Introduction
Pancreatic cancer is the fourth most common cause of
cancer deaths in the US with a five-year survival rate of <5%.
According to the American Cancer Society, in the US alone it is
estimated that 46,420 individuals will be diagnosed with and 39,590
of them will die of pancreatic cancer in 2014 (1). The low survival rate of patients
points towards an increased need for novel strategies to combat
this deadly disease. The concept of chemoprevention has recently
received significant attention as a novel strategy for pancreatic
cancer (2,3). Moreover, use of a combination of
chemopreventive agents that differ in their mode of action and
target multiple pathways has garnered recent attention (4,5).
This approach provides a means of low-dose therapy with increased
efficacy and less toxicity. However, combination therapy studies
specifically for pancreatic cancer prevention are still in its
infancy.
Numerous epidemiological and animal studies have
suggested that commonly used non-steroidal anti-inflammatory drugs
(NSAIDs) such as aspirin can reduce incidence and mortality of many
types of cancer (2,3). More recently, ibuprofen (IBU), also
an NSAID, have been reported to inhibit promotion and proliferation
of tumors in in vitro and in vivo studies (6–8).
Although promising in its chemopreventive potential, IBU’s adverse
effects such as increased gastrointestinal ulceration may prevent
long-term use (9,10). Encapsulation within nanoparticle
formulations may offer the opportunity to reduce side effects of
these drugs while maintaining high efficacy as shown in recent
literature (11,12). Lipid nanoparticles with a solid
matrix, such as solid lipid nanoparticles (SLNs) and polymeric
nanoparticles are examples of formulations which may be useful in
chemoprevention (2,3,13).
Nanosized drug delivery systems such as SLNs offer several
advantages over conventional delivery system including controlled
and sustained release of drugs, ability of the drug to cross the
mucosal barriers, decreased renal and hepatic clearance, decreased
immune recognition, increased apparent half-lives of drugs, and
increased stability and solubility (14,15).
However, the most important advantage of SLNs is that they can
increase the oral bioavailability of lipophilic drugs. The emerging
role of nanoparticles in cancer therapy and chemoprevention
justifies a need for further research in this area.
Sulforaphane (SFN) is a naturally occurring
sulfur-containing isothiocyanate found in cruciferous vegetables
such as broccoli, Brussel’s sprouts, cauliflower, and cabbage
(16). SFN has been shown to be
not only effective in preventing various chemically induced cancers
in animal models, but also inhibits the growth of established
tumors (17–19). SFN has been shown to reduce NF-κB
activity and affect expression of NF-κB mediated genes encoding
adhesion molecules, inflammatory cytokines, growth factors, and
anti-apoptotic factors (20).
In this study, IBU-loaded SLN formulations were
optimized by using i) stearic acid, ii) compritol ATO 888 and iii)
tripalmitin as the lipid matrices mixed with either a) Poloxamer
188 or b) Tween-80 as the surfactant. Particle size, entrapment
efficiency, zeta potential and in vitro drug dissolution
rates of the resulting IBU-SLNs were investigated. To date, no
other group has investigated the effects of low-dose free IBU,
IBU-SLNs or IBU-SLN combined with free SFN on pancreatic cancer
cells. Thus, we optimized IBU-SLN formulations to evaluate their
combined chemopreventive efficacy in Panc-1 and MIA PaCa-2 human
pancreatic cancer cells.
Materials and methods
Reagents
IBU was obtained from LKT Laboratories (St. Paul,
MN, USA). SFN was obtained from Santa Cruz Biotechnology (Santa
Cruz, CA, USA). Stearic acid was obtained from JT Baker (Center
Valley, PA, USA), tripalmitin from Tokyo Chemical Industry (Tokyo,
Japan) and compritol ATO 888 from Gattefossse (France). Poloxamer
188 and Tween-80 was obtained from Spectrum Chemicals (Gardena, CA,
USA). HPLC grade acetonitrile was purchased from BDH (Radnor, PA,
USA), and ortho-phosphoric acid from Fisher Scientific (Fair Lawn,
NJ, USA).
Human pancreatic cancer cell lines
Panc-1 and MIA PaCa-2 cell lines were obtained from
ATCC (Rockville, MD, USA). Cells were maintained in Dulbecco’s
modified Eagle’s medium (DMEM) containing 10% fetal bovine serum
obtained from ATCC. Cells were cultured at 37°C in a humidified
atmosphere of 5% CO2 and 95% air.
Preparation of solid lipid
nanoparticles
Ibuprofen SLNs were prepared using a hot melt
oil-in-water (o/w) emulsion technique. Stearic acid, compritol and
tripalmitin lipids were used separately to optimize the
nanoparticle formulations. Briefly, 400 mg of each lipid was melted
at 70°C. IBU (200 mg) was dissolved in the cooling melted lipid.
The water phase consisted of 2% Poloxamer or 2% Tween-80 which was
heated to the same temperature as that of the lipid phase. The
lipid phase was then added drop wise to the surfactant solution
using continuous high sheer homogenization. Thereafter, the mixture
was sonicated for 1 min using a probe-sonicator (Branson, Pomona,
CA, USA) to form an emulsion. The emulsion was cooled and the
resulting IBU-SLNs were freeze-dried in a freeze dryer (Labconco,
Kansas City, MO, USA) and subjected to particle size, encapsulation
efficiency and zeta-potential determination.
Determination of mean particle size,
polydispersity index and zeta potential
The mean particle size (z-average) and
polydispersity index (PDI) as a measure of the width of particle
size distribution is determined by photon correlation spectroscopy
using Zetasizer (Nano ZS 90, Malvern Instruments, Malvern, UK) at
25°C and 90° scattering angle. SLN formulation was diluted with
nano-pure water to weaken opalescence before measurements. The
surface charge was assessed by measuring zeta potential of SLNs
based on the Smoluchowski Equation, using the same equipment at
25°C with electric field strength of 23 V/cm (21).
Determination of % encapsulation
efficiency of IBU-SLNs
Encapsulation efficiency was determined by
dissolving 10 mg of the SLN formulation in 10 ml acetonitrile
solvent. The drug was released from the lipid into acetonitrile and
allowed to dissolve freely for 10 min in a sonicator after which it
was filtered through a 0.45-μm filter. The resulting solution was
further diluted with acetonitrile and was analyzed by using a
Shimadzu LC-20 binary HPLC system (Columbia, MD, USA). Caffeine was
used as the internal standard.
The entrapment efficiency was calculated using the
following formula: EE (%) = Amount (mg) of drug per HPLC
method/theoretical yield (mg) ×100.
In vitro drug release from IBU-SLNs
The cumulative release of IBU from SLNs was
determined in phosphate buffered saline (PBS), pH 7.4. SLNs
containing 5 mg of the IBU-SLNs was suspended in 50 ml of PBS and
placed in an incubator at 37°C with a shaking speed of 100 rpm.
Drug release from SLN was compared to PBS with blank SLNs. At
predetermined time intervals (0, 0.5, 1, 2, 4, 6, 12, 24, 48, 72
and 96 h), 1 ml of the buffer was withdrawn and replaced with
equivalent volume of fresh buffer. All samples are centrifuged at
5000 rpm for 10 min. The amount of released drug was analyzed using
HPLC. The analysis was carried out in triplicate.
Chromatographic analysis of IBU-SLNs
IBU was analyzed using a Shimadzu LC-20 binary HPLC
system. The system consists of a Restek Ultra II C-18 column
(4.6×150 mm, 5 μm) with mobile phase composed of acetonitrile and
1% ortho-phosphoric acid (60:40). The flow rate was set at 0.5
ml/min and the detection was at 220 nm using a photo diode array
detector. The retention time of IBU was 10.7 min.
Cell viability assay
The cell viability assay was performed according to
manufacturer’s protocol with the Promega CellTitre 96 Aqueous MTS
reagent (Madison, WI, USA). Briefly, 7.5×103 cells were
seeded in 96-well plates and incubated overnight. The test
compounds IBU and SFN were added and incubated for a period of 72
h. At the end of the incubation period, the growth medium was
removed followed by addition of 100 μl media consisting of 20% MTS
and 1% of phenazine methosulfate (PMS) and the mixture was
incubated for 2 h at 37 °C. MTS is bio-reduced by the cells into
formazan which could be measured at 490 nm. Thus, the quantity of
formazan product measured by absorbance is directly proportional to
the number of living cells in culture. IC50 values were
determined using GraphPad Prism software (San Diego, CA, USA). All
analysis was performed in triplicate. Each experiment was repeated
at least once.
Colony formation assay
Cells (1×104) were seeded into 24-well
plates in triplicate per data point. Cells were treated with
blank-SLN, IBU-SLN, SFN alone and in combinations IBU-SLN+SFN. Two
weeks after treatment, cells were fixed and stained with 0.5%
crystal violet (Sigma, St. Louis, MO, USA) in methanol for 5 min.
Colonies consisting of 50 or more cells were counted. The
percentage cell survival was calculated (Plating efficiency of
non-treated cultures = 1).
NF-κB DNA binding assay
The DNA-binding activity of NF-κB was quantified by
ELISA, using the TransAM NF-κB p50 transcription factor assay kit
(Active Motif, Carlsbad, CA, USA). Briefly, 30 μg of total protein
was incubated in a 96-well plates coated with immobilized
oligonucleotide for the p50 subunit. NF-κB binding to the target
oligonucleotide was detected by incubation with primary antibody
specific for the activated form of p50 (active motif), visualized
and quantified at 490 nm.
Statistical analysis
GraphPad Prism software was used for statistical
analysis and graph plotting. The results are expressed as mean ±
SEM and analyzed by one-way ANOVA followed by Tukey’s post-hoc
test. The IC50 values were calculated using nonlinear
regression and plotted as log (inhibitor) vs. response (variable
slope) curve. A probability value of ≤0.05 was considered
significant.
Results
Effect of different lipid materials on
particle size of IBU-SLNs
A summary of all formulations with their respective
preparation conditions and characterization is presented in
Table I. In order to optimize
IBU-SLN formulations, different lipid materials (stearic acid,
Compritol and tripalmitin) were used. In addition, IBU-SLNs were
prepared with drug to lipid ratio of 1:2 or 1:4, and either with 2%
Poloxamer or 2% Tween-80 in the aqueous phase. In the tested
parameters range, we observed that the mean particle diameter
(z-average) of IBU-SLNs increased with an increase in lipid
concentration from 1:2 (170–543 nm) to 1:4 (335–650 nm). Thus,
increase in lipid concentrations resulted in larger particle size.
With respect to the use of surfactant, Tween-80 based IBU-SLNs
showed smaller particle size (170–450 nm) compared to Poloxamer 188
(390–650 nm) based SLN formulations.
 | Table IOptimization of ibuprofen solid lipid
nanoparticle (IBU-SLN) formulations. |
Table I
Optimization of ibuprofen solid lipid
nanoparticle (IBU-SLN) formulations.
| Lipid | Surfactant (2%) | Drug:Lipid ratio | Particle size
(nm) | Encapsulation
efficiency (% EE) | Zeta potential
(mV) | Polydispersity index
(PDI) |
|---|
| Stearic acid | Tween-80 | 1:2 | 170 | 63 | −13.7 | 0.3 |
| | 1:4 | 450 | 66.4 | −15.8 | 0.4 |
| Poloxamer 188 | 1:2 | 485 | 69 | −13.2 | 0.3 |
| | 1:4 | 650 | 71 | −7.11 | 0.2 |
| Compritol 888
ATO | Tween-80 | 1:2 | 291 | 64 | −0.8 | 0.4 |
| | 1:4 | 350 | 69.4 | −3.76 | 0.2 |
| Poloxamer 188 | 1:2 | 543 | 70.2 | −13.2 | 0.2 |
| | 1:4 | 595 | 87 | −2.53 | 0.4 |
| Tripalmitin | Tween-80 | 1:2 | 207 | 66 | −11.5 | 0.4 |
| | 1:4 | 335 | 68 | −14.3 | 0.3 |
| Poloxamer 188 | 1:2 | 390 | 66 | −18.4 | 0.2 |
| | 1:4 | 523 | 78.5 | −5.88 | 0.4 |
Effect of various formulation parameters
on encapsulation efficiency (% EE) of IBU-SLNs
As shown in Table
I, the amount of lipid has significant effect on the
encapsulation efficiency. There was an increase in % EE from drug
to lipid ratio of 1:2 (63–70.2%) to 1:4 (66.4–87%). Specifically,
Poloxamer containing SLN formulations showed an increase in % EE
(66–87%) compared to Tween-80 (63–69.4%) based SLN
formulations.
Effect of ratio of drug to lipid on zeta
potential of IBU-SLNs
As shown in Table
I, the change of surfactant significantly affected the zeta
potential of SLNs. In the case of Poloxamer 188, the zeta-potential
charge on SLNs were reduced significantly with drug to lipid ratio
of 1:2 (−13.2 to −18.4 mV) to 1:4 (−2.53 to −7.11 mV), whereas
there was increase in zeta potential in Tween-80 formulation with
drug to lipid ratio from 1:2 (−0.8 to −13.7 mV) to 1:4 (−3.76 to
−15.8 mV).
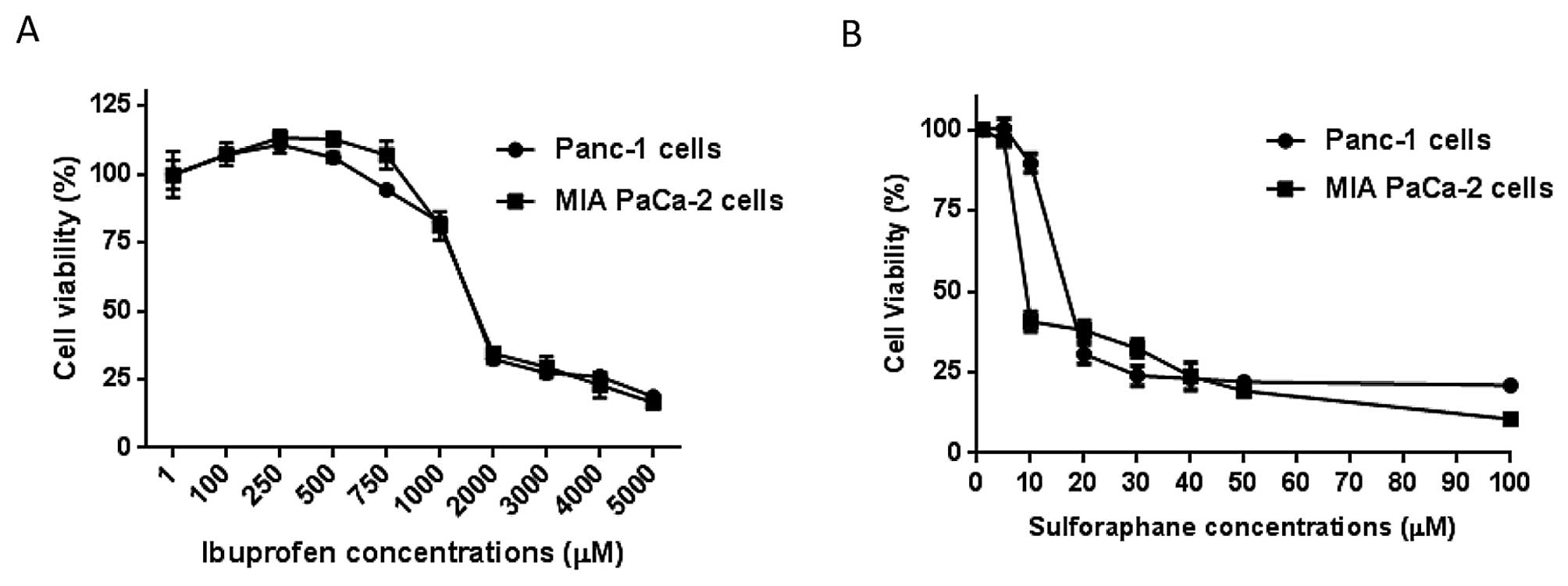
Determination of cell viability of free
IBU on pancreatic cancer cells
In order to evaluate the effect of free IBU on
pancreatic cancer, the dilution range of IBU (5–5000 μM) were added
to Panc-1 and MIA PaCa-2 cells for 72 h. Our observations indicated
a dose-dependent reduction in cell viability with IC50
concentrations of 1.25 and 1.26 mM in Panc-1 and MIA PaCa-2 cells,
respectively (Table II and
Fig. 1).
 | Table IIThe IC50 values of free
IBU and IBU-SLNs. |
Table II
The IC50 values of free
IBU and IBU-SLNs.
| Formulations | | |
|---|
| | |
|---|
| Drug | Surfactant | Drug:lipid
ratio | Panc-1 cells
(IC50 values μM) | MIA PaCa-2 cells
(IC50 values μM) |
|---|
| Free-Ibuprofen | | | 1245 | 1263 |
| IBU-stearic acid
SLNs | Poloxamer 188 | 1:2 | 113.8 | 122.6 |
| | 1:4 | 399.3 | 348.0 |
| IBU-compritol
SLNs | Poloxamer 188 | 1:2 | 408.0 | 470.4 |
| | 1:4 | 539.5 | 531.4 |
| IBU-tripalmitin
SLNs | Poloxamer 188 | 1:2 | 586.0 | 554.3 |
| | 1:4 | 639.3 | 631.7 |
Effect of IBU-SLN formulation parameters
on cell viability of pancreatic cancer cells
To verify the effect of IBU-SLNs, cell viability
assay of various formulations were carried out on pancreatic cancer
cells. As shown in Fig. 2A,
IBU-SLN formulations with Poloxamer as the surfactant and a 1:2
drug to lipid ratio showed that stearic acid based IBU-SLNs
demonstrate maximal effect, inhibiting cell proliferation by ~75%
compared to blank-SLNs (P<0.001). This formulation showed the
lowest IC50 of 113.8 and 122.6 μM in Panc-1 and MIA
PaCa-2 cells, respectively. The most striking result emerging from
this study was that the IC50 values of IBU-SLNs were at
least 10-fold lower than that of free IBU. The Compritol and
tripalmitin based SLN formulation showed ~20% decrease in cell
viability of pancreatic cancer cells, with IC50 values
in the range of 408.0–586.0 μM.
The data in Fig. 2B
represents cell viability study with Poloxamer as the surfactant
and a 1:4 drug to lipid ratio. Stearic acid based IBU-SLNs
inhibited cell viability by ~45% in pancreatic cancer cells
(P<0.01). Further analysis showed IC50 values of
399.3 and 348.0 μM in Panc-1 and MIA PaCa-2 cells, respectively.
Comparing the two results in Fig. 2A
and B, it can be seen that the IBU-SLN formulation with stearic
acid as the lipid, Poloxamer as the surfactant and a 1:2 drug to
lipid ratio showed significant reduction in cell proliferation thus
indicating maximum efficacy; subsequently, these formulations were
selected for further studies.
Effect of the combination of IBU-SLN with
SFN on pancreatic cancer cells
To examine the effect of combined regimen on cell
proliferation, Panc-1 and MIA PaCa-2 cells were treated with low
and ineffective concentrations of free-IBU (250 μM) in combination
with SFN (5 μM) for 72 h. As shown in Fig. 3A, single agents did not show
significant change in cell viability at these concentrations.
However, when used in combination at identical concentrations,
IBU+SFN showed a significant effect with a reduction in cell
viability of ~55% (P<0.01) in Panc-1 and MIA PaCa-2 cells,
respectively.
After determining the optimal formulation conditions
and dose response curves individually, IBU-SLNs (62.5 μM) and free
SFN (5 μM) were selected showing minimal inhibitory response on the
cell lines when used individually. However, when IBU SLNs+SFN
combinations were used at the same concentrations, the cell
viability was reduced by ~80% for Panc-1 and MIA PaCa-2 cells,
respectively (P<0.001; Fig.
3B). Thus, the combination of IBU-SLNs and SFN showed 4-fold
lower concentration as compared to free IBU in the reduction of
cell viability of pancreatic cancer cells.
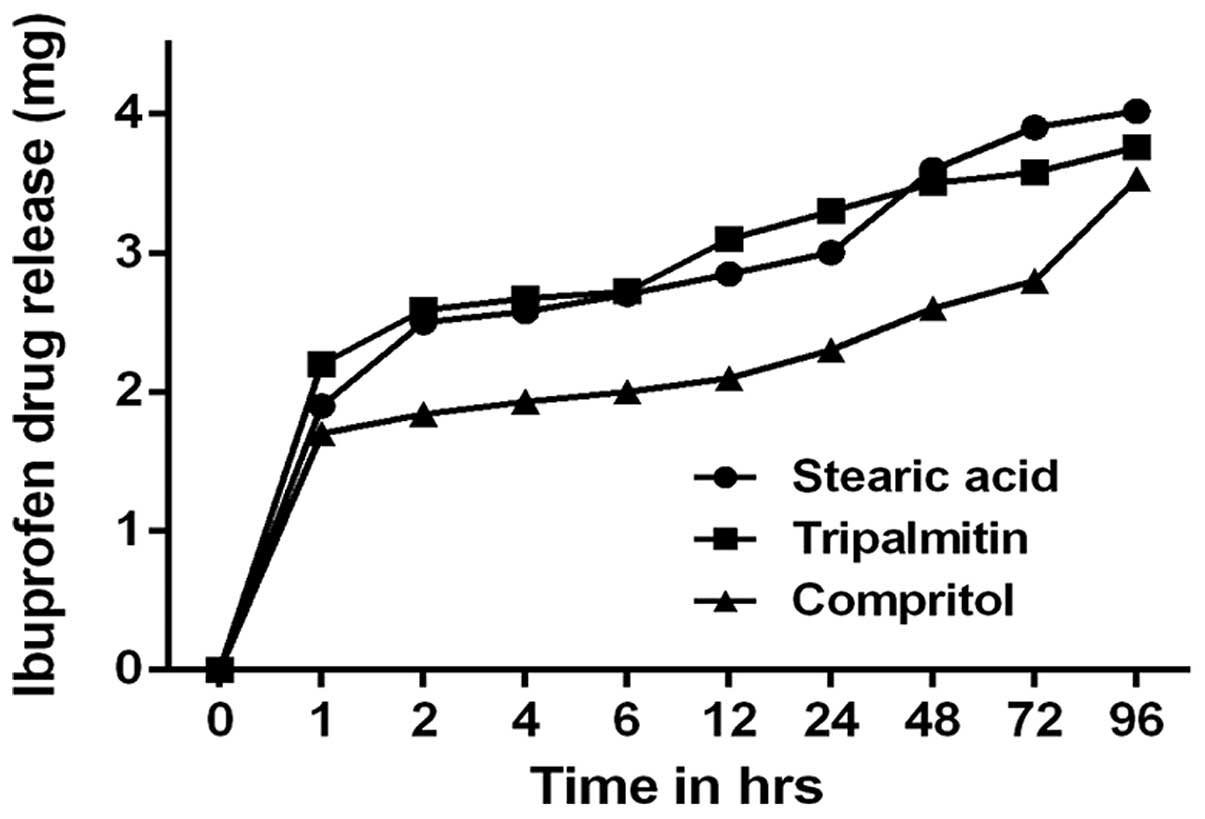
In vitro evaluation of IBU drug release
from IBU-SLNs
The in vitro drug release curve for the
IBU-SLN suspension in pH 7.4 phosphate buffer at 37°C is shown in
Fig. 4. The drug release exhibited
biphasic pattern, a burst drug release followed by sustained drug
release. The IBU release was 40% during the first 2 h;
subsequently, sustained release of IBU from optimized IBU-SLNs was
observed for the next 90 h release period.
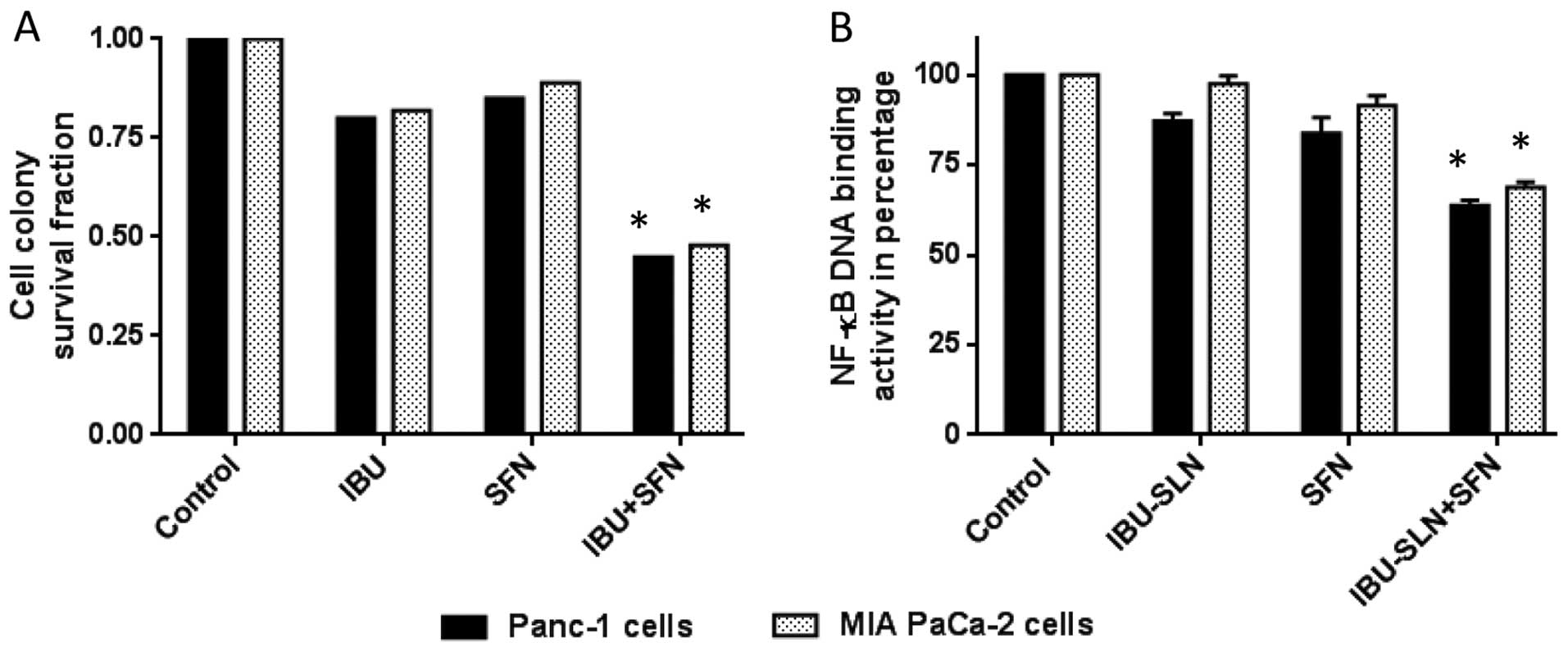
Cell colony formation assay of
IBU-SLNs
To evaluate long-term efficacy of IBU-SLNs on cell
survival, a cell colony assay was performed. The survival fraction
of the control group (blank-SLN) was set at 1 (representing 100%
cell survival) and the cell survival fraction was calculated based
on individual and combination treatment. An evaluation of cell
survival on MIA PaCa-2 cells showed survival fractions of 0.82
(IBU), and 0.89 (SFN), whereas IBU-SLN+SFN combination showed
significant decrease in the survival fraction of 0.48 (P<0.01)
(Fig. 5A). Similar results were
observed in the case of Panc-1 with low survival fractions of
cells.
Effect of IBU-SLNs in combination with
SFN on DNA binding activity of NF-κB
To gain further insight into the mechanism
associated with the combination IBU-SLN and SFN, the DNA binding
activity of the p50 subunit of the NF-κB complex was evaluated. As
shown in Fig. 5B, ~35% decrease
was observed in Panc-1 cells with the stearic acid based
formulation, whereas MIA PaCa-2 cells showed ~30% (P<0.05)
reduction in DNA binding activity of NF-κB compared to blank SLN
treatment group.
Discussion
Early diagnosis of pancreatic cancer is difficult
because it develops without any early symptoms. Low survival rate
of patients with pancreatic cancer makes this disease of great
concern (1). Millions of people
routinely take aspirin, ibuprofen and other NSAIDs for control of
pain and inflammation, or prophylaxis against cardiovascular
disease. The widespread use of these compounds has facilitated
epidemiological studies about their effect against cancer
development. Numerous epidemiological, clinical, and laboratory
studies have suggested that IBU, a commonly used NSAID, inhibits
the proliferation of various tumors (6–8).
Bonelli et al demonstrated the anti-proliferative effects of
IBU on the human gastric cancer cell MKN-45. Free IBU, at
concentration of 400–800 μM, could not inhibit cellular
proliferation in a time- and dose-dependent manner, but 200 μM
IBU-PLGA could significantly inhibit the growth of gastric cancer
MKN-45 cells (8). It is important
to note that cancer preventive doses should be well below the
therapeutic dose for the treatment of inflammatory conditions to
avoid long-term toxicity. Hence, part of our current research also
focused on the administration of low doses of IBU-SLNs to study its
chemopreventive effects against Panc-1 and MIA PaCa-2 pancreatic
cancer cells.
Appropriate choice of lipids, surfactants, and their
composition affects the particle size of IBU-SLNs, drug
encapsulation efficiency, and drug release behavior. Optimal SLN
formulation for each drug that can be obtained by investigating the
effect of process variables on the characteristics of the SLNs
(22–24). Particle size analysis of IBU-SLNs
revealed that the SLN prepared with higher lipid concentration
showed a larger particle size. To establish the effect of
surfactant type on SLNs, we used two commonly used surfactants,
Poloxamer 188 and Tween-80. We observed a lower particle size in
Tween-80 based SLNs compared to Poloxamer SLN formulations. A
possible explanation for this difference in particle size may be
the effect of hydrocarbon chain length difference of the used
surfactants (25). Thus, with
regards to particle size, the concentration of lipid and type of
emulsifier seem to be important process parameters. The results of
% EE indicated that increasing the lipid concentration also
increases encapsulation due to the presence of long chain fatty
alcohols, which could lead to the less ordered solid lipid matrix
and leave enough space to accommodate drug molecules (26). The high % EE can also be attributed
to the lipophilic nature of IBU.
Surface charge potential should play an important
role in nanoparticle stability due to electrostatic repulsion.
Since Poloxamer 188 and Tween-80 are non-ionic surfactants, lipid
cores may be responsible for the negative surface charge. The
negative charge was likely caused by the slightly ionized fatty
acids from glycerides. This may explain the changes in zeta
potential produced by the amount of lipid. Patil et al
reported that nanoparticle with zeta potential of −43 mV have the
highest cellular uptake compared with other formulations with less
negative and/or positive surface charge (27,28).
Nanoparticles show a high affinity for cellular membrane mainly due
to electrostatic interactions (29,30).
These findings agree with results obtained in this study that
IBU-SLNs with higher negative charge showed greater inhibition of
cell viability. From in vitro drug release studies (Fig. 4), we observed that 80% of IBU drug
was released in 24 h, while the later phase lasted for 96 h, which
correlates with the physiological requirement for humans. For oral
administration, both burst release and sustained drug release are
of importance to ensure quick efficacy and prolonged drug presence.
For our studies, the burst release occurred due to the presence of
the free IBU in the external phase and on the surface of the SLNs.
The lipophilic nature of the IBU could be the reason for sustained
release of the drug from internal lipidic phase after initial burst
release.
The cell viability assay showed that both free IBU
and IBU-SLNs could significantly suppress cell viability in a
dose-dependent manner. The effect of these agents was evaluated by
calculating the IC50 values of free IBU and IBU-SLNs. It
was observed that IBU-SLNs IC50 concentrations exhibited
approximately 10-fold reductions in comparison to the
IC50 of free form of IBU. Previous studies have been
reported where drug loaded aspirin, curcumin, and sulforaphane SLNs
have exhibited better cytotoxicity profile in comparison to the
free drug on in vitro and in vivo models of
pancreatic cancer (2,4,5). In
cell viability assay, DMSO and blank SLNs were used as control for
free IBU and IBU-SLNs, respectively. We found that higher
concentration of stearic acid (500 μM and above) in blank SLNs
showed ~30% reduction in cell viability, thus the cytotoxicity
effect of low concentration of IBU-SLNs was confirmed to be because
of IBU. The surfactants used as components of SLN, when tested
alone at equivalent concentrations used in SLN, did not result in
reduction of cell viability. Therefore, cytotoxic effects, induced
by SLN formulations containing specific surfactants, did not appear
to accrue from the surfactants used.
Single agent administration at low concentrations
was demonstrated to be ineffective, hence the hypothesis that two
or more chemopreventive agents when delivered at low concentrations
together, may exhibit an additive or synergistic effect against the
cancer cells was tested. This can be attributed to the
multi-factorial nature of carcinogenesis wherein cancer initiates
as a result of multiple cellular changes during a prolonged time
period. Several in vitro and in vivo studies have
shown that NSAIDs like aspirin and celecoxib have helped prevent
the progression of pancreatic cancer (2,31–33).
In our studies, the IC50 value of IBU-SLN was found to
be 113.8 μM and 122.6 μM in Panc-1 and MIA PACa-2 cells,
respectively. However, studies of ineffective and low dose of
IBU-SLN (62.5 μM) combined with SFN (5 μM) exhibited a synergistic
effect against the pancreatic cancer cells, proving to be more
efficacious at lower concentrations, reducing the concentration of
IBU-SLN by 4 times compared to free IBU (250 μM) in combination
with SFN. Therefore, using a multi-disciplinary approach, this
study investigated the synergistic effects of SLN combinations of
chemopreventive agents, namely, IBU-SLN in combination with free
SFN.
From these results, we believe that chemoprevention
is an effective way to prevent pancreatic cancer especially since
the disease cannot be diagnosed at an early stage. Using a
multi-disciplinary approach, this study investigated optimized
formulation parameters for IBU-SLNs and the synergistic effects of
a combination of IBU-SLN with free SFN. We demonstrated that this
IBU-SLN combination with SFN showed a synergistic inhibition of
cell viability in human pancreatic cancer cells. However, further
in vivo studies have to be conducted to test the efficacy of
IBU-SLN alone and in combination with SFN. Additionally, to assess
the long-term benefits of this regimen, a toxicity safety study
would be necessary. In conclusion, the preliminary results obtained
from formulation studies and cell based assays clearly demonstrate
the translational scope of developing low dose IBU encapsulated SLN
formulations to prevent pancreatic cancer.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Grandhi BK, Thakkar A, Wang J and Prabhu
S: A novel combinatorial nanotechnology-based oral chemopreventive
regimen demonstrates significant suppression of pancreatic cancer
neoplastic lesions. Cancer Prev Res. 6:1015–1025. 2013. View Article : Google Scholar
|
|
3
|
Chaudhary A, Sutaria D, Huang Y, Wang J
and Prabhu S: Chemoprevention of colon cancer in a rat
carcinogenesis model using a novel nanotechnology-based combined
treatment system. Cancer Prev Res. 4:1655–1664. 2011. View Article : Google Scholar
|
|
4
|
Thakkar A, Sutaria D, Grandhi BK, Wang J
and Prabhu S: The molecular mechanism of action of aspirin,
curcumin and sulforaphane combinations in the chemoprevention of
pancreatic cancer. Oncol Rep. 29:1671–1677. 2013.PubMed/NCBI
|
|
5
|
Sutaria D, Grandhi BK, Thakkar A, Wang J
and Prabhu S: Chemoprevention of pancreatic cancer using
solid-lipid nanoparticulate delivery of a novel aspirin, curcumin
and sulforaphane drug combination regimen. Int J Oncol.
41:2260–2268. 2012.PubMed/NCBI
|
|
6
|
Palayoor ST, Bump EA, Calderwood SK,
Bartol S and Coleman CN: Combined antitumor effect of radiation and
ibuprofen in human prostate carcinoma cells. Clin Cancer Res.
4:763–771. 1998.PubMed/NCBI
|
|
7
|
Yao M, Zhou W, Sangha S, et al: Effects of
nonselective cyclooxygenase inhibition with low-dose ibuprofen on
tumor growth, angiogenesis, metastasis, and survival in a mouse
model of colorectal cancer. Clin Cancer Res. 11:1618–1628. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bonelli P, Tuccillo FM, Federico A, et al:
Ibuprofen delivered by poly(lactic-co-glycolic acid) (PLGA)
nanoparticles to human gastric cancer cells exerts
antiproliferative activity at very low concentrations. Int J
Nanomed. 7:5683–5691. 2012. View Article : Google Scholar
|
|
9
|
Lanas A: A review of the gastrointestinal
safety data - a gastroenterologist’s perspective. Rheumatology.
49(Suppl 2): ii3–ii10. 2010. View Article : Google Scholar
|
|
10
|
Mallen SR, Essex MN and Zhang R:
Gastrointestinal tolerability of NSAIDs in elderly patients: a
pooled analysis of 21 randomized clinical trials with celecoxib and
nonselective NSAIDs. Curr Med Res Opin. 27:1359–1366. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brigger I, Dubernet C and Couvreur P:
Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev.
54:631–651. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Potta SG, Minemi S, Nukala RK, et al:
Preparation and characterization of ibuprofen solid lipid
nanoparticles with enhanced solubility. J Microencapsul. 28:74–81.
2011. View Article : Google Scholar
|
|
13
|
Kokawa A, Kondo H, Gotoda T, et al:
Increased expression of cyclooxygenase-2 in human pancreatic
neoplasms and potential for chemoprevention by cyclooxygenase
inhibitors. Cancer. 91:333–338. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
O’Driscoll CM: Lipid-based formulations
for intestinal lymphatic delivery. Eur J Pharm Sci. 15:405–415.
2002. View Article : Google Scholar
|
|
15
|
Mehnert W and Mäder K: Solid lipid
nanoparticles: production, characterization and applications. Adv
Drug Deliv Rev. 47:165–196. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matusheski NV, Juvik JA and Jeffery EH:
Heating decreases epithiospecifier protein activity and increases
sulforaphane formation in broccoli. Phytochemistry. 65:1273–1281.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fahey JW, Haristoy X, Dolan PM, et al:
Sulforaphane inhibits extracellular, intracellular, and
antibiotic-resistant strains of Helicobacter pylori and prevents
benzo[a]pyrene-induced stomach tumors. Proc Natl Acad Sci USA.
99:7610–7615. 2002. View Article : Google Scholar
|
|
18
|
Kuroiwa Y, Nishikawa A, Kitamura Y, et al:
Protective effects of benzyl isothiocyanate and sulforaphane but
not resveratrol against initiation of pancreatic carcinogenesis in
hamsters. Cancer Lett. 241:275–280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chung FL, Conaway CC, Rao CV and Reddy BS:
Chemoprevention of colonic aberrant crypt foci in Fischer rats by
sulforaphane and phenethyl isothiocyanate. Carcinogenesis.
21:2287–2291. 2000. View Article : Google Scholar
|
|
20
|
Kallifatidis G, Rausch V, Baumann B, et
al: Sulforaphane targets pancreatic tumour-initiating cells by
NF-kappaB-induced anti-apoptotic signalling. Gut. 58:949–963. 2009.
View Article : Google Scholar
|
|
21
|
Sze A, Erickson D, Ren L and Li D:
Zeta-potential measurement using the Smoluchowski equation and the
slope of the current-time relationship in electroosmotic flow. J
Colloid Interface Sci. 261:402–410. 2003. View Article : Google Scholar
|
|
22
|
Petersen S, Steiniger F, Fischer D, Fahr A
and Bunjes H: The physical state of lipid nanoparticles influences
their effect on in vitro cell viability. Eur J Pharm Biopharm.
79:150–161. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schöler N, Hahn H, Müller RH and
Liesenfeld O: Effect of lipid matrix and size of solid lipid
nanoparticles (SLN) on the viability and cytokine production of
macrophages. Int J Pharm. 231:167–176. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schöler N, Olbrich C, Tabatt K, Müller RH,
Hahn H and Liesenfeld O: Surfactant, but not the size of solid
lipid nanoparticles (SLN) influences viability and cytokine
production of macrophages. Int J Pharm. 221:57–67. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Arnarson T and Elworthy PH: Effects of
structural variations on non-ionic surfactants on micellar
properties and solubilization: surfactants containing very long
hydrocarbon chains. J Pharm Pharmacol. 33:141–144. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sanna V, Caria G and Mariani A: Effect of
lipid nanoparticles containing fatty alcohols having different
chain length on the ex vivo skin permeability of Econazole nitrate.
Powder Technol. 201:32–36. 2010. View Article : Google Scholar
|
|
27
|
Patil S, Sandberg A, Heckert E, Self W and
Seal S: Protein adsorption and cellular uptake of cerium oxide
nanoparticles as a function of zeta potential. Biomaterials.
28:4600–4607. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bernfield M, Götte M, Park PW, et al:
Functions of cell surface heparan sulfate proteoglycans. Annu Rev
Biochem. 68:729–777. 1999. View Article : Google Scholar
|
|
29
|
Win KY and Feng SS: Effects of particle
size and surface coating on cellular uptake of polymeric
nanoparticles for oral delivery of anticancer drugs. Biomaterials.
26:2713–2722. 2005. View Article : Google Scholar
|
|
30
|
Cheng H, Zhu JL, Zeng X, Jing Y, Zhang XZ
and Zhuo RX: Targeted gene delivery mediated by
folate-polyethylenimine-block-poly(ethylene glycol) with receptor
selectivity. Bioconjug Chem. 20:481–487. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Al-Wadei HA, Al-Wadei MH, Ullah MF and
Schuller HM: Celecoxib and GABA cooperatively prevent the
progression of pancreatic cancer in vitro and in xenograft models
of stress-free and stress-exposed mice. PLoS One. 7:e433762012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mukherjee P, Basu GD, Tinder TL, et al:
Progression of pancreatic adenocarcinoma is significantly impeded
with a combination of vaccine and COX-2 inhibition. J Immunol.
182:216–224. 2009. View Article : Google Scholar
|
|
33
|
Streicher SA, Yu H, Lu L, Kidd MS and
Risch HA: Case-control study of aspirin use and risk of pancreatic
cancer. Cancer Epidemiol Biomarkers Prev. 23:1254–1263. 2014.
View Article : Google Scholar : PubMed/NCBI
|