Introduction
Reactive oxygen species (ROS) are produced
endogenously through the electron transport pathway in mitochondria
as well as various metabolic pathways (1–3). ROS
are also generated in response to exogenous stimuli such as
chemical stress and irradiation, among others (2,3).
They promote tumor progression, including migration, invasiveness
and metastasis, by activating a variety of signal cascades
(4). ROS induced by
3,5,6-trichloro-2-pyridyloxyacetic acid (TPA) play an important
role in cell migration (4).
Treatment of mouse mammary epithelial cells with a low dose of
hydrogen peroxide (H2O2) resulted in
morphological changes and an increase in invasive potential
(5). Invasive potential of cells
has also been reported to be increased by oxidative stress
generated from nicotinamide adenine dinucleotide phosphate (NA DPH)
oxidase (6).
Nuclear factor-κB (NF-κB) is a transcription factor
involved in the regulation of development, cell growth, immune
response and inflammation (4,7–9).
NF-κB is activated by tumor necrosis factor-α (TNF-α) stimuli and
is associated with tumor cell survival and tumor progression
(7). NF-κB functions as an
anti-apoptotic factor, and deregulation of NF-κB is often detected
in a variety of cancer cell types (10). NF-κB activity is upregulated in
many cancer cells and contributes to tumor cell survival and tumor
progression (11–13). NF-κB is activated by ROS produced
by the mitochondrial respiratory chain (14). Exogenous treatment of
H2O2 regulates NF-κB activation through
phosphorylation of inhibitor of κB (IκB)α (15). Inhibitor of κB kinase (IKK) is also
a mediator of ROS-induced NF-κB activation (16). IKK is composed of IKKα and IKKβ,
which are catalytic kinases, and IKKγ, which is a regulatory kinase
(7). Treatment of cells with
antioxidants such as N-acetyl-L-cysteine (NAC) or pyrrolidine
dithiocarbamate (PDTC) inhibits IKK and NF-κB activation induced by
TNF-α or oxidative stress (17).
Several studies have demonstrated that constitutive NF-κB
activation results from sustained activation of upstream mediators
such as IKK or an increase in the rate of IκB degradation (18–20).
Therefore, cancer cells that show downregulation of NF-κB by IκB
are sensitive to cell death triggered by anti-cancer drugs
(21). Suppression of NF-κB
activity has also been shown to inhibit tumor cell growth in animal
models (13,22).
Reactive oxygen species modulator 1 (Romo1) is
located in mitochondria, and upregulated Romo1 expression increases
cellular ROS levels (23,24). It was suggested that ROS derived
from Romo1 expression are essential for normal cell growth
(25,26). ROS derived from Romo1 are needed
for c-Myc induction for cell cycle entry (27). Increased Romo1 expression induced
by c-Myc also plays a role in Skp2-mediated c-Myc degradation via a
negative-feedback mechanism. Romo1 is involved in cell death
triggered by serum deprivation, oxidative stress and TNF-α
(28–30). Although Romo1 is highly expressed
in a variety of cancer cells, the role of Romo1 in cancer
progression is unclear (24).
Romo1 triggers DNA damage and its expression is associated with
drug-resistance to 5-FU (31,32).
Recently, we reported that Romo1 is highly expressed in
hepatocellular carcinoma (HCC) and that overexpression of Romo1 is
associated with tumor cell invasion (24). In a subsequent experiment, Romo1
stimulated NF-κB nuclear translocation and DNA-binding activity,
and its expression was associated with the constitutive nuclear
DNA-binding activity of NF-κB (33). On the basis of these results, we
hypothesized that tumor cell invasion induced by Romo1 expression
is associated with the NF-κB signaling pathway. To verify this
hypothesis, we investigated the correlation between Romo1
expression and NF-κB activation in oxidative stress-induced tumor
cell invasion.
Materials and methods
Cell culture
Human breast cancer cell line MDA-MB-231, human
hepatocarcinoma cell line Huh-7 and the SV-40 virus-transformed
WI-38 (normal lung fibroblasts) cell line WI-38 VA13 were purchased
from the Korean Cell Line Bank (Seoul, Korea). Wild-type (WT) mouse
embryonic fibroblasts (MEF s) and IKKα−/− and
IKKβ−/− MEF s were kindly provided by Dr Inder M. Verma
(Salk Institute for Biological Studies, La Jolla, CA, USA). Huh-7,
MDA-MB-231, and WT, IKKα−/− and IKKβ−/− MEFs
were cultured in Dulbecco’s modified Eagle’s medium (DMEM)
(Gibco/Invitrogen Life Technologies, Grand Island, NY, USA)
containing 10% heat-inactivated fetal bovine serum (FBS) (Life
Technologies, Grand Island, NY, USA), 100 U/ml of penicillin, and
100 μg/ml streptomycin. WI-38 VA13 cells were cultured in Eagle’s
minimal essential medium (EMEM) (Gibco/Invitrogen Life
Technologies) supplemented with 10% FBS and antibiotics. Cells were
grown and maintained at 37°C in a humidified incubator with 5%
carbon dioxide.
Chemicals and reagents
H2O2, NAC, SB203580 (p38 MAPK
inhibitor), PD98059 (MKK1/MEK inhibitor), mouse
anti-cytosol-specific-β-actin antibody and anti-F lag (M2) antibody
were purchased from Sigma-A ldrich (St. L ouis, MO, USA). IKK-16,
rabbit polyclonal anti-I KKα antibody, mouse monoclonal anti-I KKβ
(H4) antibody and mouse polyclonal anti-p65 antibody were obtained
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Mouse
monoclonal antibody against Romo1 was obtained from OriGene
Technologies (Rockville, MD, USA). MitoSOX Red was purchased from
Molecular Probes (Eugene, OR, USA).
Cell transfection
Romo1 double-stranded small interfering RNA
(siRNA) sequences have been described previously (27,32).
Control and Romo1 siRNA were purchased from Bioneer Corp.
(Daejeon, Korea). cDNA s encoding Flag-Romo1 WT were described
previously (29). Cells were
transfected in 6-well plates or 60-mm dishes using Lipofectamine
2000 (Invitrogen Life Technologies) according to the manufacturer’s
instructions.
Invasion assay
Invasion assays were performed using polycarbonate
nucleopore membranes (Corning, Inc., Corning, NY, USA). Matrigel (1
mg/ml) was coated onto the membrane of a Transwell (6.5 mm in
diameter, 8.0 μm pore size). Cells were suspended in serum-free
media supplemented with 0.1% filtered bovine serum albumin (BSA).
Cells were seeded on the Matrigel-coated membrane matrix of the
Transwell. Cell culture media containing 10% FBS were added to the
lower chamber of the Transwell, and cells were incubated for 24 h
in a 37°C incubator. Invasive cells were fixed and stained with
Hemacolor® staining solution (Merck KGaA, Darmstadt,
Germany). The number of invasive cells was counted using light
microscopy.
Immunofluorescence assay
Cells were fixed in 4% formaldehyde in
phosphate-buffered saline (PBS), for 10 min at room temperature.
After fixation, cells were washed with PBS and treated with 0.1%
Triton X-100 in PBS for 5 min at 4°C. Cells were then treated with
blocking solution (2% BSA in PBS) for 1 h at 37°C. Cells were
incubated with primary antibodies in PBS with 1% BSA and 0.1%
Triton X-100 for 1 h at 37°C. After washing in PBS, cells were
incubated with appropriate secondary antibodies in PBS with 1% BSA
and 0.1% Triton X-100 for 30 min at 37°C. After washing in PBS,
cells were incubated with DAPI in PBS (1:10,000) for 10 min at room
temperature. Cells were then washed three times in PBS and mounted
on glass slides. Confocal analysis was performed using an Olympus
LX 50 microscope.
Measurement of ROS generation
Cellular levels of ROS were determined using MitoSOX
Red. Cells were stained with 5 μM MitoSOX Red at 37°C for 20 min.
After incubation, cells were washed with PBS, collected in
trypsin-E DTA, and suspended in PBS. Fluorescence was measured
using a FACScan flow cytometry system (BD Biosciences, Franklin
Lakes, NJ, USA).
Electrophoretic mobility shift assay
(EMSA)
Nuclear proteins were extracted using the NE-PER
® Nuclear and Cytoplasmic Extraction Reagents kit
(Pierce Biotechnology, Inc. Rockford, IL, USA), according to the
manufacturer’s instructions. EMSAs for NF-κB were performed using
the Gelshift™ Chemiluminescent EMSA kit (Active Motif, Carlsbad,
CA, USA) following the manufacturer’s instructions. Biotin
3′-end-labeled double-stranded NF-κB oligonucleotide
(5′-AGTTGAGGGGACTTTCCCAGGC-3′) was purchased from Bioneer Corp.
Nuclear protein-NF-κB-labeled oligonucleotide complexes were
separated from free NF-κB-labeled oligonucleotides by
electrophoresis through 6% (w/v) polyacrylamide gels. After
electrophoretic separation, NF-κB-labeled oligonucleotide-protein
complexes were transferred to nylon membranes. Membranes were
crosslinked, blocked and detected by chemiluminescence.
Western blot analysis
Protein extracts of cells were separated via
electrophoresis and transferred to polyvinylidene difluoride (PVDF)
membranes (Millipore, Billerica, MA, USA). After blocking with 10%
non-fat dry milk in TBST, membranes were incubated overnight with
the appropriate primary antibodies and peroxidase-conjugated
secondary antibody. Then, appropriate HRP-conjugated secondary
antibodies were added, and protein-antibody complexes were
visualized using enhanced chemiluminescence (ECL) reagents (Pierce
Biotechnology, Inc.).
RNA preparation, reverse transcription,
and polymerase chain reaction (PCR) analysis
Total cellular RNA was prepared using TRI zol
reagent (Invitrogen Life Technologies). To synthesize cDNA s,
reverse transcription reactions were performed using the following
primers: Romo1 forward, 5′-CTGTCTCAGGATCGGAATGCG-3′ and reverse,
5′-CATCGGATGCCCATCCAATG-3′; and β-actin forward,
5′-GAAATCGTGCGTGACATAGAGAG-3′ and reverse,
5′-CTAGAAGCATTTGCGGTGGACGATGGAGGGGCC-3′. Amplification was
performed using a MyCycler Thermal Cycler (Bio-Rad, Hercules, CA,
USA). Amplified PCR products were separated on a 1% agarose gel and
visualized using ethidium bromide (EtBr) staining.
Statistical analysis
All experiments were performed independently at
least three times. Data are expressed as means ± SDs, as calculated
by GraphPad PRISM version 4.02 for Windows (GraphPad Software,
Inc., San Diego, CA, USA). P<0.05 was considered statistically
significant.
Results
Romo1-induced invasion involves NF-κB
activation
Romo1 expression is known to enhance the invasive
activity of tumor cells (24).
Romo1 also contributes to constitutive activation of NF-κB
(33). To determine whether
constitutive activation of NF-κB is involved in Romo1-induced
invasion, we treated cells with the antioxidant NAC, IKK inhibitor
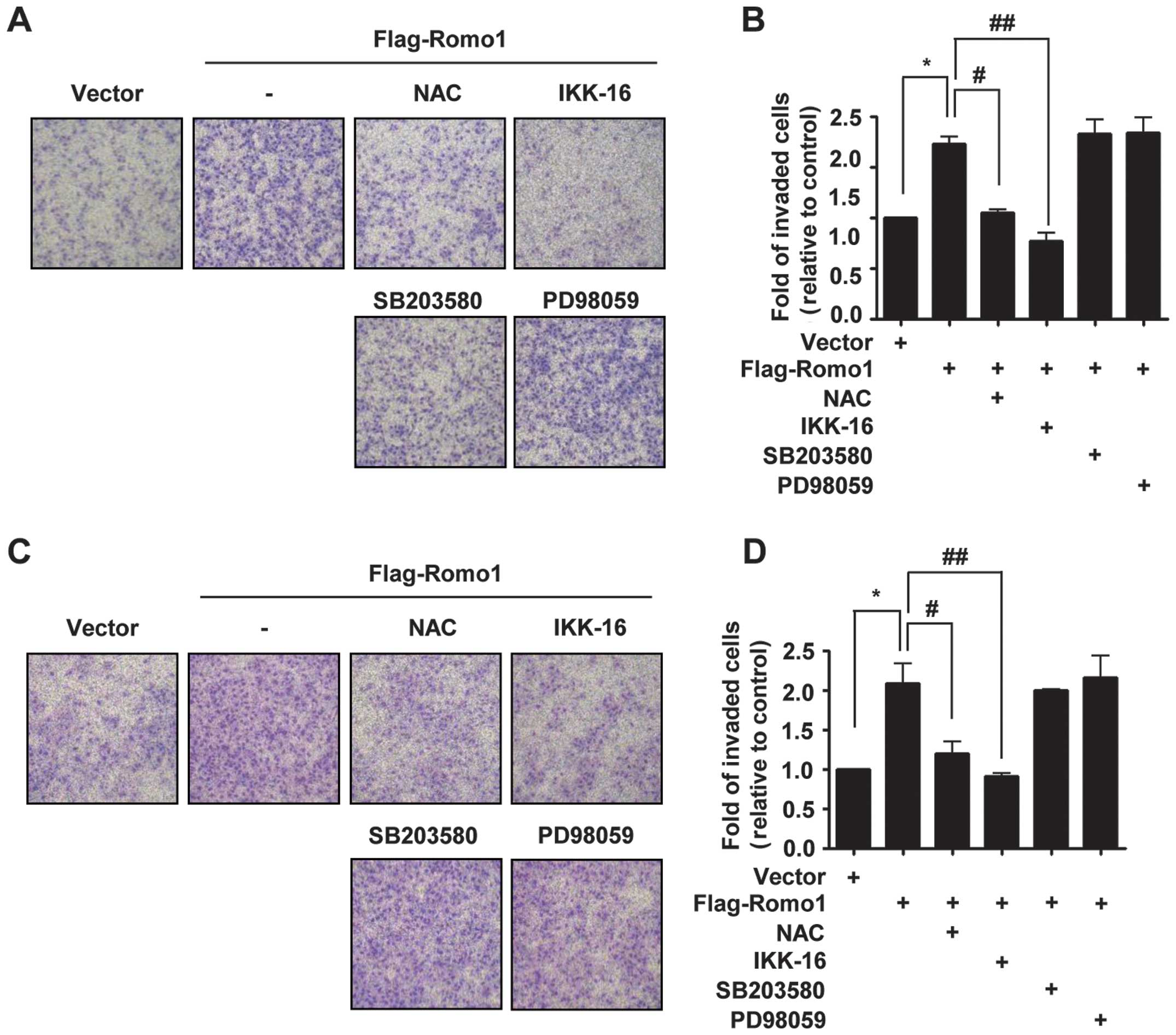
(IKK-16), p38 MAPK inhibitor (SB203580) and MKK1/MEK inhibitor
(PD98059). Although Romo1-triggered invasion was not affected by
inhibitors of p38 and MEK, it was suppressed by treatment with IKK
inhibitor or NAC in MDA-MB-231 cells (Fig. 1A). Similarly, when Huh-7 cells were
treated with NAC, IKK inhibitor, p38 inhibitor, or MEK inhibitor,
the same result was obtained (Fig.
1C). These results suggest that Romo1-induced invasion is
mediated by the NF-κB pathway.
Oxidative stress-induced NF-κB activation
and tumor cell invasion requires Romo1
Oxidative stress is known to induce cancer cell
invasion (34,35). Therefore, we explored whether Romo1
expression is required for oxidative stress-induced invasion of
tumor cells. As shown in Fig. 2A,
cell invasion triggered by H2O2 treatment was
blocked by Romo1 knockdown in MDA-MB-231 cells. Similar results
were obtained using Huh-7 cells (Fig.
2C), suggesting that Romo1 is needed for tumor cell invasion in
response to oxidative stress. Romo1 knockdown by Romo1 siRNA
was examined by RT-PCR (data not shown).
NF-κB is a major transcription factor involved in
sensing H2O2-mediated oxidative stress
(14,36). To evaluate the role of Romo1 in
chronic oxidative stress-induced NF-κB activation, we first
confirmed the pathway of activation, that is,
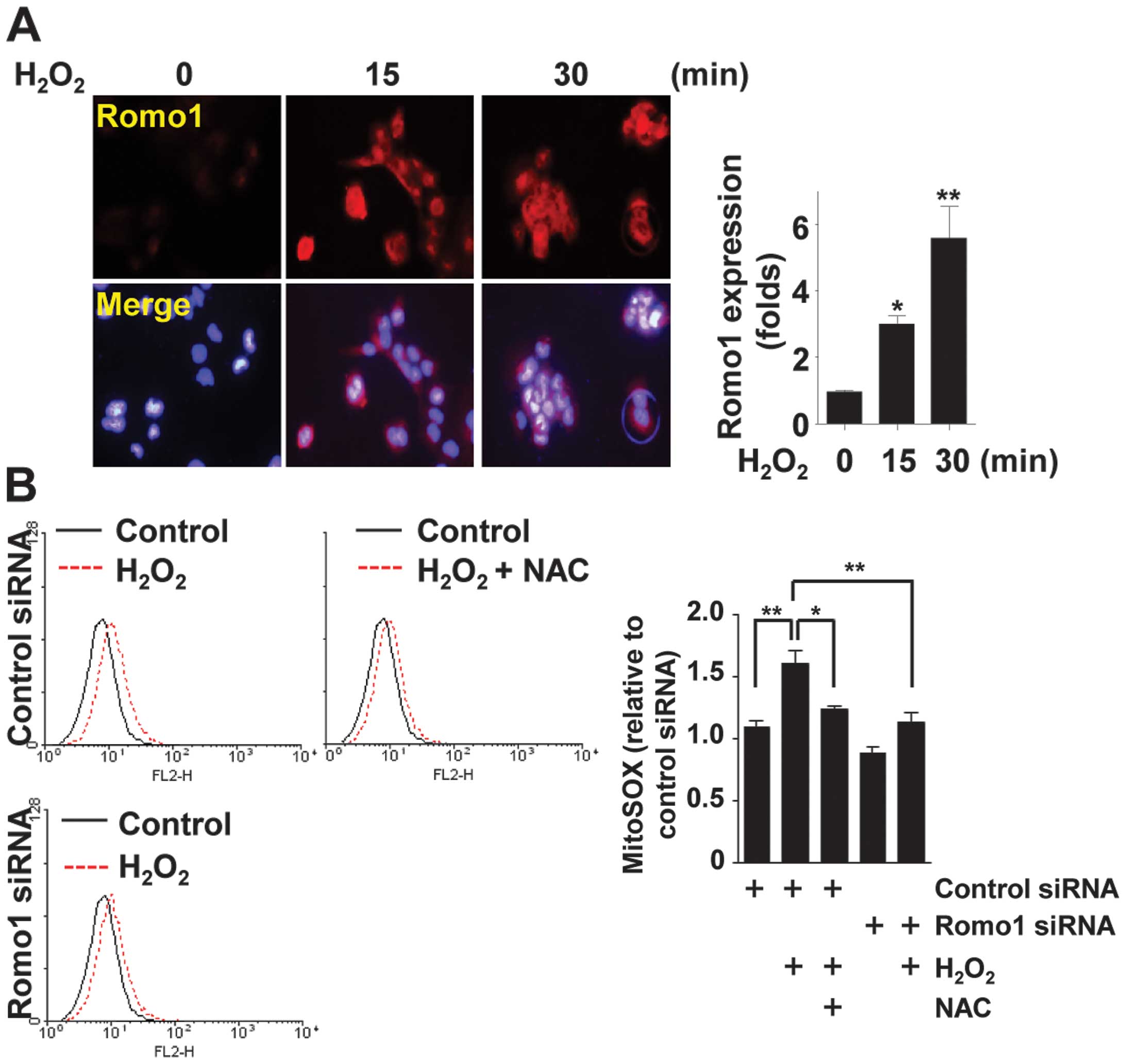
H2O2-Romo1-ROS-NF-κB. Following treatment of
WI-38 VA13 cells with H2O2, Romo1 expression
was observed to increase on fluorescence microscopy (Fig. 3A). Production of ROS following
H2O2 treatment was measured by staining cells
with MitoSOX Red (an indicator of mitochondrial superoxide). Flow
cytometric analysis showed that Romo1 depletion and NAC treatment
partially inhibited H2O2-mediated ROS
production (Fig. 3B). To clarify
the role of Romo1 in H2O2-induced NF-κB
activation, WI-38 VA13 cells were treated with
H2O2 and an EMSA was performed. As shown in
Fig. 4A, the DNA-binding activity
of NF-κB increased following H2O2 treatment,
and binding activity was sustained for up to 9 h.
H2O2-mediated NF-κB activation was suppressed
by Romo1 knockdown (Fig. 4B). This
finding was also confirmed in HEK 293 and Huh-7 cells (Fig. 4C). These results demonstrated that
oxidative stress can induce NF-κB activation through Romo1
expression.
Romo1-induced NF-κB activation and
invasion of cells involves IKK
Catalytic subunits of the IKK complex, namely IKKα
and IKKβ, are principally involved in IκBα phosphorylation
(8). To determine whether Romo1
regulates NF-κB activation via the IKK complex, we used IKKα-.or
IKKβ-deficient cells (IKKα−/− and IKKβ−/−)
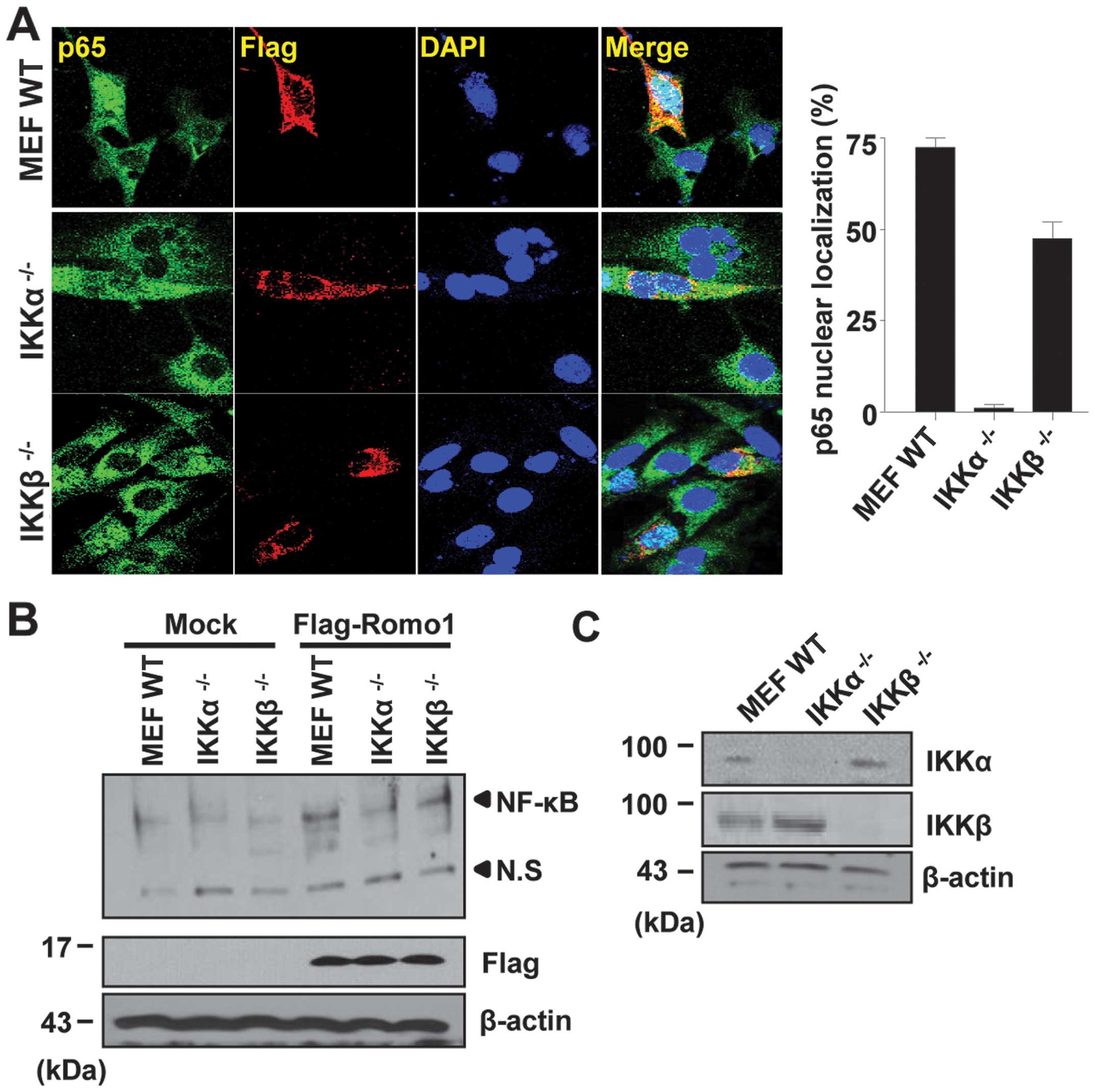
derived from primary MEF s. As shown in Fig. 5A, Romo1 expression triggered the
nuclear translocation of p65 in WT MEF s. However, the nuclear
translocation of p65 was not detectable in IKKα−/−
cells. In contrast, p65 was partially detectable in the nucleus of
IKKβ−/− cells. This result was confirmed by EMSA, and
the same result was observed, as shown in Fig. 5B. Expression of IKKα and IKKβ was
examined by western blot analysis (Fig. 5C). Together, these results
demonstrate that IKKα is an essential mediator of NF-κB activation
induced by Romo1 expression.
To further investigate the importance of IKKα in
Romo1-induced invasion, Romo1 was expressed in WT MEF,
IKKα−/− and IKKβ−/− MEF cells, and
Romo1-induced invasion was assessed. As expected,
IKKα−/− and IKKβ−/− MEF cells were less
invasive than WT MEF cells. Romo1-induced invasion was suppressed
in IKKα−/− cells and was partially suppressed in
IKKβ−/− cells (Fig.
6).
Discussion
Oxidative stress is a contributor to cancer cell
invasion (4,37). ROS are closely associated with the
NF-κB pathway and, as a result, stimulate the MMPs involved in
invasion and metastasis (4). A
variety of cellular stresses, including carcinogens, cigarette
smoke and TPA, may induce NF-κB expression as well as the
expression of pro-inflammatory genes (10,38).
Romo1 expression is similarly induced by a variety of stresses such
as TPA, H2O2 and chemotherapeutic agents
(24,29,32).
This implies that stress-induced NF-κB activation could be mediated
by Romo1 expression. In the present study,
H2O2-induced NF-κB activation was associated
with Romo1 expression (Fig. 4). In
a previous report, we demonstrated that increased NF-κB activity
was decreased by Romo1 knockdown and that Romo1 overexpression
induced translocation of NF-κB into the nucleus and its binding to
DNA (33). These results indicated
that an increase in activity of NF-κB in tumor cells is closely
related to Romo1 expression triggered by oxidative stress. Because
aberrant NF-κB activation is associated with a variety of
inflammatory diseases, drug-development efforts have targeted
components of NF-κB signaling such as IκBα degradation, IKK
activity and NF-κB binding to DNA (11,39).
Our results suggest that Romo1 is another potential therapeutic
target for diseases involving NF-κB deregulation.
NF-κB plays a key role in tumor cell invasion
(20), therefore we investigated
whether oxidative stress-induced Romo1 expression is associated
with tumor cell invasion via NF-κB signaling. In previous studies,
we showed that TPA-induced invasion of HCC is mediated by Romo1
expression and that Romo1 expression is closely related to
constitutive activation of NF-κB (24,33).
Increased NF-κB activity has been reported in many types of cancer
cells, and this deregulated NF-κB activity is responsible for cell
proliferation, progression and resistance to apoptosis of various
tumor cells (11,12,40).
In the present study, we showed that Romo1-triggered cell invasion
was suppressed by NF-κB inhibition. These results demonstrate that
Romo1-induced tumor cell invasion is mediated by NF-κB activation.
Constitutive NF-κB activation is also due to Romo1 expression
(33). A variety of stresses
induce NF-κB activation (17,41).
Romo1 expression is also enhanced by various stresses in tumor
cells (24). Therefore, we suggest
that various types of stress, particularly oxidative stress,
promote tumor cell invasion through Romo1 expression and
constitutive NF-κB activation.
It has been reported that deregulated NF-κB
activation is due to constitutive activation of an upstream
mediator, such as IKK, or an increase in the rate of IκB
degradation (18,20). IKKβ participates in most canonical
signaling pathways leading to NF-κB activation. However, IKKα may
also participate in ROS-induced NF-κB activation in TNF-α-treated
cells (17). In some cells, IKKα
plays a prominent role in regulating constitutive NF-κB activity
(19). We demonstrated in the
current study that tumor cell invasion induced by Romo1
overexpression was blocked by NAC and IKK-16 (Fig. 1). This result implied that tumor
cell invasion induced by Romo1 expression was mediated by IKK
activity. Therefore, we investigated the involvement of IKK by
performing experiments in IKKα-.or IKKβ-deficient cells. We found
that while both IKKα and IKKβ contributed to Romo1-induced NF-κB
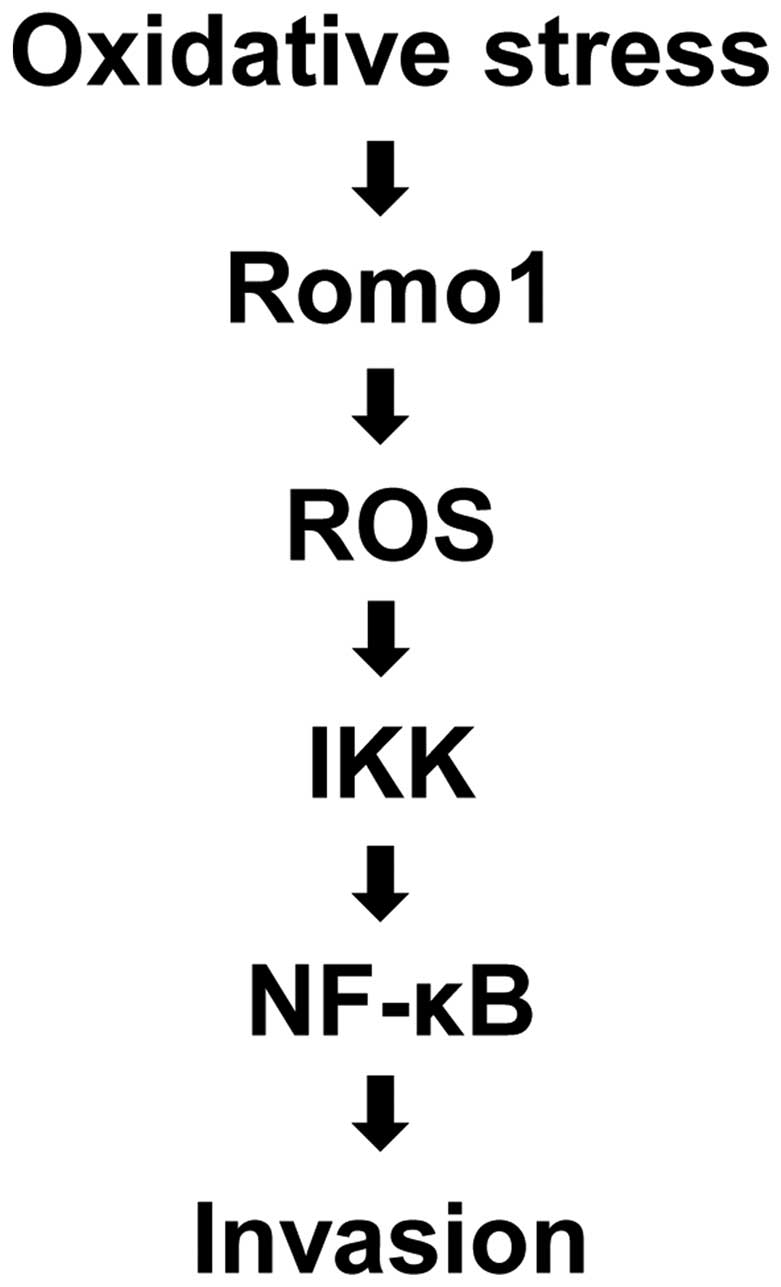
activation, IKKα was the major mediator. The putative role of Romo1
in oxidative stress-induced tumor cell invasion via the NF-κB
pathway is summarized in Fig. 7.
Based on these results and those of previous studies, we suggest
that Romo1 is an important upstream mediator of constitutive
activation of the NF-κB pathway responsible for tumor cell
invasion.
Acknowledgements
This study was supported by the National Research
Foundation of Korea (NRF) grant funded by the Korean government
(NRF-2012R1A2A2A01045800 and NRF-2013R1A1A2063171) and by a grant
from the National R&D Program for Cancer Control, Ministry for
Health, Welfare and Family Affairs, Republic of Korea
(1020180).
Abbreviations:
|
NF-κB
|
nuclear factor-κB
|
|
Romo1
|
reactive oxygen species modulator
1
|
|
IκB
|
inhibitor of κB
|
|
IKK
|
inhibitor of κB kinase
|
|
ROS
|
reactive oxygen species
|
|
WT
|
wild-type
|
|
MEF s
|
mouse embryonic fibroblasts
|
|
H2O2
|
hydrogen peroxide
|
|
NAC
|
N-acetyl-L-cysteine
|
|
EMSA
|
electrophoretic mobility shift
assay
|
|
TPA
|
12-O-tetradecanoylphorbol-13-acetate
|
References
|
1
|
Finkel T: Oxygen radicals and signaling.
Curr Opin Cell Biol. 10:248–253. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dröge W: Free radicals in the
physiological control of cell function. Physiol Rev. 82:47–95.
2002.PubMed/NCBI
|
|
3
|
Bae YS, Oh H, Rhee SG and Yoo YD:
Regulation of reactive oxygen species generation in cell signaling.
Mol Cells. 32:491–509. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu WS: The signaling mechanism of ROS in
tumor progression. Cancer Metastasis Rev. 25:695–705. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mori K, Shibanuma M and Nose K: Invasive
potential induced under long-term oxidative stress in mammary
epithelial cells. Cancer Res. 64:7464–7472. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kumar B, Koul S, Khandrika L, Meacham RB
and Koul HK: Oxidative stress is inherent in prostate cancer cells
and is required for aggressive phenotype. Cancer Res. 68:1777–1785.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baldwin AS Jr: The NF-kappa B and I kappa
B proteins: new discoveries and insights. Annu Rev Immunol.
14:649–683. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Karin M: How NF-kappaB is activated: the
role of the IkappaB kinase (IKK) complex. Oncogene. 18:6867–6874.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ghosh S and Hayden MS: New regulators of
NF-kappaB in inflammation. Nat Rev Immunol. 8:837–848. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sovak MA, Bellas RE, Kim DW, Zanieski GJ,
Rogers AE, Traish AM and Sonenshein GE: Aberrant nuclear
factor-kappaB/ Rel expression and the pathogenesis of breast
cancer. J Clin Invest. 100:2952–2960. 1997. View Article : Google Scholar
|
|
11
|
Kim HJ, Hawke N and Baldwin AS: NF-kappaB
and IKK as therapeutic targets in cancer. Cell Death Differ.
13:738–747. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sasaki N, Morisaki T, Hashizume K, et al:
Nuclear factor-kappaB p65 (RelA) transcription factor is
constitutively activated in human gastric carcinoma tissue. Clin
Cancer Res. 7:4136–4142. 2001.PubMed/NCBI
|
|
13
|
Bargou RC, Emmerich F, Krappmann D, et al:
Constitutive nuclear factor-kappaB-R elA activation is required for
proliferation and survival of Hodgkin’s disease tumor cells. J Clin
Invest. 100:2961–2969. 1997. View Article : Google Scholar
|
|
14
|
Josse C, Legrand-Poels S, Piret B, Sluse F
and Piette J: Impairment of the mitochondrial electron chain
transport prevents NF-kappa B activation by hydrogen peroxide. Free
Radic Biol Med. 25:104–112. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Morgan MJ and Liu ZG: Crosstalk of
reactive oxygen species and NF-κB signaling. Cell Res. 21:103–115.
2011. View Article : Google Scholar
|
|
16
|
Jaspers I, Zhang W, Fraser A, Samet JM and
Reed W: Hydrogen peroxide has opposing effects on IKK activity and
IkappaBalpha breakdown in airway epithelial cells. Am J Respir Cell
Mol Biol. 24:769–777. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oka S, Kamata H, Kamata K, Yagisawa H and
Hirata H: N-acetyl-cysteine suppresses TNF-induced NF-kappaB
activation through inhibition of IkappaB kinases. FE BS Lett.
472:196–202. 2000. View Article : Google Scholar
|
|
18
|
Gasparian AV, Yao YJ, Kowalczyk D, Lyakh
LA, Karseladze A, Slaga TJ and Budunova IV: The role of IKK in
constitutive activation of NF-kappaB transcription factor in
prostate carcinoma cells. J Cell Sci. 115:141–151. 2002.PubMed/NCBI
|
|
19
|
Wilson W III and Baldwin AS: Maintenance
of constitutive IkappaB kinase activity by glycogen synthase
kinase-3alpha/beta in pancreatic cancer. Cancer Res. 68:8156–8163.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miyamoto S, Chiao PJ and Verma IM:
Enhanced I kappa B alpha degradation is responsible for
constitutive NF-kappa B activity in mature murine B-cell lines. Mol
Cell Biol. 14:3276–3282. 1994.PubMed/NCBI
|
|
21
|
Wang CY, Mayo MW and Baldwin AS Jr:
TNF-and cancer therapy-induced apoptosis: potentiation by
inhibition of NF-kappaB. Science. 274:784–787. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Visconti R, Cerutti J, Battista S, et al:
Expression of the neoplastic phenotype by human thyroid carcinoma
cell lines requires NF kappaB p65 protein expression. Oncogene.
15:1987–1994. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chung YM, Kim JS and Yoo YD: A novel
protein, Romo1, induces ROS production in the mitochondria. Biochem
Biophys Res Commun. 347:649–655. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chung JS, Park S, Park SH, et al:
Overexpression of Romo1 promotes production of reactive oxygen
species and invasiveness of hepatic tumor cells. Gastroenterology.
143:1084–1094. e72012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Na AR, Chung YM, Lee SB, Park SH, Lee MS
and Yoo YD: A critical role for Romo1-derived ROS in cell
proliferation. Biochem Biophys Res Commun. 369:672–678. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chung JS, Lee SB, Park SH, Kang ST, Na AR,
Chang TS, Kim HJ and Yoo YD: Mitochondrial reactive oxygen species
originating from Romo1 exert an important role in normal cell cycle
progression by regulating p27(Kip1) expression. Free Radic Res.
43:729–737. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee SB, Kim JJ, Chung JS, Lee MS, Lee KH,
Kim BS and Do Yoo Y: Romo1 is a negative-feedback regulator of Myc.
J Cell Sci. 124:1911–1924. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shin JA, Chung JS, Cho SH, Kim HJ and Yoo
YD: Romo1 expression contributes to oxidative stress-induced death
of lung epithelial cells. Biochem Biophys Res Commun. 439:315–320.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim JJ, Lee SB, Park JK and Yoo YD:
TNF-alpha-induced ROS production triggering apoptosis is directly
linked to Romo1 and Bcl-X(L). Cell Death Differ. 17:1420–1434.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee SB, Kim JJ, Kim TW, Kim BS, Lee MS and
Yoo YD: Serum deprivation-induced reactive oxygen species
production is mediated by Romo1. Apoptosis. 15:204–218. 2010.
View Article : Google Scholar
|
|
31
|
Chung YM, Lee SB, Kim HJ, Park SH, Kim JJ,
Chung JS and Yoo YD: Replicative senescence induced by
Romo1-derived reactive oxygen species. J Biol Chem.
283:33763–33771. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hwang IT, Chung YM, Kim JJ, Chung JS, Kim
BS, Kim HJ, Kim JS and Yoo YD: Drug resistance to 5-FU linked to
reactive oxygen species modulator 1. Biochem Biophys Res Commun.
359:304–310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chung JS, Lee S and Yoo YD: Constitutive
NF-κB activation and tumor-growth promotion by Romo1-mediated
reactive oxygen species production. Biochem Biophys Res Commun.
450:1656–1661. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ho BY, Wu YM, Chang KJ and Pan TM:
Dimerumic acid inhibits SW620 cell invasion by attenuating
H2O2-mediated MMP-7 expression via JNK/C-Jun
and ERK/C-Fos activation in an AP-1-dependent manner. Int J Biol
Sci. 7:869–880. 2011. View Article : Google Scholar
|
|
35
|
Liu Z, Li S, Cai Y, Wang A, He Q, Zheng C,
Zhao T, Ding X and Zhou X: Manganese superoxide dismutase induces
migration and invasion of tongue squamous cell carcinoma via
H2O2-dependent Snail signaling. Free Radic
Biol Med. 53:44–50. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schreck R, Rieber P and Baeuerle PA:
Reactive oxygen intermediates as apparently widely used messengers
in the activation of the NF-kappa B transcription factor and HIV-1.
EMBO J. 10:2247–2258. 1991.PubMed/NCBI
|
|
37
|
Nonaka Y, Iwagaki H, Kimura T, Fuchimoto S
and Orita K: Effect of reactive oxygen intermediates on the in
vitro invasive capacity of tumor cells and liver metastasis in
mice. Int J Cancer. 54:983–986. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Staal FJ, Roederer M and Herzenberg LA and
Herzenberg LA: Intracellular thiols regulate activation of nuclear
factor kappa B and transcription of human immunodeficiency virus.
Proc Natl Acad Sci USA. 87:9943–9947. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ahn KS and Aggarwal BB: Transcription
factor NF-kappaB: a sensor for smoke and stress signals. Ann N Y
Acad Sci. 1056:218–233. 2005. View Article : Google Scholar
|
|
40
|
Rayet B and Gélinas C: Aberrant rel/nfkb
genes and activity in human cancer. Oncogene. 18:6938–6947. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kamata H, Manabe T, Oka S, Kamata K and
Hirata H: Hydrogen peroxide activates IkappaB kinases through
phosphorylation of serine residues in the activation loops. FE BS
Lett. 519:231–237. 2002. View Article : Google Scholar
|