Introduction
Colorectal cancer (CRC) is one of the most common
malignant tumors of the digestive tract, and the third most
malignant tumor in 2013 (1).
According to the statistics, the incidence of annual new cases
worldwide reached one million and nearly half of the deaths, and
the occurrence of CRC was reported to occur in a dominantly
inherited pattern (2). The
traditional treatment of CRC is generally drugs, surgery and
chemotherapy, but the effects of these methods remain
unsatisfactory. By contrast, the progress of molecular biology has
explained that several genes are involved in the carcinogenesis and
development of CRC. Hence, efforts should be focused on taking
advantage of the genomic imprinting systems to targeted therapy for
cancers mediated by oncolytic adenovirus vector.
Insulin like growth factor 2 (IGF2) is
located on chromosome 11p15.5 and expressed predominantly from the
paternal allele. IGF2 gene is closely linked to the
H19 gene, both IGF2 and H19 reciprocally
regulated imprinted genes and shared enhancers, cis-acting
regulatory elements such as the imprinting control region (ICR)
(3). Most imprinted genes form
clusters or imprinting domains. The ICRs are similar to
differentially methylated regions (DMRs). The IGF2 imprinted
gene has a momentous function in cell growth, proliferation,
differentiation, transformation, apoptosis and the growth and
development of embryos, placental formation and metabolism
(4) and regulated by enhancers,
DMRs, promoter and the transcriptional regulator CCCTC-binding
factor (CTCF) (5). Importantly, we
successfully came into a new stage to use targeted therapy for
malignant tumor based on the loss of IGF2 system (6).
One of the most critical issues of gene therapy is
choosing an effective vehicle to deliver therapeutic genes safely
and efficiently into target cells. Early region 1A (E1A) is
the first viral gene expressed after human adenovirus (HAdV)
infection (7). E1A can
influence the cell cycle and prevent apoptosis, making sure viral
replication effectively. The adenovirus as a vector for gene
therapy has been rapidly developed because of the simple structure,
wide host range, high infection rate, easy cultivation and
purification (8). Ad5 is commonly
used as a gene transfer vector and oncolytic virotherapy using
adenoviruses has potential value for therapeutic benefits in
malignant mesothelioma. As oncolytic adenovirus particularly
replicates and proliferates in tumor cells, the antioncogenes may
have an obvious increase in replication, and its expression level
may improve hundreds of times to achieve the purpose of killing the
tumor cells. The recombinant adenovirus vectors were firstly used
as gene therapy in 1985 (9,10).
Now, the recombinant adenoviruses provide a common system for both
gene expression studies and therapeutic applications (8).
In the aforementioned studies, the DT-A gene,
which was constructed by a recombinant replication adenovirus
carrying the IGF2 imprinting system, was specially expressed
in the tumor cells (6). In the
present study, we further evaluated the efficacy of gene therapy
for CRC by constructing the conditional replication-competent
adenovirus to provide a novel therapeutic strategy.
Materials and methods
Ethics statement and nude mice
The present study was carried out in strict
accordance with the Guide for the Care and Use of Laboratory
Animals of the US National Institutes of Health, and the study
protocol was approved by the Committee on the Ethics of Animal
Experiments of Nanjing Medical University (SYXK2009-0015). All
surgeries were performed under sodium pentobarbital anesthesia, and
suffering was minimized as much as possible. Female athymic nude
mice at 4 weeks old were obtained from the Experimental Animal
Center of University of Yangzhou, Yangzhou, China. The animals were
housed in SPF-free facilities with 12-h light-dark cycles and
standard pellet feed and water ad libitum.
Cell lines and culture conditions
Human embryonic kidney 293 cells (HEK293) were
obtained from the American Type Culture Collection (ATTCC,
Manassas, VA, USA). Human colorectal carcinoma cell lines (HCT-8,
HT-29 and SW480) and human gastric epithelial cells (GES-1) were
purchased by the Shanghai Cell Collection, Chinese Academy of
Sciences (Shanghai, China). All the cell lines except SW480 were
cultivated in Dulbecco’s modified Eagle’s medium (DMEM; Gibco-BRL,
Grand Island, NY, USA) with 10% fetal bovine serum (FBS; Gibco) and
SW480 cell line was maintained in RPMI-1640 (Invitrogen, Carlsbad,
CA, USA) with 10% FBS. All the cells were incubated under
humidified conditions of 95% air and 5% CO2 at 37°C.
Plasmid construction and adenovirus
packaging
In the present study, the original adenovirus
shuttle plasmid (pDC-312) was used. The mouse H19 enhancer
exon 1 (258 bp) and exon 2 (360 bp) and DMR exon 1–2 (429 bp), exon
3 (207 bp) and exon 4 (156 bp) were amplified by PCR from the mouse
genomic DNA, respectively, and then the two fragments were linked
to a single fragment by PCR. The DMD was cloned downstream of the
enhancer by the restriction endonuclease EcoRI and
NheI. The mouse H19 promoter (302 bp) was amplified
by PCR from mouse genomic DNA with the primers shown in Table I. We used restriction endonuclease
SalI/HindIII to clone downstream of the enhancer-DMD.
The human adenovirus E1A segment (1013 bp) was amplified by PCR
from a TOPK plasmid, which was benevolently provided by Dr Ji-Fan
Hu (Stanford University Medical School, Stanford, CA, USA) and the
primers are indicated in Table I.
The enhanced green fluorescent protein (EGFP) reporter gene
from the pEGFP-C1 vector (Clontech Laboratories Inc., Mountain
View, CA, USA) and the E1A gene were inserted downstream of
H19 promoter by using restriction endonuclease BamHI
and HindIII to construct pDC312-enhancer-DMD-H19-EGFP and
pDC312-enhancer-DMD-H19-E1A, respectively, which were confirmed by
DNA sequencing. The adenovirus Ad312-E1A was constructed by
homologous recombination techniques utilizing
pDC312-enhancer-DMD-H19-E1A and the adeno-virus packaging plasmid
PBHGLOX1, 3CRE.
 | Table IThe primers of H19-promoter,
E1A and β-actin. |
Table I
The primers of H19-promoter,
E1A and β-actin.
| Target gene | Primers |
|---|
|
H19-promoter | Forward:
5′-AAGTCGACCACCGTTCTATGAAGGGCTTCAGCA-3′ |
| Reverse:
5′-AGAAGCTTGCCCGGGCTTTTTCTAACTG-3′ |
| E1A | Forward:
5′-CCCGGATCCGGGCCCTATGAGACATATTATCT-3′ |
| Reverse:
5′-CGCGTCGACCGCAATCACAGGTTTACACCTTA-3′ |
| β-actin | Sense:
5′-CTGGAACGGTGAAGGTGACA-3′ |
| Antisense:
5′-AAGGGACTTCCTGTAACAACGCA-3′ |
The plasmid carrying E1A gene and the
adenovirus vector Ad5 were transfected into HEK293 with liposome
Lipofectamine™ 2000 (Invitrogen-Life Technologies, Carlsbad, CA,
USA). The Ad312-EGFP is a standard replication deficient adenovirus
and constructed by cotransfection of the adenovirus shuttle vector
covering EGFP with a deleted E1A/B adenoviral backbone vector. The
culture solution was changed after 4–6 h and the cytopathic effect
(CPE) was continuously observed through the transfection. The CPE
was observed after ~10 days and the abnormal cells and supernatant
were collected, frozen and thawed at −80°C/37°C three times and
centrifuged at 2,500 rpm for 15 min. The supernatant was Ad-E1A and
Ad-EGFP. The adenoviruses were plaque purified and propagated in
HEK293, then by a CsCl gradient according to standard techniques
purified again. Functional particle titers of all adenoviruses were
identified using a plaque assay in HEK293. The explicit control
adenovirus (H101) was benevolently supplied by Dr Sheng-Fang Ge
(Shanghai Jiao Tong University School of Medicine, Shanghai,
China).
Virus infection
The four cell lines were seeded in 96-well plates at
a density of 1,000/well for the Cell Counting kit-8 (CCK-8; Dojindo
Laboratories, Kumamoto, Japan) assay and in 6-well plates at a
density of 106/well for RT-PCR, western blot analysis
and flow cytometric analysis. The cells were incubated with various
concentrations of H101, AdpDC312-E1A and AdpDC312-EGFP with
serum-free DMEM at 37°C for 60 min. After the incubation period, a
normal growth medium replaced the serum-free DMEM with the viruses.
The infected cells were expected to continue to be cultured at 37°C
for further assays.
The EGFP expression analysis in the
constructed plasmids
Four kinds of cells (HCT-8, HT-29, SW480 and GES-1)
were infected with adenoviral vectors (Ad-EGFP, 10 PFU/cell). EGFP
expression was examined at 48 h after infection using a
fluorescence inversion microscope system (excitation 450–490 nm
type 108; Nikon).
The E1A expression analysis in virus
infected cells by real-time PCR
E1A mRNA expression was determined by
real-time PCR (RT-PCR; Applied Biosystems, Waltham, MA, USA). Four
types of cells (HCT-8, HT-29, SW480 and GES-1) were, respectively,
infected with Ad312-E1A (10 plaques forming units/cell). Total RNA
was extracted using TRIzol (Invitrogen-Life Technologies) followed
by the manufacturer’s instructions. The first strand cDNA synthesis
was performed in a whole volume of 25 μl: 2 μg RNA, 0.5 μg
up-primer and down-primer, 200 units of M-MLV reverse
transcriptase. The cDNA was then amplified in 50 μl reaction
volumes containing 0.4 μmol/l of up-primer and down-primer as well
as 1.25 U Taq DNA polymers (Takara, Dalian, China) with the
conditions of pre-denaturation at 94°C for 5 min, subsequently, by
35 cycles of 94°C for 40 sec, 60°C for 40 sec and 72°C for 1 min
and a final extension of 72°C for 7 min. The PCR products with 1013
bp were electrophoresed on a 1% agarose gel with ethidium bromide
before visualizing under UV light.
The E1A expression analysis by western
blot analysis
Western blot analysis was performed to evaluate the
E1A protein level. The HCT-8, HT-29, SW480 and GES-1 cells were
washed three times with ice-cold PBS, and the cells were suspended
in lysis buffer. Cell lysates were collected through 12,000 rpm, 5
min at 4°C and then separated by 12% sodium dodecyl sulfate
polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto
polyvinylidene difluoride (PVDF). Then blocked with 5% non-fat dry
milk in TBST buffer overnight at 4°C, with mouse anti-E1A
antibodies (1:1,000) and rabbit anti-human β-actin antibodies
(1:500) incubated at 24°C for 2 h, and washed by shaking with TBST
solution. The proteins were visualized by ECL (Wuhan Boster
Biological Technology, Ltd., Wuhan, China). Western blot analyses
were performed at least three times.
Cytotoxic effect of E1A by CCK-8
assay
CCK-8 assay was based on the ability of viable cells
using a Cell Counting kit-8 (CCK-8; Dojindo Laboratories). The
cells were seeded in 96-well plates at a density of 1,000
cells/wells and were infected with recombinant adenoviral vectors
(10 PFU/cell) the next day. Following incubation for 72 h, the
cells were assayed with CCK-8 reagents by measuring absorbances at
450 nm with a microplate reader (Bio-Rad Laboratories, Richmond,
CA, USA) to cell growth and viability. All samples were assayed in
quadruplicate and the experiments were repeated three times.
Quantative evaluation of apoptosis assay
by flow cytometry
The apoptosis assay was performed by flow cytometry
after double staining with Annexin V fluorescein isothiocyanate
(FITC) apoptosis detection kit (Nanjing Keygen Biotech, Co., Ltd.,
Nanjing, China) to discriminate early apoptosis (single Annexin
V-positive) and double Annexin V/propidium iodide-positive (PI)
differentiated necrotic cells. The 6-well dishes were inoculated
with 1×106 cells/well and then infected with 10 PFU/cell
of Ad312-E1A, Ad312-EGFP and H101. Cell apoptosis was analyzed at
72 h after infection.
Treatment of tumor-bearing nude mice with
Ad312-E1A
Tumor xenografts were established by oxter injection
of 5×107 HT-29 cells into the 4-week-old female athymic
nude mice (the Experimental Animal Center of University of
Yangzhou, Yangzhou, China). The tumor volume was measured by the
formula: Volume = 1/2× (length × width2). When tumors
had grown to 100 mm3, the xenografted mice were randomly
divided into four groups of twelve mice in each group. The
Ad312-E1A, Ad312-EGFP, H101 received intratumoral injections of
109 PFU every other day. The tumor was calculated by
vernier calipers every two days. All the mice were euthanized at 1
month post-inoculation. The mice were euthanized by cervical
dislocation at a predetermined interval of observation. The
harvested tumors were fixed in 40% buffered formalin, sectioned at
5–7 mm and stained with H&E.
Immunohistochemistry analysis
The tumors were stored in 10% formalin and embedded
in paraffin for staining, and then incubated at 4°C overnight with
the mouse anti-human E1A antibody (1:50 dilution; Abcam, Boston,
MA, USA) to detect E1A protein expression. The sections were rinsed
in PBS-T (0.05% Triton X-100 in PBS) with a goat anti-mouse
secondary antibody (1:500 dilution) incubation for 60 min at room
temperature and then incubated with streptavidin-horseradish
peroxidase (BD Biosciences, San Jose, CA, USA), diaminobenzidine
substrate to form the colorimetric reaction. The positive cells
were calculated in six random fields at 400 magnifications with a
light microscope. Only distinct staining cells were counted and the
positivity rate was utilized to grade the expression levels.
TUNEL assay
Apoptosis of the tumor cells was detected by the
terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end
labeling (TUNEL) and performed with In Situ Cell Death Detection
kit (Roche, Mannheim, Germany) with the manufacturer’s direction.
The tumors were filled with 10% formaldehyde and paraffin-embedded
sections were prepared to stain apoptotic cells. The number of
TUNEL-positive cells was calculated in six random fields at 400
magnification with a light microscope, and then the apoptosis index
for each field was calculated as the percent of TUNEL-positive
cells relative to the total.
Statistical analysis
IBM SPSS Statistics software version 20.0 (IBM,
Armonk, NY, USA) was used to analyze the data. Every assay was
performed at least three times. All experimental data were
expressed as the mean ± standard deviation and assessed by the
Student’s t-tests and the one-way ANOVA. The results of in
vivo survival experiments were assessed by GraphPad Prism 5
(GraphPad software, San Diego, CA, USA). P<0.05 was considered
to be statistically significant.
Results
Construction and characterization of the
oncolytic adenovirus Ad312-E1A
We constructed a conditional replication-competent
adenovirus to gene therapy for cancer cells on loss of IGF2
imprinting and p53 mutations as shown in Table II, and cells infected with H101
served as a positive control. Enhancer-DMD fragment (1376 bp) was
verified through the restriction endonuclease SalI and
XbaI from the recombinant plasmids pDC312-enhancer-DMD. The
H19 fragment (302 bp) was confirmed through the restriction
endonuclease HindIII and SalI from the recombinant
plasmid pDC312-enhancer-DMD-H19. In addition, E1A (1013 bp) and
EGFP (718 bp) fragment were, respectively, verified through the
restriction endonuclease BamHI and HindIII from both
pDC312-enhancer-DMD-H19-E1A and pDC312-enhancer-DMD-H19-EGFP, which
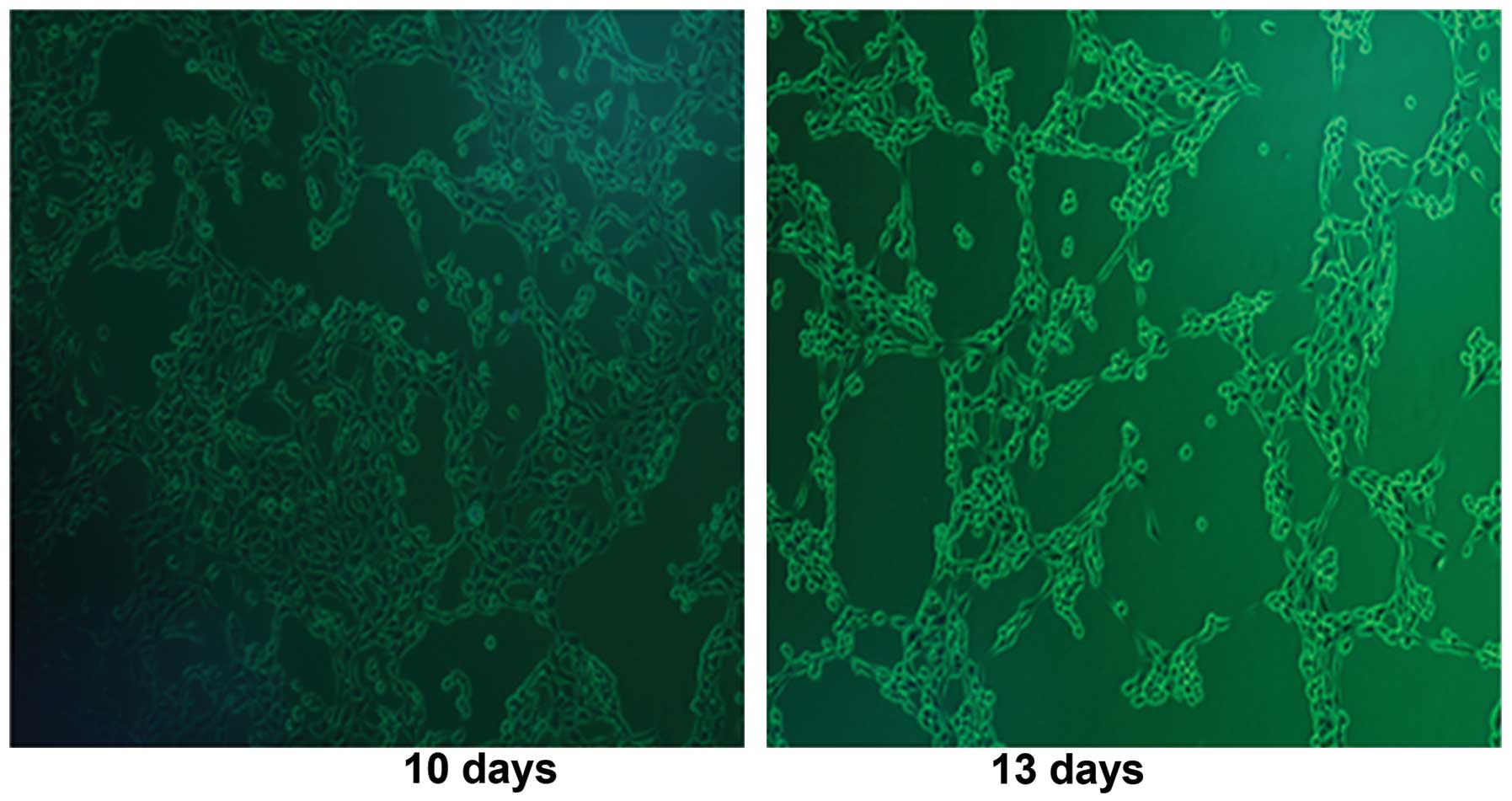
were transfected into HEK293 cells, respectively. We observed CPE
in HEK293 cells (Fig. 1) after
infection with Ad312-E1A at day 10 and day 13. The successful
construction of oncolytic adenovirus Ad312-E1A and Ad312-EGFP was
confirmed and regulated by the IGF2 imprinting systems, and
the Ad312-E1A expressed efficiently and replicated selectively in
HCT-8 and HT-29 (IGF2 LOI), except for SW480 and GES-1
(IGF2 MOI). Targeted therapy for CRC was mediated-by
oncolytic adenovirus vector, which was based on the IGF2 LOI
genomic imprinting systems.
 | Table IIGenomic imprinting of IGF2 and
analysis of p53 mutation. |
Table II
Genomic imprinting of IGF2 and
analysis of p53 mutation.
| Cell line | Source of cell | p53
status | IGF2
imprinting |
|---|
| HT-29 | Colon cancer | Mutation | LOI |
| HCT-8 | Colon cancer | Wild | LOI |
| SW480 | Colon cancer | Wide | MOI |
| GES-1 | Human gastric
epithelial cell | Wide | MOI |
EGFP protein expression in different
cells
An EGFP reporter gene was utilized to examine
the applicability of the expression system. After infection of the
four cell types with Ad312-EGFP (10 PFU/cell) for 48 h, EGFP
gene expression was found in LOI cells (HCT-8 and HT-29), but
negative or only weakly positive EGFP was observed in MOI
cells (SW480 and GES-1) for maintained normal IGF2
imprinting system (Fig. 2). Hence,
the virus gene therapy system only expressed the reporter gene in
the tumor cells with IGF2 LOI.
E1A mRNA transcript and protein
expression
We tested E1A mRNA and protein expression in
HCT-8, HT-29, SW480 and GES-1 cells which were, respectively,
infected with Ad312-E1A and H101 (10 PFU/cell). The expression of
E1A mRNA and protein were determined by RT-PCR and western
blot analysis 48 h after infection, respectively. As shown in
Fig. 3, in the Ad312-E1A group,
E1A mRNA and protein were expressed in LOI cells (HCT-8 and
HT-29), not in MOI cells (SW480) or normal cell (GES-1). The p53
mutant cells (HT-29) infected with H101 lead to high E1A
expression in mRNA and protein level, which was hardly expressed in
p53 wild-type cells (HCT-8, SW480 and GES-1), indicating that H101
expressed only in cell lines with p53 mutant.
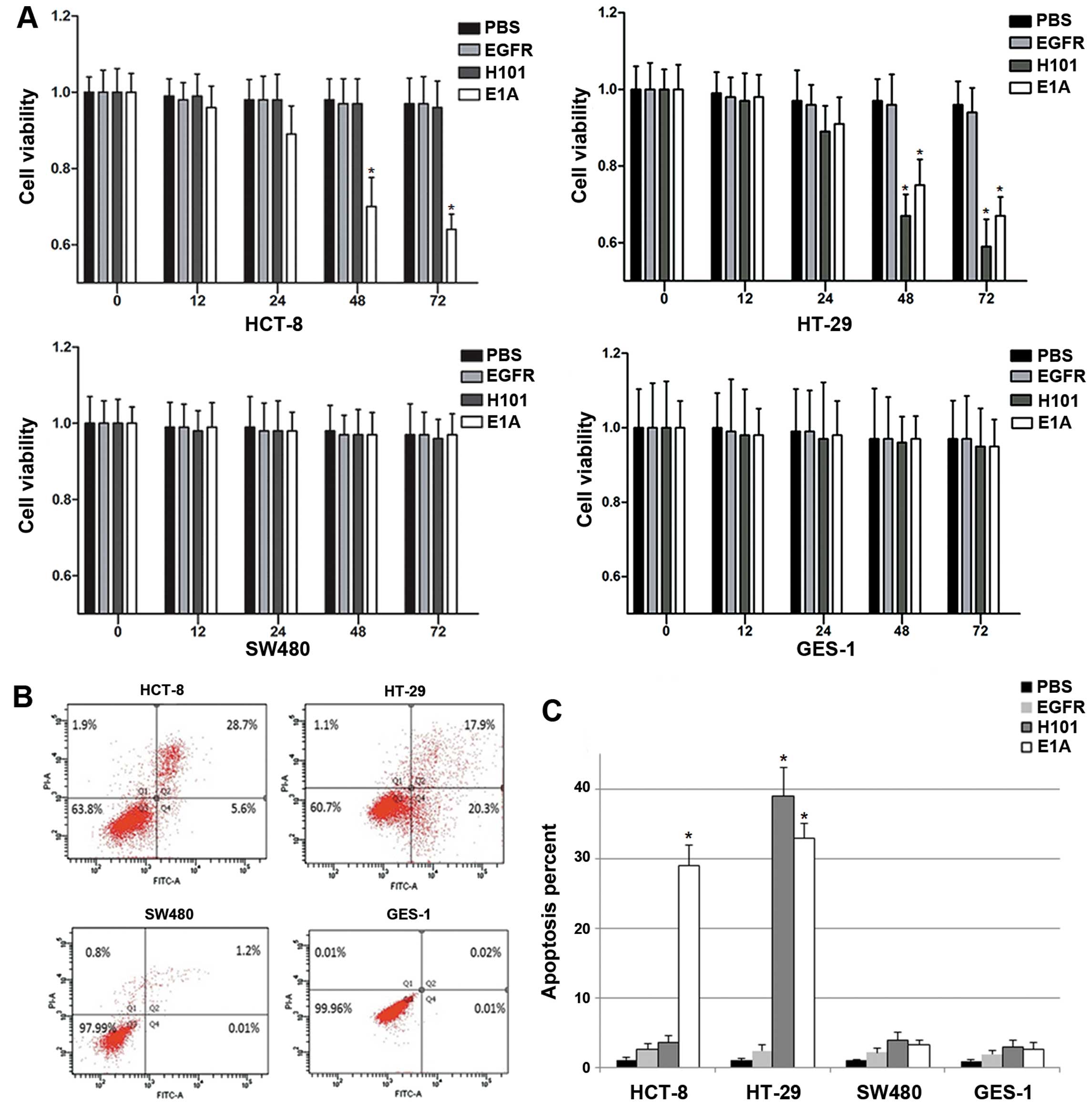
Growth inhibition and cytotoxicity of
different cell lines by Ad312-E1A infection
The cytotoxic effects of adenoviral vectors
expressing E1A were observed in four cell types. The groups
were treated with oncolytic adenovirus (10 PFU/cell) Ad312 EGFP,
Ad312-E1A, H101 and PBS at 72 h, their viability was assessed by
CCK-8 assay (shown in Fig. 4A).
The result of CCK-8 assay revealed that the cell viability of the
LOI cells (HCT-8 and HT-29) infected with Ad312-E1A was
significantly reduced when compared with the MOI cells (SW480 and
GES-1) (P<0.05), which still had higher cell viability. In the
same way, the viability of p53 mutant cell line (HT-29) infected
with oncolytic adenovirus H101 was also significantly decreased in
contrast to the p53 wild cell lines (HCT-8, SW480 and GES-1)
(P<0.05), which had obviously stronger cell viability.
Cell apoptosis induced by the
Ad312-E1A
Apoptosis in the four cell types was calculated
using flow cytometry 72 h after Ad312-E1A infection. To evaluate
the cytopathic effect of adenoviral infection on the cells,
apoptosis of cells infected with Ad312-EGFP was measured as the
negative control (Fig. 4B). The
resutls indicated that the apoptosis rate in LOI cell lines (HCT-8
and HT-29) infected with Ad312-E1A (10 PFU/cell) was significantly
higher than that in the control group (P<0.05). However, there
was no significant difference of apoptosis ratio between MOI cells
(SW480 and GES-1) infected with Ad312-E1A (10 PFU/cell) and the
negative control group (P>0.05). To evaluate the cytopathic
effect of H101 and Ad312-E1A on the p53 mutant cells (HT-29),
similar experimental procedure was applied, and the results showed
no obvious difference between the two groups (P>0.05), as shown
in Fig. 4C.
In vivo antitumor effect of the
Ad312-E1A
The antitumor effect of the recombinant adenoviral
was tested in nude mice transplanted with HT-29 cells. We measured
tumor volume once every three days for 30 days after injecting
Ad312-E1A, H101, Ad312-EGFP and PBS (n=12 per group), and the
average volume of the tumors were 432, 498, 2132 and 2233
mm3, as shown in Fig.
5A. After 30 days of injection, the tumor tissues were
harvested, as shown in Fig. 5B.
Although no significant difference existed between infected with
Ad312-E1A and H101 groups, a significant antitumor efficacy was
shown in these two groups compared with that in PBS and Ad312-EGFP
groups (P<0.01). In addition, the average survival time of the
four groups treated with Ad312-E1A, H101, Ad312-EGFP and PBS were
149, 152, 60 and 55 days, as shown in Fig. 5C. In short, the above results
showed that the survival time of group with infection of Ad312-EGFP
was not significantly prolonged compared with the group injected
with PBS (P>0.05), and that the survival time of the group
injected with the Ad312-E1A and H101 was obviously prolonged
compared with PBS group (P<0.05).
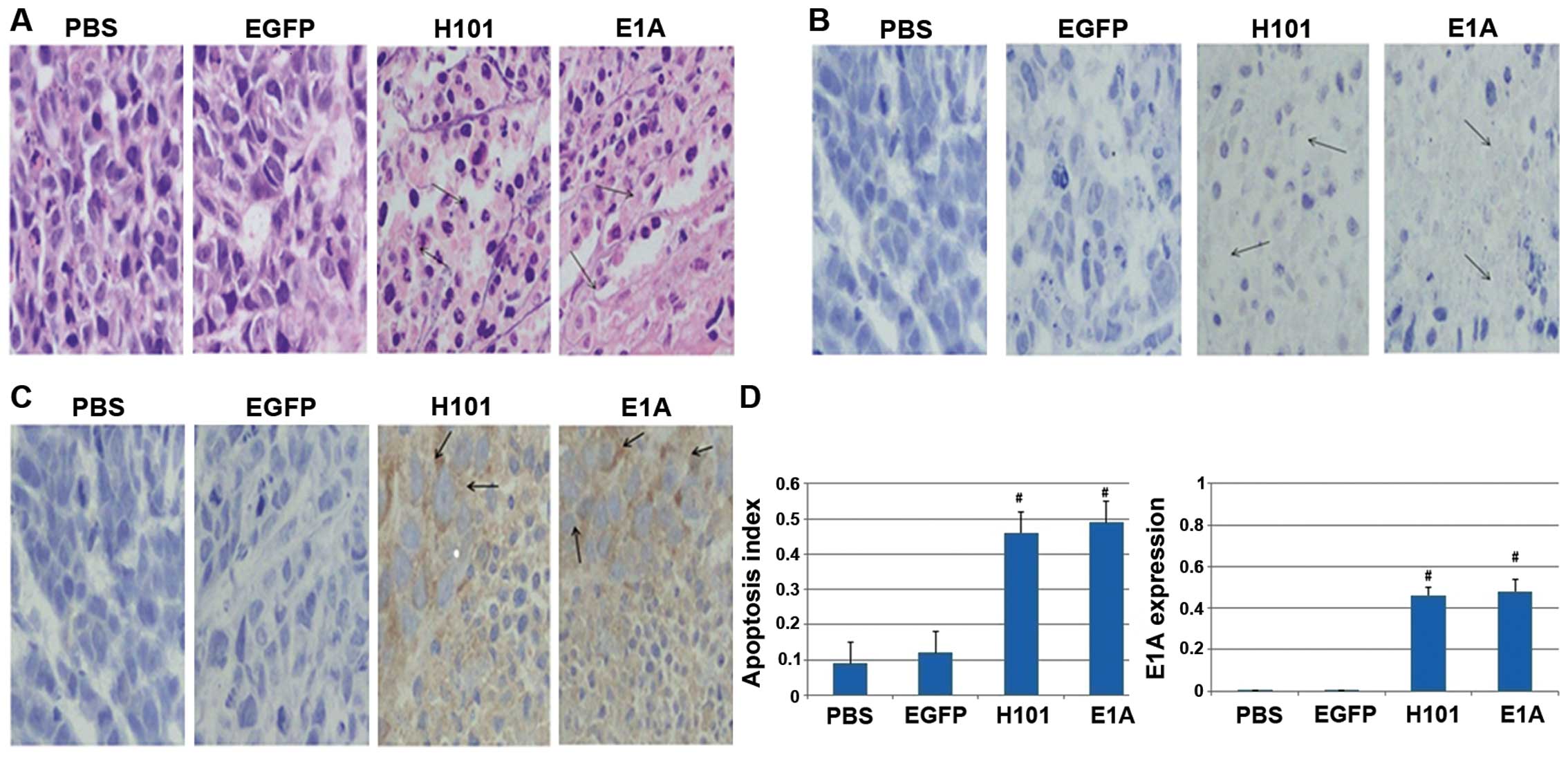
Immunohistology by TUNEL assay
To detect apoptosis in tumors from mice injected
with Ad312-E1A, Ad312-EGFP, H101 and PBS, TUNEL assay was applied
after the tumor tissue obtained, the number of apoptotic bodies in
tumor tissue after staining with hematoxylin and eosin (H&E)
was higher in the two groups with Ad312-E1A and H101 infection than
in the group treated with Ad312-EGFP and PBS (shown in Fig. 6A and B). Furthermore, the
expression of E1A protein was confirmed in tumor tissue from mice
injected with Ad312-E1A or H101, as shown in Fig. 6C. In addition, the apoptosis index,
represented by the percentage of TUNEL-positive cells, in the group
of H101 (0.63±0.04) and E1A (0.59±0.05) was higher than that in
group of PBS (0.19±0.06), EGFP (0.27±0.02), separately (P<0.01).
Moreover, the expression rate of E1A in tumour tissue of the four
groups was 0.009±0.001 in PBS group, 0.01±0.002 in Ad312-EGFP
group, 0.43±0.06 in H101 group and 0.48±0.08 in Ad312-E1A group,
respectively, as shown in Fig. 6D.
In conclusion, cell apoptotic indexes of tumour tissue from
xenograft mice in groups treated with both Ad312-E1A and H101 were
obviously higher than in the groups treated with the PBS and EGFP,
separately (P<0.05), and the apoptotic indexes of xenograft
tumors treated with Ad312-E1A had no difference compared with the
H101 group (P>0.05).
Discussion
Genomic imprinting is involved in hominoid
epigenetic regulation. In recent years, IGF2, characterized
by genomic imprinting, is an important autocrine/paracrine growth
factor in tumors for its mitogenic and antiapoptotic functions
(11). However, it is also
reported that the imprinting status of the IGF2 gene is
closely associated with somatic overgrowth and embryonal tumors
related to several different malignancies in human (12–18).
Hence, in the present study, the novel replication-selective
adenovirus Ad312-E1A and Ad312-EGFP were constructed based on loss
of the IGF2 imprinting, and the data based on the oncolytic
adenovirus Ad312-E1A displayed a meaningfully effect on suppressing
tumor growth both in vitro and in vivo, indicating
that replication-selective adenoviruses carrying the IGF2
imprinting system can be used as a new type of antitumor agent with
a high therapeutic potential of clinical treatment.
For IGF2 gene, in general, only paternal
alleles expressed and maternal alleles closed, which is known as
the maintenance of imprinting (MOI); however, the imprinted
IGF2 had abnormal expression by the reactivation of
suppressed maternal IGF2 allele unregulation (19,20),
which is known as loss of imprinting (LOI), triggered by the
abnormal binding of insulator CTCF to H19 ICR (21–23),
which caused by the impaired function of CTCF or the
hypomethylation status of DMR in the ICR region.
Overexpression of IGF2 accelerated the growth
of tumor cells causing tumorigenesis by influencing a bioactive
peptide to promote mitosis function (24).
The IGF2 LOI was detected in tumor tissues
and cell lines by several methods according to the published
reports. In the Reeve and Feinberg laboratories, the imprinted
IGF2, transcribed exclusively from paternal allele while
maternal allele remains silent, could be identified by the
restriction fragment length polymorphism (RFLP) method applied for
the detection of the an ApaI digestion single nucleoside
polymorphism (SNP) in exon 9 of IGF2 (25,26).
The ribonuclease protection assays (RPA) method, a sensitive
technique for detection and quantification of RNA expression, has
been reported to detect the imprinting status of the IGF2
gene (27,28). Previous studies based on chromatin
conformation capture (3C) method or chromatin immunoprecipitation
(ChIP) have confirmed the imprinting status of IGF2 in tumor
cell lines (IGF2 LOI: HCT-8, HRT18, HT-29, HCT15, T84,
Caco-2 and SW1222; IGF2 MOI: SW1116, SW480, HCT116 and
LIM1215) (29–31). In the present study, the
IGF2 LOI cell lines (HCT-8 and HT-29) and IGF2 MOI
cell lines (SW480 and GES-1) were utilized for gene therapy.
IGF2 LOI as a hallmark of various human
neoplasms has been widely investigated in somatic overgrowth and
embryonal tumors related to breast (12), prostate (13), lung cancer (14), renal cell carcinoma (15), esophageal (16,17),
ovarian cancer (18) and Wilms’
tumor (26). Moreover, Baba et
al (31) demonstrated an
association between IGF2 DMR hypomethylation and clinical
prognosis, and expounded its potential role as a prognostic
biomarker in more than 1,000 patients of CRC. In addition,
upregulation expression of IGF2 has been detected in CRC
(32,33), indicating IGF2 LOI may serve
as a potential biomarker in diagnosis of CRC (34).
IGF2 LOI involved in the pathology of cancers
has trigged research interest. The present study carried out gene
therapy based on oncolytic virus H101 and Ad312-E1A for CRC in
vivo and in vitro. H101, an adenovirus with the E1B-55KD
and pretrial E3 deleted, is the oncolytic adenovirus with the most
extensive investigation in that it could selectively replicate in
tumor cells rather than in normal cells, resulting in specific
tumor cytolysis, and was first applied in clinical treatment of
squamous cell carcinoma of head and neck in China (35). Ad312-E1A, a tumoricidal gene, is
constructed by the replication-defective adenovirus Ad312, which
has been verified as a promising vector system for the treatment of
malignant diseases by influencing the cell cycle, preventing
apoptosis, and making sure of viral replication (36). In the present study, Ad312-E1A was
observed to be safe in xenografts in mice, in vivo.
Regardless of the fact that, the reconstruction virus combined with
IGF2 LOI and Ad312-E1A or H101 was confirmed with antitumor
efficacy, the gene therapy based on them should be addressed with
some caution. Firstly, the oncolytic adenovirus Ad312-E1A, which
carried loss of IGF2 imprinting system, has a positive
effect on the cells with IGF2 LOI. Moreover, the oncolytic
virus H101 was expressed in p53 mutant cells with IGF2 LOI
(HT-29). Secondly, the reconstruction virus discussed in the study
involved in a small number of CRC, and further research is required
using other types of cancer on the loss of IGF2 imprinting,
such as Wilms’ tumor, leukemia, osteosarcoma, leiomyosarcoma,
breast cancer, lung cancer and hepatoma.
In conclusion, in the present study, the
conditionally replicative adenovirus Ad312-E1A, which carried loss
of IGF2 imprinting system, has a positive effect on CRC cell
lines with IGF2 LOI, indicating that the gene therapy based
on Ad312-E1A and IGF2 LOI could act as a novel strategy for
CRC therapy.
Acknowledgements
The present study was supported by grants from The
National Nature Science Foundation of China (no. 81172141 and
81200401), the Nanjing Science and Technology Committee Project
(no. 201108025), the Nanjing Medical Technology Development Project
(no. ZKX11025), the Nanjing Health Young Talent Project, Jiangsu
Provincial Key Medical Talents to S.K.W., and the Nanjing Medical
Science and Technique Development Foundation to Y.Q.P. (no.
QRX11255 and YKK13107) and B.S.H. (no. QRX11254).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Houlston RS, Collins A, Slack J and Morton
NE: Dominant genes for colorectal cancer are not rare. Ann Hum
Genet. 56:99–103. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fu VX, Dobosy JR, Desotelle JA, et al:
Aging and cancer-related loss of insulin-like growth factor 2
imprinting in the mouse and human prostate. Cancer Res.
68:6797–6802. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Engstrom W, Shokrai A, Otte K, et al:
Transcriptional regulation and biological significance of the
insulin like growth factor II gene. Cell Prolif. 31:173–189. 1998.
View Article : Google Scholar
|
|
5
|
Engel N, Thorvaldsen JL and Bartolomei MS:
CTCF binding sites promote transcription initiation and prevent DNA
methylation on the maternal allele at the imprinted H19/Igf2 locus.
Hum Mol Genet. 15:2945–2954. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pan Y, He B, Li T, et al: Targeted tumor
gene therapy based on loss of IGF2 imprinting. Cancer Biol Ther.
10:290–298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nevins JR, Ginsberg HS, Blanchard JM,
Wilson MC and Darnell JE Jr: Regulation of the primary expression
of the early adenovirus transcription units. J Virol. 32:727–733.
1979.PubMed/NCBI
|
|
8
|
Breyer B, Jiang W, Cheng H, et al:
Adenoviral vector-mediated gene transfer for human gene therapy.
Curr Gene Ther. 1:149–162. 2001. View Article : Google Scholar
|
|
9
|
Yamada M, Lewis JA and Grodzicker T:
Overproduction of the protein product of a nonselected foreign gene
carried by an adenovirus vector. Proc Natl Acad Sci USA.
82:3567–3571. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ballay A, Levrero M, Buendia MA, Tiollais
P and Perricaudet M: In vitro and in vivo synthesis of the
hepatitis B virus surface antigen and of the receptor for
polymerized human serum albumin from recombinant human
adenoviruses. EMBO J. 4:3861–3865. 1985.PubMed/NCBI
|
|
11
|
Pollak MN, Schernhammer ES and Hankinson
SE: Insulin-like growth factors and neoplasia. Nat Rev Cancer.
4:505–518. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ito Y, Koessler T, Ibrahim AE, et al:
Somatically acquired hypomethylation of IGF2 in breast and
colorectal cancer. Hum Mol Genet. 17:2633–2643. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kaneda A and Feinberg AP: Loss of
imprinting of IGF2: a common epigenetic modifier of intestinal
tumor risk. Cancer Res. 65:11236–11240. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Suzuki H, Ueda R and Takahashi T: Altered
imprinting in lung cancer. Nat Genet. 6:332–333. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oda H, Kume H, Shimizu Y, Inoue T and
Ishikawa T: Loss of imprinting of igf2 in renal-cell carcinomas.
Int J Cancer. 75:343–346. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mori M, Inoue H, Shiraishi T, et al:
Relaxation of insulin-like growth factor 2 gene imprinting in
esophageal cancer. Int J Cancer. 68:441–446. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hibi K, Nakamura H, Hirai A, et al: Loss
of H19 imprinting in esophageal cancer. Cancer Res. 56:480–482.
1996.PubMed/NCBI
|
|
18
|
Yun K, Fukumoto M and Jinno Y: Monoallelic
expression of the insulin-like growth factor-2 gene in ovarian
cancer. Am J Pathol. 148:1081–1087. 1996.PubMed/NCBI
|
|
19
|
Li T, Hu JF, Qiu X, et al: CTCF regulates
allelic expression of Igf2 by orchestrating a promoter-polycomb
repressive complex 2 intrachromosomal loop. Mol Cell Biol.
28:6473–6482. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rakha EA, Pinder SE, Paish CE and Ellis
IO: Expression of the transcription factor CTCF in invasive breast
cancer: a candidate gene located at 16q22.1. Br J Cancer.
91:1591–1596. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang Y, Hu JF, Ulaner GA, et al:
Epigenetic regulation of Igf2/H19 imprinting at CTCF insulator
binding sites. J Cell Biochem. 90:1038–1055. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Paradowska A, Fenic I, Konrad L, et al:
Aberrant epigenetic modifications in the CTCF binding domain of the
IGF2/H19 gene in prostate cancer compared with benign prostate
hyperplasia. Int J Oncol. 35:87–96. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Szabo PE, Tang SH, Silva FJ, Tsark WM and
Mann JR: Role of CTCF binding sites in the Igf2/H19 imprinting
control region. Mol Cell Biol. 24:4791–4800. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu M, Roth A, Yu M, et al: The IGF2
intronic miR-483 selectively enhances transcription from IGF2 fetal
promoters and enhances tumorigenesis. Genes Dev. 27:2543–2548.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rainier S, Johnson LA, Dobry CJ, Ping AJ,
Grundy PE and Feinberg AP: Relaxation of imprinted genes in human
cancer. Nature. 362:747–749. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ogawa O, Eccles MR, Szeto J, et al:
Relaxation of insulin-like growth factor II gene imprinting
implicated in Wilms’ tumour. Nature. 362:749–751. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ekstrom TJ, Cui H, Li X and Ohlsson R:
Promoter-specific IGF2 imprinting status and its plasticity during
human liver development. Development. 121:309–316. 1995.PubMed/NCBI
|
|
28
|
Ohlsson R, Nystrom A, Pfeifer-Ohlsson S,
et al: IGF2 is parentally imprinted during human embryogenesis and
in the Beckwith-Wiedemann syndrome. Nat Genet. 4:94–97. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakagawa H, Chadwick RB, Peltomaki P,
Plass C, Nakamura Y and de La Chapelle A: Loss of imprinting of the
insulin-like growth factor II gene occurs by biallelic methylation
in a core region of H19-associated CTCF-binding sites in colorectal
cancer. Proc Natl Acad Sci USA. 98:591–596. 2001. View Article : Google Scholar :
|
|
30
|
Cui H: Loss of imprinting of IGF2 as an
epigenetic marker for the risk of human cancer. Dis Markers.
23:105–112. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Baba Y, Nosho K, Shima K, et al:
Hypomethylation of the IGF2 DMR in colorectal tumors, detected by
bisulfite pyrosequencing, is associated with poor prognosis.
Gastroenterology. 139:1855–1864. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tricoli JV, Rall LB, Karakousis CP, et al:
Enhanced levels of insulin-like growth factor messenger RNA in
human colon carcinomas and liposarcomas. Cancer Res. 46:6169–6173.
1986.PubMed/NCBI
|
|
33
|
Lambert S, Vivario J, Boniver J and
Gol-Winkler R: Abnormal expression and structural modification of
the insulin-like growth-factor-II gene in human colorectal tumors.
Int J Cancer. 46:405–410. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cui H, Cruz-Correa M, Giardiello FM, et
al: Loss of IGF2 imprinting: a potential marker of colorectal
cancer risk. Science. 299:1753–1755. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Garber K: China approves world’s first
oncolytic virus therapy for cancer treatment. J Natl Cancer Inst.
98:298–300. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Holm PS, Lage H, Bergmann S, et al:
Multidrug-resistant cancer cells facilitate E1-independent
adenoviral replication: impact for cancer gene therapy. Cancer Res.
64:322–328. 2004. View Article : Google Scholar : PubMed/NCBI
|