Introduction
Despite its decreasing incidence over the past few
decades, gastric cancer remains the most common gastrointestinal
tract cancer worldwide (1).
Additionally, it is still the fourth most commonly diagnosed cancer
and the second most deadly cancer (2,3). Due
to cancer tissue invasion and metastasis, chemotherapy and
radiation therapy cannot completely cure cancer, nor significantly
improve the quality of life of patients with cancer metastasis.
Thus, novel therapies targeting the aberrant molecules that lead to
cancer invasion or metastasis are needed.
Recepteur d’origine Nantais (RON), a member of c-Met
family of scatter factor receptors, plays an important role in the
progression, invasion, and metastasis of gastric carcinoma
(4). RON is initially synthesized
as a single chain precursor, a 170-kDa pro-RON, which is
subsequently cleaved into a 150-kDa β chain and a 40-kDa α chain.
The α chain is completely extracellular, whereas the β chain
contains the intracellular tyrosine kinase and traverses the cell
membrane (5).
Macrophage-stimulating protein (MSP) is the only ligand identified
for RON. Through ligand binding, RON is activated and mediates
multiple signaling cascades involved in cell motility, adhesion,
migration, and invasion, including the mitogen-activated protein
kinase (MAPK), phosphatidylinostiol-3 kinase (PI3K)/Akt, β-catenin,
and nuclear factor-κB (NF-κB) (6).
Several human tumor tissues show increased RON expression,
including tumors of the stomach, breast, colon, lung, liver,
kidney, ovary, pancreas, bladder, and prostate (4,6,7).
Gene expression analyses indicate that high expressions of RON are
associated with metastatic cancer (8). Since RON plays an essential role in
multiple processes involved in cancer progression and metastasis,
it is an attractive target for RON molecular-based cancer
therapy.
Because of the crucial role of RON in cancer
progression and metastasis, and the multi-anticancer functions of
flavonoids, it is of great significance to know the effects of
flavonoids on RON expression, so that selected flavonoids can be
used in cancer therapy to target the metastasis-related molecules.
However, the effect of flavonoids on RON is not well studied,
especially in gastric cancer cells. Thus, the aims of this study
were to screen potential RON inhibitors from natural flavonoids in
gastric cancer, and to examine their underlying mechanisms. Our
previous study investigated and reported that EGCG, a kind of
flavanol, inhibited gastric cancer cell invasion via suppression of
RON expression (9). In the present
study, four kinds of flavonoids, kaempferol, quercetin, genistein
and chrysin were examined for an inhibitory effect on RON
expression.
Kaempferol,
3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one, a
natural flavonol, has been isolated from tea, broccoli, Delphinium,
grapefruit, cabbage, kale, beans, endive, leek, tomato,
strawberries, grapes, Brussels sprouts, apples, and other plant
sources (10). Kaempferol
consumption in tea and broccoli has been associated with a reduced
risk of heart disease (11).
Quercetin,
2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one, a kind
of natural flavonol, is found in fruits, vegetables, leaves, and
grains. It can be used as an ingredient in supplements, beverages,
or food. Laboratory studies have investigated the potential of
quercetin for use in anticancer applications (12,13).
Genistein, 4′,5,7-trihydroxyisoflavoneis, a
phytoestrogen, belongs to the category of isoflavones. Genistein
has been identified as an angiogenesis inhibitor, and has been
found to inhibit the uncontrolled cell growth of cancer (14,15).
Chrysin, 5,7-dihydroxyflavone, a type of naturally
occurring flavonoid, has been known to inhibit tumor progression
and angiogenesis (16,17). It has been demonstrated that
chrysin suppresses IL-6-induced angiogenesis through
down-regulation of the soluble interleukin-6
receptor/gp130/JAK1/STAT3/VEGF signaling pathway (16). Recently, chrysin was reported to
inhibit insulin-induced HIF-1α highly expression, increase
ubiquitination of HIF-1α, and interfere with interaction between
HIF-1α and heat shock protein 90 (18).
In the four kinds of flavonoids, only chrysin
effectively suppresses RON expression. Therefore, we focused on
investigating the effect of chrysin on RON expression and cell
invasion, and examined the underlying mechanisms, taking advantage
of chrysin’s ability to inhibit gastric cancer invasion via
targeting RON.
Materials and methods
Cell culture and culture conditions
The AGS human gastric cancer cell line was obtained
from the American Type Culture Collection (Manassas, VA, USA). The
cells were cultured in RPMI-1640 (Hyclone; South Logan, UT, USA)
supplemented with 10% fetal bovine serum (FBS) and 0.6%
penicillin-streptomycin (Hyclone) at 37°C in an atmosphere
containing 5% CO2. In order to test the effect of
chrysin, genistein, quercetin, kaempferol or BAY-11-7082 (BAY) on
endogenous RON or Egr-1 expression, when the confluence reached
50%, we co-incubated the cells with chrysin or BAY in serum-reduced
(1% FBS) RPMI-1640 media. To determine the effects of chrysin on
PMA-induced RON expression, the cells were pretreated by different
concentrations of chrysin and then treated by PMA. RPMI-1640, FBS,
and penicillin-streptomycin were obtained from Thermo Scientific,
USA. PMA, chrysin, genistein, quercetin, and kaempferol were
obtained from Sigma-Aldrich, St. Louis, MO, USA; BAY was purchased
from Calbiochem, Merck KGaA, Darmstadt, Germany.
Cell viability
Cells (5×103) were incubated in a 96-well
plate with RPMI media containing 0–100 μM chrysin for 24 h, and
cell respiration was determined by an established
3-[4,5-dimethylthiazol-2-yl]c-2,5-diphenyltetrazolium bromide (MTT,
Sigma-Aldrich) assay. After the cell incubation, 10 μl of 5 mg/ml
MTT was added to each well of the 96-well plates and incubated at
37°C for 2 h. The formazan granules obtained were dissolved in 100%
dimethyl sulfoxide, and absorbance at 570 nm was detected with a
96-well ELISA reader (BioTek Instruments, Winooski, VT, USA).
Reverse transcription-PCR
Total RNA was extracted from the AGS cells using the
TRIzol reagent (Invitrogen, Carlsbad, CA, USA). One microgram of
total RNA was used for first-strand complementary DNA synthesis
using random primers and M-MLV transcriptase (Promega, Madison, WI,
USA). The complementary DNA was subjected to PCR amplification with
primer sets for GAPDH and RON using PCR master mix solution
(iNtRON, Korea). The specific primers sequences were as follows:
GAPDH sense, 5′-TTGTTGCCATCAATGAC CCC-3′ and GAPDH antisense,
5′-TGACAAAGTGGTCGTTG AGG-3′ (836 bp); RON sense,
5′-ACGGCTTAGCGCCACTG AGC-3′, RON antisense,
5′-CATGTGTGCCACTGTGACGT-3′ (550 bp); Egr-1 sense,
5′-CAGTGGCCTAGTGAGCATGA-3′, and Egr-1 antisense,
5′-CCGCAAGTGGATCTTGGTAT-3′ (767 bp). The PCR conditions were as
follows: denaturation at 94°C for 30 sec, annealing at 52°C for 20
sec and extension at 72°C for 30 sec, repeat 32 cycles, using a
Thermal Cycler (T100, Bio-Rad, USA, Hercules, CA, USA).
Western blot analysis
Cells were washed in phosphate-buffered saline
(PBS), detached using trypsin-EDTA buffer, and stored at −70°C
until needed. The protein was extracted with RIPA buffer [1% NP-40,
0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS)] and
protease inhibitors (aprotinin, leupeptin,
phenylmethanesulfonylfluoride (PMSF), benzamidine, trypsin
inhibitor, and sodium orthovanadate). The protein (50 μg) was then
separated by 10% SDS-polyacrylamide gel electrophoresis and
transferred to Immobilon-P membranes (Millipore Corp., Billarica,
MA, USA). The membranes were blocked in a 0.1% Tween-20 in
Tris-buffered saline (TBST) solution containing 5% skim milk,
incubated with primary antibody in a blocking solution overnight at
4°C, and washed three times with TBST at 10-min intervals.
Horseradish peroxidase-conjugated secondary antibody (#7074, Cell
Signaling Technology, Danvers, MA, USA) 1:5000 was used to detect
the immunoreactive proteins by chemiluminescence. The following
primary antibodies (1:1000) were used: rabbit anti-human RON
(sc-25781, Santa Cruz Biotechnology, Inc., CA, USA), rabbit
anti-human Egr-1 (sc-110, Santa Cruz), rabbit anti-human
phospho-NF-κB-p65 (#3033, Cell Signaling Technology) and rabbit
anti-human phospho-IκBα (#9241, Cell Signaling Technology). The
total protein levels were assayed by washing the blotted membrane
with a stripping solution composed of 100 mM 2-mercaptoethanol, 2%
SDS, and 62.5 mM Tris-HCl (pH 6.7) for 30 min at 50°C, and the
membrane was then re-probed with either anti-β-actin (#4967, Cell
Signaling Technology) or rabbit anti-human IκBα (#9242, Cell
Signaling Technology).
RON promoter reporter construct
The sequence of RON promoter fragment, approximately
3 kb in length, was synthesized from human genomic DNA (Promega) by
PCR using the primer 5′-GGTACCTAGCTGACC-3′ (forward) and 5′-GGG
CCAAATTTAAGC-3′ (reverse). The amplified PCR products were ligated
into the T&A Vector (RBC Bioscience, Saskatoon, SK, Canada),
and then digested with restriction endonuclease KpnI and
BglII (Takara, Japan). The products were ligated into the
KpnI and BglII sites of the pGL3-Basic Vector
(Promega). The final construct was the RON promoter reporter
(pGL-3-RON).
Measurement of RON promoter activity
The transcriptional regulation of RON was examined
by the transient transfection of a RON promoter-luciferase reporter
construct (pGL3-RON). AGS cells were seeded and grown until they
reached 70% confluence. Then, the pGL3-RON promoter plasmids were
transfected into the cells using FuGENE 6 (Promega) according to
the manufacturer’s protocol. PRL-TK was transfected as an internal
control. Cells were incubated in the transfection medium for 12 h
and then treated with PMA for 4 h. The effects of chrysin on RON
promoter activity were determined by pretreating cells with chrysin
for 1 h prior to the addition of PMA. Co-transfection studies were
performed in the presence or absence of the expression vector
encoding the full length cDNA coding human Egr-1, which was kindly
provided by Dr Young Han Lee (Konkuk University, Seoul, Korea).
Additionally, in order to check the role of NF-κB on RON
expression, the co-transfection studies were performed in the
presence or absence of the expression vector encoding the dominant
negative mutant IKKα, IKKβ, or NIK, which were kindly provided by
Dr D.W. Ballard (19) and W.C.
Greene (20). The cells were
harvested with a Cell Culture Lysis reagent (Promega), and the
luciferase activities were determined using a luminometer (Centro
XS lb960 Microplate Luminometer, Berthold Technologies, USA)
according to the manufacturer’s protocol.
Transient transfection of Egr-1 promoter
reporter
The Egr-1 promoter reporter plasmid was kindly
provided by Dr Young Han Lee (Konkuk University). When AGS cells
reached 60–70% confluence, they were washed by Opti-MEM medium and
transfected with a pGL-3 vector containing the Egr-1 promoter
reporter using FuGENE 6 (Promega) according to the manufacturer’s
protocol. Reporter-transfected cells were pretreated with chrysin
for 1 h, treated with 100 nM PMA for 4 h, and then luciferase
activity was measured using a luminometer.
Transient transfection of NF-κB
reporter
The NF-κB reporter plasmid was purchased from
Clontech (Palo Alto, CA, USA). AGS cells were washed in Opti-MEM
medium and transfected with the pGL-3-NF-κB reporter plasmid using
FuGENE 6 (Promega) according to the manufacturer’s protocol.
Reporter-transfected cells were pretreated with chrysin for 1 h,
treated with 100 nM PMA for 4 h, and then the luciferase activity
was measured using a luminometer.
Small interfering RNA transfection
Gene silencing was performed by using human Egr-1
(sc-29303, Santa Cruz) and RON sequence-specific duplex siRNA
(sc-36434, Santa Cruz). Briefly, for each transfection reaction in
two separate tubes, 20 nM siRNA oligonucleotides and 2 μl
Lipofectamine RNAi MAX (Invitrogen) were respectively mixed with
100 μl serum-free Optimem (Hyclone). Then, we mixed the two tubes,
incubating for 5 min at room temperature to form the
RNA-lipofectamine complex, and then they were added to cell plates
with Opti-MEM medium. After incubation for 6 h, the medium was
replaced with normal growth medium.
Matrigel invasion assay
The cell invasion assay was carried out using the
10-well chemotasis chamber (Neuro Probe, USA) with 8 μM membrane
pore (Neuro Probe) with 10% FBS containing RPMI as the
chemoattractant in the lower chamber. AGS cells (105) in
250 μl were added to upper chamber with PMA with the presence of
chrysin or RON antibody and allowed to invade the Matrigel for 24
h. The non-invading cells on the upper surface of each membrane
were removed from the chamber with a cotton swab, and the invading
cells on the lower surface of each membrane were stained with the
Quick-Diff kit (Becton-Dickinson, Franklin Lakes, NJ, USA). After
two washes with water, the chambers were allowed to air-dry. The
number of invading cells was counted using a phase-contrast
microscope (Olympus IX50, Japan).
Statistics
Data are shown as mean ± SD, and represent the mean
of at least three separate experiments performed in triplicate.
Differences between data sets were determined by the t-test.
Differences described as significant in the text correspond to
P<0.05.
Results
Chrysin inhibits endogenous RON in
gastric cancer AGS cells
To determine the effect of flavones in endogenous
RON expression in human gastric cancer AGS cells, the cells were
incubated with chrysin, genistein, quercetin, and kaempferol
(Fig. 1A), and the levels of RON
mRNA were determined by RT-PCR analysis. Interestingly, as shown in
Fig. 1B, among the four
flavonoids, only chrysin inhibited RON expression in AGS cells.
Next, we verified the inhibitory effect of chrysin against RON
expression by incubating the cells with different concentration of
chrysin. The RT-PCR results showed that chrysin decreases RON mRNA
in a dose-dependent manner (Fig.
1C). Additionally, western blotting result showed that chrysin
decreased RON protein in a dose-dependent manner (Fig. 1D). Collectively, these results
demonstrated that chrysin suppresses the expression of endogenous
RON in human gastric cancer AGS cells. The concentrations of
chrysin used in the above study did not affect cell viability
(Fig. 1E). These results indicated
that chrysin inhibited endogenous RON expression in a
dose-dependent manner in AGS cells.
Chrysin inhibits PMA-induced RON in
gastric cancer AGS cells
In order to test the suppressive effect of chrysin
against inducible RON, AGS cells pretreated with chrysin were
incubated with PMA, and then RT-PCR and western blot analysis were
performed. PMA-induced RON mRNA was inhibited by chrysin in a
dose-dependent manner (Fig. 2A).
Additionally, the PMA-upregulated RON protein was also suppressed
by chrysin in a dose-dependent manner (Fig. 2B). Next, we checked the inhibitory
effect of chrysin against RON through a promoter activity assay. As
shown in Fig. 2C, the activated
RON promoter was suppressed by chrysin in a dose-dependent manner.
The above results demonstrated that chrysin inhibits the
PMA-induced RON in human gastric cancer AGS cells.
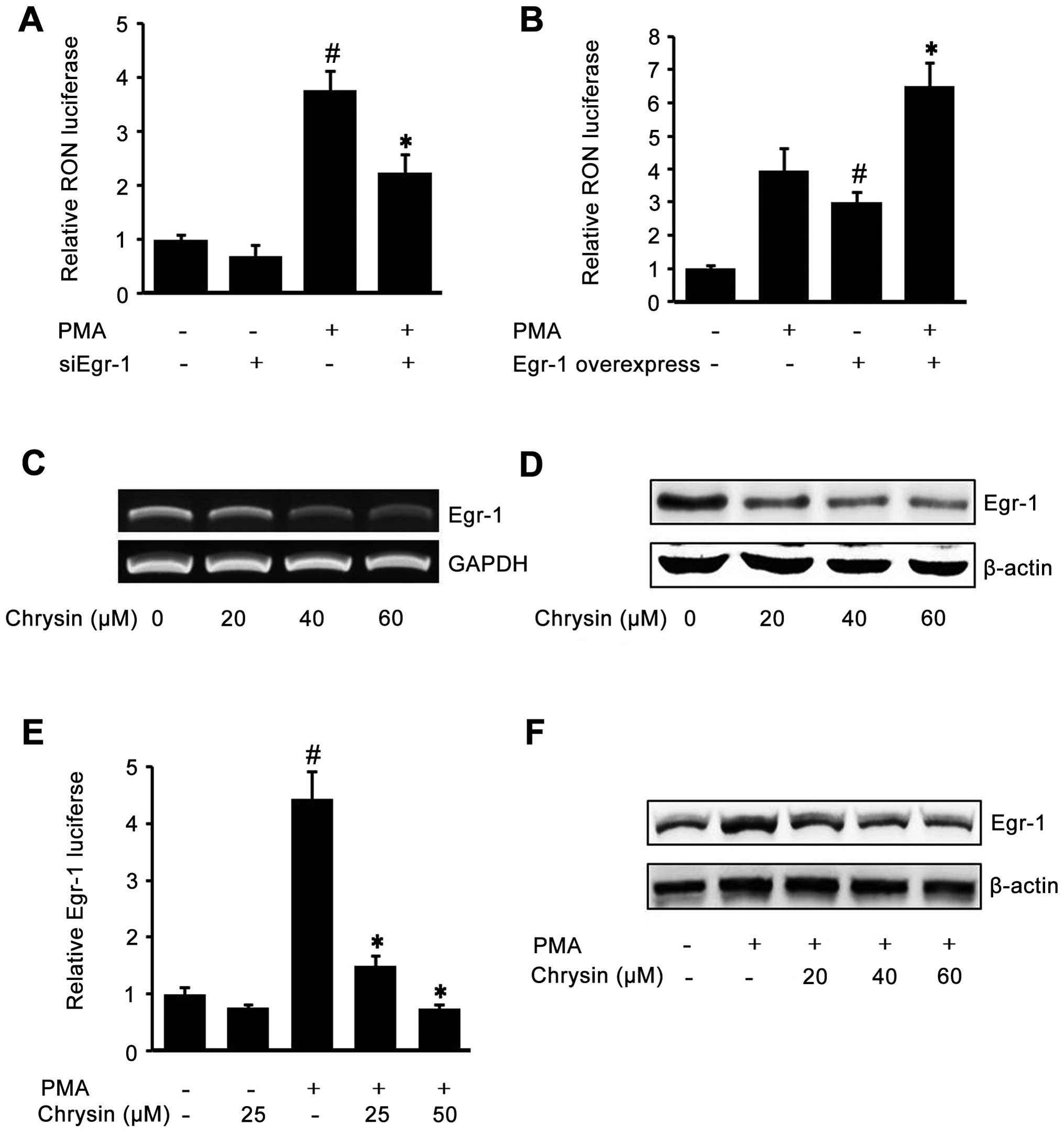
Role of Egr-1 in the inhibition of RON by
chrysin in gastric cancer AGS cells
To check the role of Egr-1 on RON expression, AGS
cells were transiently co-transfected with siEgr-1 and RON promoter
reporter (pGL-3-RON), and then the cells were treated with PMA. As
shown in Fig. 3A, the
siEgr-1-transfected cells showed lower RON promoter activity in
both PMA-treated cells and untreated cells. Next, we verified the
role of Egr-1 on RON expression by co-transfecting the Egr-1
expression construct and pGL-3-RON. As shown in Fig. 3B, the cells transfected with the
Egr-1 expression construct showed higher RON promoter activity in
both PMA-treated cells and untreated cells. Fig. 3A and B demonstrated that Egr-1
played a crucial role in RON expression. So, we hypothesized that
chrysin may inhibit RON by inhibiting Egr-1. To test this
hypothesis, we checked the effect of chrysin on endogenous Egr-1.
After incubating cells with different concentration of chrysin,
RT-PCR and western blotting were performed to check the Egr-1 mRNA
and protein levels. As shown in Fig.
3C and D, chrysin suppressed endogenous Egr-1 mRNA and protein
in a dose-dependent manner. Subsequently, in order to check the
effect of chrysin on inducible Egr-1 activity, we transfected the
Egr-1 reporter construct and stimulated the cells with PMA. PMA
drastically induced Egr-1 reporter lucif-erase activity, which was
inhibited by chrysin (Fig. 3E).
Finally, to examine the inhibitory effect of chrysin on PMA-induced
Egr-1 expression, western blotting was employed. As shown in
Fig. 3F, chrysin inhibited
PMA-upregulated Egr-1 protein in a dose-dependent manner. These
results indicated that one of the chrysin mechanisms of inhibition
of RON is through the suppression of Egr-1, a critically required
transcription factor of RON expression.
Role of NF-κB in the inhibition of RON by
chrysin in gastric cancer AGS cells
To check the role of transcription factor NF-κB on
RON expression, we first treated the cells with different
concentrations of NF-κB inhibitor BAY-11-7082. As shown in Fig. 4A, BAY decreased endogenous RON
expression, indicating that NF-κB may play an important role in RON
expression. To further confirm the role of NF-κB on RON expression,
dominant negative mutant expression constructs were co-transfected
with pGL-3-RON into AGS cells. As shown in Fig. 4B, the expression of dominant
negative mutant forms of NIK, IKK-α and IKK-β, resulted in a
decrease of PMA-induced RON promoter activity indicating the
essential role of NF-κB on RON expression. Next, we checked whether
chrysin was able to affect NF-κB by transfection of a pGL3-NF-κB
luciferase construct. As shown in Fig.
4C, chrysin inhibited PMA-induced NF-κB activity in a
dose-dependent manner. Subsequently, to check the effect of chrysin
on NF-κB-related proteins p65 and IκBα, western blotting was
performed: chrysin inhibited the endogenous phosphorylated NF-κB
p65 and IκBα (Fig. 4D); and the
PMA-induced NF-κB p65 and IκBα phosphorylation was also suppressed
by chrysin in a dose-dependent manner (Fig. 4E and F).
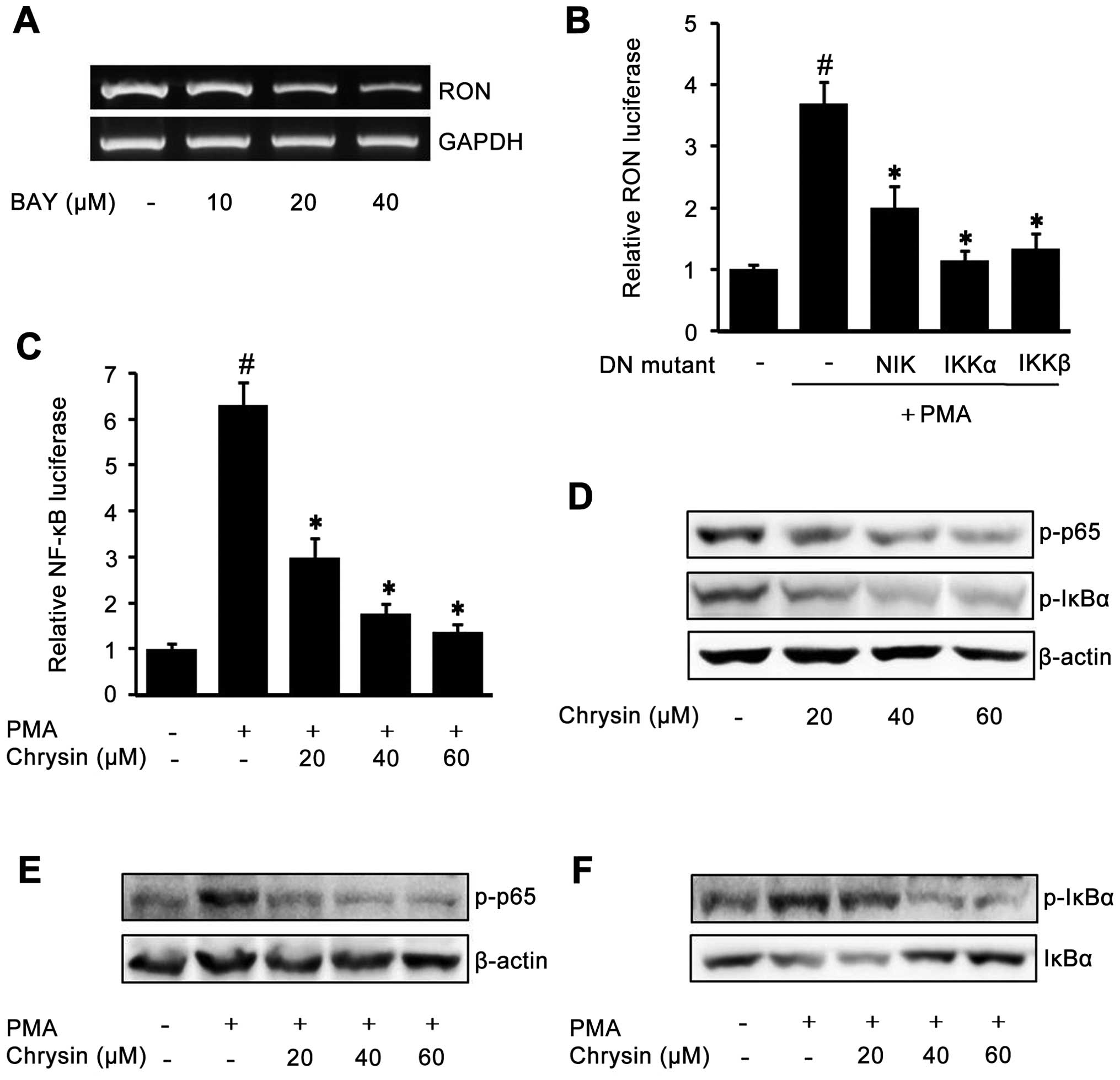 | Figure 4The role of NF-κB in the inhibition
of RON by chrysin in gastric cancer AGS cells. When cell confluence
was 50%, they were incubated with the indicated concentrations of
BAY-11-7082 (BAY) for 24 h in RPMI with 1% FBS. After incubation,
RON mRNA was examined by RT-PCR (A). An expression vector encoding
dominant negative mutant NIK, IKKα, IKKβ, or empty vector was
co-transfected with a pGL-3-RON promoter construct into AGS cells.
After incubation with 100 nM PMA for 4 h, the luciferase activity
was determined using a luminometer. The data represent the mean ±
SD from triplicate measurements. #P<0.05 versus
control; *P<0.05 versus PMA only (B). The
NF-κB-luciferase reporter construct transfected cells were
pretreated with the indicated concentrations of chrysin for 1 h,
then they were incubated with 100 nM PMA for 4 h and the NF-κB
luciferase activity was determined using a luminometer. The data
represent the mean ± SD from triplicate measurements.
#P<0.05 versus control; *P<0.05 versus
PMA only (C). When the confluence of cells was 50%, they were
incubated with the indicated concentrations of chrysin for 24 h in
RPMI media containing 1% FBS. After cell harvest, western blotting
was employed to examine the phosphorylated p65 and IκBα (D). AGS
cells pretreated with the indicated concentrations of chrysin for 1
h were treated with 100 nM PMA for 15 min. After incubation,
western blotting was performed to analyze the phosphorylated NF-κB
p65 (E); as well as phosphorylated IκBα and total IκBα (F). |
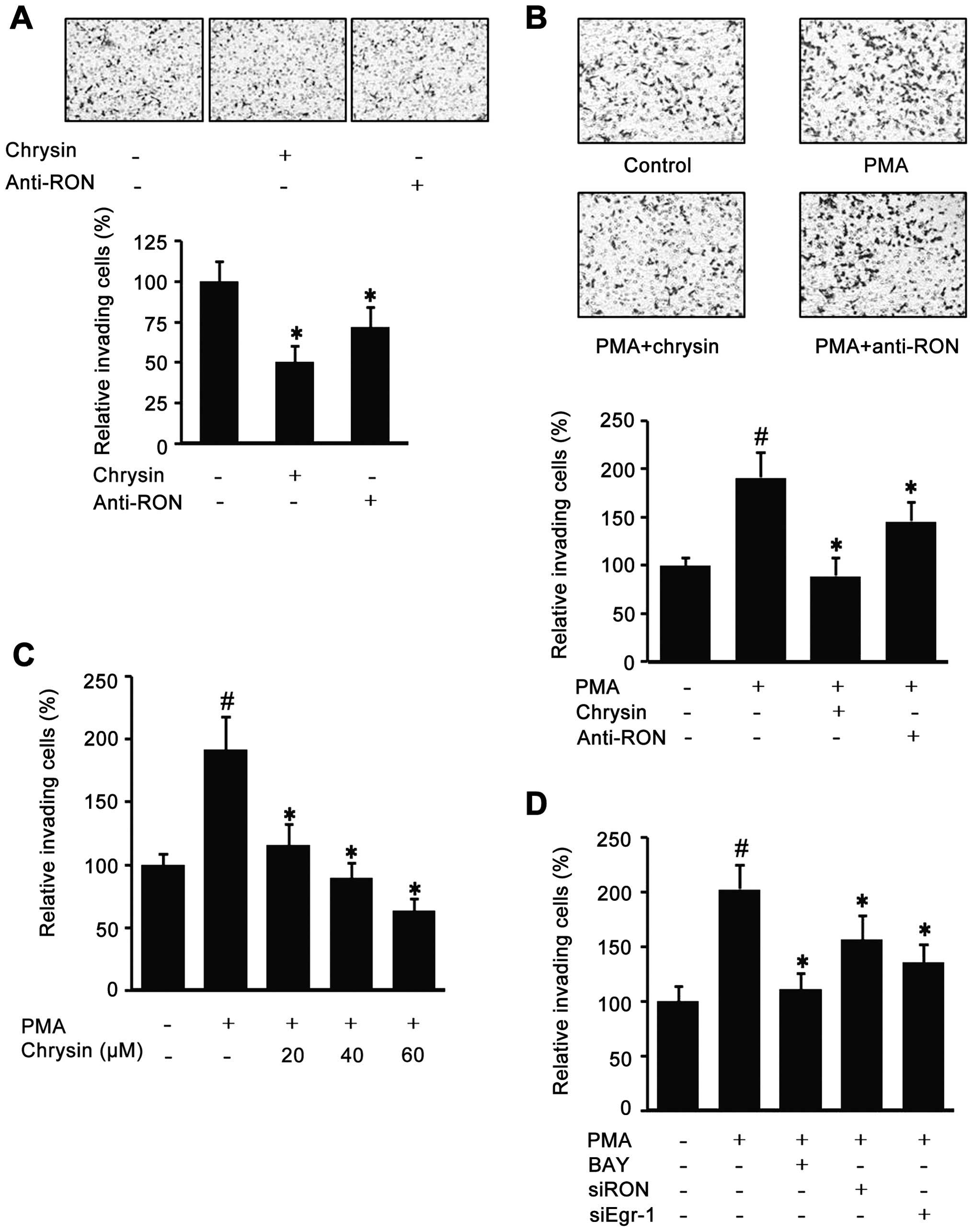
Chrysin inhibits gastric cancer AGS cell
invasion
Since chrysin could decrease endogenous RON, the
effect of chrysin in AGS cell invasion was checked with a modified
Boyden Chamber method. As shown in Fig. 5A, the cells co-incubated with
chrysin or RON antibody had decreased the Matrigel invasiveness.
Additionally, because it has been suggested that highly expressed
RON is important for the invasive phenotype of cancer cells, the
role of PMA-induced RON in AGS cell invasion was evaluated with a
modified Boyden Chamber method. As shown in Fig. 5B, the PMA-induced cell invasion was
suppressed by chrysin and RON antibody. Fig. 5C indicates that chrysin suppressed
PMA-induced invasion in a dose-dependent manner. Additionally,
BAY-treated cells partially lost their PMA-induced invasiveness and
the si-Egr-1 and si-RON transfected cells also showed lower
invasiveness (Fig. 5D). These
results suggest that chrysin may reduce cancer invasiveness via the
suppression of RON expression.
Discussion
RON is a kind of tyrosine kinase receptor which
regulates multiple processes involved in tumor progression and
metastasis (21,22). Generally, RON is relatively low in
normal cells. However, the levels of RON expression in malignant
cancer cells increase several-fold compared with normal cells. The
aberrant expression of RON has been observed in many kinds of
cancer cells, and is associated with malignancy and poor clinical
outcome in various cancers including gastric cancer (7,23).
Because of the importance of RON in cancer initiation and
progression, more and more investigators are considering RON to be
an important therapeutic target.
In this study, which focused on the inhibition of
RON, we demonstrated that chrysin inhibited both endogenous and
inducible RON expression by regulating Egr-1 and NF-κB in gastric
cancer cells, and that these events may contribute to develop a new
cancer therapy. The reasons we are interested in chrysin as an
anticancer agent, are the following: i) chrysin is a naturally
occurring flavone with low cytotoxicity (24); ii) it was discovered that chrysin
has an anti-inflammation function, such as inhibition of COX-2
(25) and TNF-α (26); iii) chrysin reduces the
proliferation and induces apoptosis in the various cancers, such as
prostate cancer cells (27) and
leukemia cells (28); iv) in our
preliminary experiment, among the four flavonoids, chrysin was
unique in its ability to inhibit RON expression. Although chrysin
administration inhibits carcinogenesis using several mechanisms,
our present finding, in a novel angle, suggests that chrysin may
exert its anti-invasion effects by inhibiting RON expression.
Previously, we investigated the role of RON in the
course of gastric cancer cell invasion and we identified the
essential regulatory elements of Egr-1 that are critically required
for oncogenic RON expression (4).
Egr-1 is an inducible early response transcription factor that
recognizes 9 bp targeting DNA sites via three zinc finger domains
and activates genes in response to cellular stimuli such as
cytokines, growth factors, synaptic signals, and vascular stress,
and modulates cell proliferation, inflammation, and apoptosis in
various cells (29,30). In the present study, using the
Egr-1 siRNA and Egr-1 expression construct, we confirmed the
essential role of Egr-1 for RON gene transcription. Interestingly,
chrysin suppressed the Egr-1 mRNA and protein, as well as the Egr-1
transcription factor activity (Fig.
3C–F), indicating that the interruption of Egr-1 by chrysin
plays a critical role in suppressing RON gene expression. This
result is consistent with the previous report that genistein, a
flavone with a similar structure to chrysin, inhibits the surface
IgM and IgD-crosslinking-induced Egr-1 expression in a
dose-dependent manner in the human B lymphoma cell line B104
(31).
Besides Egr-1, we found that NF-κB is another
essential transcription factor involved in RON expression
regulation. NF-κB, a protein complex that controls DNA
transcription, is found in almost all animal cell types and is
involved in cellular responses to stimuli, such as stress,
cytokines, and free radicals, and plays a key role in regulating
the immune response to infection. Incorrect regulation of NF-κB has
been linked to inflammatory, and autoimmune diseases, and cancer
(32–34). Consistent with that reported by
Narasimhan and Ammanamanchi, there are two putative NF-κB p65
subunit binding sites on the RON promoter (35), and we also demonstrated that NF-κB
is critically required for RON gene transcription through a
specific inhibitor and dominant negative plasmid transfection
assays (Fig. 4A and B). We found
chrysin suppressed PMA-induced NF-κB activity by inhibiting IκBα
and NF-κB phosphorylation (Fig.
4C–F). A similar finding was reported, that chrysin inhibited
the activation of NF-κB, which is involved in regulation of the
iNOS and COX-2 in BV-2 microglia cells (36). We infer that the mechanism of
chrysin inhibiting NF-κB may be through the inactivation of IκB
kinase (IKK). Another report is consistent with our hypothesis:
apigenin and luteolin, two kinds of flavones with similar
structures to chrysin, inhibit IκBα degradation, NF-κB DNA-protein
binding, and NF-κB luciferase activity via actively inhibiting IKK
activity (37).
Concluding, this study finds that chrysin is able to
suppress RON expression and cell invasion in gastric cancer AGS
cells, which suggests that the downregulation of RON by chrysin is
involved in decreasing cell invasion. The inhibition mechanism may
be that chrysin inhibits RON expression by suppressing the
activation of transcription factor Egr-1 and NF-κB. However, we did
not exclude other possible mechanisms. These findings may provide
useful evidence for developing new anticancer therapeutics for
gastric cancer.
Acknowledgements
This study was supported by a research grant
(0720570) from the National Cancer Center, by a Basic Science
Research Program grant through the National Research Foundation of
Korea (NRF) funded by the Ministry of Education, Science, and
Technology (2010-0009910), and by a Medical Research Center
(2012-000-9442) grant from the Korean Science and Engineering
Foundation.
Abbreviations:
|
RON
|
Recepteur d’origine Nantais
|
|
PMA
|
phorbol 12-myristate 13-acetate
|
|
Egr-1
|
early growth response protein 1
|
References
|
1
|
Yamashita K, Sakuramoto S, Nemoto M,
Shibata T, Mieno H, Katada N, Kikuchi S and Watanabe M: Trend in
gastric cancer: 35 years of surgical experience in Japan. World J
Gastroenterol. 17:3390–3397. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Orditura M, Galizia G, Sforza V,
Gambardella V, Fabozzi A, Laterza MM, Andreozzi F, Ventriglia J,
Savastano B and Mabilia A: Treatment of gastric cancer. World J
Gastroenterol. 20:1635–1649. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee KE, Park JS, Khoi PN, Joo YE, Lee YH
and Jung YD: Upregulation of Recepteur d’origine Nantais tyrosine
kinase and cell invasiveness via early growth response-1 in gastric
cancer cells. J Cell Biochem. 113:1217–1223. 2012. View Article : Google Scholar
|
|
5
|
Wang MH, Lee W, Luo YL, Weis M and Yao HP:
Altered expression of the RON receptor tyrosine kinase in various
epithelial cancers and its contribution to tumourigenic phenotypes
in thyroid cancer cells. J Pathol. 213:402–411. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim S, Yoon TM, Lee DH, Park Y-L, Lee K-H,
Lim SC, Joo Y-E and Lee JK: RON (Recepteur d’origine Nantais)
expression and its association with tumor progression in laryngeal
squamous cell carcinoma. Auris Nasus Larynx. 41:201–206. 2014.
View Article : Google Scholar
|
|
7
|
Leonis MA, Thobe MN and Waltz SE:
Ron-receptor tyrosine kinase in tumorigenesis and metastasis.
Future Oncol. 3:441–448. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thangasamy A, Rogge J and Ammanamanchi S:
Recepteur d’origine Nantais tyrosine kinase is a direct target of
hypoxia-inducible factor-1α-mediated invasion of breast carcinoma
cells. J Biol Chem. 284:14001–14010. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park JS, Khoi PN, Joo YE, Lee YH, Lang SA,
Stoeltzing O and Jung YD: EGCG inhibits Recepteur d’origine Nantais
expression by suppressing Egr-1 in gastric cancer cells. Int J
Oncol. 42:1120–1126. 2013.PubMed/NCBI
|
|
10
|
Lin C-W, Chen P-N, Chen M-K, Yang W-E,
Tang C-H, Yang S-F and Hsieh Y-S: Kaempferol reduces matrix
metalloproteinase-2 expression by down-regulating ERK1/2 and the
activator protein-1 signaling pathways in oral cancer cells. PLoS
One. 8:e808832013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Calderon-Montano JM, Burgos-Morón E,
Pérez-Guerrero C and López-Lázaro M: A review on the dietary
flavonoid kaempferol. Mini Rev Med Chem. 11:298–344. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Murakami A, Ashida H and Terao J:
Multitargeted cancer prevention by quercetin. Cancer Lett.
269:315–325. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin C-W, Hou W-C, Shen S-C, Juan S-H, Ko
C-H, Wang L-M and Chen Y-C: Quercetin inhibition of tumor invasion
via suppressing PKCδ/ERK/AP-1-dependent matrix metalloproteinase-9
activation in breast carcinoma cells. Carcinogenesis. 29:1807–1815.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakamura Y, Yogosawa S, Izutani Y,
Watanabe H, Otsuji E and Sakai T: A combination of indol-3-carbinol
and genistein synergistically induces apoptosis in human colon
cancer HT-29 cells by inhibiting Akt phosphorylation and
progression of autophagy. Mol Cancer. 8:1476–4598. 2009. View Article : Google Scholar
|
|
15
|
Kim SH, Kim SH, Kim YB, Jeon YT, Lee SC
and Song YS: Genistein inhibits cell growth by modulating various
mitogen-activated protein kinases and AKT in cervical cancer cells.
Ann NY Acad Sci. 1171:495–500. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin C-M, Shyu K-G, Wang B-W, Chang H, Chen
Y-H and Chiu J-H: Chrysin suppresses IL-6-induced angiogenesis via
down-regulation of JAK1/STAT3 and VEGF: an in vitro and in ovo
approach. J Agric Food Chem. 58:7082–7087. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lirdprapamongkol K, Sakurai H, Abdelhamed
S, Yokoyama S, Maruyama T, Athikomkulchai S, Viriyaroj A, Awale S,
Yagita H and Ruchirawat S: A flavonoid chrysin suppresses hypoxic
survival and metastatic growth of mouse breast cancer cells. Oncol
Rep. 30:2357–2364. 2013.PubMed/NCBI
|
|
18
|
Fu B, Xue J, Li Z, Shi X, Jiang B-H and
Fang J: Chrysin inhibits expression of hypoxia-inducible factor-1α
through reducing hypoxia-inducible factor-1α stability and
inhibiting its protein synthesis. Mol Cancer Ther. 6:220–226. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McKinsey TA, Brockman JA, Scherer DC,
Al-Murrani SW, Green PL and Ballard DW: Inactivation of IkappaBbeta
by the tax protein of human T-cell leukemia virus type 1: a
potential mechanism for constitutive induction of NF-kappaB. Mol
Cell Biol. 16:2083–2090. 1996.PubMed/NCBI
|
|
20
|
Geleziunas R, Ferrell S, Lin X, Mu Y,
Cunningham ET, Grant M, Connelly MA, Hambor JE, Marcu KB and Greene
WC: Human T-cell leukemia virus type 1 Tax induction of NF-κB
involves activation of the IκB kinase α (IKKα) and IKKβ cellular
kinases. Mol Cell Biol. 18:5157–5165. 1998.PubMed/NCBI
|
|
21
|
Snuderl M, Fazlollahi L, Le LP, Nitta M,
Zhelyazkova BH, Davidson CJ, Akhavanfard S, Cahill DP, Aldape KD
and Betensky RA: Mosaic amplification of multiple receptor tyrosine
kinase genes in glioblastoma. Cancer Cell. 20:810–817. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lemmon MA and Schlessinger J: Cell
signaling by receptor tyrosine kinases. Cell. 141:1117–1134. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou D, Pan G, Zheng C, Zheng J, Yian L
and Teng X: Expression of the RON receptor tyrosine kinase and its
association with gastric carcinoma versus normal gastric tissues.
BMC Cancer. 8:3532008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brechbuhl HM, Kachadourian R, Min E, Chan
D and Day BJ: Chrysin enhances doxorubicin-induced cytotoxicity in
human lung epithelial cancer cell lines: the role of glutathione.
Toxicol Appl Pharmacol. 258:1–9. 2012. View Article : Google Scholar :
|
|
25
|
Khan MS, Devaraj H and Devaraj N: Chrysin
abrogates early hepatocarcinogenesis and induces apoptosis in
N-nitrosodiethylamine-induced preneoplastic nodules in rats.
Toxicol Appl Pharmacol. 251:85–94. 2011. View Article : Google Scholar
|
|
26
|
Ahad A, Ganai AA, Mujeeb M and Siddiqui
WA: Chrysin, an anti-inflammatory molecule, abrogates renal
dysfunction in type 2 diabetic rats. Toxicol Appl Pharmacol.
279:1–7. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Samarghandian S, Afshari JT and Davoodi S:
Chrysin reduces proliferation and induces apoptosis in the human
prostate cancer cell line pc-3. Clinics. 66:1073–1079. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Khoo BY, Chua SL and Balaram P: Apoptotic
effects of chrysin in human cancer cell lines. Int J Mol Sci.
11:2188–2199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zandarashvili L, Vuzman D, Esadze A,
Takayama Y, Sahu D, Levy Y and Iwahara J: Asymmetrical roles of
zinc fingers in dynamic DNA-scanning process by the inducible
transcription factor Egr-1. Proc Natl Acad Sci USA.
109:E1724–E1732. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kwon O, Soung NK, Thimmegowda N, Jeong SJ,
Jang JH, Moon D-O, Chung JK, Lee KS, Kwon YT and Erikson RL:
Patulin induces colorectal cancer cells apoptosis through EGR-1
dependent ATF3 up-regulation. Cell Signal. 24:943–950. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kanazashi S, Hata D, Ishigami T, Jung EY,
Shintaku N, Sumimoto S, Heike T, Katamura K and Mayumi M: Induction
of phosphatidylinositol turnover and EGR-1 mRNA expression by
crosslinking of surface IgM and IgD in the human B cell line B104.
Mol Immunol. 31:21–30. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Perkins ND: Integrating cell-signalling
pathways with NF-κB and IKK function. Nat Rev Mol Cell Biol.
8:49–62. 2007. View
Article : Google Scholar
|
|
33
|
Gilmore TD: Introduction to NF-κB:
players, pathways, perspectives. Oncogene. 25:6680–6684. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim S and Joo Y-E: Theaflavin inhibits
LPS-induced IL-6, MCP-1, and ICAM-1 expression in bone
marrow-derived macrophages through the blockade of NF-κB and MAPK
signaling pathways. Chonnam Med J. 47:104–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Narasimhan M and Ammanamanchi S: Curcumin
blocks RON tyrosine kinase-mediated invasion of breast carcinoma
cells. Cancer Res. 68:5185–5192. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ha SK, Moon E and Kim SY: Chrysin
suppresses LPS-stimulated proinflammatory responses by blocking
NF-κB and JNK activations in microglia cells. Neurosci Lett.
485:143–147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen C-C, Chow M-P, Huang W-C, Lin Y-C and
Chang Y-J: Flavonoids inhibit tumor necrosis factor-α-induced
up-regulation of intercellular adhesion molecule-1 (ICAM-1) in
respiratory epithelial cells through activator protein-1 and
nuclear factor-κB: structure-activity relationships. Mol Pharmacol.
66:683–693. 2004.PubMed/NCBI
|