Introduction
More than 500,000 new cases of head and neck
squamous cell carcinoma (HNSCC) occurred in 2008 worldwide. Oral
squamous cell carcinoma (OSCC) is the most frequently occurring
cancer among HNSCCs and is associated with a mortality rate of
approximately 50%, as reported in 2008 (1). Despite the increasing knowledge of
OSCC pathogenesis, as well as advances in chemotherapy,
radiotherapy, and surgery, little improvement in the relative
survival rate has been observed in OSCC during the past several
decades (2). Therefore, a greater
understanding of the pathogenesis of OSCC is needed for the
development of optimal therapeutic approaches.
Cancer cells acquire abnormalities in multiple
oncogenes and tumor-suppressor genes. Overexpression and
constitutive activation of some oncogenes support the
proliferation, invasion, and metastasis of cancer cells.
Inactivation of a single critical oncogene can induce cancer cells
to differentiate into cells with a normal phenotype or to undergo
apoptosis. This dependence on oncogenes for maintaining the cancer
phenotype is an Achilles heel for cancer cells, which can be
exploited in cancer therapy (3).
In HNSCCs, including OSCC, the overexpression of
epidermal growth factor receptor (EGFR) correlates with lymph node
metastasis, risk of locoregional recurrence, and poor prognosis
(4,5). Cetuximab, which targets EGFR, is used
for patients with local advanced, recurrent, or metastatic HNSCC.
Radiotherapy or platinum-based chemotherapy plus cetuximab improve
locoregional control and overall survival (6,7).
However, cetuximab is the only available molecular targeted drug
for the treatment of OSCC. Thus, we previously attempted to
identify useful target molecules for the treatment of OSCC using
microarray analysis, and we identified 465 cancer-related genes
that were commonly over-expressed in human OSCC cell lines
(8). Among these genes,
overexpression of ribonucleotide reductase M2 (RRM2), which is
targeted by gemcitabine (GEM), has been shown to be involved in
tumor progression in human malignancies (9).
Ribonucleotide reductase (RR) catalyzes the
conversion of ribonucleotide 5′-diphosphates to their
2′-deoxynucleotide form required for DNA synthesis. RRM2 is the
catalytic subunit of RR and modulates its enzymatic activity
(10–12). GEM binds to RRM2, replaces cytidine
during DNA replication, and inhibits RR, hence reducing
deoxynucleotide pools; moreover, GEM also competes with
deoxycytidine triphosphate for incorporation into elongating DNA
strands and halts DNA polymerization (13). GEM has been used for the treatment
of pancreatic cancer, biliary tract cancer, lung cancer, breast
cancer, bladder cancer, and ovarian cancer. Furthermore, GEM has
been applied in preclinical and clinical studies for head and neck
cancer including OSCC (14–19).
Therefore, in this study, we examined the role of
RRM2 in OSCC using GEM, which targets RRM2, and RNA interference
(RNAi). Our data show that RRM2 is an important molecular target
that is involved in the pathogenesis of OSCC and that it could be a
promising target for the treatment of OSCC.
Materials and methods
Cells and cell culture
We used four human OSCC cell lines: green
fluorescent protein (GFP)-SAS (20), Ca9-22, HSC2, and HSC3, as
previously described (21). All
cell lines were maintained in Dulbecco’s modified Eagle’s medium
(DMEM; Wako, Osaka, Japan) supplemented with 10% fetal bovine serum
(FBS; Biosource, Camarillo, CA, USA), 100 U/ml penicillin, and 100
μg/ml streptomycin (Wako), referred to here as complete medium.
Primary cultured cells were established from OSCC tumors harvested
from patients. Tumor tissues, including adjacent normal tissues,
were surgically excised and rinsed several times with complete
medium. The tumor and adjacent normal tissues were individually cut
into small fragments and dissociated at 37°C for 2 h with 0.1%
collagenase (Wako). The cell suspension was filtered through a cell
strainer with 70-μm nylon mesh (BD, Franklin Lakes, NJ, USA). The
cells were collected by centrifugation, resuspended in keratinocyte
serum-free medium (K-SFM; Life Technologies, Carlsbad, CA, USA),
seeded onto plastic tissue culture dishes, and grown in an
incubator with a humidified atmosphere of 95% air and 5%
CO2 at 37°C.
Small molecule compound
Gemcitabine hydrochloride (GEM; Wako) was dissolved
in nuclease-free water to a stock concentration of 10 mM and stored
at 4°C until use.
Samples from patients
Twenty-five OSCC tissues from patients were obtained
at the Ehime University Hospital from September 2008 to May 2014.
The tissues were collected from resected specimens of primary
tumors (17 men and 8 women; average age, 68.04 years). Three
primary cultured cells were derived from OSCC of the lower gingiva
(from a 55-year-old man, T4N2bM0), tongue (from a 57-year-old man,
rT1N0M0), and skin metastasis (from a 61-year-old man, rT0N0M1).
The Institutional Review Board (IRB) at Ehime University Hospital
approved this study. All patients were enrolled after providing
written informed consent.
Western blot analysis
Cells (5×105 for GFP-SAS, Ca9-22, HSC2,
and HSC3) were grown in monolayers and lysed with lysis buffer [0.5
M EDTA (Dojindo, Kumamoto, Japan) and 1% NP-40 (Nacalai Tesque,
Kyoto, Japan) in phosphate-buffered saline (PBS; Wako) containing a
protease inhibitor and phosphatase inhibitor (Roche Diagnostics,
Basel, Switzerland)]. Tissues were homogenized in 500 μl of lysis
buffer with the use of a TissueLyser system (Qiagen, Valencia, CA,
USA). The samples were centrifuged at 15,000 × g for 15 min at 4°C,
and supernatants were electrophoresed on SDS-polyacrylamide gels,
followed by transfer to polyvinylidene difluoride membranes
(Millipore, Bedford, MA, USA). The membranes were blocked with 5%
nonfat dried milk (Wako) in 1X TBS-T [25 mM Tris-HCl, 125 mM NaCl,
and 0.1% Tween-20 (Sigma-Aldrich, St. Louis, MO, USA)] for 15 min
at 37°C. Membranes were then probed with monoclonal mouse
anti-human RRM2 antibodies (Abnova, Taipei, Taiwan; diluted 1:500)
or monoclonal mouse anti-β-tubulin antibodies (BD; diluted 1:1000)
in 5% nonfat dried milk in 1X TBS-T for 1 h at room temperature,
followed by treatment with horseradish perioxidase-conjugated
secondary antibodies against mouse IgG (GE Healthcare,
Buckinghamshire, UK) for 1 h at room temperature. The immune
complexes were visualized using enhanced chemiluminescence (ECL)
Prime western blotting detection reagent (GE Healthcare). The
density of visualized immune complexes was quantified using an
LAS-3000 system (Fujifilm, Tokyo, Japan).
Transfection with synthetic small
interfering RNA (siRNA)
We used Silencer Select siRNA targeting RRM2
(siRRM2) and Silencer Select Negative Control (siNT) purchased from
Life Technologies. Transfections were performed with Lipofectamine
RNAiMAX (Life Technologies) mixed with 5 nM siRNAs for western
blotting and cell growth assays.
Cell growth assay
Cells (2×103 GFP-SAS cells,
3×103 Ca9-22, HSC2, and HSC3 cells) were seeded into
96-well plates in complete medium with 5 nM synthetic siRNAs and
0.2% Lipofectamine RNAiMAX in a final volume of 100 μl. GEM was
added to each well at different concentrations ranging from 1 to
1000 nM. After 72 h, cell growth was evaluated by WST-8 assay (Cell
Counting Kit-8; Dojindo).
In vitro collagen gel droplet embedded
culture drug sensitivity test (CD-DST)
The chemosensitivity of OSCC was evaluated using
CD-DST kit (Primastar®; Kurabo, Osaka, Japan) according
to manufacturer’s protocol as described previously (22). The anticancer drugs tested in the
CD-DST were GEM (Eli Lilly Japan, Kobe, Japan; 8.0 μg/ml for 1 h),
cisplatin (CDDP; Bristol-Myers Squibb, Tokyo, Japan; 0.2 μg/ml for
24 h), 5-fluorouracil (5-FU; Kyowa Hakko Kirin, Tokyo, Japan; 1.0
μg/ml for 24 h) and 0.1 μg/ml docetaxel (DOC; Sanofi, Tokyo, Japan;
0.1 μg/ml for 24 h). In vitro sensitivity was expressed as
the T/C (%), where T was the total volume of the treated group and
C was the total volume of the control group. A T/C of 50% or less
to each anticancer drug was regarded as in vitro sensitive
(23).
Real-time quantitative reverse
transcriptional polymerase chain reaction (qRT-PCR)
Total RNA was extracted by lysing OSCC tissues with
ISOGEN reagent (NipponGene, Toyama, Japan) after homogenization
with a TissueLyser (Qiagen). The relative quantity of mRNA was
determined using SYBR Green and the comparative CT method (ΔCT
method). Hydroxymethylbilane synthase (HMBS) was used as an
internal control. PCR amplification was performed in a 10 μl final
reaction mixture containing 5 μl of 2X One Step SYBR RT-PCR Buffer
4, 0.4 μl of PrimeScript One Step Enzyme Mix 2 (Takara, Otsu,
Japan), 0.4 μl of PCR forward and reverse primers (10 μM), 0.2 μl
of ROX reference Dye II (×50), 2.6 μl of RNase-free
dH2O, and 1 μl of total RNA (100 ng/μl). The
thermal-cycling conditions were as follows: reverse transcription
at 42°C for 5 min and 95°C 10 sec, followed by 40 cycles at 95°C
for 5 sec and 60°C 30 sec. SYBR Green I fluorescence was detected
with ViiA™7 (Life Technologies). The sequences of primers used were
as follows: RRM2: forward 5′-TGC GTC GAT ATT CTG GCT CAA G-3′ and
reverse 5′-CCG ATG GTT TGT GTA CCA GGT G-3′; HMBS: forward 5′-CAT
GCA GGC TAC CAT CCA TGT C-3′ and reverse 5′-GTT ACG AGC AGT GAT GCC
TAC CAA-3′.
Statistical analysis
All in vitro experiments were performed in
triplicate and repeated three times. All statistical analyses were
performed using GraphPad Prism software, version 5.04 (GraphPad
Software, San Diego, CA, USA). Student’s t-tests were used to
determine the significance of differences between the groups.
Log-rank tests were used to analyze survival rates. Differences
with P-values of <0.05 were considered statistically
significant.
Results
Overexpression of RRM2 protein in
OSCC
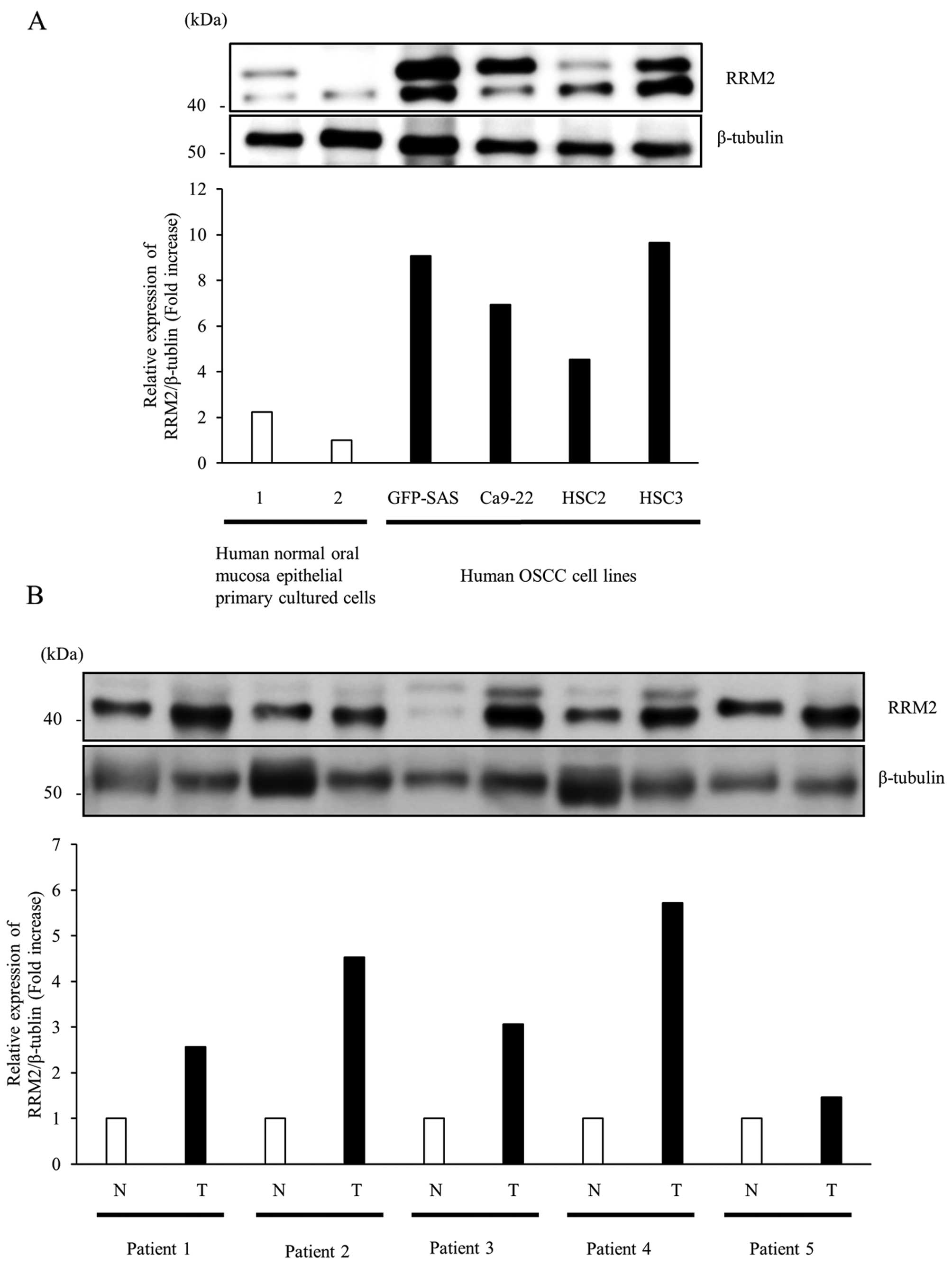
First, we examined the expression of RRM2 protein in
four human OSCC cell lines and human normal oral mucosa epithelial
primary cultured cells by western blotting. The expression levels
of RRM2 protein in all OSCC cells were >2-fold higher than those
in human normal oral mucosa epithelial cells (Fig. 1A). Subsequently, we compared the
expression levels of RRM2 protein in OSCC tumors and adjacent
normal mucosa tissues from the same patient; in these samples, we
found 1.5- to 5.7-fold higher expression in RRM2 protein in tumor
tissues than in normal tissues (Fig.
1B). Thus, these data supported that RRM2 protein was
overexpressed in OSCC tissues and cultured cells.
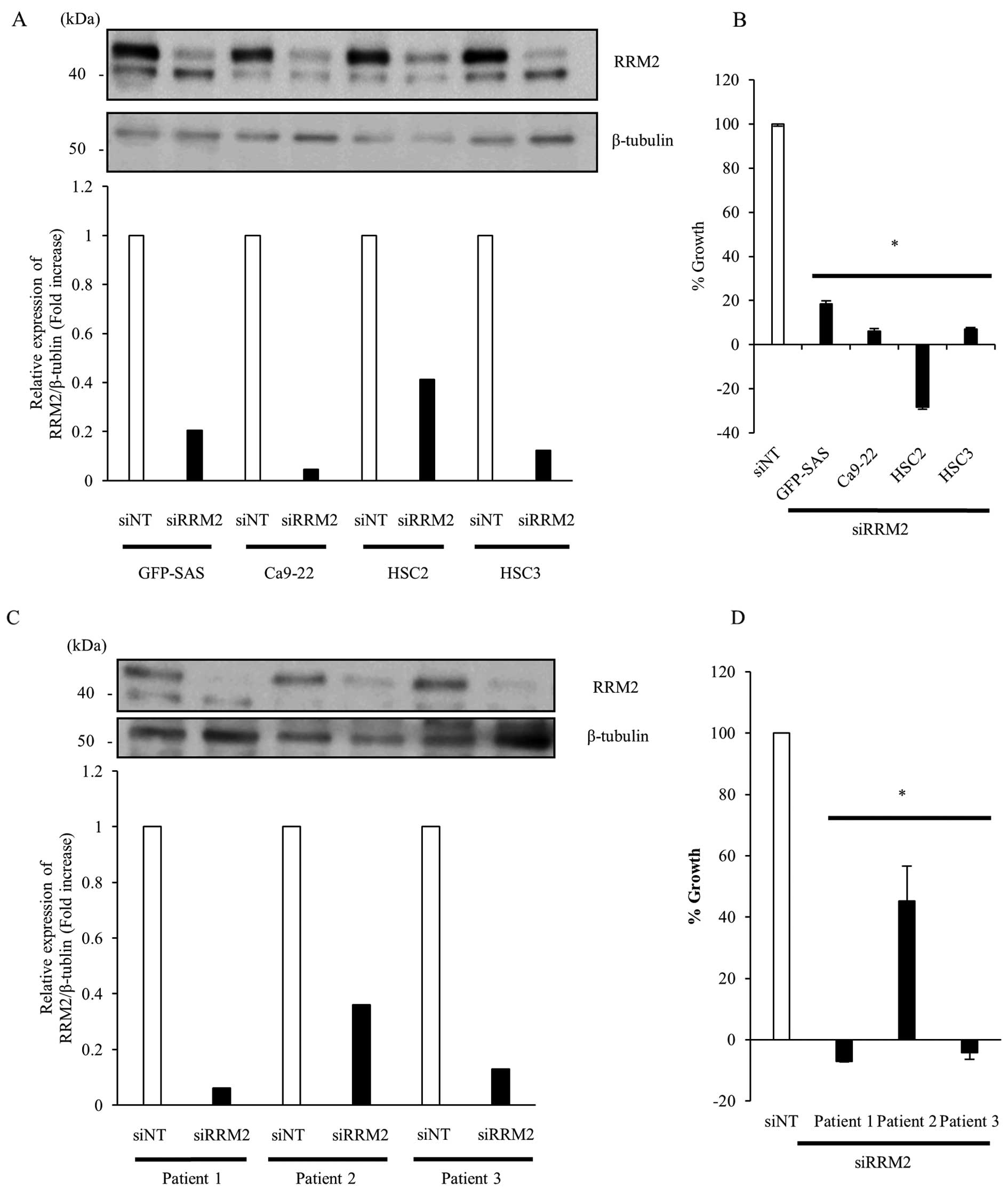
Growth inhibitory effects of siRRM2 in
human OSCC cells in vitro
To clarify the function of RRM2 in the proliferation
of human OSCC cells, we transfected GFP-SAS, Ca9-22, HSC2, and HSC3
cells with 5 nM siRRM2. Targeting RRM2 by RNAi suppressed the
expression of RRM2 protein by >58% compared to siNT and
significantly inhibited the growth of human OSCC cell lines by
>81.5% (Fig. 2A and B).
Furthermore, in primary cultured cells obtained from culture of
resected tumor tissues from OSCC patients, knockdown of RRM2
expression also suppressed the growth of OSCC primary cultured
cells by >54.8% (Fig. 2C and
D).
Antitumor effects of GEM in human OSCC
cells in vitro
Next, we examined the effects of GEM on the
inhibition of RRM2 activity on the growth of human OSCC cell lines
and primary cultured cells. In both types of cells, GEM markedly
reduced the cell growth rate in a concentration-dependent manner.
The growth rate of all OSCC cells was decrease >83.0% following
treatment with 1000 nM GEM (Fig. 3A
and B).
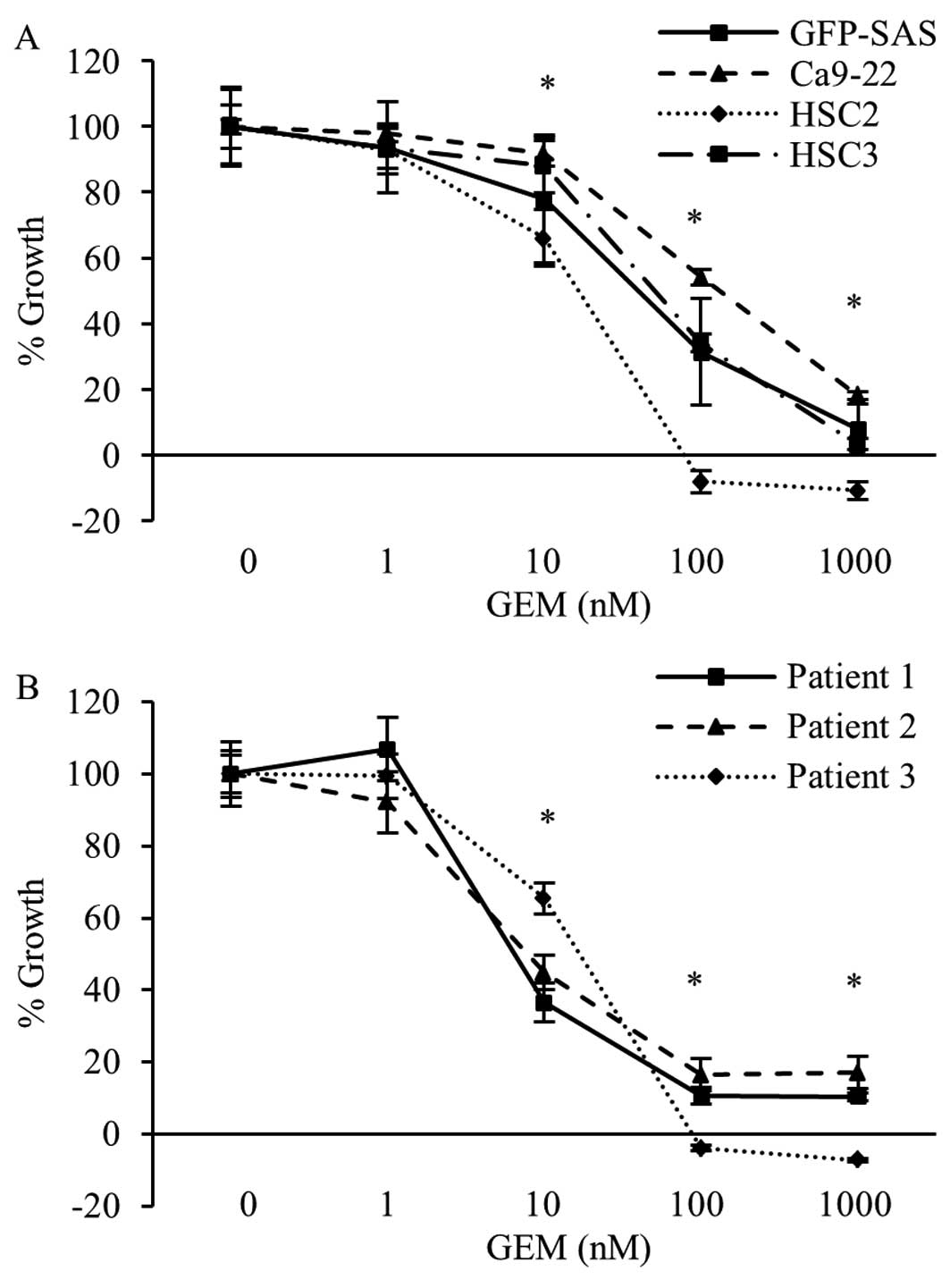 | Figure 3Antiproliferative effects of GEM in
human OSCC cells in vitro. Human OSCC cell lines, i.e.,
GFP-SAS, Ca9-22, HSC2 and HSC3 (A), and three primary cultured
cells (B) were seeded in complete medium. Twenty-four hours later,
GEM was added to each well at final concentrations of 0, 1, 10,
100, or 1000 nM. After 72 h, cell viability was evaluated by WST-8
assay. Bars denote the SDs of samples analyzed in triplicate.
*P<0.01 compared to the control culture. |
In vitro chemosensitivity of OSCC
Next, we evaluated the chemosensitivity of 25 OSCC
tumors to GEM, CDDP, 5-FU, and DOC using CD-DST. From this
analysis, we found that 16 cases (64%) were sensitive to GEM, with
an average T/C value of 42.6%. OSCC tumors were more sensitive to
GEM and DOC than to CDDP and 5-FU (Table I).
 | Table ICD-DST in OSCC cases (n=25). |
Table I
CD-DST in OSCC cases (n=25).
| Anticancer drugs | GEM | CDDP | 5-FU | DOC |
|---|
| Average T/C (%) | 42.6±30.5 | 73.5±21.7 | 82.4±17.5 | 41.1±24.5 |
| Sensitive case
(n) | 16 | 1 | 5 | 18 |
| P-value | | <0.001 | <0.001 | 0.697 |
Clinical significance of GEM sensitivity
and RRM2 mRNA expression in OSCC
Finally, we examined RRM2 mRNA expression
levels by qRT-PCR in OSCC cases and investigated the relationship
between GEM sensitivity, RRM2 mRNA expression levels, and
prognosis (overall, disease-specific and disease-free survival) in
OSCC cases. However, there were no significant correlations or
differences among these factors (data not shown).
Discussion
In this study, we examined the expression and
function of RRM2 in OSCC. Our data demonstrated that RRM2 was
over-expressed in OSCC cells and primary culture cells derived from
patient tumors and that inhibition of RRM2 via GEM or siRRM2
suppressed cell growth of OSCC cells. Thus, RRM2 may represent a
novel target for anticancer therapy in OSCC.
RRM2 overexpression has been shown to be
significantly associated with tumor progression or survival in many
types of cancer (9,24–26).
Ectopic expression of RRM2 induces membrane-associated Raf1
expression and MAPK2 and Rac-1 activation, resulting in enhanced
metastatic potential in a xenograft model (27). In addition, a previous study showed
that HNSCC cells overexpress RRM2 and that knockdown of RRM2 by
RNAi significantly reduces the growth of HNSCC cells in
vitro and in vivo (28). Herein, we showed that RRM2 was
overexpressed in OSCC and demonstrated the growth inhibitory
effects of targeting RRM2 by RNAi in human OSCC cells. A recent
study showed that knockdown of RRM2 expression promotes apoptosis
by suppressing Bcl-2 protein expression in HNSCC and non-small cell
lung cancer (NSCLC) cells. RRM2 suppression contributes to the
instability of Bcl-2 or causes Bcl-2 to remain unprotected from
degradation. Thus, RRM2 may be an attractive interventional target
to downregulate Bcl-2, resulting in the induction of
mitochondria-mediated intrinsic apoptosis (26). Therefore, targeting RRM2 may be an
appropriate therapeutic approach for the treatment of these
cancers.
GEM, triapine, and GTI-2040 are known RRM2
inhibitors (29–31). Triapine and GTI-2040 have been
evaluated in phase I clinical trials (30,31).
GEM competes with RRM2 for replicating DNA and is a potential
candidate for RRM2 inhibition that has been approved by the US Food
and Drug Administration for the treatment of NSCLC, pancreatic
cancer, ovarian cancer, and breast cancer. However, GEM has not
been approved for the treatment of OSCC.
Currently, CDDP-based chemotherapy is generally the
first-line treatment for inoperable recurrent or metastatic OSCC
because we cannot predict the efficacy of chemotherapy. CD-DST can
mimic in vivo tumor growth using type I collagen to create a
three-dimensional culture system and individually evaluate the
response to chemotherapeutic drugs. Several clinical studies have
reported that CD-DST may be useful when devising optimal treatment
strategies for NSCLC (23),
ovarian (32), gastrointestinal
(33), or colon cancer (34). In patients with HNSCC, CD-DST can
be also used to predict CDDP sensitivity and guide individualized
chemotherapy (35). Thus, CD-DST
has been approved for use in the evaluation of cancer treatments by
the Ministry of Health, Labour and Welfare of Japan. In the present
study, CD-DST indicated that GEM and DOC had more potent anti-tumor
activity against OSCC than CDDP and 5-FU. CDDP plus 5-FU (PF)
chemotherapy is the most widely used therapy in the treatment of
patients with OSCC. The resistance of tumor cells to PF remains a
major cause of treatment failure. Therefore, GEM may be a useful
second-line chemotherapy for PF-resistant OSCC.
The relationship between RRM2 mRNA expression
levels and the response to GEM in the clinical setting has been
investigated in various cancers. In pancreatic cancer, the response
rate to GEM is significantly higher in patients with low
RRM2 mRNA expression in biopsy specimens (36). Furthermore, in patients with lung
adenocarcinoma, low levels of RRM2 mRNA are associated with
the response to GEM plus DOC (24). These results indicate the
possibility that expression levels of RRM2 mRNA could
predict chemosensitivity to GEM. However, there was no significant
correlation between GEM sensitivity and RRM2 mRNA expression
levels in patients with OSCC in this study. Thus, there is still no
molecular marker available to predict the response to GEM in
OSCC.
In conclusion, our data suggest that RRM2 plays a
critical role in supporting the growth of human OSCC cells, and
agents targeting RRM2, such as GEM, appear to be a potentially
useful therapeutic approach for OSCC.
Acknowledgements
This work was supported, in part, by JSPS KAKENHI
(grant no. 17689057).
Abbreviations:
|
CD-DST
|
collagen gel droplet embedded culture
drug sensitivity test
|
|
GEM
|
gemcitabine
|
|
HNSCC
|
head and neck squamous cell
carcinoma
|
|
OSCC
|
oral squamous cell carcinoma
|
|
RRM2
|
ribonucleotide reductase M2
|
|
siRRM2
|
small interfering RNA specific for
RRM2
|
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
2
|
Gupta S, Kong W, Peng Y, Miao Q and
Mackillop WJ: Temporal trends in the incidence and survival of
cancers of the upper aerodigestive tract in Ontario and the United
States. Int J Cancer. 125:2159–2165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weinstein IB: Cancer. Addiction to
oncogenes - the Achilles heal of cancer. Science. 297:63–64. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ang KK, Berkey BA, Tu X, Zhang HZ, Katz R,
Hammond EH, Fu KK and Milas L: Impact of epidermal growth factor
receptor expression on survival and pattern of relapse in patients
with advanced head and neck carcinoma. Cancer Res. 62:7350–7356.
2002.PubMed/NCBI
|
|
5
|
Rubin Grandis J, Melhem MF, Gooding WE,
Day R, Holst VA, Wagener MM, Drenning SD and Tweardy DJ: Levels of
TGF-α and EGFR protein in head and neck squamous cell carcinoma and
patient survival. J Natl Cancer Inst. 90:824–832. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bonner JA, Harari PM, Giralt J, et al:
Radiotherapy plus cetuximab for squamous-cell carcinoma of the head
and neck. N Engl J Med. 354:567–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vermorken JB, Mesia R, Rivera F, et al:
Platinum-based chemotherapy plus cetuximab in head and neck cancer.
N Engl J Med. 359:1116–1127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tanaka H, Nakashiro K, Iwamoto K, Tokuzen
N, Fujita Y, Shirakawa R, Oka R, Goda H and Hamakawa H: Targeting
Aurora kinase A suppresses the growth of human oral squamous cell
carcinoma cells in vitro and in vivo. Oral Oncol. 49:551–559. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Furuta E, Okuda H, Kobayashi A and Watabe
K: Metabolic genes in cancer: Their roles in tumor progression and
clinical implications. Biochim Biophys Acta. 1805:141–152.
2010.PubMed/NCBI
|
|
10
|
Duxbury MS, Ito H, Zinner MJ, Ashley SW
and Whang EE: RNA interference targeting the M2 subunit of
ribonucleotide reductase enhances pancreatic adenocarcinoma
chemosensitivity to gemcitabine. Oncogene. 23:1539–1548. 2004.
View Article : Google Scholar
|
|
11
|
Eriksson S and Martin DW Jr:
Ribonucleotide reductase in cultured mouse lymphoma cells. Cell
cycle-dependent variation in the activity of subunit protein M2. J
Biol Chem. 256:9436–9440. 1981.PubMed/NCBI
|
|
12
|
Thelander M, Gräslund A and Thelander L:
Subunit M2 of mammalian ribonucleotide reductase. Characterization
of a homogeneous protein isolated from M2-overproducing mouse
cells. J Biol Chem. 260:2737–2741. 1985.PubMed/NCBI
|
|
13
|
Plunkett W, Huang P, Xu YZ, Heinemann V,
Grunewald R and Gandhi V: Gemcitabine: Metabolism, mechanisms of
action, and self-potentiation. Semin Oncol. 22(Suppl 11): 3–10.
1995.PubMed/NCBI
|
|
14
|
Aguilar-Ponce J, Granados-García M,
Villavicencio V, et al: Phase II trial of gemcitabine concurrent
with radiation for locally advanced squamous cell carcinoma of the
head and neck. Ann Oncol. 15:301–306. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Braakhuis BJM, van Dongen GAMS, Vermorken
JB and Snow GB: Preclinical in vivo activity of
2′,2′-difluorode-oxycytidine (Gemcitabine) against human head and
neck cancer. Cancer Res. 51:211–214. 1991.PubMed/NCBI
|
|
16
|
Senkal CE, Ponnusamy S, Rossi MJ, et al:
Potent antitumor activity of a novel cationic pyridinium-ceramide
alone or in combination with gemcitabine against human head and
neck squamous cell carcinomas in vitro and in vivo. J Pharmacol Exp
Ther. 317:1188–1199. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saddoughi SA, Garrett-Mayer E, Chaudhary
U, et al: Results of a phase II trial of gemcitabine plus
doxorubicin in patients with recurrent head and neck cancers: Serum
C18-ceramide as a novel biomarker for monitoring
response. Clin Cancer Res. 17:6097–6105. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fury MG, Haque S, Stambuk H, Shen R,
Carlson D and Pfister D: A phase 2 study of pemetrexed plus
gemcitabine every 2 weeks for patients with recurrent or metastatic
head and neck squamous cell cancer. Cancer. 117:795–801. 2011.
View Article : Google Scholar
|
|
19
|
van Herpen CML, Locati LD, Buter J, et al:
Phase II study on gemcitabine in recurrent and/or metastatic
adenoid cystic carcinoma of the head and neck (EORTC 24982). Eur J
Cancer. 44:2542–2545. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shintani S, Mihara M, Nakahara Y, Aida T,
Tachikawa T and Hamakawa H: Lymph node metastasis of oral cancer
visualized in live tissue by green fluorescent protein expression.
Oral Oncol. 38:664–669. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shintani S, Hamakawa H, Nakashiro K,
Shirota T, Hatori M, Tanaka M, Kuroshita Y and Kurokawa Y: Friend
leukaemia insertion (Fli)-1 is a prediction marker candidate for
radiotherapy resistant oral squamous cell carcinoma. Int J Oral
Maxillofac Surg. 39:1115–1119. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kobayashi H, Higashiyama M, Minamigawa K,
Tanisaka K, Takano T, Yokouchi H, Kodama K and Hata T: Examination
of in vitro chemosensitivity test using collagen gel droplet
culture method with colorimetric endpoint quantification. Jpn J
Cancer Res. 92:203–210. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Higashiyama M, Oda K, Okami J, Maeda J,
Kodama K, Imamura F, Minamikawa K, Takano T and Kobayashi H:
Prediction of chemotherapeutic effect on postoperative recurrence
by in vitro anticancer drug sensitivity testing in non-small cell
lung cancer patients. Lung Cancer. 68:472–477. 2010. View Article : Google Scholar
|
|
24
|
Souglakos J, Boukovinas I, Taron M, et al:
Ribonucleotide reductase subunits M1 and M2 mRNA expression levels
and clinical outcome of lung adenocarcinoma patients treated with
docetaxel/gemcitabine. Br J Cancer. 98:1710–1715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morikawa T, Maeda D, Kume H, Homma Y and
Fukayama M: Ribonucleotide reductase M2 subunit is a novel
diagnostic marker and a potential therapeutic target in bladder
cancer. Histopathology. 57:885–892. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rahman MA, Amin ARMR, Wang D, et al: RRM2
regulates Bcl-2 in head and neck and lung cancers: A potential
target for cancer therapy. Clin Cancer Res. 19:3416–3428. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fan H, Villegas C and Wright JA:
Ribonucleotide reductase R2 component is a novel malignancy
determinant that cooperates with activated oncogenes to determine
transformation and malignant potential. Proc Natl Acad Sci USA.
93:14036–14040. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rahman MA, Amin ARMR, Wang X, et al:
Systemic delivery of siRNA nanoparticles targeting RRM2 suppresses
head and neck tumor growth. J Control Release. 159:384–392. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shao J, Zhou B, Chu B and Yen Y:
Ribonucleotide reductase inhibitors and future drug design. Curr
Cancer Drug Targets. 6:409–431. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wadler S, Makower D, Clairmont C, Lambert
P, Fehn K and Sznol M: Phase I and pharmacokinetic study of the
ribonucleotide reductase inhibitor,
3-aminopyridine-2-carboxaldehyde thiosemicarbazone, administered by
96-hour intravenous continuous infusion. J Clin Oncol.
22:1553–1563. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Desai AA, Schilsky RL, Young A, Janisch L,
Stadler WM, Vogelzang NJ, Cadden S, Wright JA and Ratain MJ: A
phase I study of antisense oligonucleotide GTI-2040 given by
continuous intravenous infusion in patients with advanced solid
tumors. Ann Oncol. 16:958–965. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yabushita H, Ohnishi M, Komiyama M, Mori T
and Noguchi M, Kishida T, Noguchi Y, Sawaguchi K and Noguchi M:
Usefulness of collagen gel droplet embedded culture drug
sensitivity testing in ovarian cancer. Oncol Rep. 12:307–311.
2004.PubMed/NCBI
|
|
33
|
Mori S, Kunieda K, Sugiyama Y and Saji S:
Prediction of 5-fluorouracil and cisplatin synergism for advanced
gastrointestinal cancers using a collagen gel droplet embedded
culture. Surg Today. 33:577–583. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mekata E, Sonoda H, Shimizu T, Tatsuta T,
Yamaguchi T, Endo Y and Tani T: Clinical predictive value of in
vitro anti-cancer drug sensitivity test for the therapeutic effect
of adjuvant chemotherapy in patients with stage II–III colorectal
cancer. Mol Clin Oncol. 1:763–767. 2013.
|
|
35
|
Zhang ZP, Sun YL, Fu L, Gu F, Zhang L and
Hao XS: Correlation of Notch1 expression and activation to
cisplatin-sensitivity of head and neck squamous cell carcinoma. Ai
Zheng. 28:100–103. 2009.PubMed/NCBI
|
|
36
|
Itoi T, Sofuni A, Fukushima N, Itokawa F,
Tsuchiya T, Kurihara T, Moriyasu F, Tsuchida A and Kasuya K:
Ribonucleotide reductase subunit M2 mRNA expression in pretreatment
biopsies obtained from unresectable pancreatic carcinomas. J
Gastroenterol. 42:389–394. 2007. View Article : Google Scholar : PubMed/NCBI
|