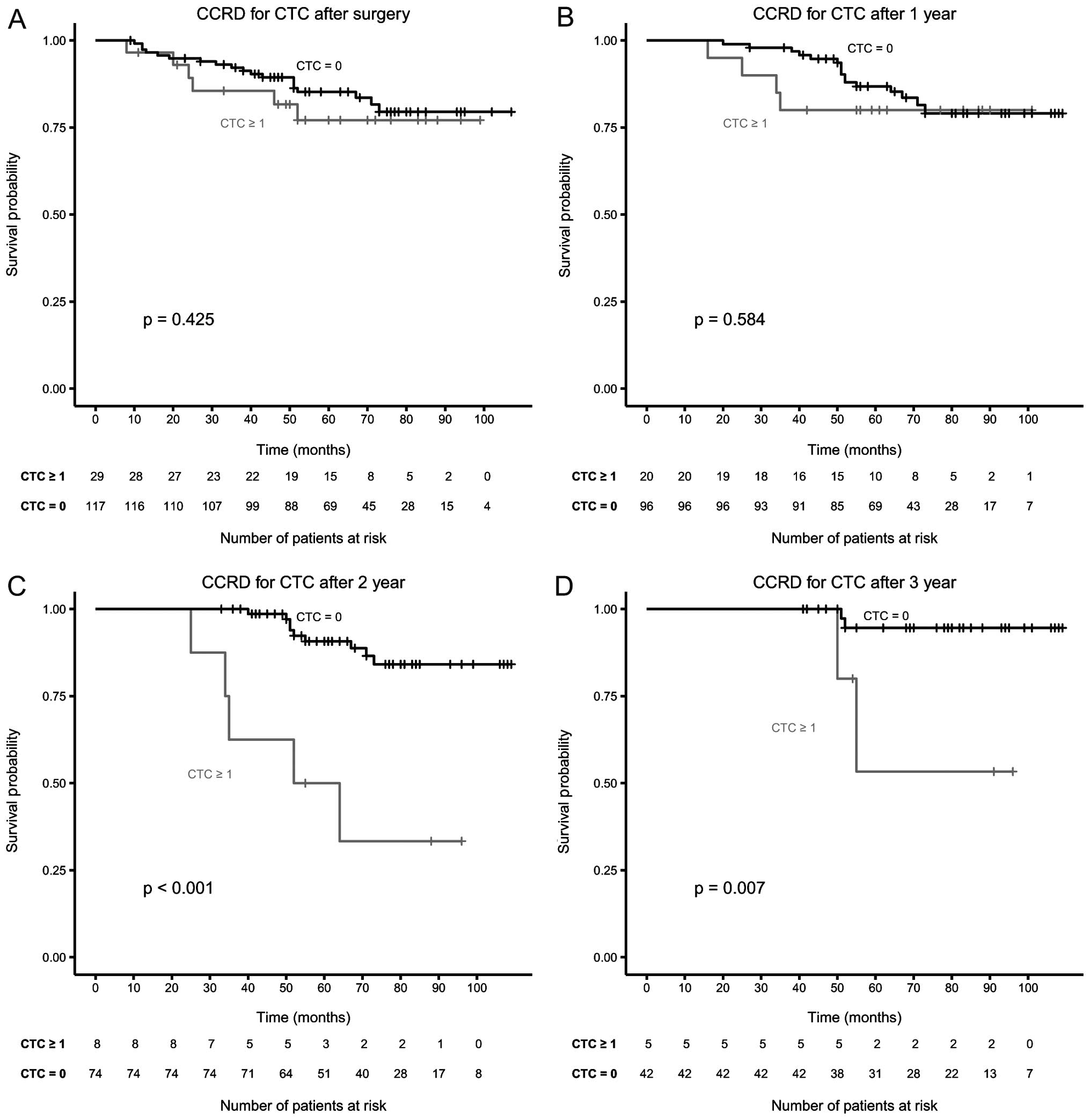
shown in Fig. 1. The frequency of
CTC 1–12 weeks after surgery decreased only slightly (from 24 to
20%), but their presence was no longer significant, likewise CTC
were not significant 1 year after surgery. Two and 3 years after
surgery the presence of CTC again was highly significant for CCRD
as shown in the Kaplan-Meier curves of Fig. 2. For the subgroup of patients that
received adjuvant therapy the presence of CTC before surgery and
after completion of therapy was significant for RFS and CCRD, as
listed in Table III. The
subgroup of patients that did not receive adjuvant therapy (70% of
the 183 patients) did not have a significant relation between CTC
and RFS (P=0.126) and CCRD (P=0.118) before surgery.
 | Table IIIRelation between the presence of CTC
and recurrence-free survival (RFS) or colon cancer related death
(CCRD). For each time-point during follow-up (FU) the RFS and colon
cancer related death (CCRD) for the before surgery draw of the same
patients is shown in italics. |
Table III
Relation between the presence of CTC
and recurrence-free survival (RFS) or colon cancer related death
(CCRD). For each time-point during follow-up (FU) the RFS and colon
cancer related death (CCRD) for the before surgery draw of the same
patients is shown in italics.
| N | Unfavorable
(%) | RFS P-value | CCRD P-value |
|---|
| Before surgery | 183 | 24 | 0.014 | 0.002 |
| After surgery | 143 | 20 | 0.940 | 0.425 |
| (before surgery
draw) | | 22 | 0.252 | 0.029 |
| After adjuvant
TX | 42 | 24 | 0.027 | 0.009 |
| (before surgery
draw) | | 24 | 0.056 | 0.016 |
| After 1 year | 116 | 17 | 0.722 | 0.584 |
| (before surgery
draw) | | 20 | 0.056 | 0.001 |
| After 2 years | 82 | 10 | 0.001 | <0.001 |
| (before surgery
draw) | | 22 | 0.194 | 0.196 |
| After 3 years | 47 | 11 | 0.091 | 0.007 |
| (before surgery
draw) | | 17 | 0.838 | 0.717 |
| After 4 years | 16 | 19 | 0.034 | 0.004 |
| (before surgery
draw) | | 25 | 1.000 | 0.595 |
Multivariate analysis
Only the presence of CTC before surgery was included
in the multivariate analysis. The univariate significant parameters
CTC, N-stage and T-stage were also included in a Cox proportional
hazards model. The multivariate regression was performed with a
conditional stepwise elimination of the least significant
parameters. For RFS and CCRD CTC and N-stage remained as
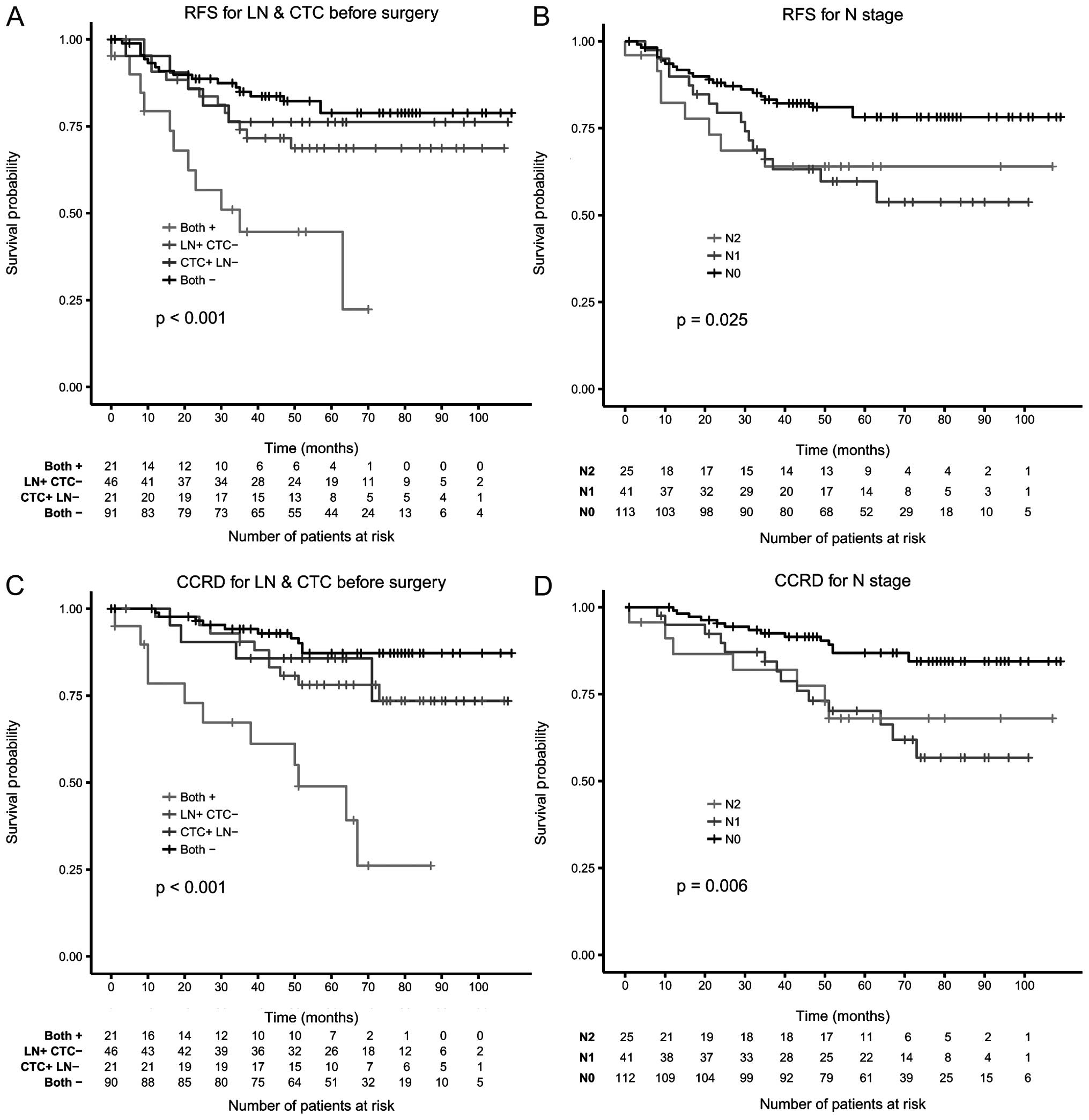
significant predictors in the model (Table IV). In Fig. 3 both CTC and lymph nodes were
combined to define at risk status in Kaplan-Meier curves for RFS
and CCRD.
 | Table IVMultivariate Cox proportional hazard
regression analysis of all univariate significant parameters with a
conditional stepwise elimination of least significant parameters
for recurrence-free survival (RFS) and colon cancer related death
(CCRD). |
Table IV
Multivariate Cox proportional hazard
regression analysis of all univariate significant parameters with a
conditional stepwise elimination of least significant parameters
for recurrence-free survival (RFS) and colon cancer related death
(CCRD).
| Variables | Categories | P-value | Hazard ratio | 95% CI |
|---|
| RFS | CTC | Yes vs. no | 0.030 | 1.96 | 1.06–3.61 |
| N-Stage | N0 vs. N1 | 0.021 | 2.21 | 1.12–4.08 |
| N-Stage | N0 vs. N2 | 0.084 | 2.12 | 0.91–4.59 |
| CCRD | CTC | Yes vs. no | 0.002 | 2.88 | 1.46–5.66 |
| N-Stage | N0 vs. N1 | 0.007 | 2.78 | 1.32–5.84 |
| N-Stage | N0 vs. N2 | 0.034 | 2.68 | 1.08–6.65 |
Discussion
Tumor cells present in the blood before surgical
intervention reflect the invasion of the primary tumor in the
bloodstream and these CTC have the potential to generate distant
metastasis. Determination of CTC could be of value in determining
which patients are at risk for recurrence after surgery and if
validated could determine which patients need adjuvant treatment.
The primary aim of the present study was to determine the number of
CTC before surgery and correlate them to disease recurrence in
patients with newly diagnosed CRC without distant metastatic
disease at presentation or perioperatively. Secondary endpoints
were to determine the correlation between CTC presence and
recurrence-free survival, colon cancer related death before and
during 4 years of follow-up after surgery.
Our results showed a significant correlation between
the presence of CTC before surgery and RFS and CCRD. In
multivariate comparison of established risk factors determined
perioperatively, only the N-stage and the presence of CTC before
surgery remained significant for both RFS and CCRD (Table IV). Presence of both lymph node
involvement and CTC clearly identifies a subgroup of patients with
extremely high risk for disease recurrence and death from colon
cancer indicating that current treatment is not sufficient for this
group of patients. The presence of only one of those risk factors
was much less predictive (Figs. 1
and 3). Separation of the patients
into those who received and those who did not receive adjuvant
therapy showed that the presence of CTC before surgery was only
significant for those patients who did receive adjuvant therapy.
The latter suggests a role for CTC, suggesting a more aggressive
therapy rather than providing therapy to those patients that
currently are not receiving adjuvant therapy. The presence of CTC
during follow-up in the group of patients without CTC before
surgery and not receiving adjuvant therapy suggests that the
sensitivity of the CellTracks technology for the detection of CTC
in this group of patients before surgery is insufficient.
The significant relation between the presence of CTC
and the potential for disease recurrence has also been shown for
patients undergoing surgery for primary breast cancer (9,10,14,19,20).
The reported frequency of CTC in these studies are quite similar
and a model predicted that the sensitivity of CTC detection will
need to reach 9±6 CTC/l blood to detect a tumor before the
formation of distant metastasis (21). Our observations on the significant
association between the presence of CTC and lower progression free
survival and death is in agreement with those in metastatic
colorectal cancer (8,13,22).
The prevalence of CTC detected by the CellSearch system in 7.5 ml
of blood in metastatic colorectal cancer is low. To increase the
possibility to find CTC in early colorectal cancer the volume of
blood was increased from 7.5 to 30 ml by running four CellSearch
tests in parallel. Based on statistical analysis it was already
suggested that the presence of ≥1 CTC was associated with a poor
outcome (23). Based on the low
prevalence and the possible significance of 1 CTC the cut-off point
was set at ≥1 CTC in 30 ml of blood. The low prevalence of CTC
detected by the CellSearch system in non-metastatic colorectal
cancer has been confirmed in other studies (24–26).
The variation in CTC frequency in these reports can likely be
contributed to the inclusion of different stages of the disease and
the absence in most studies of random inclusion of normal samples
during the study period. For non-metastatic colon cancer low
numbers of CTCs in 7.5 ml have been correlated to N-stage in other
studies (27). We found no
correlation to N-stage but did find a correlation to T-stage.
In a recent study, the CTC frequency from patients
with metastatic colorectal, prostate and breast cancer was modeled
and predicted that 99% of patients had at least one CTC in 5 liters
of blood before initiation of therapy for metastatic disease
(28). In the same report each
10-fold increase in CTC was associated with a 6.6-month decrease in
survival (28). To detect CTC in
all patients with micro and or macro metastatic disease the volume
of blood analyzed will need to be increased substantially and the
specificity of detection improved. An increase in blood volume,
however, will also result in a higher false positive number of CTC.
In the present study 7 patients with benign colorectal disease had
one and 1 patient had two CTC detected in 30 ml of blood. Detection
of cells with the CTC phenotype in 7.5 ml of blood in patients with
benign colorectal disease using the CellSearch system and other
systems have also been reported by others (29). Whether or not these cells are
indeed tumor cells or are an artifact of the current detection
technologies will need to be investigated. Surprisingly, the
presence of CTC from blood drawn 4–92 (28 mean) days and 1 year
after surgery was not significantly related to RFS and CCRD. In
contrast, the presence of CTC from blood drawn 2, 3 and 4 years
after surgery was significantly correlated with RFS and CCRD
(except after 3 years for RFS). CTC measured before surgery from
the same patient group was not significantly related to RFS and
CCRD. This suggests that residual disease directly after surgery
+/− adjuvant therapy and one year later while in situ and
progressing is shedding tumor cells into the peripheral blood and
for those patients that ultimately recur the shedding of tumor
cells by the micro-metastasis until year two after surgery is not
sufficient to be detected with the CTC detection technology used in
the present study. An alternative explanation for the presence of
CTC after surgery that are not related to outcome rises from the
observation that a large number of CTC are released during surgery
(30). These tumor cells may just
not have the potential to form distant metastasis. The number of
approaches available for CTC detection has grown since the start of
the present study (31),
evaluation of these different approaches in controlled clinical
studies will show whether the advances are sufficient to detect
tumor cells in all patients with disseminated disease. The latter
will allow treatment on the basis of presence of disease rather
than on an increased risk profile.
In summary, the present study demonstrates that the
presence of CTC in patients with non-metastatic CRC before surgery
is associated with a statistically significant higher risk of
disease recurrence and shorter recurrence-free survival and a
higher colon cancer related death. Presence of CTC also has a
significant impact on the disease course when measured 2 to 4 years
after surgery but not within the first year after surgery. Further
improvement of sensitivity and specificity of CTC detection
technology is warranted as it has the promise to be incorporated in
the diagnostic armamentarium to determine which patients do and do
not need adjuvant therapy.
Acknowledgements
The present study was supported by the Immunicon
Corporation, responsible for the development of the CellSearch
system.
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shah S, Haddad R and Al-Sukhni W: Surgical
resection of hepatic and pulmonary metastases from colorectal
carcinoma. J Am Coll Surg. 202:468–475. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Safi F and Beyer HG: The value of
follow-up after curative surgery of colorectal carcinoma. Cancer
Detect Prev. 17:417–424. 1993.PubMed/NCBI
|
|
4
|
André T, Boni C, Navarro M, et al:
Improved overall survival with oxaliplatin, fluorouracil, and
leucovorin as adjuvant treatment in stage II or III colon cancer in
the MOSAIC trial. J Clin Oncol. 27:3109–3016. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Allard WJ, Matera J, Miller MC, et al:
Tumor cells circulate in the peripheral blood of all major
carcinomas but not in healthy subjects or patients with
nonmalignant diseases. Clin Cancer Res. 10:6897–6904. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cristofanilli M and Budd G: Circulating
tumor cells, disease progression, and survival in metastatic breast
cancer. Engl J Med. 351:781–791. 2004. View Article : Google Scholar
|
|
7
|
De Bono JS, Scher HI, Montgomery RB, et
al: Circulating tumor cells predict survival benefit from treatment
in metastatic castration-resistant prostate cancer. Clin Cancer
Res. 14:6302–6309. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cohen SJ, Punt CJA, Iannotti N, et al:
Relationship of circulating tumor cells to tumor response,
progression-free survival, and overall survival in patients with
metastatic colorectal cancer. J Clin Oncol. 26:3213–3221. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Franken B, de Groot MR, Mastboom WJB,
Vermes I, van der Palen J, Tibbe AGJ and Terstappen LWMM:
Circulating tumor cells, disease recurrence and survival in newly
diagnosed breast cancer. Breast Cancer Res. 14:R1332012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van Dalum G, van der Stam GJ, Tibbe AGJ,
et al: Circulating tumor cells before and during follow-up after
breast cancer surgery. Int J Oncol. 46:407–413. 2015.
|
|
11
|
Liu MC, Shields PG, Warren RD, et al:
Circulating tumor cells: a useful predictor of treatment efficacy
in metastatic breast cancer. J Clin Oncol. 27:5153–5159. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pierga JY, Hajage D, Bachelot T, et al:
High independent prognostic and predictive value of circulating
tumor cells compared with serum tumor markers in a large
prospective trial in first-line chemotherapy for metastatic breast
cancer patients. Ann Oncol. 23:618–624. 2012. View Article : Google Scholar
|
|
13
|
Tol J, Koopman M, Miller MC, et al:
Circulating tumour cells early predict progression-free and overall
survival in advanced colorectal cancer patients treated with
chemotherapy and targeted agents. Ann Oncol. 21:1006–1012. 2010.
View Article : Google Scholar
|
|
14
|
Lucci A, Hall CS, Lodhi AK, et al:
Circulating tumour cells in non-metastatic breast cancer: a
prospective study. Lancet Oncol. 13:688–695. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bidard FC, Mathiot C, Delaloge S, et al:
Single circulating tumor cell detection and overall survival in
non-metastatic breast cancer. Ann Oncol. 21:729–733. 2010.
View Article : Google Scholar
|
|
16
|
Martín M, Custodio S, de Las Casas MLM, et
al: Circulating tumor cells following first chemotherapy cycle: an
early and strong predictor of outcome in patients with metastatic
breast cancer. Oncologist. 18:917–923. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
https://www.oncoline.nl. Integr
Kankercent Ned. 2013
|
|
18
|
R Core Team. R: A Language and Environment
for Statistical Computing. 2013
|
|
19
|
Jueckstock JK, Rack BK, Zwingers T, Hepp
PGM, Schneeweiss A, Beckmann MW, Lichtenegger W, Sommer HL, Pantel
K, Tesch H, Forstbauer H, Lorenz R, Rezai M, Neugebauer JK,
Andergassen U, Friese K and Janni W: Prognostic relevance of
circulating tumor cells (CTC) before adjuvant chemotherapy in
patients with breast cancer: results of the German SUCCESS trial. J
Clin Oncol. 29(Suppl): 10332011.
|
|
20
|
Pierga JY, Bidard FC, Mathiot C, et al:
Circulating tumor cell detection predicts early metastatic relapse
after neoadjuvant chemotherapy in large operable and locally
advanced breast cancer in a phase II randomized trial. Clin Cancer
Res. 14:7004–7010. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Coumans FA, Siesling S and Terstappen LW:
Detection of cancer before distant metastasis. BMC Cancer.
13:2832013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cohen SJ, Punt CJ, Iannotti N, et al:
Prognostic significance of circulating tumor cells in patients with
metastatic colorectal cancer. Ann Oncol. 20:1223–1229. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tibbe AGJ, Miller MC and Terstappen LWMM:
Statistical considerations for enumeration of circulating tumor
cells. Cytometry A. 71:154–162. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thorsteinsson M, Soletormos G and Jess P:
Low number of detectable circulating tumor cells in non-metastatic
colon cancer. Anticancer Res. 31:613–617. 2011.PubMed/NCBI
|
|
25
|
Sastre J, Maestro ML, Puente J, et al:
Circulating tumor cells in colorectal cancer: correlation with
clinical and pathological variables. Ann Oncol. 19:935–938. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hiraiwa K, Takeuchi H, Hasegawa H, et al:
Clinical significance of circulating tumor cells in blood from
patients with gastrointestinal cancers. Ann Surg Oncol.
15:3092–3100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gazzaniga P, Gianni W and Raimondi C:
Circulating tumor cells in high-risk nonmetastatic colorectal
cancer. Tumor Biol. 34:2507–2509. 2013. View Article : Google Scholar
|
|
28
|
Coumans FA, Ligthart ST, Uhr JW and
Terstappen LWMM: Challenges in the enumeration and phenotyping of
CTC. Clin Cancer Res. 18:5711–5718. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pantel K, Denève E, Nocca D, et al:
Circulating epithelial cells in patients with benign colon
diseases. Clin Chem. 58:936–940. 2012. View Article : Google Scholar
|
|
30
|
Wind J, Tuynman JB, Tibbe AG, Swennenhuis
JF, Richel DJ, van Berge Henegouwen MI and Bemelman WA: Circulating
tumour cells during laparoscopic and open surgery for primary
colonic cancer in portal and peripheral blood. Eur J Surg Oncol.
35:942–950. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bednarz-Knoll N, Alix-Panabières C and
Pantel K: Clinical relevance and biology of circulating tumor
cells. Breast Cancer Res. 13:2282011. View
Article : Google Scholar : PubMed/NCBI
|