Introduction
Endocrine therapy is up-front systemic therapy for
breast cancer patients with estrogen receptor (ER)-positive tumors.
The antiestrogen tamoxifen has been the key endocrine agent since
the 1970s and the meta-analysis after 15 years follow-up of 5 years
adjuvant tamoxifen therapy revealed reduction in both recurrence
risk and death (1). The
third-generation aromatase inhibitors (AIs) letrozole, anastrozole
and exemestane were introduced in the 1990s and proved superior to
tamoxifen both in advanced disease and in the adjuvant setting
(2–5). Consequently, the recommended
first-line endocrine therapy for postmenopausal women with
ER-positive disease is treatment with a third-generation AI
(6). Both primary (de novo)
and secondary (acquired) resistance occur, limiting the benefit of
AI therapy. This emphasizes the need for early identification of
resistance and new treatment options for patients with resistant
tumors. Clinical data demonstrating improved outcome by combining
AI therapy with HER2 targeted therapy or with PI3K/AKT/mTOR
inhibitors (7–9) show that these pathways play important
roles in AI-resistant cell growth. However, at present there are no
tools to select the patients for specific therapy, and the
heterogeneity of clinical resistance (10,11)
underscores the need for extensive clinical and basic research to
disclose the underlying molecular mechanisms for resistance to
AIs.
The estrogen supply for postmenopausal breast tumors
originates from circulatory uptake and local synthesis in both
tumor cells and surrounding tissue, e.g., adipocytes, fibroblasts
and inflammatory cells (12–15).
The resistance mechanisms to AIs may differ in tumors depending on
systemic estrogen supply and tumors in which the carcinoma cells
are able to utilize the endogenous aromatase enzyme for estrogen
supply. Cell models, mimicking AI resistance in tumors depending on
systemically delivered estrogen, have been established from
estrogen-responsive breast cancer cells, which have been adapted to
grow under long-term estrogen deprivation (LTED) (16–20).
LTED cells grow estrogen independently and cell growth has been
shown to occur primarily via cross-talk between ER and growth
factor receptor signaling pathways, including HER2, IGF-IR and PI3K
(21–26). AIs have no effect on growth of LTED
cells, whereas antiestrogens, and in particular the ER down
modulator fulvestrant, inhibit growth of LTED cells (21,22,27).
The importance of local synthesis versus circulatory uptake for
supply of estrogen to the cancer cells has been debated and
although most breast carcinoma cells express aromatase, most
studies point towards a major importance of uptake of circulatory
estrogen (13–15). Treatment with AIs results in total
suppression of whole body estrogen synthesis, but the cancer cells
have the capability to increase local estrogen production.
Therefore, a model with endogenous estrogen synthesis is
warranted.
Surrogate models with aromatase overexpressing
breast cancer cells have been developed by stable introduction of
aromatase cDNA (25,28–30).
Cell lines and xenografts with acquired resistance to AIs have been
established by long-term AI treatment of MCF-7 and T47D cells with
exogenous over-expression of aromatase (25,31,32).
Genome-wide analyses have revealed that the expression profiles for
the MCF-7 sublines resistant to the non-steroidal compounds
letrozole and anastrozole were very similar whereas a different
profile was observed in cell lines with acquired resistance to the
steroidal inhibitor exemestane (31,33).
Ligand-independent activation of ER was found in both letrozole and
anastrozole-resistant cell lines (31), whereas exemestane appeared to act
as a weak agonist in exemestane-resistant cell lines, resulting in
e.g., induction of the EGFR ligand amphiregulin and activation of
EGFR signaling (33,34). A xenograft model with aromatase
overexpressing breast cancer cells has disclosed adaptive changes
resulting in activation of alternate signaling pathways due to
increased expression of e.g., EGFR, HER2 and IGF-IR (25).
The regulation of the endogenous aromatase gene
(CYP19A1) is very complex (12) and may play a key role in AI
resistance. We have discovered culture conditions for MCF-7 cells,
under which the growth is dependent on conversion of androgen to
estrogen via the endogenous aromatase enzyme (35). Treatment with AIs totally
suppressed cell growth under these culture conditions (35,36).
However, during long-term treatment with AIs, a small subpopulation
of the cells survived and slowly resumed growth. From such cells,
we have been able to establish AI-resistant cell lines. These cell
lines are unique models, which mimic acquired AI resistance in
tumors utilizing endogenous aromatase activity to obtain estrogen
stimulated cell growth. This report is an initial characterization
of our panel of letrozole-, exemestane- and anastrozole-resistant
cell lines, presenting the expression of HER receptors, ER and
ER-regulated proteins, and growth response to treatment with AIs,
tamoxifen and fulvestrant.
Materials and methods
Cell lines and culture conditions
The parental cell line for the AI-resistant cell
lines was MCF-7 subline 0.5 (MCF-7/S0.5), which originates from
MCF-7 cells from the Human Cell Culture Bank (Mason Research
Institute, Rockville, MD, USA) that have been stepwise adapted to
grow with 0.5% fetal calf serum (FCS) (37). The MCF-7/S0.5 (MCF-7) cells were
maintained at 37°C in humidified air with 5% CO2 in
phenol red-free DMEM/F12 medium (Life Technologies, Carlsbad, CA,
USA) supplemented with 1% heat inactivated FCS (Life Technologies),
2 mM GlutaMAX™-1 (Life Technologies) and 6 ng/ml insulin
(Sigma-Aldrich, St. Louis, MO, USA). In order to obtain growth,
which depends on conversion of androgen to estrogen via the
endogenous aromatase enzyme, MCF-7 cells were transferred to medium
with 10% newborn calf serum (NCS) (Life Technologies) and
10−7 M testosterone (Sigma-Aldrich) as described
previously (35). AI-resistant
cell lines were established from MCF-7 cells grown in medium with
10% NCS and 10−7 M testosterone. A culture of MCF-7
cells were treated with AI [10−6 M letrozole,
10−7 M anastrozole or 10−7 M exemetane
(Selleck Chemicals, Munich, Germany)] for one week, trypsinized and
seeded in serial dilutions in 24-well plates. Single colonies were
transferred to new wells and gradually expanded in medium with AI.
After ~2–3 months, the isolated colonies gave rise to AI-resistant
cell lines, which could be grown in AI-containing medium with a
weekly split ratio of ~1:25. The MCF-7 cell line was authenticated
in January 2014 by DNA profiling using short tandem repeat loci
performed by Leibniz-Institut DSMZ (Braunschweig, Germany) and
found to be matching the genetic profile reported for the MCF-7
cell line (DSMZ ACC 115).
Growth experiments
For dose-response growth experiments, MCF-7 cells
were seeded in 24-well plates (Nunc) in their standard growth
medium and after one day, they were switched from 1% FCS to 10%
NCS. On day 2, treatment was initiated and renewed on day 5. On day
7, cell number was determined by a crystal violet colorimetric
assay (38). One week prior to the
dose-response growth experiments, AI and testosterone were
withdrawn from the growth medium for resistant cell lines. The
AI-resistant cell lines were seeded in medium with 10% NCS,
experimental medium added on day 2, renewed on day 5 and cell
number determined on day 7 as described above. For combination of
AI and antiestrogen treatment, the AI-resistant cell lines were
seeded in their standard growth medium and treatment from days 2 to
7 with 10−6 M tamoxifen (Sigma-Aldrich) and
10−7 M fulvestrant (ICI 182.780; Tocris Bioscience,
Bristol, UK) was performed in standard growth medium with the
respective AI. All growth experiments were performed with four
sample replicates or more and repeated at least twice with similar
results.
Western blot analysis
Lysates (RIPA buffer; 100 nM NaCl, 20 mM Tris base,
1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 1 nM EDTA, pH
8.0) were prepared from early passages (5 and 6) of four cell lines
resistant to each of the three AIs; letrozole, anastrozole and
exemestane grown in their standard growth medium and from the
selected AI-resistant cell lines; LetR-1,
LetR-3, ExeR-1 and ExeR-3 grown in
standard growth medium in passages between 15–30. Lysates from
MCF-7 cells grown in standard growth medium with 1% FCS or grown
for 21 weeks with 10% NCS + 10−7 M testosterone were
used for comparison. For analysis of estrogen-regulated proteins,
lysates were prepared from MCF-7 cells and from AI-resistant cell
lines (withdrawn from AI and testosterone for one week) grown for
five days with 10% NCS and 10% NCS supplemented with estradiol
(Sigma-Aldrich), testosterone and testosterone in combination with
AIs. SDS-PAGE page and immunoblotting were performed as described
previously (39). Antibodies used
against the proteins were: β-actin (A5441) from Sigma-Aldrich);
Bcl-2 (M0887), EGFR (M7298), HER2 (A0485) and HER3 (M7297) from
Dako (Glostrup, Denmark); ERα (RM-9101), progesterone receptor
(PR-A and PR-B) (RM-9102) and Hsp70 (MS-482-PO) from Neomarkers
(Fremont, CA, USA) and HER4 (4795) from Cell Signaling Technology
(Danvers, MA, USA). Blots were washed four times with TBS/0.1%
Tween-20 followed by incubation for 1 h with species-specific
peroxidase-conjugated secondary antibodies (Dako). Detection was
done using ECLplus reagent (GE Healthcare) and a Fujifilm image
reader (LAS1000).
Quantitative RT-PCR
MCF-7 cells were grown with 10% NCS +
10−7 M testosterone for five days and AI-resistant cell
lines were grown in their standard medium. RNA was isolated using
PureLink Micro-to-Midi Total RNA Purification system (Life
Technologies) and reverse transcribed to cDNA by the High-Capacity
RNA-to-cDNA kit (Life Technologies). The real-time PCR analysis was
performed with Power SYBR® Green PCR Master Mix (Life
Technologies) using a Rotor-Gene 3000 (Corbett Life Science,
Sidney, Australia). All experiments were conducted in accordance to
the manufacturer’s recommendations. Results were calculated based
on a real-time RT-PCR relative quantification strategy and
presented as mean relative gene expression levels, compared to the
parental MCF-7 cells. Primers and programs for CYP19A1 were as
previously described by Díaz-Cruz et al (40).
Statistical analysis
Two-tailed t-test with Bonferroni adjusted p-values
for multiple group comparisons was used. The level of statistical
significance was set to p<0.05, and indicated by asterisks in
the figures.
Results
Testosterone stimulation of MCF-7
cells
To study the effect of AIs and acquired AI
resistance, a model system in which cell growth is stimulated by
estradiol produced via aromatase-mediated conversion of
testosterone is required. Newborn calf serum (NCS) contains low
amount of estrogenic activity and MCF-7 cells require estrogen
supplementation to grow continuously in 10% NCS (35). Both estradiol and testosterone
exerted dose-dependent growth stimulation of MCF-7 cells in medium
with 10% NCS (Fig. 1). Maximal
growth stimulation of 13-fold was obtained with estradiol
concentrations from 10−11 M (Fig. 1A), whereas maximal stimulation of
8-fold was seen with testosterone in concentrations of 0.1–1.0 μM
(Fig. 1B).
Establishment of AI-resistant cell lines
and determination of ER, PR, Bcl-2, HER receptors and CYP19A1
mRNA
The testosterone stimulation of MCF-7 cell growth
can be completely abrogated by addition of the third-generation
AIs, letrozole, anastrozole and exemestane (36), but after long-term treatment
colonies of cells grow out. We have selected four cell lines
resistant to each of the three AIs, letrozole, anastrozole and
exemestane, from isolated single colonies from cultures treated for
long-term (≥2 months) with 10−6 M letrozole,
10−7 M anastrozole and 10−7 M exemestane,
respectively (see Materials and methods). An initial analysis for
expression of ER and the ER-regulated proteins; progesterone
receptor (PR-A and PR-B) and Bcl-2 as well as the HER receptors,
was performed on the cells harvested after 2.5 months with the
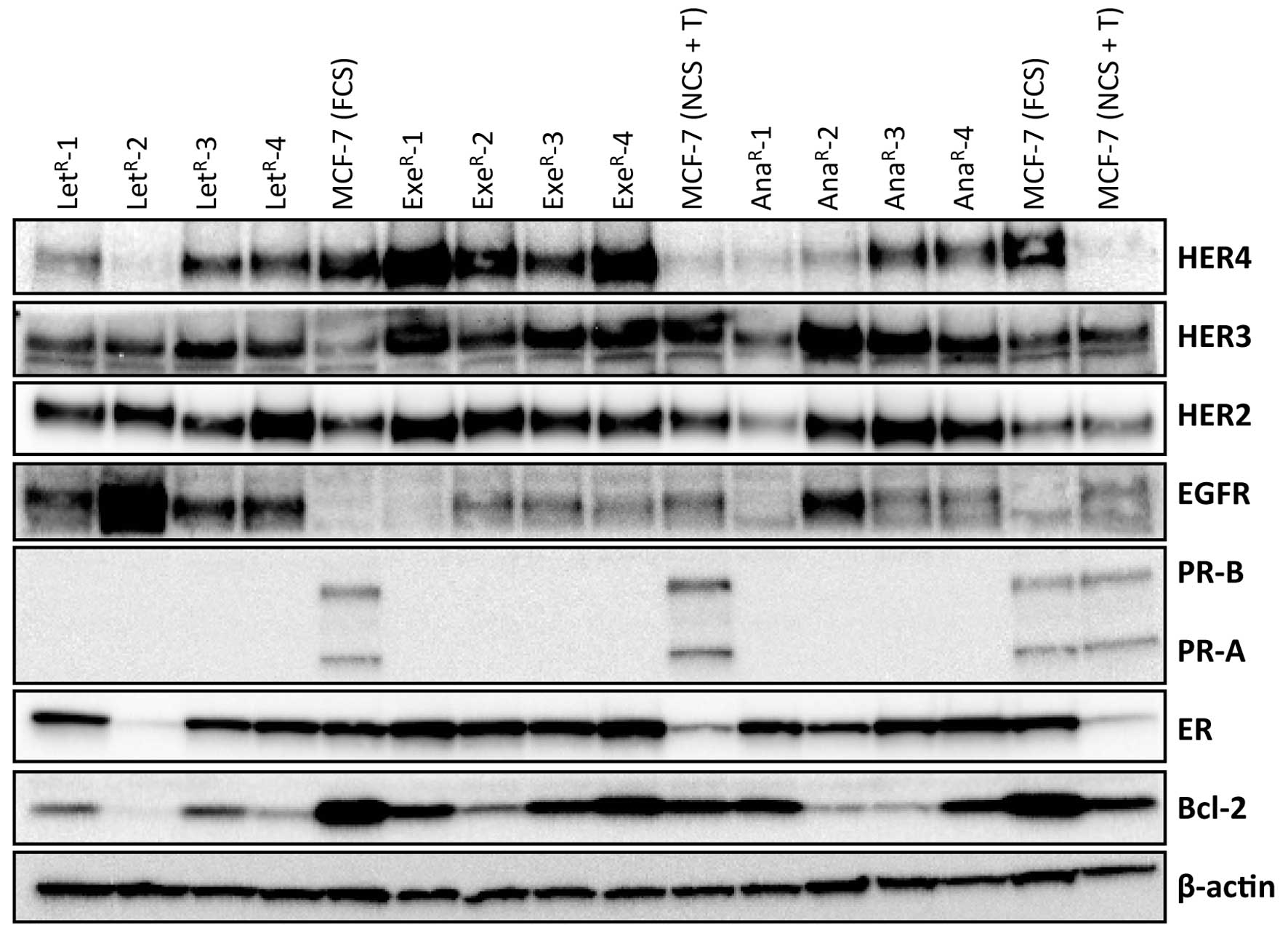
respective AI (Fig. 2). All but
one AI-resistant cell line maintained ER expression and the level
of ER was comparable or higher than in parental MCF-7 grown with 1%
FCS. MCF-7 cells grown with 10% NCS + 10−7 M
testosterone had very low level of ER (Fig. 3B). PR-B and PR-A were not
detectable in the resistant cell lines which were grown
continuously in medium with testosterone and AI (Fig. 2). Bcl-2 level was lower in
resistant cell lines than in MCF-7 cells grown under standard
conditions with 1% FCS. EGFR level was low in MCF-7 cells and also
in exemestane-resistant cell lines, whereas increased level of EGFR
was seen in all four letrozole-resistant cell lines and in one
anastrozole-resistant cell line. Noteworthy, the
letrozole-resistant cell line with highest EGFR expression had a
very low ER level. HER2 and HER3 were increased in most
AI-resistant cell lines compared with MCF-7. In contrast, HER4 was
reduced in letrozole- and anastrozole-resistant cell lines compared
with parental MCF-7 cells grown in 1% FCS, whereas HER4 was
increased in exemestane-resistant cell lines.
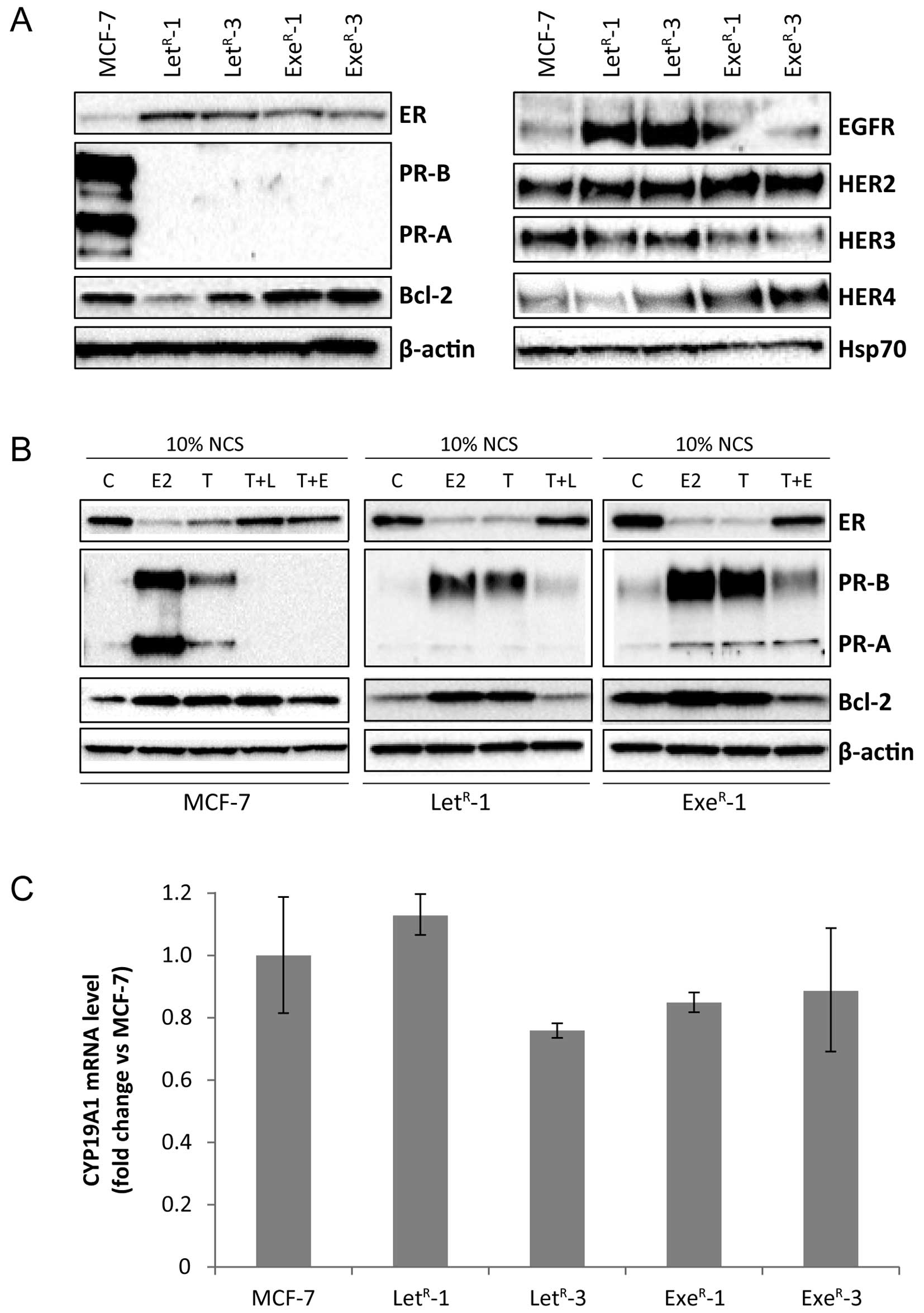 | Figure 3Expression of ER, PR, Bcl-2, HER
receptors and CYP19A1 mRNA in MCF-7 and AI-resistant cell lines.
(A) Western blot analysis of lysates from MCF-7 cells grown with
10% NCS + 10−7 M testosterone (MCF-7) and
LetR-1, LetR-3, ExeR-1 and
ExeR-3 grown in their standard growth medium with
10−6 M letrozole and 10−7 M exemestane,
respectively. β-actin and Hsp70 were used as loading controls. (B)
Western blot analysis of lysates from MCF-7, LetR-1 and
ExeR-1 cells grown for five days in 10% NCS (C) or 10%
NCS + 10−12 M estradiol (E2), 10% NCS + 10−7
M testosterone (T), 10% NCS + 10−7 M testosterone +
10−6 M letrozole (T + L), 10% NCS + 10−7 M
testosterone + 10−7 M exemestane (T+E).
LetR-1 and ExeR-1 cells were withdrawn from
testosterone and their respective AI one week before onset of
experiment. β-actin was used as loading control. (C) CYP19A1 mRNA
level in MCF-7 cells grown with 10% NCS + 10−7 M
testosterone for five days and AI-resistant cell lines grown in
their standard medium determined by quantitative RT-PCR. |
For further analyses, we selected two cell lines
resistant to the non-steroidal AI letrozole, LetR-1 and
LetR-3, and two cell lines resistant to the steroidal AI
exemestane, ExeR-1 and ExeR-3. Initially, we
tested the stability regarding expression of ER, PR, Bcl-2 and HER
receptors. Fig. 3A is a
representative experiment with determination of protein expression
in AI-resistant cells from passages in which they have obtained
stable growth rates in presence of the respective AI. The resistant
cell lines maintained ER expression and the level of ER was higher
in both the letrozole- and exemestane-resistant cell lines compared
with MCF-7 cells grown with 10% NCS + 10−7 M
testosterone. It should be mentioned that the ER content varies
with the growth conditions and that ER level was low in MCF-7 cells
grown with 10% NCS + testosterone as can be seen in Fig. 2. PR-A and PR-B were expressed at
high levels in MCF-7 cells grown with 10% NCS + testosterone and PR
continued to be undetectable in the AI-resistant cell lines grown
in medium with their respective AI, whereas Bcl-2 level varied
between the AI-resistant cell lines (Fig. 3A). EGFR level was significantly
higher in letrozole-resistant cell lines compared with MCF-7 and
exemestane-resistant cell lines. HER2 level appeared to be slightly
higher in resistant cells, whereas HER3 was expressed at level
comparable with MCF-7. As also found in the initial analysis
(Fig. 2), HER4 expression was
higher in exemestane-resistant cell lines compared with MCF-7 and
letrozole-resistant cell lines (Fig.
3A). In order to explore whether ER was functional, AI was
withdrawn from the AI-resistant cell lines for one week and the
cells were treated with estradiol, testosterone or testosterone in
combination with AI for five days. Estradiol and testosterone
induced expression of PR-A and PR-B as well as Bcl-2 in MCF-7 cells
and the two AI-resistant cell lines, LetR-1 and
ExeR-1 (Fig. 3B). The
ER level was reduced in MCF-7, LetR-1 and
ExeR-1 grown with estradiol and with testosterone as
expected due to reduced stability of estradiol-bound ER. PR-A and
PR-B were hardly detectable in MCF-7 cells grown with 10% NCS and
when treated with exemestane and letrozole. PR-A and PR-B were
expressed at low level in AI-resistant cells grown with 10% NCS and
also expressed at low level in 10% NCS + testosterone and the
respective AI. Bcl-2 was expressed at comparable level in control
cells with 10% NCS and in cells grown with testosterone and AI
(Fig. 3B). These data demonstrate
that ER is functional in the AI-resistant cell lines and that the
AIs inhibit the conversion of testosterone to estradiol.
Aromatase expression is highly regulated at the
transcriptional level (12), and
to investigate whether the AI-resistant cell lines displayed
aberrant aromatase expression, we measured CYP19A1 mRNA
expression using real-time RT-PCR. The analysis revealed similar
CYP19A1 mRNA level in MCF-7, LetR-1,
LetR-3, ExeR-1 and ExeR-3 cells
(Fig. 3C).
AI-resistant cell lines display low
degree of androgen responsiveness and cross-resistance between
letrozole and exemestane
MCF-7 cells grown with 10% NCS displayed ~6–10-fold
stimulation with 10−7 M testosterone, and letrozole and
exemestane exerted a dose-dependent growth inhibition with maximum
inhibition to the basal level with 10−7 M and
10−6 M AI (Fig. 4). The
growth rate of the AI-resistant cell lines increased gradually
during the first 15 weeks in medium with AI and then a constant
cell population doubling time of ~30 h was achieved (corresponding
to a weekly split ratio ~1:25). In comparison, the weekly split
ratio for MCF-7 cells grown with 1% FCS is 1:40 (data not shown).
To test whether the AI-resistant cell lines maintain the ability to
be androgen-stimulated, testosterone and AI were withdrawn for one
week from the AI-resistant cell lines before onset of the
experiments shown in Fig. 4.
Testosterone increased growth of all AI-resistant cell lines, mean
stimulation was 2.7-, 2.5-, 1.4- and 3.3-fold for
LetR-1, LetR-3, ExeR-1 and
ExeR-3, respectively. A dose-dependent inhibition of the
testosterone-stimulated cell growth was seen for AI-resistant cell
lines treated with letrozole (Fig. 4A
and D) and exemestane (Fig. 4B and
C). AI treatment of the AI-resistant cell lines abrogated the
testosterone-induced cell growth, but did not exert growth arrest
as in MCF-7 cells. Noteworthy, the cell number in AI-resistant cell
lines grown with 10% NCS was significantly higher than for MCF-7
cells and in contrast to MCF-7 cells, the AI-resistant cell lines
could be propagated continuously in medium with 10% NCS (data not
shown).
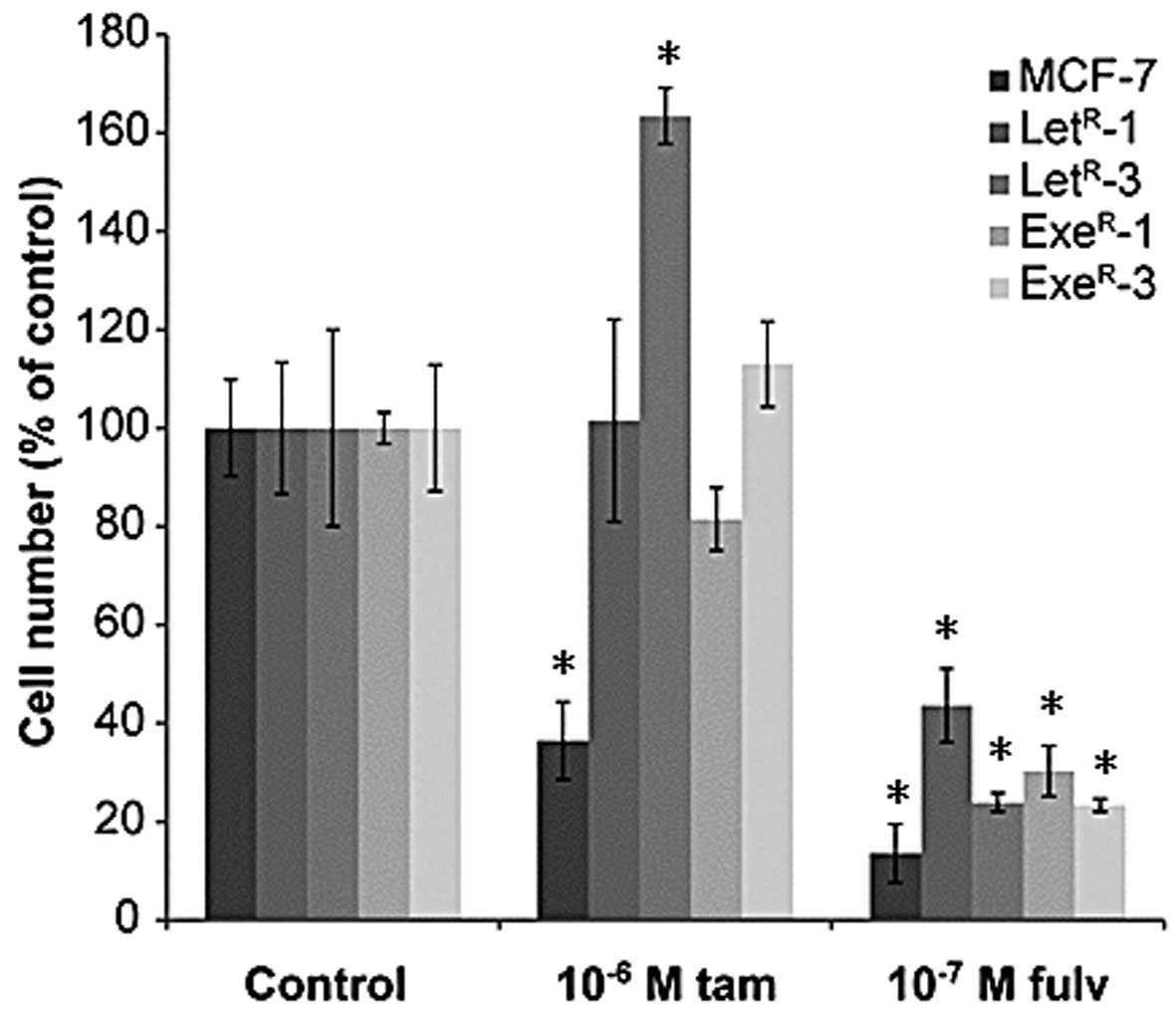
Fulvestrant, but not tamoxifen, exerts
complete growth arrest of AI-resistant cell lines
Tamoxifen is a weak estrogen antagonist and in
medium with 10% NCS + 10−7 M testosterone, a
dose-dependent growth inhibition of MCF-7 cells was seen. However,
growth was not completely arrested after five days treatment with
10−6 M tamoxifen (Fig. 5A
and B) as it is normally observed with MCF-7 cells grown in
their standard medium with 1% FCS (41). Fulvestrant exerted complete growth
arrest of MCF-7 cells at concentrations from 10−9 M
(Fig. 5C and D). Dose-response
growth experiments with antiestrogens were performed with
AI-resistant cell lines withdrawn from AI treatment for one week
(Fig. 5) and with cells grown with
AI (Fig. 6). In AI-resistant cell
lines grown with 10% NCS + 10−7 M testosterone and
withdrawn from AI treatment for one week, tamoxifen induced a
dose-dependent growth inhibition down to the level seen in 10% NCS
(Fig. 5A and B). Fulvestrant also
inhibited growth of AI-resistant cell lines in a dose-dependent
manner, but in contrast to tamoxifen, fulvestrant reduced growth of
the AI-resistant cell lines to below the level in NCS medium and
down to the level seen in MCF-7 cells treated with 10−7
M fulvestrant (Fig. 5C and D).
Tamoxifen treatment of AI-resistant cell lines grown in their
standard growth medium with their respective AI had no effect or a
stimulatory effect, whereas fulvestrant suppressed growth totally,
as seen in MCF-7 cells (Fig. 6).
To determine whether AI-resistant cell lines were completely growth
arrested with fulvestrant, cultures with LetR-1 and
ExeR-1 cells were treated with 10−7 M
fulvestrant for one week, split and treated for further one week
with fulvestrant. After a total of 14 days of treatment with
10−7 M fulvestrant, no viable LetR-1 or
ExeR-1 cells could be detected.
Discussion
Adjuvant treatment with AIs postpones or abrogates
development of advanced disease, and patients with advanced disease
benefit from treatment. However, most patients with advanced
disease will eventually progress, and some patients receiving
adjuvant AIs progress during therapy. To find new treatment options
and new biomarkers for resistant tumors, it is important to gain
knowledge of the molecular mechanisms involved in resistance to
AIs. In this report, we describe a new series of breast cancer cell
lines with acquired resistance to the clinically relevant AIs
letrozole, exemestane and anastrozole, which may be useful models
for studies of the resistance mechanisms. The resistant cell lines
were developed from MCF-7 cells grown under conditions at which the
extracellular estrogen supply via serum was low and growth
stimulation was mediated via conversion of testosterone to
estradiol by the endogenous aromatase enzyme. Long-term treatment
of MCF-7 cells with letrozole, exemestane or anastrozole appeared
to inhibit growth completely, but some cells survived treatment and
gave rise to outgrowth of colonies, which could be isolated and
propagated continuously in presence of the respective AI.
Initially, the growth rate was slow, but a gradual increase in
growth rate was observed during the first 15 weeks of treatment
until constant growth rate was achieved. We believe that the
surviving colonies arise from cells with inherent resistance,
whereas acquired changes may be responsible for the observed
increase in growth rate during long-term propagation.
A switch from ER-driven growth to involvement of HER
receptor-driven growth has been described for
antiestrogen-resistant breast cancer cells (41–44),
and model systems have indicated that resistance to AIs may involve
cross-talk between ER and growth factor signaling pathways, and
ligand-independent activation of ER (27, 45–47).
The initial analysis of expression of ER, estrogen-regulated
proteins and HER receptors revealed that ER expression was severely
reduced in only one (8%) of twelve AI-resistant cell lines, and a
similar low fraction of patients were found to be ER-negative at
relapse or progression after AI treatment (11). In general, HER receptor level was
higher in AI-resistant cell lines than in parental cells, in
particular EGFR was increased in the letrozole-resistant cell lines
and HER4 was increased in exemestane-resistant cell lines, pointing
to involvement of the HER receptor system in AI resistance. Two
letrozole and two exemestane resistant cell lines were selected for
further studies and the expression pattern of ER, ER-regulated
proteins and HER receptors did not change significantly during
longer time propagation of the cell lines. ER was functional with
respect to stimulation of expression of PR and Bcl-2, and ER
protein expression was reduced in both MCF-7 cells and AI-resistant
cell lines grown with estradiol and testosterone. Downregulation of
ER expression in MCF-7 cells grown with estradiol and with
testosterone has been observed before (35,48)
and may be explained by destabilization of the ER protein upon
binding to estradiol (49). The
testosterone-induced expression of the estrogen-regulated proteins
PR-A, PR-B and Bcl-2 in the AI-resistant cell lines verified that
AI-resistant cells were able to convert testosterone to estradiol
via the aromatase enzyme. AI treatment significantly reduced PR
expression in AI-resistant cell lines, but PR expression was not
totally blocked as in MCF-7 cells, suggesting that the AIs may not
completely block the aromatase activity or that PR may be induced
by ligand-independent activation of ER. A similar low PR expression
was found in AI-resistant cell lines grown with NCS alone,
supporting a ligand-independent activation of ER. Upregulation of
aroma-tase gene expression could result in insufficient inhibition
of the aromatase enzyme in the AI-resistant cells and explain the
transcriptional activity of ER. However, the observed similar level
of CYP19A1 mRNA in parental MCF-7 cells and in the four
tested AI-resistant cell lines does not support this mechanism of
resistance.
Cell number in both the letrozole- and the
exemestane-resistant cell lines increased 20–25 times during one
week in the standard growth medium with the respective AI, and all
resistant cell lines displayed a low degree of responsiveness to
growth stimulation with testosterone when AI and testosterone were
withdrawn for one week. The ability of the AI-resistant cell lines
to grow continuously in presence of AI and also in medium with 10%
NCS demonstrates that the cell lines have acquired ability to grow
without estrogen stimulation, whereas the observed growth response
to testosterone indicates that AI-resistant cell lines have
progressed from estrogen-dependent to estrogen-responsive cell
growth. Letrozole, exemestane and tamoxifen could abrogate the
testosterone-mediated growth stimulation, but could not reduce
growth to below the level in standard growth medium with AI. In
contrast, fulvestrant inhibited growth of the AI-resistant cell
lines to below the level in 10% NCS and down to the level of MCF-7
cells which were completely growth arrested by treatment with
fulvestrant. To investigate whether fulvestrant completely blocked
growth of AI-resistant cell lines, AI-resistant cells treated for
one week with fulvestrant were trypsinized and seeded in new
culture flasks, but no growth was observed, demonstrating that
fulvestrant exerted complete growth arrest of AI-resistant cell
lines and supporting that growth of the AI-resistant cell lines
depends primarily on ER. The severe growth inhibition seen with
fulvestrant treatment of AI-resistant cell lines in their standard
growth medium with AI also supports that ER drives growth of the
AI-resistant cell lines. The importance of ER-mediated growth and
effect of fulvestrant treatment have also been found in
exemestane-resistant cell lines derived from aromatase
overexpressing MCF-7 cells (34),
in MCF-7 breast tumor xenograft with letrozole-resistant aromatase
overexpressing cells (50), and in
the LTED model system (26).
Clinical benefit rates of 32% were found in two phase III studies
with fulvestrant treatment of patients with advanced disease after
progression on a non-steroidal AI (51,52),
supporting that ER may also be an important driver of metastatic
breast cancer cells in patients relapsing from AI therapy.
Letrozole and exemestane abrogated the
testosterone-mediated growth of both letrozole- and
exemestane-resistant cell lines but could not arrest cell growth,
demonstrating cross-resistance between the non-steroidal AI
letrozole and the steroidal AI exemestane. This is in contrast to
clinical studies in which sequential treatment from a non-steroidal
to a steroidal AI or vice versa has resulted in clinical
benefit for 30–50% of the patients (53). It should be mentioned that the
studies included low number of patients (mean 54) and objective
response rates were low, average in 8 studies was 11%. Furthermore,
mechanisms explaining lack of cross-resistance have not been
documented (53), but lack of
effective uptake in tumor tissue of particular compounds has been
suggested as a possibility (54)
and patients may metabolize the compounds differently. Such
mechanisms will not be disclosed in our in vitro cell
culture model.
Tamoxifen was able to abrogate the
testosterone-mediated growth stimulation of the AI-resistant cell
lines but could not reduce growth further, demonstrating that
tamoxifen is able to inhibit the estrogen-mediated but not the
presumed ligand-independent activation of ER. In line with this, we
have recently found that ER is the main driver of growth of
tamoxifen-resistant cell lines (41). The multi-targeting kinase
inhibitors sorafenib and nilotinib could restore the sensitivity of
tamoxifen-resistant cell lines to tamoxifen, indicating that growth
of tamoxifen-resistant cells occurs via ligand-independent
activation of the ER (55). More
direct evidence of the inability of tamoxifen to inhibit
ligand-independently activated ER was obtained in a study showing
that Aurora kinase A upon phosphorylation of ER renders breast
cancer cells less sensitive to treatment with tamoxifen (56). In agreement with this, we have
shown that Aurora kinase A plays a major role for growth of
tamoxifen-resistant breast cancer cell lines, and that inhibition
of Aurora kinase A restores the sensitivity to tamoxifen treatment
(57). Whether Aurora kinase A
plays a major role by ligand-independent activation of ER in AI
resistance is under investigation. It should be mentioned that
about one third of patients recurring from tamoxifen treatment
benefit from treatment with AI (58,59),
showing that different mechanisms for tamoxifen and AI resistance
also exist.
In conclusion, this report presents a large series
of AI-resistant breast cancer cell lines derived from MCF-7 cells,
which can be used as models to unravel the molecular mechanisms for
growth of breast cancer in patients who recur after an initial
response to AI therapy. We found that the majority of AI-resistant
cell lines maintained ER expression and function, whereas HER
receptor expression was increased. The complete growth inhibition
of the AI-resistant cell lines by treatment with the ER down
modulator fulvestrant demonstrates that ER is the main driver of
growth of AI-resistant cell lines, supporting the potential of
fulvestrant therapy for AI-resistant breast cancer.
Acknowledgements
We thank Birgit Reiter for excellent technical
assistance. This study was supported by the Danish Cancer Society,
A Race Against Breast Cancer, Danish Cancer Research Foundation,
Astrid Thaysen’s Grant, Wedell-Wedellsborg’s Foundation, and Harboe
Foundation.
References
|
1
|
Early Breast Cancer Trialists’
Collaborative Group. Davies C, Godwin J, et al: Relevance of breast
cancer hormone receptors and other factors to the efficacy of
adjuvant tamoxifen: patient-level meta-analysis of randomised
trials. Lancet. 378:771–784. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mouridsen H, Gershanovich M, Sun Y, et al:
Superior efficacy of letrozole versus tamoxifen as first-line
therapy for postmenopausal women with advanced breast cancer:
results of a phase III study of the International Letrozole Breast
Cancer Group. J Clin Oncol. 19:2596–2606. 2001.PubMed/NCBI
|
|
3
|
Nabholtz JM, Buzdar A, Pollak M, et al:
Anastrozole is superior to tamoxifen as first-line therapy for
advanced breast cancer in postmenopausal women: results of a North
American multicenter randomized trial. Arimidex Study Group J Clin
Oncol. 18:3758–3767. 2000.
|
|
4
|
Paridaens RJ, Dirix LY, Beex LV, et al:
Phase III study comparing exemestane with tamoxifen as first-line
hormonal treatment of metastatic breast cancer in postmenopausal
women: the European Organisation for Research and Treatment of
Cancer Breast Cancer Cooperative Group. J Clin Oncol. 26:4883–4890.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dowsett M, Cuzick J, Ingle J, et al:
Meta-analysis of breast cancer outcomes in adjuvant trials of
aromatase inhibitors versus tamoxifen. J Clin Oncol. 28:509–518.
2010. View Article : Google Scholar
|
|
6
|
Goldhirsch A, Wood WC, Coates AS, et al:
Strategies for subtypes - dealing with the diversity of breast
cancer: highlights of the St. Gallen International Expert Consensus
on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol.
22:1736–1747. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schwartzberg LS, Franco SX, Florance A,
O’Rourke L, Maltzman J and Johnston S: Lapatinib plus letrozole as
first-line therapy for HER-2+ hormone receptor-positive
metastatic breast cancer. Oncologist. 15:122–129. 2010. View Article : Google Scholar
|
|
8
|
Baselga J, Campone M, Piccart M, et al:
Everolimus in post-menopausal hormone-receptor-positive advanced
breast cancer. N Engl J Med. 366:520–529. 2012. View Article : Google Scholar
|
|
9
|
Burris HA III: Overcoming acquired
resistance to anticancer therapy: focus on the PI3K/AKT/mTOR
pathway. Cancer Chemother Pharmacol. 71:829–842. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miller WR: Aromatase inhibitors:
prediction of response and nature of resistance. Expert Opin
Pharmacother. 11:1873–1887. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arnedos M, Drury S, Afentakis M, et al:
Biomarker changes associated with the development of resistance to
aromatase inhibitors (AIs) in estrogen receptor-positive breast
cancer. Ann Oncol. 25:605–610. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bulun SE, Sebastian S, Takayama K, Suzuki
T, Sasano H and Shozu M: The human CYP19 (aromatase P450) gene:
update on physiologic roles and genomic organization of promoters.
J Steroid Biochem Mol Biol. 86:219–224. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lykkesfeldt AE, Henriksen KL, Rasmussen
BB, et al: In situ aromatase expression in primary tumor is
associated with estrogen receptor expression but is not predictive
of response to endocrine therapy in advanced breast cancer. BMC
Cancer. 9:1852009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Haynes BP, Straume AH, Geisler J, et al:
Intratumoral estrogen disposition in breast cancer. Clin Cancer
Res. 16:1790–1801. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lonning PE, Haynes BP, Straume AH, et al:
Recent data on intratumor estrogens in breast cancer. Steroids.
76:786–791. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Briand P and Lykkesfeldt AE: Long-term
cultivation of a human breast cancer cell line, MCF-7, in a
chemically defined medium. Effect of estradiol. Anticancer Res.
6:85–90. 1986.PubMed/NCBI
|
|
17
|
Masamura S, Santner SJ, Heitjan DF and
Santen RJ: Estrogen deprivation causes estradiol hypersensitivity
in human breast cancer cells. J Clin Endocrinol Metab.
80:2918–2925. 1995.PubMed/NCBI
|
|
18
|
Chan CM, Martin LA, Johnston SR, Ali S and
Dowsett M: Molecular changes associated with the acquisition of
oestrogen hypersensitivity in MCF-7 breast cancer cells on
long-term oestrogen deprivation. J Steroid Biochem Mol Biol.
81:333–341. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Coutts AS and Murphy LC: Elevated
mitogen-activated protein kinase activity in estrogen-nonresponsive
human breast cancer cells. Cancer Res. 58:4071–4074.
1998.PubMed/NCBI
|
|
20
|
Pink JJ, Jiang SY, Fritsch M and Jordan
VC: An estrogen-independent MCF-7 breast cancer cell line which
contains a novel 80-kilodalton estrogen receptor-related protein.
Cancer Res. 55:2583–2590. 1995.PubMed/NCBI
|
|
21
|
Martin LA, Farmer I, Johnston SR, Ali S,
Marshall C and Dowsett M: Enhanced estrogen receptor (ER) alpha,
ERBB2, and MAPK signal transduction pathways operate during the
adaptation of MCF-7 cells to long term estrogen deprivation. J Biol
Chem. 278:30458–30468. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jensen J, Kitlen JW, Briand P, Labrie F
and Lykkesfeldt AE: Effect of antiestrogens and aromatase inhibitor
on basal growth of the human breast cancer cell line MCF-7 in
serum-free medium. J Steroid Biochem Mol Biol. 84:469–478. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Santen RJ, Song RX, Zhang Z, et al:
Long-term estradiol deprivation in breast cancer cells up-regulates
growth factor signaling and enhances estrogen sensitivity. Endocr
Relat Cancer. 12(Suppl 1): S61–S73. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Staka CM, Nicholson RI and Gee JM:
Acquired resistance to oestrogen deprivation: role for growth
factor signalling kinases/oestrogen receptor cross-talk revealed in
new MCF-7X model. Endocr Relat Cancer. 12(Suppl 1): S85–S97. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sabnis G and Brodie A: Adaptive changes
results in activation of alternate signaling pathways and
resistance to aromatase inhibitor resistance. Mol Cell Endocrinol.
340:142–147. 2011. View Article : Google Scholar
|
|
26
|
Martin LA, Ghazoui Z, Weigel MT, et al: An
in vitro model showing adaptation to long-term oestrogen
deprivation highlights the clinical potential for targeting kinase
pathways in combination with aromatase inhibition. Steroids.
76:772–776. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miller TW, Balko JM, Fox EM, et al:
ERalpha-dependent E2F transcription can mediate resistance to
estrogen deprivation in human breast cancer. Cancer Discov.
1:338–351. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou DJ, Pompon D and Chen SA: Stable
expression of human aromatase complementary DNA in mammalian cells:
a useful system for aromatase inhibitor screening. Cancer Res.
50:6949–6954. 1990.PubMed/NCBI
|
|
29
|
Sun XZ, Zhou D and Chen S: Autocrine and
paracrine actions of breast tumor aromatase. A three-dimensional
cell culture study involving aromatase transfected MCF-7 and T-47D
cells. J Steroid Biochem Mol Biol. 63:29–36. 1997. View Article : Google Scholar
|
|
30
|
Macaulay VM, Nicholls JE, Gledhill J,
Rowlands MG, Dowsett M and Ashworth A: Biological effects of stable
overexpression of aromatase in human hormone-dependent breast
cancer cells. Br J Cancer. 69:77–83. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Masri S, Phung S, Wang X, et al:
Genome-wide analysis of aromatase inhibitor-resistant,
tamoxifen-resistant, and long-term estrogen-deprived cells reveals
a role for estrogen receptor. Cancer Res. 68:4910–4918. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brodie A, Jelovac D, Sabnis G, Long B,
Macedo L and Goloubeva O: Model systems: mechanisms involved in the
loss of sensitivity to letrozole. J Steroid Biochem Mol Biol.
95:41–48. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Masri S, Phung S, Wang X and Chen SA:
Molecular characterization of aromatase inhibitor-resistant,
tamoxifen-resistant and LTEDaro cell lines. J Steroid Biochem Mol
Biol. 118:277–282. 2010. View Article : Google Scholar :
|
|
34
|
Wang X, Masri S, Phung S and Chen SU: The
role of amphi-regulin in exemestane-resistant breast cancer cells:
evidence of an autocrine loop. Cancer Res. 68:2259–2265. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sonne-Hansen K and Lykkesfeldt AE:
Endogenous aromatization of testosterone results in growth
stimulation of the human MCF-7 breast cancer cell line. J Steroid
Biochem Mol Biol. 93:25–34. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lundqvist J, Hansen SK and Lykkesfeldt AE:
Vitamin D analog EB1089 inhibits aromatase expression by
dissociation of comodulator WSTF from the CYP19A1 promoter-a new
regulatory pathway for aromatase. Biochim Biophys Acta. 1833.40–47.
2013.
|
|
37
|
Briand P and Lykkesfeldt AE: Effect of
estrogen and anti-estrogen on the human breast cancer cell-line
MCF-7 adapted to growth at low serum concentration. Cancer Res.
44:1114–1119. 1984.PubMed/NCBI
|
|
38
|
Lundholt BK, Briand P and Lykkesfeldt AE:
Growth inhibition and growth stimulation by estradiol of estrogen
receptor transfected human breast epithelial cell lines involve
different pathways. Breast Cancer Res Treat. 67:199–214. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Larsen MS, Yde CW, Christensen IJ and
Lykkesfeldt AE: Carboplatin treatment of antiestrogen-resistant
breast cancer cells. Int J Oncol. 41:1863–1870. 2012.PubMed/NCBI
|
|
40
|
Diaz-Cruz ES, Shapiro CL and Brueggemeier
RW: Cyclo-oxygenase inhibitors suppress aromatase expression and
activity in breast cancer cells. J Clin Endocrinol Metab.
90:2563–2570. 2005. View Article : Google Scholar
|
|
41
|
Thrane S, Lykkesfeldt AE, Larsen MS,
Sorensen BS and Yde CW: Estrogen receptor alpha is the major
driving factor for growth in tamoxifen-resistant breast cancer and
supported by HER/ERK signaling. Breast Cancer Res Treat. 139:71–80.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Frogne T, Benjaminsen RV, Sonne-Hansen K,
et al: Activation of ErbB3, EGFR and Erk is essential for growth of
human breast cancer cell lines with acquired resistance to
fulvestrant. Breast Cancer Res Treat. 114:263–275. 2009. View Article : Google Scholar :
|
|
43
|
Nicholson RI, Hutcheson IR, Jones HE, et
al: Growth factor signalling in endocrine and anti-growth factor
resistant breast cancer. Rev Endocr Metab Disord. 8:241–253. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Morrison G, Fu X, Shea M, et al:
Therapeutic potential of the dual EGFR/HER2 inhibitor AZD8931 in
circumventing endocrine resistance. Breast Cancer Res Treat.
144:263–272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Weigel MT GZ, Dunbier A, Pancholi S,
Dowsett M and Martin LA: Preclinical and clinical studies of
estrogen deprivation support the PDGF/Abl pathway as a novel
therapeutic target for overcoming endocrine resistance in breast
cancer. Breast Cancer Res. 14:R782012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Brodie A, Macedo L and Sabnis G: Aromatase
resistance mechanisms in model systems in vivo. J Steroid Biochem
Mol Biol. 118:283–287. 2010. View Article : Google Scholar
|
|
47
|
Liu S, Meng X, Chen H, et al: Targeting
tyrosine-kinases and estrogen receptor abrogates resistance to
endocrine therapy in breast cancer. Oncotarget. 5:9049–9064.
2014.PubMed/NCBI
|
|
48
|
Jensen BL, Skouv J, Lundholt BK and
Lykkesfeldt AE: Differential regulation of specific genes in MCF-7
and the ICI 182780-resistant cell line MCF-7/182(R)-6. Br J Cancer.
79:386–392. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dauvois S, Danielian PS, White R and
Parker MG: Antiestrogen ICI 164,384 reduces cellular estrogen
receptor content by increasing its turnover. Proc Natl Acad Sci
USA. 89:4037–4041. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Long BJ, Jelovac D, Thiantanawat A and
Brodie AM: The effect of second-line antiestrogen therapy on breast
tumor growth after first-line treatment with the aromatase
inhibitor letrozole: long-term studies using the intratumoral
aromatase postmenopausal breast cancer model. Clin Cancer Res.
8:2378–2388. 2002.PubMed/NCBI
|
|
51
|
Chia S, Gradishar W, Mauriac L, et al:
Double-blind, randomized placebo controlled trial of fulvestrant
compared with exemestane after prior nonsteroidal aromatase
inhibitor therapy in post-menopausal women with hormone
receptor-positive, advanced breast cancer: results from EFECT. J
Clin Oncol. 26:1664–1670. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Johnston SR, Kilburn LS, Ellis P, et al:
Fulvestrant plus anastrozole or placebo versus exemestane alone
after progression on non-steroidal aromatase inhibitors in
postmenopausal patients with hormone-receptor-positive locally
advanced or metastatic breast cancer (SoFEA): a composite,
multicentre, phase 3 randomised trial. Lancet Oncol. 14:989–998.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Miller WR and Larionov AA: Understanding
the mechanisms of aromatase inhibitor resistance. Breast Cancer
Res. 14:2012012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lonning PE: Lack of complete
cross-resistance between different aromatase inhibitors; a real
finding in search for an explanation? Eur J Cancer. 45:527–535.
2009. View Article : Google Scholar
|
|
55
|
Pedersen AM, Thrane S, Lykkesfeldt AE and
Yde CW: Sorafenib and nilotinib resensitize tamoxifen resistant
breast cancer cells to tamoxifen treatment via estrogen receptor
alpha. Int J Oncol. 45:2167–2175. 2014.PubMed/NCBI
|
|
56
|
Zheng XQ, Guo JP, Yang H, et al: Aurora-A
is a determinant of tamoxifen sensitivity through phosphorylation
of ERalpha in breast cancer. Oncogene. 33:4985–4996. 2014.
View Article : Google Scholar
|
|
57
|
Thrane S, Pedersen AM, Thomsen MB, et al:
A kinase inhibitor screen identifies Mcl-1 and Aurora kinase A as
novel treatment targets in antiestrogen-resistant breast cancer
cells. Oncogene. Nov 3–2014.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Buzdar AU, Jonat W, Howell A, et al:
Anastrozole versus megestrol acetate in the treatment of
postmenopausal women with advanced breast carcinoma: results of a
survival update based on a combined analysis of data from two
mature phase III trials. Arimidex Study Group Cancer. 83:1142–1152.
1998.
|
|
59
|
Dombernowsky P, Smith I, Falkson G, et al:
Letrozole, a new oral aromatase inhibitor for advanced breast
cancer: double-blind randomized trial showing a dose effect and
improved efficacy and tolerability compared with megestrol acetate.
J Clin Oncol. 16:453–461. 1998.PubMed/NCBI
|