Introduction
Breast cancer is the most commonly diagnosed cancer
in women in the United States, while colorectal cancer is one of
the top three most commonly diagnosed cancers among men and women
(1–3). It is estimated that there will be
136,830 new colorectal cancer cases and 235,030 new breast cancer
cases in 2014 in the USA (4–6). The
incidence rates for breast and colorectal cancer have been
declining consistently in recent years among all age groups and
ethnicities (7–9). The decreasing incidence trends in
breast and colorectal cancer have been attributed to earlier
detection and more advanced treatment (10–14).
Although cancer incidence rates have declined since the early
1990’s, the burden of cancer and its complications remains high and
has a significant impact on human health (15–17).
A number of studies have examined Surveillance,
Epidemiology and End Results (SEER) data for national cancer
incidence trends and variations by age, ethnicity, cancer stage and
other factors in the USA (18–23).
However, the SEER data do not include Texas, a large and diverse
state. The Texas Cancer Registry collects data on cancer in Texas.
Few studies have examined the cancer incidence trends in the state
of Texas (24–26), and no study has ever been conducted
to compare the temporal trends of breast and colorectal cancer
incidence in Texas with those of SEER. Therefore, in this study, we
used the National Cancer Institute’s SEER data and Texas Cancer
Registry (TCR) data to examine whether the overall incidence trends
for both breast and colorectal cancer have similar patterns in the
TCR and in SEER areas over the past 17 years from 1995 to 2011
(2,27). We also examined the variations in
cancer incidence rates by age, gender, ethnicity, tumor stage and
tumor grade in the TCR and SEER areas. The findings from this
parallel comparison are expected to provide a significant overview
of cancer incidence trends at the state and national level, and
also to identify important factors associated with a decreasing
risk of cancer, which are critical to enhance cancer prevention and
control.
Materials and methods
Data sources
The SEER (Surveillance, Epidemiology and End
Results) public-use dataset and the TCR (Texas Cancer Registry)
limited-use dataset were utilized for this study (2,27).
The SEER program, supported by the National Cancer Institute,
includes population-based tumor registries in selected geographic
areas in the USA. Because of our study comparison between the TCR
and SEER for cancer cases in 1995 through 2011, we selected the
following 9 SEER areas: Atlanta, Connecticut, Detroit, Hawaii,
Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound and
Utah, accounting for ~9.4% of the USA population (2). By the year of 2010, there were 18
SEER registries that covered 26.2% of the USA population (2). Because our study aimed to compare the
incidence trends in breast and colorectal cancer from 1995 to 2011,
only these nine registries, which had complete records on tumor
stage and tumor grade in the study period, were included. The SEER
registries ascertain all newly diagnosed (incident) cancer cases
from multiple reporting sources such as hospitals, outpatient
clinics, laboratories, private medical practitioners,
nursing/convalescent homes/hospices, autopsy reports and death
certificates (2). The TCR is a
statewide and population-based cancer registry and is Gold
Certified by the North American Association of Central Cancer
Registries (27). The TCR dataset
was determined to cover at least 95% statewide data for all cancer
cases diagnosed from 1995 through 2011 in Texas. The denominator of
population data used to calculate incidence rates were acquired
from the U.S. Census Bureau’s Population Estimates Program
(28). The Committee for the
Protection of Human Subjects at the University of Texas Health
Science Center in Houston approved this study.
Study population
We identified all women diagnosed with breast cancer
and men and women diagnosed with colorectal cancer as their primary
tumor in 1995–2011 from both SEER and TCR. In the 9 SEER
registries, 328,142 patients with breast cancer and 224,511
patients with colorectal cancer were included. In Texas, 243,695
women with breast cancer and 155,551 patients with colorectal
cancer were included.
Study variables
The primary outcome of interest was the incidence
rates of breast and colorectal cancer from 1995 to 2011. Incidence
rates were defined as the number of new cases in one calendar year
divided by the total population at risk in the same year (29). Breast cancer cases were identified
using the ‘Primary Site’ variable in both SEER and TCR, coded as
C500–C509 according to ‘International Classification of Diseases
for Oncology, Third Edition (ICD-O-3), and Topography Section
(2,7)’. Colorectal cancer cases were coded as
C180–C189, C199, C209, and C260 (2). According to the methods by Wu et
al in counting total colorectal cancer cases, colon included
the cecum (C180), appendix (C181), ascending colon (C182), hepatic
flexure (C183), transverse colon (C184), splenic flexure (C185),
descending colon (C186), sigmoid colon (C187), and large intestine,
NOS(C188–C189,C260) (30). The
rectum included the rectosigmoid junction (C199) and the rectum-not
otherwise specified (C209).
The independent variables of interest in this study
included age, gender, ethnicity, tumor stage and tumor grade. Age
was classified according to five categories with <50, 50–59,
60–69, 70–79 and ≥80 years. Gender was a binary variable with male
and female, but for breast cancer, only women were included because
of the rarity of diagnosis in men. Ethnicity was categorized as
white, black and other. Tumor stage was classified as localized,
regional, distant and unknown. Localized stage was confined within
the breast and colon. Regional stage was defined as tumor
involvement of the regional lymph nodes, primarily those in the
axilla, to be involved. Distant stage was defined as the cancer
metastatic to other parts of the body as well. The unknown stage
was defined as having missing information of cancer status
(31). Tumor grade represented the
level of differentiation, in which poorly differentiated cancer
cells usually divide more quickly and therefore represent more
aggressive malignancies (32).
Tumor grade at diagnosis was stratified into four categories:
differentiated, moderately differentiated, poorly differentiated
and undetermined.
Statistical analysis
Annual incidence rates of breast and colorectal
cancer cases per 100,000 persons were age-adjusted to the 2000 US
standard population stratified by five age groups. The incidence
rates were computed by age-group, gender, race, tumor stage, and
tumor grade for breast and colorectal cancer separately. We used
the SEER*Stat software to calculate incidence rates by
dividing the number of new cancer cases in each category over the
total population at risk (33).
The SAS statistical software was used to calculate incidence rates
with 95% confidence intervals for TCR data. Poisson regression
model with population size specific to demographic groups as offset
variable were used to determine the association between incidence
rates and potential risk factors. Covariates included age-group,
gender, race, tumor stage and tumor grade. In order to determine
the temporal relationship, risk ratios between two cancer incidence
rates were calculated and adjusted by potential confounders. The
assumption of the Poisson model were examined by examining constant
variance plots of the variables. No specific pattern was detected
in the outputs indicating that the constant variance was valid.
Results
Table I presents
the total number of patients diagnosed with breast and colorectal
cancer in Texas and in 9 SEER areas. From 1995 to 2011, there were
243,695 new breast cancer cases and 155,551 colorectal cancer cases
in Texas, and 328,142 breast cancer cases and 224,511 colorectal
cancer cases in 9 SEER areas. The mean age for breast cancer was
60.5 years in the TCR and 61.7 years in SEER, and the mean age for
colorectal cancer was 67.2 years in the TCR and 69.2 years in SEER.
The age distribution of these cancer cases was similar overall in
the TCR and SEER, although the proportion of younger patients
appeared to be slightly higher in Texas. Over 50% of new breast
cancer cases occurred in those aged ≥60 years, while >70% of new
colorectal cancer cases occurred in those aged ≥60 years. There
were a slightly more male colorectal cancer cases in the TCR and
SEER, and >80% of patients were white. The overall distribution
by tumor stage and tumor grade was similar between Texas and SEER,
but the proportion of cancer cases with unknown tumor stage and
undetermined tumor grade was higher in the TCR than that in
SEER.
 | Table INumber (proportion) of patients with
breast and CRC in the Texas Cancer Registry (TCR) and SEER,
1995–2011, stratified by patient and tumor factors. |
Table I
Number (proportion) of patients with
breast and CRC in the Texas Cancer Registry (TCR) and SEER,
1995–2011, stratified by patient and tumor factors.
| No. (%) of cancer
cases |
|---|
|
|
|---|
| Patient and tumor
characteristics | TCR (%) | SEER (%) | TCR (%) | SEER (%) |
|---|
| Mean age | 60.46 | 61.73 | 67.15 | 69.16 |
| Age (years) |
| <50 | 60017 (24.63) | 74066 (22.57) | 17359 (11.16) | 20190 (8.99) |
| 50–59 | 59481 (24.41) | 76676 (23.37) | 28088 (18.06) | 35423 (15.78) |
| 60–69 | 55660 (22.84) | 72686 (22.15) | 37406 (24.05) | 48532 (21.62) |
| 70–79 | 43744 (17.95) | 62866 (19.16) | 40976 (26.34) | 62987 (28.06) |
| ≥80 | 24793 (10.17) | 41848 (12.75) | 31722 (20.39) | 57379 (25.56) |
| Gender |
| Male | | | 82571 (53.08) | 113388 (50.50) |
| Female | 243695 (100) | 328142 (100) | 72980 (46.92) | 111123 (49.50) |
| Race/ethnicity |
| White | 210903 (86.54) | 269958 (82.65) | 132793 (85.37) | 181261 (81.14) |
| Black | 26161 (10.74) | 30525 (9.35) | 19428 (12.49) | 22513 (10.08) |
| Other (American
Indian/Asian/Pacific Islander) | 6631 (2.72) | 26135 (8.00) | 3330 (2.14) | 19609 (8.78) |
| Tumor stage |
| Localized | 149999 (61.55) | 207998 (63.39) | 57800 (37.16) | 88825 (39.56) |
| Regional | 61325 (25.16) | 96063 (29.27) | 52374 (33.67) | 81824 (36.45) |
| Distant | 11567 (4.75) | 15808 (4.82) | 26594 (17.10) | 41776 (18.61) |
| Unknown | 20804 (8.54) | 8273 (2.52) | 18783 (12.08) | 12086 (5.38) |
| Tumor grade |
| I: Well
differentiated | 34324 (14.08) | 63902 (19.47) | 11562 (7.43) | 18962 (8.45) |
| II: Moderately
differentiated | 77260 (31.70) | 123943 (37.77) | 84543 (54.35) | 128657 (57.31) |
| III: Poorly
differentiated | 75475 (30.97) | 100962 (30.77) | 23173 (14.90) | 36499 (16.26) |
| IV:
Undetermined | 56636 (23.24) | 39335 (11.99) | 36273 (23.32) | 40393 (17.99) |
| Total | 243695 | 328142 | 155551 | 224511 |
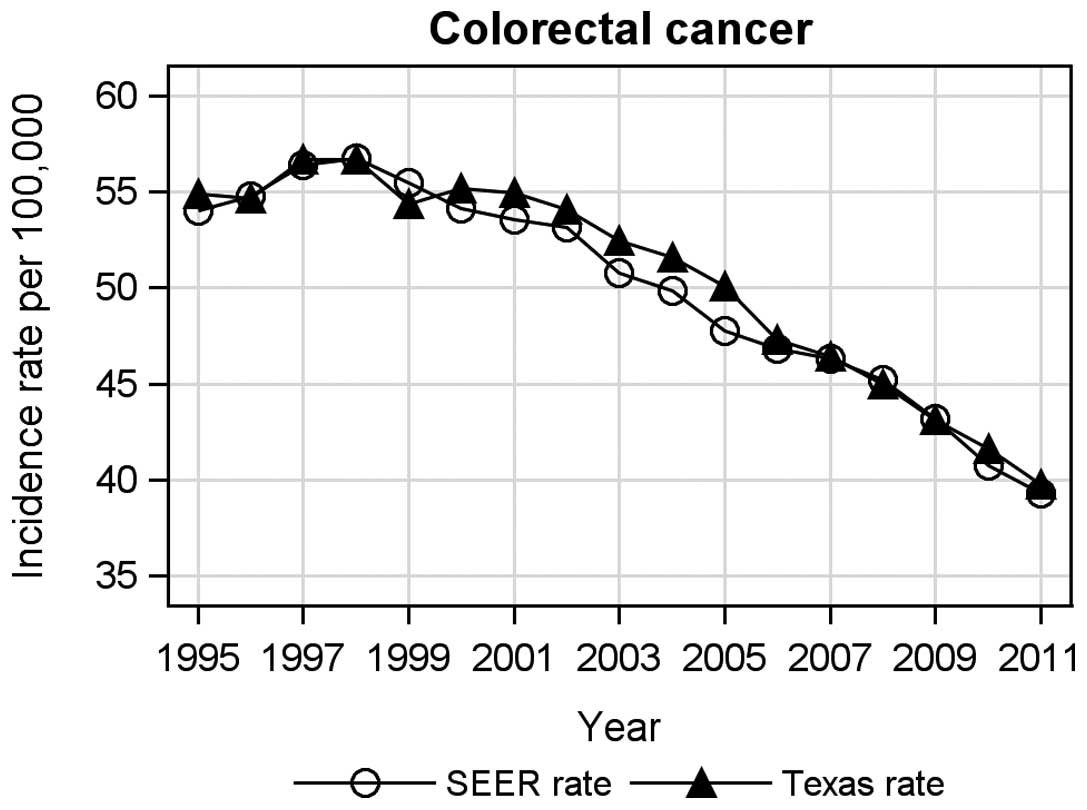
Fig. 1 presents
parallel comparisons of age-adjusted incidence trends from 1995 to
2011 for breast cancer between the TCR and SEER areas, whereas
Fig. 2 presents the age-adjusted
incidence trends from 1995 to 2011 for colorectal cancer in the TCR
as compared to SEER. The overall incidence trends and changing
patterns over the 17-year periods for breast and colorectal cancer
were almost identical between the TCR and SEER areas. Specifically,
breast cancer incidence increased from 1995 to 2001, decreased from
2002 to 2006, and then remained relatively stable from 2007 to 2011
(Fig. 1). The increased breast
cancer incidence in 1995–2001 was consistent with the time period
when the widespread use of screening program was implemented
(34). For colorectal cancer, the
incidence increased in the first three years between 1995 and 1997,
and then decreased continuously from 1998 to 2011 in both Texas and
SEER areas.
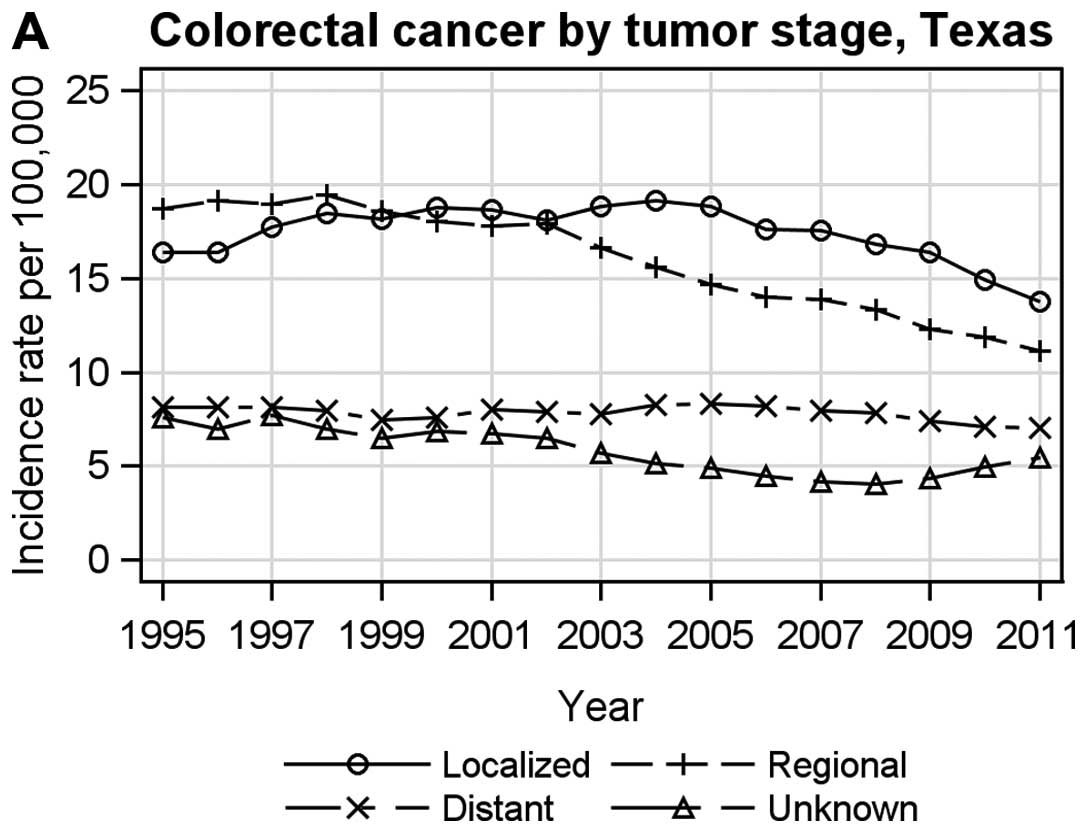
Figs. 3 and
4 present the age-adjusted tumor
stage-specific incidence rates for breast and colorectal cancer in
the TCR and SEER. For breast cancer, tumor stage-specific incidence
rates were similar between the TCR (Fig. 3A) and SEER (Fig. 3B), in which incidence for localized
breast cancer increased early on and then decreased, while the
incidence for distant stage breast cancer was stable with a slight
increase over time. For colorectal cancer, the incidence for all
stages decreased over time in the TCR (Fig. 4A) and in SEER (Fig. 4B) except for an increase for
unknown stage colorectal cancer in the TCR from 2008 to 2011.
Table II presents
the age-adjusted incidence rates of breast cancer and the relative
risks of cancer incidence by patient and tumor characteristics in
the TCR and SEER. The overall age-adjusted breast cancer incidence
in 1995–2011 was 134.74 per 100,000 in the TCR and 131.78 per
100,000 in SEER. The specific incidence rates and relative risks by
age, gender and ethnicity were also similar between in the TCR and
SEER after adjusting for tumor stage and grade. For example, as
compared to those <50 years of age, women ≥60 were >9 times
more likely to develop breast cancer, whereas American Indians and
Asian-Pacific Islanders were significantly less likely to develop
breast cancer and African Americans had a marginally lower risk of
developing breast cancer as compared to whites.
 | Table IIAge-adjusted incidence rates of
breast cancer in the Texas Cancer Registry (TCR) and in SEER,
1995–2011. |
Table II
Age-adjusted incidence rates of
breast cancer in the Texas Cancer Registry (TCR) and in SEER,
1995–2011.
| Breast cancer in
TCR | Breast cancer in
SEER |
|---|
|
|
|
|---|
|
Characteristics | Incidence (95%
CI)a | RR (95% CI)b | Incidence (95%
CI)a | RR (95% CI)b |
|---|
| Age (years) |
| <50 | 43.03
(42.24–43.82) | 1.00
(Reference) | 44.17
(43.85–44.49) | 1.00
(Reference) |
| 50–59 | 287.02
(274.65–299.38) | 6.58
(6.50–6.65) | 267.75
(265.86–269.66) | 6.16
(6.10–6.23) |
| 60–69 | 405.00
(392.57–417.44) | 9.34
(9.23–9.45) | 394.61
(391.75–397.50) | 9.29
(9.20–9.39) |
| 70–79 | 461.24
(444.70–477.78) | 10.63
(10.50–10.76) | 463.28
(459.66–466.92) | 11.74
(11.62–11.87) |
| ≥80 | 404.07
(389.27–418.87) | 9.27
(9.14–9.41) | 419.42
(415.40–423.46) | 12.06
(11.91–12.20) |
| Race/ethnicity |
| White | 136.14
(131.91–140.37) | 1.00 | 136.45
(135.93–136.97) | 1.00 |
| Black | 131.90
(129.40–134.40) | 0.98
(0.97–0.99) | 123.33
(121.93–124.75) | 0.93
(0.92–0.94) |
| Other (American
Indian/Asian/Pacific Islander) | 98.51
(89.74–107.27) | 0.77
(0.75–0.79) | 96.75
(95.58–97.94) | 0.72
(0.71–0.73) |
| Total | 134.74
(130.88–138.60) | | 131.78
(131.32–132.23) | |
Similarly, Table
III presents the age-adjusted incidence rates of colorectal
cancer and relative risks of cancer incidence stratified by patient
characteristics in the TCR and SEER after adjusting for tumor stage
and grade. Overall age adjusted colorectal cancer incidence rate
from 1995 to 2011 was 50.52 per 100,000 in the TCR and 49.44 per
100,000 in SEER. Because the mean age for developing colorectal
cancer was older than that for breast cancer, those aged 60–69
years were >23 times more likely to develop colorectal cancer
and those aged ≥80 years were >58 times more likely to develop
this disease than those <50 years of age. The risk of developing
colorectal cancer was noted to be significantly lower in women than
in men. African Americans had a higher risk of developing
colorectal cancer as compared to whites.
 | Table IIIAge-adjusted incidence rates of CRC
in the Texas Cancer Registry (TCR) and in SEER, 1995–2011. |
Table III
Age-adjusted incidence rates of CRC
in the Texas Cancer Registry (TCR) and in SEER, 1995–2011.
| Colorectal cancer
in TCR | Colorectal cancer
in SEER |
|---|
|
|
|
|---|
|
Characteristics | Incidence (95%
CI)a | RR (95% CI)b | Incidence (95%
CI)a | RR (95% CI)b |
|---|
| Age (years) |
| <50 | 6.11
(5.92–6.30) | 1.00
(Reference) | 6.03
(5.95–6.12) | 1.00
(Reference) |
| 50–59 | 68.46
(67.00–69.92) | 11.29
(11.07–11.50) | 63.13
(62.47–63.79) | 10.60
(10.42–10.79) |
| 60–69 | 145.38
(137.06–153.69) | 23.76
(23.33–24.19) | 139.91
(138.67–141.16) | 23.37
(22.98–23.76) |
| 70–79 | 245.37
(225.95–264.79) | 41.05
(40.33–41.79) | 262.84
(260.79–264.90) | 45.09
(44.38–45.82) |
| ≥80 | 342.33
(314.44–370.23) | 58.68
(57.60–59.78) | 371.32
(368.29–374.37) | 65.51
(64.46–66.59) |
| Gender |
| Male | 60.78
(57.26–64.29) | 1.00 | 57.49
(57.15–57.83) | 1.00 |
| Female | 42.70
(40.18–45.21) | 0.70
(0.69–0.71) | 43.12
(42.87–43.38) | 0.75
(0.74–0.76) |
| Race/ethnicity |
| White | 49.37
(46.52–52.22) | 1.00 | 48.87
(48.65–49.10) | 1.00 |
| Black | 64.84
(61.68–67.99) | 1.35
(1.33–1.37) | 58.43
(57.65–59.22) | 1.21
(1.19–1.22) |
| Other (American
Indian/Asian/Pacific Islander) | 36.69
(33.71–39.67) | 0.75
(0.72–0.77) | 42.67
(42.07–43.27) | 0.88
(0.86–0.89) |
| Total | 50.52
(47.67–53.38) | | 49.44
(49.23–49.64) | |
Discussion
This parallel comparison study between the TCR and
SEER reported a number of significant findings. The overall
incidence trends for both breast and colorectal cancer were noted
to have similar patterns from 1995 to 2011. The breast and
colorectal cancer incidence rates by age, gender, ethnicity, tumor
stage and tumor grade in the TCR were also similar to those in SEER
areas. These cancer incidence trends over time and variations by
other factors are important findings when monitoring cancer
progress, assessing the success of cancer prevention and control,
and identifying high-risk populations for additional intervention.
Furthermore, the identical cancer incidence trends reported in this
comparative study can also be viewed as important evidence of the
validity of the TCR’s incidence data. This is because the National
Cancer Institute’s SEER has been established since 1973 and is
often considered the gold standard in cancer registries (2).
This study demonstrated that breast cancer incidence
increased from 1995 to 2001, decreased from 2002 to 2006, and then
was relatively stable from 2007 to 2011. The interval of increased
breast cancer incidence was consistent with the time period when
widespread use of early detection for breast cancer such as
screening mammography programs were implemented. According to a
study by Swan et al, the increased use of mammography during
the late 1990’s resulted in a dramatically increased number of
breast cancer cases among females in the USA (35). Many other studies also supported
this finding (36,37). After the peak time increase in
2001, breast cancer incidence continued to decline and became
relatively stable over the past several years. A number of studies
have suggested that the decreased incidence rates may be
attributable to reduction in the use of peri-menopausal hormone
therapy, decreases in utilization of mammography and decreases in
the number of preclinical cases detected by screening in recent
years (38–44).
Similarly, we found that colorectal cancer incidence
increased from 1995 to 1997 and then continued to decrease from
1998 to 2011 in both the TCR and SEER areas. We did not observe any
dramatically increasing trend period associated with colorectal
cancer screening. Several previous studies have reported similar
findings and conclusions (7,8,29).
This may be related to the nature and gradual adoption of screening
tools for colorectal cancer over time (13,14).
The U.S. Preventive Service Task Force has recommended that fecal
occult blood testing and sigmoidoscopy be used for colorectal
cancer screening since the 1990s for persons aged ≥50 (45). The federal Medicare program began
covering the cost of colonoscopy screening for colorectal cancer
since 2001 for individuals with an average-risk of developing
colorectal cancer (46).
Colorectal cancer is known to occur later in life with a mean age
at 70 years (68 for men and 72 for women) (47,48).
We found that men had a higher risk of developing colorectal cancer
than women in both the TCR and SEER. Our finding is consistent with
the study by Cook et al who concluded that males had much
higher risk (RR, 1.36) for colorectal cancer than females from 1995
to 2004 (49). Differences in
colorectal cancer incidence rates stratified by ethnicity can
largely be explained by differences in education level, smoking
status and health insurance status (50–55).
In our analysis, the black population had highest colorectal cancer
incidence rates. Possible explanations include a larger percentage
of the smoking population in blacks; the highest prevalence of
cigarette smoking is also known to occur among individuals with
high school or lower education (56–58).
Although TCR and SEER datasets are known to be
comprehensive and of high quality, a number of factors may have
affected the findings. First, we were unable to verify specific
populations by year in each registry, which might have resulted in
biased calculations for the annual incidence rates. Second, we
studied the 9 SEER areas that accounted for ~9% of the USA
population, and therefore the results may not be generalizable to
all SEER areas or to the entire USA population. Furthermore, a
number of important known risk factors for breast and colorectal
cancer such as smoking, family history, physical exercise and
environmental factors are not included in these datasets and cannot
be studied. The differences in these factors may have affected the
cancer incidence comparisons.
In conclusion, breast and colorectal cancer
incidence trends from 1995 to 2011 were almost identical between
the TCR and SEER areas. Breast cancer incidence increased in
1995–2001 and decreased afterwards, while colorectal cancer
incidence decreased continuously from 1998 to 2011. Older age was a
significant risk factor for the high risk of developing cancer,
particularly for colorectal cancer. The cancer risk also varied
according to gender and race/ethnicity. Additional studies may be
needed to explore smaller geographical areas within these
registries and environmental factors associated with the changing
incidence trends.
Acknowledgements
This study was supported by a grant from the Cancer
Prevention and Research Institute of Texas (RP130051). We
acknowledge the efforts of the Texas Cancer Registry and the
National Cancer Institute in the creation of these databases. The
interpretation and reporting of these data are the sole
responsibilities of the authors.
References
|
1
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011. CA Cancer J Clin. 61:212–236. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
National Cancer Institute. Surveillance,
Epidemiology, and End Results (SEER): SEER limited-use data,
1973–2011 (9 registries databases). http://seer.cancer.gov.
Accessed May 29, 2014
|
|
3
|
DeSantis C, Siegel R, Bandi P and Jemal A:
Breast cancer statistics, 2011. CA Cancer J Clin. 61:408–418. 2011.
View Article : Google Scholar
|
|
4
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siegel R, DeSantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
American Cancer Society. Cancer facts and
figures. 2014, Atlanta: http://www.cancer.org/.
Accessed July 29, 2014
|
|
7
|
Edwards BK, Ward E, Kohler BA, et al:
Annual report to the nation on the status of cancer, 1975–2006,
featuring colorectal cancer trends and impact of interventions
(risk factors, screening, and treatment) to reduce future rates.
Cancer. 116:544–573. 2010. View Article : Google Scholar
|
|
8
|
Ries LA, Wingo PA, Miller DS, et al: The
annual report to the nation on the status of cancer, 1973–1997,
with a special section on colorectal cancer. Cancer. 88:2398–2424.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bray F, McCarron P and Parkin DM: The
changing global patterns of female breast cancer incidence and
mortality. Breast Cancer Res. 6:229–239. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Berry DA, Cronin KA, Plevritis SK, et al:
Effect of screening and adjuvant therapy on mortality from breast
cancer. N Engl J Med. 353:1784–1792. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Coughlin SS, Costanza ME, Fernandez ME, et
al: CDC-funded intervention research aimed at promoting colorectal
cancer screening in communities. Cancer. 107(Suppl 5): 1196–1204.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheng L, Eng C, Nieman LZ, Kapadia AS and
Du XL: Trends in colorectal cancer incidence by anatomic site and
disease stage in the united states from 1976 to 2005. Am J Clin
Oncol. 34:573–580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wulfkuhle JD, Liotta LA and Petricoin EF:
Proteomic applications for the early detection of cancer. Nat Rev
Cancer. 3:267–275. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
McKean-Cowdin R, Feigelson HS, Ross RK,
Pike MC and Henderson BE: Declining cancer rates in the 1990s. J
Clin Oncol. 18:2258–2268. 2000.PubMed/NCBI
|
|
15
|
Weir HK, Thun MJ, Hankey BF, et al: Annual
report to the nation on the status of cancer, 1975–2000, featuring
the uses of surveillance data for cancer prevention and control. J
Natl Cancer Inst. 95:1276–1299. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jemal A, Clegg LX, Ward E, et al: Annual
report to the nation on the status of cancer, 1975–2001 with a
special feature regarding survival. Cancer. 101:3–27. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ghafoor A, Jemal A, Ward E, Cokkinides V,
Smith R and Thun M: Trends in breast cancer by race and ethnicity.
CA Cancer J Clin. 53:342–355. 2003. View Article : Google Scholar
|
|
18
|
Pruitt SL, Shim MJ, Mullen PD, Vernon SW
and Amick BC III: Association of area socioeconomic status and
breast, cervical, and colorectal cancer screening: a systematic
review. Cancer Epidemiol Biomarkers Prev. 18:2579–2599. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Berry J, Bumpers K, Ogunlade V, et al:
Examining racial disparities in colorectal cancer care. J Psychosoc
Oncol. 27:59–83. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
White A, Liu C, Xia R, et al: Racial
disparities and treatment trends in a large cohort of elderly
african americans and caucasians with colorectal cancer, 1991 to
2002. Cancer. 113:3400–3409. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fowble BL, Schultz DJ, Overmoyer B, et al:
The influence of young age on outcome in early stage breast cancer.
Int J Radiat Oncol Biol Phys. 30:23–33. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chlebowski RT, Chen Z, Anderson GL, et al:
Ethnicity and breast cancer: Factors influencing differences in
incidence and outcome. J Natl Cancer Inst. 97:439–448. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
De Bruijn K, Arends L, Hansen B, Leeflang
S, Ruiter R and van Eijck C: Systematic review and meta-analysis of
the association between diabetes mellitus and incidence and
mortality in breast and colorectal cancer. Br J Surg.
100:1421–1429. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Coyle YM, Hynan LS, Euhus DM and
Minhajuddin AT: An ecological study of the association of
environmental chemicals on breast cancer incidence in texas. Breast
Cancer Res Treat. 92:107–114. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Coyle YM, Minahjuddin AT, Hynan LS and
Minna JD: An ecological study of the association of metal air
pollutants with lung cancer incidence in texas. J Thorac Oncol.
1:654–661. 2006. View Article : Google Scholar
|
|
26
|
Whitworth KW, Symanski E and Coker AL:
Childhood lympho-hematopoietic cancer incidence and hazardous air
pollutants in southeast texas, 1995–2004. Environ Health Perspect.
116:1576–1580. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Texas Cancer Registry. Epidemiology and
Surveillance branch, Texas Department of State Health Services, 211
E. 7th street, suite 325, Austin, TX 78701. http://www.dshs.state.tx.us/tcr/default.shtm/.
Accessed May 28, 2014
|
|
28
|
U.S. Census Bureau. American community
survey (ACS), 2014. http://www.census.gov/topics/population/.
Accessed June 11, 2014
|
|
29
|
Ries L, Melbert D, Krapcho M, et al: SEER
cancer statistics review, 1975–2005. Bethesda, MD: National Cancer
Institute; pp. 1975–2005. 2008
|
|
30
|
Wu XC, Chen VW, Steele B, et al:
Subsite-specific incidence rate and stage of disease in colorectal
cancer by race, gender, and age group in the united states,
1992–1997. Cancer. 92:2547–2554. 2001. View Article : Google Scholar
|
|
31
|
Ries LAG, Fritz AG and Hurlbut A: SEER
summary staging manual-2000: codes and coding instructions.
National Cancer Institute, SEER Program, NIH Pub. 62-84. 2007
|
|
32
|
Klassen AC, Curriero F, Kulldorff M,
Alberg AJ, Platz EA and Neloms ST: Missing stage and grade in
Maryland prostate cancer surveillance data, 1992–1997. Am J Prev
Med. 30:S77–S87. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
National Cancer Institute. Surveillance
epidemiology and end results (SEER). surveillance research program,
National Cancer Institute SEER*Stat software. Updated
version 8.1.5. 2014
|
|
34
|
Smith RA, Saslow D, Sawyer KA, et al:
American Cancer Society guidelines for breast cancer screening:
update 2003. CA Cancer J Clin. 53:141–169. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Swan J, Breen N, Coates RJ, Rimer BK and
Lee NC: Progress in cancer screening practices in the United
States. Cancer. 97:1528–1540. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jorgensen KJ and Gotzsche PC:
Overdiagnosis in publicly organised mammography screening
programmes: systematic review of incidence trends. BMJ.
339:b25872009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miller BA, Feuer EJ and Hankey BF: Recent
incidence trends for breast cancer in women and the relevance of
early detection: an update. CA Cancer J Clin. 43:27–41. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Althuis MD, Dozier JM, Anderson WF, Devesa
SS and Brinton LA: Global trends in breast cancer incidence and
mortality 1973–1997. Int J Epidemiol. 34:405–412. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ravdin PM, Cronin KA, Howlader N, et al:
The decrease in breast-cancer incidence in 2003 in the United
States. N Engl J Med. 356:1670–1674. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cronin KA, Ravdin PM and Edwards BK:
Sustained lower rates of breast cancer in the united states. Breast
Cancer Res Treat. 117:223–224. 2009. View Article : Google Scholar
|
|
41
|
Glass AG, Lacey JV Jr, Carreon JD and
Hoover RN: Breast cancer incidence, 1980–2006 combined roles of
menopausal hormone therapy, screening mammography, and estrogen
receptor status. J Natl Cancer Inst. 99:1152–1161. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Breen N, Cronin AK, Meissner HI, et al:
Reported drop in mammography. Cancer. 109:2405–2409. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jemal A, Ward E and Thun MJ: Recent trends
in breast cancer incidence rates by age and tumor characteristics
among U.S. women. Breast Cancer Res. 9:R282007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pelucchi C, Levi F and La Vecchia C: The
rise and fall in menopausal hormone therapy and breast cancer
incidence. Breast. 19:198–201. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
U.S. Preventive Services Task Force.
Screening for colorectal cancer: U.S. preventive services task
force recommendation statement. Ann Intern Med. 149:627–637. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gross CP, Andersen MS, Krumholz HM, McAvay
GJ, Proctor D and Tinetti ME: Relation between medicare screening
reimbursement and stage at diagnosis for older patients with colon
cancer. JAMA. 296:2815–2822. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Slater G, Papatestas AE, Tartter PI,
Mulvihill M and Aufses AH Jr: Age distribution of right- and
left-sided colorectal cancers. Am J Gastroenterol. 77:63–66.
1982.PubMed/NCBI
|
|
48
|
American Cancer Society. Colorectal cancer
facts & figures 2011–2013, Atlanta. http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-028312.pdfacs/groups/content/@epidemiologysurveilance/documents/document/acspc-028312.pdf.
Accessed July 8, 2014
|
|
49
|
Cook MB, Dawsey SM, Freedman ND, et al:
Sex disparities in cancer incidence by period and age. Cancer
Epidemiol Biomarkers Prev. 18:1174–1182. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Goel MS, Wee CC, McCarthy EP, Davis RB,
Ngo-Metzger Q and Phillips RS: Racial and ethnic disparities in
cancer screening. J Gen Intern Med. 18:1028–1035. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Belasco EJ, Gong G, Pence B and Wilkes E:
The impact of rural health care accessibility on cancer-related
behaviors and outcomes. Appl Health Econ Health Policy. 12:461–470.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Leuven E, Plug E and Rønning M: Education
and Cancer Risk, IZA Discussion Paper, No. 7956. 2014, http://www.econstor.eu/bitstream/10419/93310/1/dp7956.pdf.
Accessed July 8, 2014
|
|
53
|
Parajuli R, Bjerkaas E, Tverdal A, et al:
The increased risk of colon cancer due to cigarette smoking may be
greater in women than men. Cancer Epidemiol Biomarkers Prev.
22:862–871. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hurley S, Goldberg D, Nelson DO, et al:
Risk of colorectal cancer associated with active smoking among
female teachers. Cancer Causes Control. 24:1291–1304. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tillmans LS, Vierkant RA, Wang AH, et al:
Associations between cigarette smoking, hormone therapy, and folate
intake with incident colorectal cancer by TP53 protein expression
level in a population-based cohort of older women. Cancer Epidemiol
Biomarkers Prev. 23:350–355. 2014. View Article : Google Scholar :
|
|
56
|
US Department of Health and Human
Services. Tobacco use among US racial/ethnic minority groups -
African Americans, American indians and Alaska natives, Asian
Americans and Pacific islanders, and Hispanics: a report of the
surgeon general. National Center for Chronic Disease Prevention and
Health Promotion, Office on Smoking and Health. pp. 901998
|
|
57
|
Fiore MC, Novotny TE, Pierce JP,
Hatziandreu EJ, Patel KM and Davis RM: Trends in cigarette smoking
in the United States: the changing influence of gender and race.
JAMA. 261:49–55. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Novotny TE, Warner KE, Kendrick JS and
Remington PL: Smoking by blacks and whites: socioeconomic and
demographic differences. Am J Public Health. 78:1187–1189. 1988.
View Article : Google Scholar : PubMed/NCBI
|