Introduction
Pancreatic cancer, of which ~85% are ductal
adenocarcinomas, remains one of the most aggressive and deadly
malignancies worldwide (1). In the
USA, pancreatic cancer is diagnosed in ~46,000 people annually,
with 40,000 of these patients dying (2). Surgical resection is a potential
curative therapy for pancreatic cancer; however, ~80% of patients
are diagnosed too late to be operated on because of vague symptoms
and lack of an efficient screening method to detect pancreatic
cancer at an earlier stage (1).
Even patients who have undergone resection have a poor prognosis
because pancreatic cancers often relapse. Patients with
unresectable or metastatic pancreatic cancer usually receive
chemotherapy with gemcitabine alone, or are subject to combination
chemotherapy such as gemcitabine plus albumin-bound paclitaxel
particles, or a FOLFIRINOX regimen (oxaliplatin, irinotecan,
fluorouracil and leucovorin) (3–5).
Although the efficacy of the FOLFIRINOX regimen in patients results
in considerable improvement, it often causes adverse side effects
such as fatigue, neutropenia, anemia, vomiting, diarrhea, and
sensory neuropathy (4,6). These adverse effects occur because
anticancer drugs affect cancer cells and normal cells. There is an
urgent need for the development of drugs that can selectively
target and kill malignant cells without adversely affecting
patients. Antibody-drug conjugates (ADCs) are drugs developed
through the conjugation of an anticancer agent to a monoclonal
antibody (mAb), thereby making them selective for certain cells
(7). Antibodies for ADCs must be
internalized by the target cells, but few methods are available for
screening the ability of mAbs to be internalized by cells. We
recently developed a recombinant protein, DT3C, comprising
diphtheria toxin (DT) lacking the receptor-binding domain but
containing the C1, C2, and C3 domains of Streptococcus
protein G (3C) (8). When a
mAb-DT3C conjugate, which functions like an ADC in vitro,
reduces the viability of cancer cells, the mAb being tested must
have been internalized by target cells. This screening method using
DT3C has enabled us to develop a new mAb that recognizes mucin 13
(MUC13), and is internalized by pancreatic cancer cells.
Histologically, most pancreatic cancers are ductal
adenocarcinomas. Glandular epithelia, from which adenocarcinoma
cells arise, produce mucin, a macromolecule glycoprotein. The mucin
family of proteins covers the apical surface of the respiratory,
gastrointestinal, or genital tract; they can be categorized as
secreted or transmembrane type proteins (9,10).
It appears that transmembrane mucins, whose expression is often
increased in cancer cells, play an important role in the
development or maintenance of adenocarcinomas (9–12).
MUC13, our focus in the current study, is a transmembrane mucin and
is expressed at the mRNA and protein levels in several normal
glandular epithelia such as the intestine, colon or pancreatic duct
(13). Expression levels of MUC13
appear to be upregulated in gastric, colonic, pancreatic, and
ovarian cancers. Increased expression of MUC13 seems to enhance the
proliferation, migration, or invasion of cancer cells (14–21).
To the best of our knowledge, no reports have shown that MUC13 is a
candidate target molecule that ADCs can bind to. Additionally,
there are only two reports that reveal the expression of MUC13 in
pancreatic cancers (18,20). Our objective was to determine if
MUC13 could be a target molecule in pancreatic cancer therapy, and
we have found that pancreatic carcinoma tissues examined expressed
more MUC13 than that in normal tissues, and that anti-MUC13
mAb-DT3C conjugates induced cell death in pancreatic cancer
cells.
Materials and methods
Ethics
The experimental procedures were approved by the
Institutional Review Board at Sapporo Medical University.
Cell lines
We used the human TCC-PAN2 pancreatic cancer cell
line (Japanese Collection of Research Bioresources Cell Bank,
Osaka, Japan), the mouse myeloma P3U1 cell line (Japanese
Collection of Research Bioresources Cell Bank), and the CHO-K1
Chinese hamster ovary cell line (American Type Culture Collection,
Manassas, VA, USA). All cell lines were cultured in RPMI-1640 or
DMEM supplemented with 10% (v/v) Super Low IgG-FBS (Hyclone, Thermo
Scientific, Waltham, MA, USA), 1 mM sodium pyruvate (Life
Technologies Japan, Tokyo, Japan), and 1% (v/v)
streptomycin-penicillin-glutamine solution (Life Technologies) at
37°C/5% CO2.
Production of DT3C
DT3C was produced and purified as described
previously (8).
Production and screening of
hybridomas
We intraperitoneally injected a Balb/c mouse with
phosphate-buffered saline (PBS) containing 5×106
TCC-PAN2 cells every 7 days. A final booster injection was
administered after 7 days with the same number of the cells
(Fig. 1A). Two days after the
final injection the mouse was sacrificed, and 7.75×107
splenocytes were fused with 1.55×107 P3U1 cells using
polyethylene glycol. When the hybridomas had grown to ~50%
confluence, the culture supernatant fluid that was likely to
contain polyclonal antibodies was tested for antibody
internalization. The supernatant from each well, and DT3C, were
incubated in 96-well microplates at room temperature for 30 min to
form Ab-DT3C conjugates. TCC-PAN2 cells were then added to each
well at a concentration of 1×104 cells/well and
incubated for 72 h to evaluate the degree of survival for TCC-PAN2
cells in each well in the presence of Ab-DT3C conjugates. The
number of viable cells after treatment was then estimated using a
WST-1 assay (Roche Diagnostics, Indianapolis, IN, USA). The
hybridomas that induced extensive cell death were selected and
cloned by limiting dilution. The mouse mAb was purified using
Protein G Sepharose 4 Fast Flow (GE Healthcare Japan, Tokyo,
Japan). A Mouse Monoclonal Antibody Isotyping kit (Roche Applied
Science, Mannheim, Germany) was used to identify the antibody
isotype.
Immunoprecipitation and mass
spectrometry
We prepared TCC-PAN2 cells and biotinylated their
surfaces using a Biotinylation kit (Thermo Scientific). Membranes
were solubilized on ice for 30 min in 1 ml of buffer comprising 1%
(v/v) NP40, 50 mM Tris-HCl pH 7.6, 150 mM NaCl, and a protease
inhibitor cocktail (Roche Applied Science). A mouse mAb, TCC56,
which we identified and was extensively analyzed in this study, and
a control mouse IgG3 (BD Biosciences, Tokyo, Japan) were used.
Details of these experiments have been described previously
(22,23).
Confirmation of mass spectrometry
results
MUC13 or control cDNA were ligated into the pEZ-M02
expression vector (GeneCopoeia, Rockville, MD, USA). The
recombinant plasmid was transfected into CHO-K1 cells using FuGENE
HD Transfection reagent (Promega KK, Tokyo, Japan). At 48 h
post-transfection, transfected cells were trypsinized, washed and
suspended in staining buffer (2% FBS/PBS) containing saturating
amounts of anti-MUC13 (TCC56) or negative control mouse IgG1 (clone
P3; eBioScience, Affymetrix Japan, Tokyo, Japan). The reactivity of
each mAb was analyzed by flow cytometry using a FACSCalibur (BD
Biosciences).
Evaluating mAb internalization by
cells
A mAb and DT3C were incubated at room temperature
for 30 min to generate the mAb-DT3C conjugate. TCC-PAN2 cells were
seeded and incubated in the presence of the mAb-DT3C conjugate for
120 h. The viability of cancer cells was assessed using a WST-1
assay. We determined, and used a quantity of mAb required for
sufficient formation of mAb-DT3C conjugates at each DT3C
concentration tested. In theory, each conjugate consisted of one
mAb molecule (150 kDa) and two DT3Cs (140 kDa) (8).
Primary pancreatic cancer tissues
Patients (n=156) were operated on for pancreatic
ductal cancer at Sapporo Medical University Hospital between August
2001 and February 2013. We selected 40 patients whose tumors were
resected completely (R0 resection) and who were followed up after
surgery on a regular basis. The patient group comprised 22 men and
18 women, with a median age of 69 years (range, 41–84 years). All
tumor slides were stained with hematoxylin and eosin (HE), and
reviewed by one of the authors (Yuji Sakuma) to verify diagnosis.
We classified 35 tumors as well differentiated (n=17), moderately
differentiated (n=14), and poorly differentiated (n=4) carcinomas,
and designated these as invasive ductal adenocarcinomas. The other
tumors (n=5) were adenosquamous cell carcinomas according to
histological results. Pathological examination confirmed that all
tumors were completely resected, 25 of the 40 patients died within
two years after surgery, while the remaining patients (n=15)
survived for three years or more without evidence of recurrence
after the operation.
MUC13 immunohistochemistry
Immunohistochemical staining for MUC13 was performed
on formalin-fixed, paraffin-embedded (FFPE) pancreatic cancer
tissue sections. We selected a representative FFPE tissue block
from each patient that contained non-cancerous and cancerous
pancreatic tissue to precisely evaluate MUC13 expression in both
regions. Whole tissue sections were retrieved using Novocastra
Epitope Retrieval Solutions pH 6.0 (Leica Biosystems, Nussloch,
Germany) at 100°C for 20 min. An anti-MUC13 mAb (clone ppz0020),
which was developed previously (14), was used as a primary antibody at 10
μg/ml, and immunohistochemical staining conducted using a Leica
BOND-MAX (Leica). Immunohistochemical staining of normal pancreatic
ducts was used as an internal positive control. MUC13 membrane
staining was scored based on intensity (0, none; 1, weak; 2,
intermediate; 3, strong) and proportion of carcinoma or normal
ductal cells expressing the molecule (0, none; 1, <1/100; 2,
1/100–1/10; 3, 1/10–1/3; 4, 1/3–2/3; 5, >2/3) using the Allred
scoring method (24).
Statistical analysis
We used the Wilcoxon signed-ranks test to evaluate
the differences in Allred scores for MUC13 expression between
cancerous and non-cancerous ductal cells. Differences in Allred
score among the four tumor subtypes were evaluated by the
Kruskal-Wallis test. We used the Kaplan-Meier method to estimate
overall survival; the survival curves between the high expression
(Allred score ≥6) and low expression (≤5) groups of patients were
compared using the log-rank test. A P-value <0.05 was considered
significant. All statistical calculations were performed with JMP
software (JMP for Windows version 7; SAS Institute Japan; Tokyo,
Japan).
Results
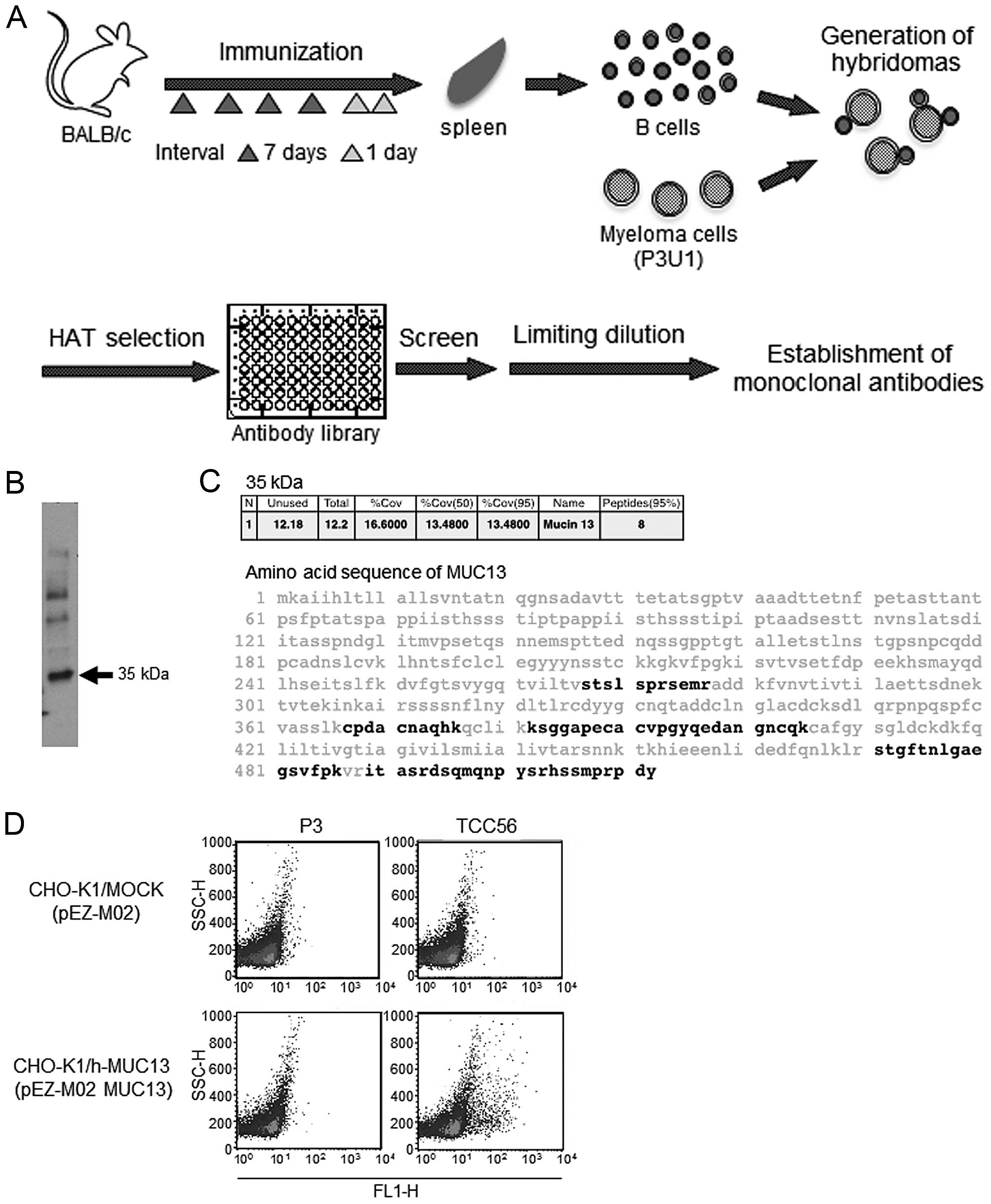
Establishment of anti-MUC13 mAb
TCC56
We cloned hybridomas from wells where Abs contained
in each well and DT3C conjugate (Ab-DT3C conjugates) had
substantially decreased viability of TCC-PAN2 cells. Cell viability
in each well reflected the efficiency of internalization for
Ab-DT3C conjugates into TCC-PAN2 cells. We consequently established
a hybridoma secreting mAb TCC56 (Fig.
1A), which was an IgG3κ isotype. Biotinylated proteins were at
35 kDa by immunoprecipitation using mAb TCC56 under reducing
conditions (Fig. 1B). MUC13 was
identified as a possible candidate of the 35 kDa molecule by mass
spectrometry (Fig. 1C). Flow
cytometry results indicated that mAb TCC56 reacted with
transfectants expressing MUC13 (Fig.
1D).
The mAb TCC56-DT3C conjugate kills
TCC-PAN2 cells
We confirmed that the MUC13 protein was expressed in
TCC-PAN2 cells by flow cytometry (Fig.
2A). We assessed to what degree the TCC56-DT3C conjugate could
induce cell death in TCC-PAN2 cells. The TCC56-DT3C conjugate
decreased the viability of TCC-PAN2 cells in a
concentration-dependent manner; viability was <20% at 0.1 μg/ml
DT3C, while neither DT3C alone nor an IgG3-DT3C (control) conjugate
was able to induce cell death even at 1 μg/ml DT3C (Fig. 2B).
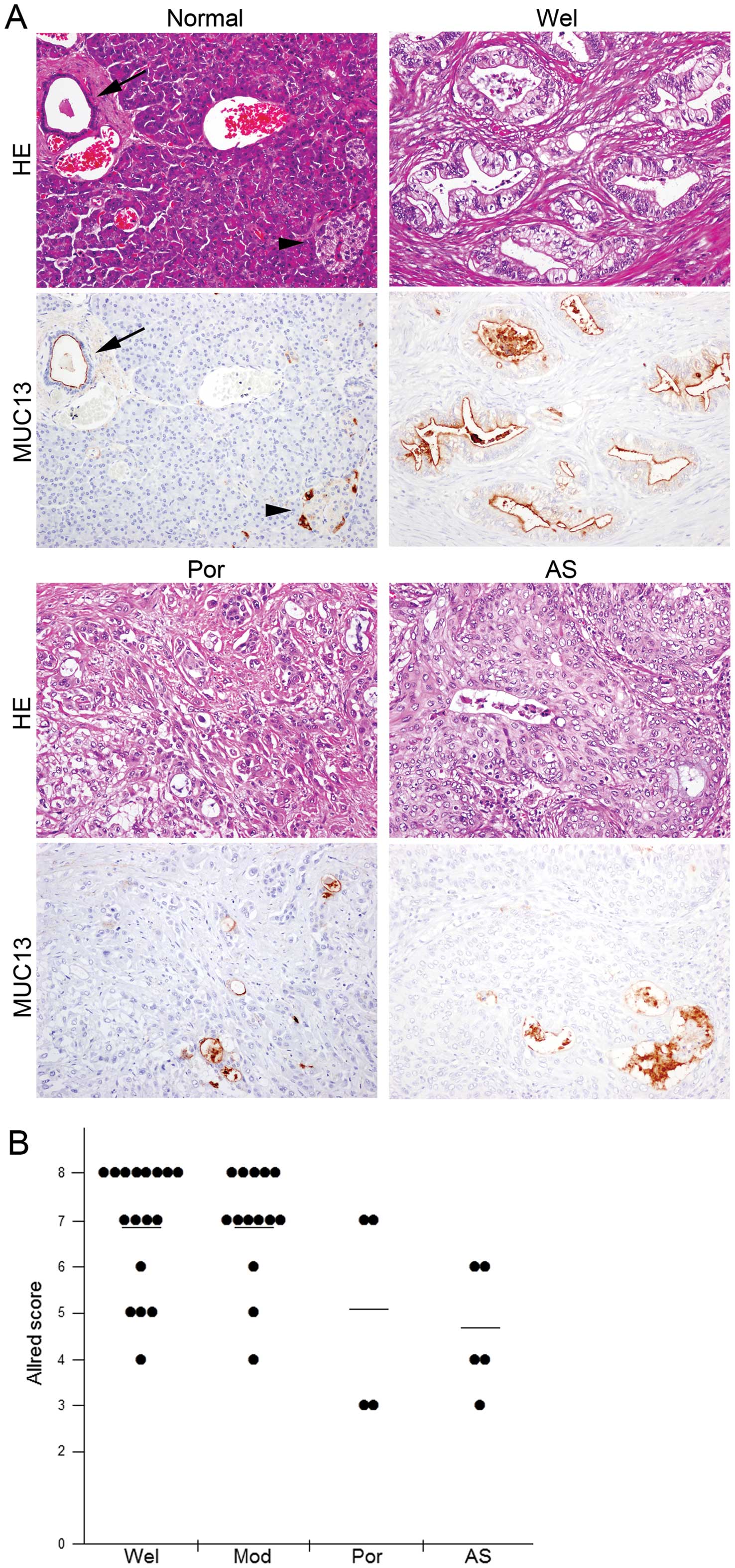
Pancreatic ductal adenocarcinoma cells
express higher levels of MUC13
In non-cancerous pancreatic tissues, MUC13
expression was observed in ductal cells and some islet cells. While
the protein was localized at the apical membrane of ductal cells,
islet cells positive for MUC13 staining showed it was present
throughout the cytoplasm, but was not observed at the membrane
(Fig. 3A), suggesting that the
expression observed in islet cells was due to non-specific
staining. In contrast, of all 40 cancer tissues examined MUC13 was
expressed in varying degrees and was confined to the apical
membrane of glands where adenocarcinoma cells formed (Fig. 3A). Expression levels of MUC13
varied substantially among histological subtypes (p=0.0122); well
or moderately differentiated ductal adenocarcinomas expressed MUC13
at higher levels than in poorly differentiated ductal
adenocarcinomas or adenosquamous cell carcinomas (Fig. 3). We did not observe MUC13
expression in squamous cell carcinoma components of the five
adenosquamous cell carcinomas examined. Expression levels of MUC13
in carcinoma tissues were slightly, but significantly higher than
those in normal tissues (tumor vs. normal: 6.4±1.6 vs. 5.6±0.9,
p=0.0023).
Pancreatic carcinomas with low expression
levels of MUC13 were associated with poor outcome
We examined if expression levels of MUC13 affected
clinical outcome in patients with pancreatic cancers. Pancreatic
carcinomas with lower MUC13 expression levels (Allred score ≤5,
n=11) were weakly associated with unfavorable outcomes compared
with cancers exhibiting higher expression levels of MUC13 (Allred
score ≥6, n=29), however this difference was not statistically
significant (p=0.3241) (Fig.
4).
Discussion
We have developed a new anti-MUC13 mAb TCC56 to be
internalized by cells using DT3C-based screening. Increased
expression of MUC13 is reported for several carcinomas, including
pancreatic cancer, therefore we hypothesized that MUC13 is a likely
potential target molecule for cancer therapy (14–21).
If an ADC comprising mAb TCC56 and a potent anti-cancer drug was
produced, the new ADC could be effective for several cancers
expressing MUC13. We have also demonstrated that all 40 pancreatic
cancer tissues examined in this study expressed MUC13 at least
partially, and that the expression levels of MUC13 in
adenocarcinoma cells were significantly greater than those in
normal ductal cells. Our findings suggest MUC13 is a candidate
target molecule for treatment with ADCs. Our immunohistochemistry
results revealed that well or moderately differentiated
adenocarcinomas (n=31) expressed MUC13 to greater extent than in
poorly differentiated adenocarcinomas or adenosquamous cell
carcinomas (n=9). The MUC13 expression patterns observed suggest
that an anti-MUC13 mAb-based drug could be applied to a wide range
of pancreatic cancers. Cancers with low MUC13 expression levels
appear to be somewhat more aggressive than those with high MUC13
expression levels led us to surmise that MUC13 did not play a
critical role in the survival of pancreatic cancer cells. We
attempted to repress MUC13 expression in TCC-PAN2 cells using RNA
interference techniques, however MUC13 expression was barely
affected by the MUC13 short interfering RNA molecules we used,
possibly because of very low transfection efficiencies (data not
shown).
We postulated that MUC13 could not be targeted by
ADCs, including anti-MUC13 mAbs, as its localization was confined
to the apical membrane of cells. However, an ADC targeting an
apical antigen has been developed previously and exhibited evidence
of antitumor activity in phase I/II clinical trials involving
patients with non-small cell lung cancers or ovarian cancers
(25,26). The apical antigen targeted is a
pH-sensitive sodium-dependent phosphate transporter encoded by the
SLC34A2 gene, which is also known as NaPi2b (27,28).
These data raise the possibility that ADCs, including anti-MUC13
mAb TCC56, could affect cases where MUC13 is clearly expressed in
cancer cells.
In conclusion, we have developed a new anti-MUC13
mAb that could be efficiently internalized by cells. We have also
demonstrated that MUC13 expression levels in pancreatic cancer
tissues were higher than those in normal tissues, and that well or
moderately differentiated ductal adenocarcinomas clearly expressed
the protein. Our combined results suggest that MUC13 is a target
molecule for pancreatic cancer treatment. ADCs, including
anti-MUC13 mAbs, are promising anticancer agents that could
alleviate the adverse effects of various chemotherapies.
Acknowledgements
We are grateful to Dr Hirofumi Hamada (former
professor at Sapporo Medical University) for developing a sensitive
screening method for selecting antibodies to be internalized by
cells. We also thank Drs Mami Yamaguchi and Michitoshi Kimura
(Sapporo Medical University School of Medicine) for assistance with
immunohistochemistry.
References
|
1
|
Ryan DP, Hong TS and Bardeesy N:
Pancreatic adenocarcinoma. N Engl J Med. 371:1039–1049. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rossi ML, Rehman AA and Gondi CS:
Therapeutic options for the management of pancreatic cancer. World
J Gastroenterol. 20:11142–11159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Michael M and Moore M: Clinical experience
with gemcitabine in pancreatic carcinoma. Oncology (Williston
Park). 11:1615–1622. 1997.
|
|
4
|
Kindler HL: A new direction for pancreatic
cncer treatment: FOLFIRINOX in context. Am Soc Clin Oncol Educ
Book. 2012:232–237. 2012.
|
|
5
|
Von Hoff DD, Ervin T, Arena FP, et al:
Increased survival in pancreatic cancer with nab-paclitaxel plus
gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Conroy T, Desseigne F, Ychou M, et al:
FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N
Engl J Med. 364:1817–1825. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Panowksi S, Bhakta S, Raab H, Polakis P
and Junutula JR: Site-specific antibody drug conjugates for cancer
therapy. MAbs. 6:34–45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamaguchi M, Nishii Y, Nakamura K, Aoki H,
Hirai S, Uchida H, Sakuma Y and Hamada H: Development of a
sensitive screening method for selecting monoclonal antibodies to
be internalized by cells. Biochem Biophys Res Commun. 454:600–603.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hollingsworth MA and Swanson BJ: Mucins in
cancer: protection and control of the cell surface. Nat Rev Cancer.
4:45–60. 2004. View
Article : Google Scholar
|
|
10
|
Kufe DW: Mucins in cancer: function,
prognosis and therapy. Nat Rev Cancer. 9:874–885. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jonckheere N, Skrypek N and Van Seuningen
I: Mucins and pancreatic cancer. Cancers. 2:1794–1812. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kaur S, Kumar S, Momi N, Sasson AR and
Batra SK: Mucins in pancreatic cancer and its microenvironment. Nat
Rev Gastroenterol Hepatol. 10:607–620. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Williams SJ, Wreschner DH, Tran M, Eyre
HJ, Sutherland GR and McGuckin MA: MUC13, a novel human cell
surface mucin expressed by epithelial and hemopoietic cells. J Biol
Chem. 276:18327–18336. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shimamura T, Ito H, Shibahara J, et al:
Overexpression of MUC13 is associated with intestinal-type gastric
cancer. Cancer Sci. 96:265–273. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Walsh MD, Young JP, Leggett BA, Williams
SH, Jass JR and McGuckin MA: The MUC13 cell surface mucin is highly
expressed by human colorectal carcinomas. Hum Pathol. 38:883–892.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chauhan SC, Vannatta K, Ebeling MC, et al:
Expression and functions of transmembrane mucin MUC13 in ovarian
cancer. Cancer Res. 69:765–774. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maher DM, Gupta BK, Nagata S, Jaggi M and
Chauhan SC: Mucin 13: structure, function, and potential roles in
cancer pathogenesis. Mol Cancer Res. 9:531–537. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chauhan SC, Ebeling MC, Maher DM, Koch MD,
Watanabe A, Aburatani H, Lio Y and Jaggi M: MUC13 mucin augments
pancreatic tumorigenesis. Mol Cancer Ther. 11:24–33. 2012.
View Article : Google Scholar
|
|
19
|
Gupta BK, Maher DM, Ebeling MC, et al:
Increased expression and aberrant localization of mucin 13 in
metastatic colon cancer. J Histochem Cytochem. 60:822–831. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khan S, Ebeling MC, Zaman MS, et al:
MicroRNA-145 targets MUC13 and suppresses growth and invasion of
pancreatic cancer. Oncotarget. 5:7599–7609. 2014.PubMed/NCBI
|
|
21
|
Gupta BK, Maher DM, Ebeling MC, Stephenson
PD, Puumala SE, Koch MR, Aburatani H, Jaggi M and Chauhan SC:
Functions and regulation of MUC13 mucin in colon cancer cells. J
Gastroenterol. 49:1378–1391. 2014. View Article : Google Scholar
|
|
22
|
Suzuki K, Nakamura K, Kato K, et al:
Exploration of target molecules for prostate cancer gene therapy.
Prostate. 67:1163–1173. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ishii K, Nakamura K, Kawaguchi S, et al:
Selective gene transfer into neurons via Na, K-ATPase beta1.
Targeting gene transfer with monoclonal antibody and adenovirus
vector. J Gene Med. 10:597–609. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Allred DC, Harvey JM, Berardo M and Clark
GM: Prognostic and predictive factors in breast cancer by
immunohistochemical analysis. Mod Pathol. 11:155–168.
1998.PubMed/NCBI
|
|
25
|
Burris HA, Gordon MS, Gerber DE, et al: A
phase I study of DNIB0600A, an antibody-drug-conjugate (ADC)
targeting NaPi2b, in patients (pts) with non-small cell lung cancer
or platinum-resistant ovarian cancer (OC). J Clin Oncol. 32(Suppl):
25042014.
|
|
26
|
Ritter G, Yin B, Murray A, et al: Membrane
transporter NaPi2b (SCL34A1) epitope for antibody therapy,
antibodies directed thereto, and target for cancer therapy.
US8603474. Issued December 10, 2013.
|
|
27
|
Murer H, Forster I and Biber J: The sodium
phosphate cotransporter family SLC34. Pflugers Arch. 447:763–767.
2004. View Article : Google Scholar
|
|
28
|
Filonenko V, Gout T, Usenko VS, Lyzogubov
VV, Shyian M and Kiyamova R: Immunohistochemical analysis of NaPi2b
PROTEIN (MX35 antigen) expression and subcellular localization in
human normal and cancer tissues. Exp Oncol. 33:157–161.
2011.PubMed/NCBI
|