Introduction
Epithelial ovarian cancer (EOC) is an asymptotic
lethal disease that is frequently diagnosed at late stages in women
(1). Initial responsiveness to
chemotherapy and surgery is often thwarted by inherent or acquired
resistance, resulting in early recurrence and premature death
(2). Approximately 22,000 women
will be diagnosed with EOC this year in the US alone, and more than
14,000 will succumb to their disease (3). Because first-line platinum-taxane and
second-line dox-topotecan therapies often fail, third-line
chemotherapy options are urgently needed.
American cranberry (Vaccinium macrocarpon)
has received attention in our laboratories and elsewhere because of
the potential for cranberry A-type proanthocyanidins (PACs) and
flavonols to treat upper urinary tract infections and cancer
(4–6). However, the isolation of pure PACs
and flavonol constituents has remained an unmet challenge that
frequently impedes broad-spectrum screening for biological
activity. Semi-pure cranberry PACs have consistently exhibited
potent anti-proliferative activity against various cancer cells
in vitro (6–8). We had previously developed an
iterative but efficient HPLC and mass spectrometry-based approach
to generate high-purity polymeric PAC fractions from cranberries
(9). Purified PACs have exhibited
cytotoxic effects against a panel of gynecologic cancer and
neuroblastoma cells in our laboratories (9–11).
PACs exerted these cytotoxic effects via cell cycle arrest,
production of lethal levels of intracellular reactive oxygen
species (ROS), and induction of pro-apoptotic signal transductions
at low microgram concentrations (10,11).
Further optimization of the purification and a detailed
investigation of the mechanism of anti-proliferative action have
been pursued in our laboratories since purified PACs became
accessible.
In this study, we further elaborate analytical
methodology to isolate and purify individual flavonols and PACs of
cranberry for broad-spectrum biological activity screening studies.
We also describe the two most active leads, PAC DP-9 and quercetin
aglycone, in SKOV-3 and OVCAR-8 ovarian cancer cells, and we
characterize their anti-proliferative efficacy and mechanism of
cell cycle arrest, induction of apoptotic activities, and
inhibition of oncogenes and DNA repair machinery. The multifaceted
anti-proliferative properties exerted by these two cranberry
flavonoids highlight their potential for treatment of ovarian
cancer.
Materials and methods
Plant material
Cranberry fruits of cultivar ‘Stevens’ were
harvested from the Philip E. Marucci Center for Blueberry and
Cranberry Research and Extension and kept frozen at −20°C before
use.
Reagents and LC-MS instrumentation
All solvents were purchased from EMD Millipore
(Billercia, MA, USA). Sephadex® LH-20 was obtained from
GE Healthcare Bio-Science (Piscataway, NJ, USA), and
BakerBound® Diol was obtained from Avantor Performance
Materials (Center Valley, PA, USA). LC-MS spectra were obtained
with a Dionex UltiMate® 3000 LC system (Thermal
Scientific, Sunnyvale, CA, USA) including the UltiMate 3000 RS
Pump, UltiMate 3000 RS Autosampler, UltiMate 3000 RS Column
Compartment and UltiMate 3000 RS Diode Array Detector coupled with
Applied Biosystems API 3000TM triple quad LC-MS/MS mass
spectrometer (AB SCIEX, Framingham, MA, USA). Previously described
HPLC methods for flavonol and PAC identification (12,13)
were modified slightly for LC-MS analysis. Structure and purity of
flavonols and PACs were determined by HPLC-PDA/Fluorescence and/or
LC-MS.
Extraction and isolation of individual
cranberry flavonols and PACs
Crude flavonoids were extracted and further
separated in a Sephadex LH-20 column as previously described
(14). Individual cranberry
flavonols were isolated using a semi-preparative HPLC system as
described previously (14).
Individual PACs were isolated with a regular Diol gravity column
chromatography as previously reported (9). Eight flavonols were isolated and
characterized as myricetin-3-galactoside, quercetin-3-galactoside,
quercetin-3-glucoside, quercetin-3-xylopyranoside,
quercetin-3-arabinopyranosdie, quercetin-3-arabinofuranoside,
quercetin-3-rhamnopyranoside and quercetin aglycone. Eleven
cranberry A-type PACs from dimer to polymer 12 (named as PAC DP-2
to PAC DP-12) were isolated and characterized. Purity of all
isolated cranberry flavonoids was > 95% (w/w) based on HPLC and
LC-MS analysis.
Cell lines and cell culture
SKOV-3 and OVCAR-8 cells (ovarian epithelial
adenocarcinoma) were purchased from ATCC (Manassas, VA, USA). Cells
were cultured with Dulbecco’s modified Eagle’s medium (DMEM, Life
Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine
serum (Life Technologies), 100 μg/ml streptomycin and 100 μg/ml
penicillin (Life Technologies) in an incubator at 37°C, 5%
CO2 and 95% humidity. For all assays, cells were allowed
to attach for 24 h prior to treatment.
Cell viability assay
Cells (5,000/well) were seeded in 96-well flat
bottom plates (USA Scientific, Orlando, FL, USA) and treated with
various concentrations of flavonoids for 72 h. Cell viability was
determined by CellTiter 96® Aqueous One Solution assay
(Promega, Madison, WI, USA) following the manufacturer’s protocol.
Experiments were performed in triplicate; data are expressed as
mean of triplicate measurements (mean ± SD) in percentage of
untreated cells (100%). SPSS Statistics 19 (IBM Corp., Armonk, NY,
USA) was used to perform ANOVA with linear regression between cell
viability and compound concentration, calculate IC50
value of each cranberry flavonoid, and conduct Student’s t-tests
and calculate p-values based on mean cell viability for each
treatment.
DNA fragmentation analysis
DNA fragmentation as a hallmark of apoptosis was
studied using the Roche In Situ Cell Death Detection kit
(Branford, CT, USA) (TUNEL assay). SKOV-3 and OVCAR-8 cells
(2×104/well) were seeded in Lab-Tek 8-well chamber glass
slides (Nalge Nunc., Naperville, IL, USA) and treated with 50 μg/ml
PAC DP-9, 25 μg/ml quercetin aglycone, or DMSO vehicle control for
12 h. Cells were fixed with 10% neutral buffered formalin, and
stained according to the manufacturer’s protocol. Slides were then
cover-slipped with Vectashield mounting medium with DAPI (Vector
Laboratories, Burlingame, CA, USA). Images were acquired on a Nikon
E800 upright microscope (Nikon Instruments, Inc., Melville, NY,
USA) using SPOT Advanced Software (SPOT Imaging Solutions, Sterling
Heights, MA, USA).
Western blot analysis
Cells (3×106/dish) were seeded into
100-mm2 tissue culture dishes and treated with
individual cranberry flavonoids. Cells were then lysed with cell
lysis buffer (Cell Signaling Technology, Inc., Danvers, MA, USA)
supplemented with 5 μl/ml phenylmethylsulfonyl fluoride
(Sigma-Aldrich, St. Louis, MO, USA). Protein concentrations of cell
lysates were determined with Pierce BCA protein assay kit (Pierce
Technology, Rockford, IL, USA). Gel electrophoresis was performed
in NuPAGE Gel system (Life Technologies) according to the
manufacturer’s instructions. Separated proteins were transferred
onto a nitrocellulose membrane, which was then blocked with 5%
non-fat milk in PBS-Tween buffer and probed against various primary
antibodies (cleaved caspase-3 no. 9664, cleaved PARP no. 5625, EGFR
no. 4267, phospho-EGFR no. 3777, phospho-c-Raf no. 9427,
phospho-ERK1/2 no. 4370, phospho-p53 no. 9286, p18 INK4C no. 2896,
p21 Waf1/Cip1 no. 2947, p27 Kip1 no. 3686, CDK2 no. 2546, cyclin D1
no. 2926, cyclin D3 no. 2936, β-actin no. 8457, β-tubulin no. 2128;
Cell Signaling Technology). Protein bands were visualized using
horseradish peroxidase conjugated anti-rabbit or anti-mouse
secondary antibody (Cell Signaling Technology) and Pierce ECL
Western Blotting Substrate, and documented by Bio-Rad Gel Doc
system (Bio-Rad, Hercules, CA, USA).
Immunofluorescence microscopy
analysis
SKOV-3 and OVCAR-8 cells (2×104/well)
were seeded in Lab-Tek 8-well chamber slides and treated with
individual cranberry flavonoids overnight for 12 h. Cells were
fixed with 10% neutral buffered formalin, washed 3 times with
PBS-Tween 0.1% and blocked with 5% horse serum (Vector
Laboratories) in PBS-Tween for 30 min. Blocked cells were incubated
with various primary antibodies (EGFR no. 4267, p21 Waf1/Cip1 no.
2947, phospho-ERK1/2no.4370,DNA-PKno.4602, Cell Signaling
Technology; and MEK no. sc-166197, phospho-histone H3 no. sc-12927,
cyclin D1 no. sc-246, Santa Cruz Biotechnology, Dallas, TX, USA) at
4°C overnight. Probed proteins were visualized with DyLight 488/594
(Thermo Scientific, Waltham, MA, USA) or Alexa Fluor®
594 (Cell Signaling Technology) secondary antibodies, and nuclei
were stained with Vectorshield® mounting medium with
DAPI (Vector Laboratories). Images were acquired on Olympus
FSX100® (Olympus America Inc., Center Valley, PA, USA;
for EGFR) or Nikon E800 upright (for other proteins) microscope
system.
Co-immunoprecipitation analysis
SKOV-3 and OVCAR-8 cells were cultured in 100
mm2 tissue culture dishes to 80% confluency. Cells were
lysed and protein concentration was quantified as described
earlier. Lysate containing 500 μg protein was incubated with target
antibody or control IgG antibody (Cell Signaling Technology) for 4
h with rotation at 4°C. Protein G sepharose (75 μl) (50% slurry, GE
Healthcare Life Sciences) was added to the lysate and incubated at
4°C overnight. After incubation, beads were washed with 500 μl
lysis buffer 3 times and re-suspended in 40 μl Laemmli buffer (2X,
Bio-Rad), vortexed, and heated to 95°C for 5 min. Suspension was
centrifuged to collect supernatant for western blot analysis.
Cell cycle analysis
SKOV-3 and OVCAR-8 cells (3×105/well)
were seeded in 6-well plates and treated with a series of
concentrations of individual cranberry flavonoids (0–100 μg/ml,
24–48 h). After trypsinization, cells were collected and fixed in
ice-cold 70% ethanol and stained with solution containing propidium
iodide (Sigma-Aldrich; 0.1 mg/ml), sodium citrate (Sigma-Aldrich; 2
mg/ml) and Triton X-100 (Sigma-Aldrich; 1 μl/ml). Cell counting
data were acquired in an Accuri® C6 Flow Cytometer (BD
Biosciences, San Jose, CA, USA) and analyzed with ModFit LT
software (Verity Software House, Inc., Topsham, ME, USA).
Results
Individual cranberry flavonoids display
differential cytotoxicity against ovarian cancer cell lines
Individual cranberry flavonols and PACs exhibited
different levels of cytotoxicity against SKOV-3 and OVCAR-8
cell-lines (Fig. 2).
Quercetin-3-xylopyranoside did not show cytotoxicity against SKOV-3
and OVCAR-8 cells, while myricetin-3-galactoside and
quercetin-3-arabinopyranoside were cytotoxic to OVCAR-8 cells
(IC50, 130 and 212 μg/ml) but non-toxic to SKOV-3 cells
at treatment concentrations. Compared to quercetin glycosides,
quercetin aglycone exhibited higher cytotoxicity against the two
cancer cell lines (IC50, 83 and 61 μg/ml for SKOV-3 and
OVCAR-8 cells, respectively).
Cranberry PAC DP-3, DP-7, and DP-10
exhibited less cytotoxicity against both SKOV-3 and OVCAR-8 cells
than other PACs
PAC DP-5 and DP-12 were more effective against
SKOV-3 cells (IC50, 126 μg/ml and 162 μg/ml for DP-5 and
DP-12, respectively) than OVCAR-8 cells. Compared to other
cytotoxic PAC molecules, PAC DP-9 exhibited the highest activity
against SKOV-3 cells (IC50, 82 μg/ml) and relatively
high cytotoxicity against OVCAR-8 cells (IC50, 138
μg/ml). Based on these results, quercetin aglycone and PAC DP-9
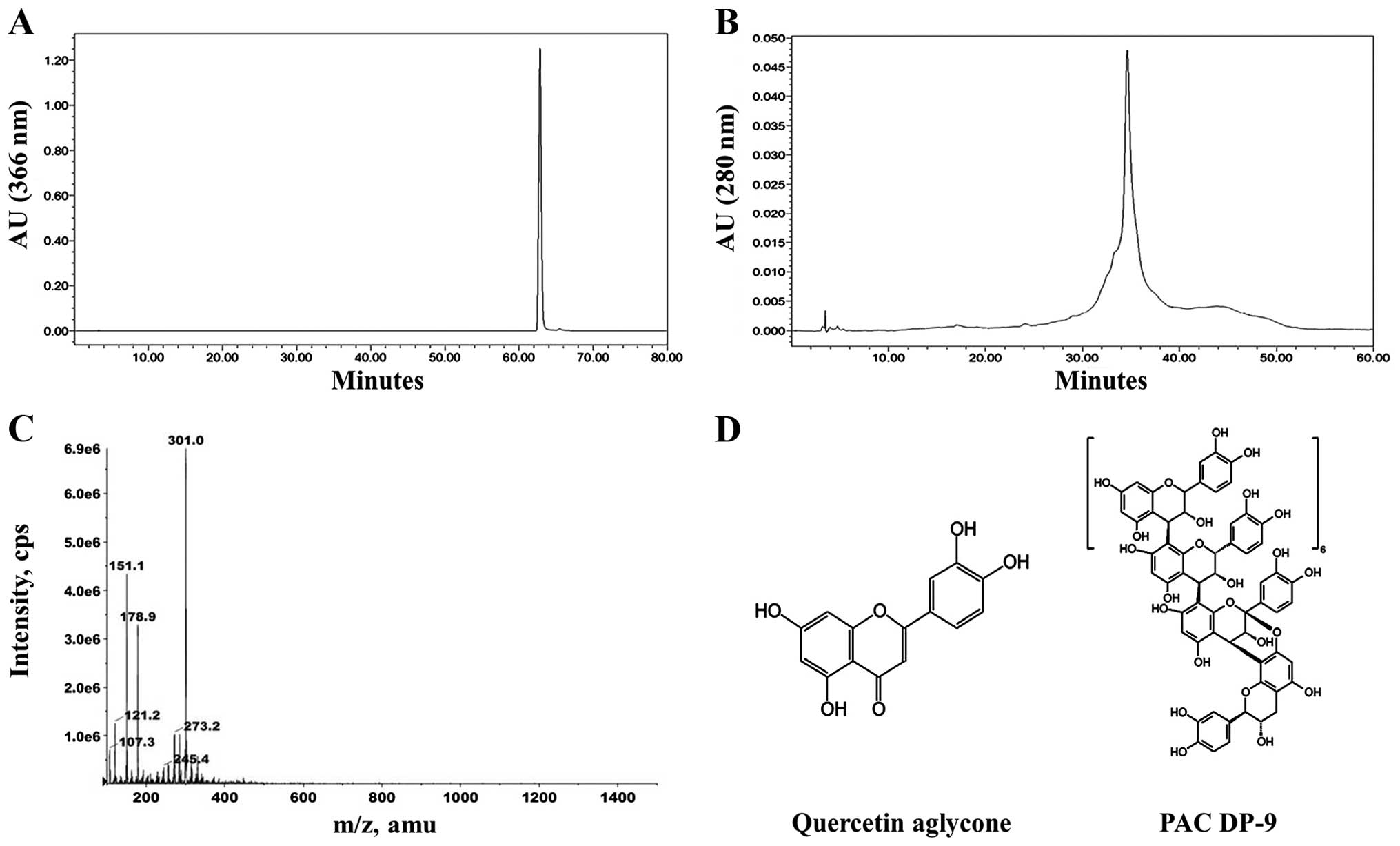
(Fig. 1) were selected as lead
candidates for our studies in SKOV-3 and OVCAR-8 cells.
Cytotoxicity values for individual flavonoids and statistical
analysis including p-values and correlation coefficients are
provided in Table I.
 | Table ICorrelation coefficient (r), p-value
and IC50 value of individual cranberry PACs and
flavonols against SKOV-3 and OVCAR-8 cells.a |
Table I
Correlation coefficient (r), p-value
and IC50 value of individual cranberry PACs and
flavonols against SKOV-3 and OVCAR-8 cells.a
| SKOV-3 | OVCAR-8 |
|---|
|
|
|
|---|
| Compound | Correlation
coefficient | p-value | IC50
(μg/ml) | Correlation
coefficient | p-value | IC50
(μg/ml) |
|---|
| DP-2 | −0.917 | 0.0000263 | 194.8 | −0.919 | 0.0000239 | 143.1 |
| DP-3 | −0.804 | 0.00163 | 210.8 | −0.513 | 0.0882 | ND |
| DP-4 | −0.923 | 0.0000185 | 200.0 | −0.936 | 0.00000741 | 164.1 |
| DP-5 | −0.983 |
1.15×10−8 | 126.3 | −0.738 | 0.00616 | 209.9 |
| DP-6 | −0.929 | 0.0000129 | 201.1 | −0.9 | 0.0000674 | 152.6 |
| DP-7 | −0.821 | 0.00106 | 288.7 | −0.736 | 0.00631 | 211.2 |
| DP-8 | −0.944 | 0.00000397 | 137.9 | −0.945 | 0.00000356 | 125.7 |
| DP-9 | −0.939 | 0.00000584 | 81.9 | −0.923 | 0.0000189 | 137.8 |
| DP-10 | −0.494 | 0.103 | ND | −0.565 | 0.0558 | ND |
| DP-11 | −0.966 |
3.53×10−7 | 165.9 | −0.931 | 0.0000107 | 146.9 |
| DP-12 | −0.824 | 0.000978 | 162.6 | −0.791 | 0.00216 | 248.5 |
| M-Gal | 0.331 | 0.293 | ND | −0.78 | 0.00275 | 130.7 |
| Q-Gal | −0.833 | 0.000757 | 164.8 | −0.896 | 0.000082 | 132.7 |
| Q-Glu | −0.899 | 0.0000684 | 160.6 | −0.929 | 0.000013 | 160.6 |
| Q-A-P | −0.665 | 0.0182 | 410.2 | −0.777 | 0.00293 | 212.7 |
| Q-A-F | −0.791 | 0.00217 | 173.5 | −0.908 | 0.0000435 | 171.2 |
| Q-Xylo | 0.095 | 0.77 | ND | −0.266 | 0.404 | ND |
| Q-Rhamno | −0.879 | 0.000167 | 207.4 | −0.945 | 0.00000369 | 163.4 |
| Q-Aglycone | −0.982 |
1.45×10−8 | 83.2 | −0.966 |
3.48×10−7 | 61.1 |
Quercetin aglycone and PAC DP-9 induced
apoptosis and increased cisplatin sensitivity in SKOV-3 and OVCAR-8
cells
To examine whether apoptosis was induced in ovarian
cancer cells upon treatment with cranberry flavonoids, western blot
and DNA fragmentation analysis (TUNEL assay) were carried. As shown
in Fig. 3A, quercetin aglycone
induced caspase-3 activation in both SKOV-3 and OVCAR-8 cells, and
PAC DP-9 led to caspase-3 and cleaved-PARP expression specifically
in SKOV-3 cells.
TUNEL assay was performed to detect
apoptosis-induced DNA fragmentation. Quercetin aglycone and PAC
DP-9 treated cells showed apoptosis-induced DNA fragmentation,
which was detected in both SKOV-3 and OVCAR-8 cells after 12 h of
treatment (Fig. 3B). Both western
blot and DNA fragmentation analysis confirmed apoptosis induction
in ovarian cancer cells exposed to these two cranberry
flavonoids.
To investigate whether quercetin aglycone and PAC
DP-9 could sensitize ovarian cancer cells to cisplatin, cells were
pretreated with subtoxic concentrations of quercetin aglycone or
PAC DP-9 for 6 h, exposed to cisplatin overnight for 12 h, and cell
viability was analyzed. As shown in Fig. 3C, while subtoxic concentrations of
quercetin aglycone or PAC DP-9 alone did not reduce SKOV-3 and
OVCAR-8 cell viability, their pretreatment significantly reduced
viability of cisplatin-treated SKOV-3 and OVCAR-8 cells, with
quercetin aglycone acting more strongly than PAC DP-9. Thus,
quercetin aglycone and PAC DP-9 at low concentrations sensitize the
response of cisplatin-resistant ovarian cancer cells to cisplatin,
resulting in enhanced cytotoxicity.
PAC DP-9 downregulated expression and
activation of epidermal growth factor receptor (EGFR) in SKOV-3
cells and induced EGFR nuclear translocation
Elevated EGFR expression results in poor prognosis
in lung, breast and ovarian cancer (15). EGFR expression in quercetin
aglycone or PAC DP-9 treated ovarian cancer cells was analyzed by
immunoblotting and immunofluorescence microscopy. To eliminate the
effect of serum growth factors on cellular EGFR regulation, ovarian
cancer cells were serum-deprived for 4 h prior to treatment, and
low concentrations (5–40 μg/ml) of cranberry flavonoids were
applied with serum-free medium to avoid cell toxicity. After 12 h
of treatment, quercetin aglycone did not affect phosphorylated-EGFR
levels in either SKOV-3 or OVCAR-8 cells, but PAC DP-9 induced a
dose-dependent downregulation of both phosphorylated-EGFR and total
EGFR in SKOV-3 cells (Fig. 4A). As
shown in Fig. 4B, EGFR was
expressed predominantly at the cell membrane in the untreated
controls, and exhibited nuclear translocation within 3 h of
treatment with PAC DP-9 in a dose-dependent manner such that lower
concentrations (12.5–25 μg/ml) induced EGFR peri-nuclear
localization in SKOV-3 cells, and higher concentrations (50–200
μg/ml) of PAC DP-9 mediated EGFR nuclear translocation.
Quercetin aglycone and PAC DP-9
downregulated pro-survival MAP kinase proteins in ovarian cancer
cells
We examined changes in MAP kinase signaling
regulation exerted by quercetin aglycone and PAC DP-9 in ovarian
cancer cells. Expression of MEK (Fig.
5A) and phospho-ERK1/2 (Fig.
5B) was downregulated after 12 h of treatment with quercetin
aglycone or PAC DP-9, indicating inhibition of pro-survival
MAPK-ERK signal transduction by cranberry flavonoids. Similarly,
treatment with quercetin aglycone and PAC-9 led to rapid,
significant downregulation of phospho-p42/22 MAPK and phospho-c-Raf
in OVCAR-8 cells within 6 h (Fig.
6A), demonstrating their inhibitory effect on the activated
pro-survival MAPK pathway.
Quercetin aglycone and PAC DP-9 affected
cell cycle progression of ovarian cancer cells
Immunofluorescence microscopy analysis of quercetin
aglycone and PAC DP-9 treatment revealed downregulation of cyclin
D1 and upregulation of p21 in SKOV-3 and OVCAR-8 cells (Fig. 5D and F). Phospho-histone H3
(Fig. 5C) and DNA-dependent
protein kinase (DNA-PK, Fig. 5E),
proteins often overexpressed in ovarian cancer cells, were also
downregulated in SKOV-3 and OVCAR-8 cells upon treatment with
cranberry flavonoids. Based on immunoblot study, phospho-p53 and
p21 expression were first downregulated after 6 h of treatment with
quercetin aglycone in OVCAR-8 cells and then exhibited sustained
upregulation (Fig. 6A). Three
other cell cycle regulators, p18, p27, and CDK2 were also
upregulated within 6–24 h of treatment with quercetin aglycone.
Independent of p53 expression, SKOV-3 cells exhibited rapid
upregulation of cell cycle inhibitor p21 after 6 h of treatment of
quercetin aglycone, together with upregulation of p18, p27, and
CDK2 within 24 h of treatment. Similarly, PAC DP-9 induced
upregulation of p21 in both SKOV-3 and OVCAR-8 cells within 6–24
h-treatment regardless of p53 expression level. CDK2 expression was
also upregulated in SKOV-3 and OVCAR-8 cells after 24 h of PAC DP-9
treatment.
Because immunofluorescence microscopy and western
blot analysis showed inhibition of SKOV-3 cellular EGFR expression
by PAC DP-9 (Fig. 4),
co-immunoprecipitation (Co-IP) was utilized to examine potential
protein-protein interactions involved with EGFR and cell cycle
regulatory factors, including p18, p21, p27, CDK2, cyclin D1 and
cyclin D3. While most of the probed proteins did not show a
positive signal, CDK2 signal was detected in both SKOV-3 and
OVCAR-8 EGFR Co-IP samples. As shown in Fig. 6B, CDK2 was recovered in both
positive controls and two Co-IP samples as well as in the OVCAR-8
negative control, indicating a false-positive signal in the OVCAR-8
Co-IP sample. The absence of CDK2 signal in SKOV-3 negative control
confirmed direct interaction between CDK2 and EGFR in SKOV-3
cells.
Both western blot and Co-IP studies indicate a
direct effect of cranberry flavonoids on ovarian cancer cell
progression. Subpopulations of propidium iodide-stained SKOV-3 and
OVCAR-8 ovarian cancer cells treated with cranberry flavonoids were
analyzed by flow cytometry. As illustrated in Fig. 6C and D, quercetin aglycone or PAC
DP-9 treatment for 24 h in SKOV-3 cells led to G2/M-phase arrest
dose-dependently. The G2/M subpopulation increased from 10.81 to
16.52% for SKOV-3 cells treated with 50 μg/ml quercetin aglycone
and further increased to 32.35% after treatment with 100 μg/ml
quercetin aglycone. Similarly, 50 and 100 μg/ml PAC DP-9 caused an
increase in the SKOV-3 cell G2/M subpopulation to 17.69 and 29.63%,
respectively. After 48 h of treatment in SKOV-3 cells, PAC DP-9
exhibited similar dose-dependent G2/M-phase arrest, whereas 50 and
100 μg/ml quercetin aglycone led to an increase in S subpopulation
from 22.01 to 45.54 and 50.35%, respectively, with no significant
change in G2/M subpopulation. This suggests that quercetin aglycone
caused S/G2-phase arrest in SKOV-3 cells after 48 h of
treatment.
Quercetin aglycone treatment for 24 and 48 h in
OVCAR-8 cells caused G1/S-phase arrest. At 50 μg/ml, quercetin
aglycone led to retention of 75.69 and 86.12% of cells in G0/G1
phase after 24 and 48 h of treatment, respectively, and the
subpopulation of S-phase cells decreased from 38.67 to 12.61% after
24 h and from 26.35 to 6.45% after 48 h (Fig. 6D). PAC DP-9 showed a similar effect
in OVCAR-8 cells, thus confirming that G1/S-phase arrest was caused
by the two compounds in OVCAR-8 ovarian cancer cells.
Discussion
Cranberry phenolic extracts have shown in
vitro anticancer and chemo-preventive properties in different
cancer cell lines (5–11). Research on individual phenolic
compounds of cranberry has been limited by the difficulty of
compound isolation due to complex structural variations, including
degree-of-polymerization, linkage type, position of the double
linkage between constituent units, and attachment with sugar
moieties (9,12). Serial application of Dionex
UltiMate® 3000 LC system consisting of UltiMate 3000 RS
Pump, UltiMate 3000 RS Autosampler, UltiMate 3000 RS Column
Compartment, and UltiMate 3000 RS Diode Array Detector coupled with
Applied Biosystems API 3000 triple quad LC-MS/MS mass spectrometer
has allowed the reproducible isolation and characterization of
individual PACs with up to the 10th degree of polymerization in
>95% purity in our laboratories. So far, we have successfully
isolated 19 high-purity individual cranberry flavonoids,
characterized their structures by LC-APCI-MS, and used them as
primary materials to determine bioactivity.
SKOV-3 and OVCAR-8 ovarian cell lines, which possess
several key oncogenic hallmarks such as p53 mutation, EGFR
overexpression, and cisplatin resistance (16–18),
were employed as an in vitro cell culture model to determine
the mechanism of action of cranberry flavonoids. Individual PACs
DP-2 to DP-4 failed to show strong cytotoxicity against SKOV-3
cells, and high molecular weight PAC polymers generally exhibited
stronger cytotoxicity similar to what we showed earlier (9). Quercetin aglycone emerged as the most
cytotoxic of the tested cranberry flavonols. Shen et al
showed that quercetin aglycone exhibited significantly higher
cytotoxicity against human promyelocytic leukemia cells HL-60
compared to its rutinose and rhamnose glycosides (19). Murota et al also reported
that quercetin aglycone was much more efficiently absorbed by
Caco-2 colorectal adenocarcinoma cells than its glycosides
(20). Both quercetin aglycone and
PAC DP-9 induced ovarian cancer cell death through apoptotic
events, and subtoxic concentrations of the two compounds
significantly increased the efficacy of cisplatin against ovarian
cancer cells. Because developed resistance to platinum-based drugs
is one of the major causes of mortality in ovarian cancer (2), the observation that cranberry
flavonoids can sensitize drug-resistant ovarian cancer cells to
cisplatin provides an opportunity to improve the efficacy of
platinum chemotherapies and reduce side effects associated with
cisplatin.
EGFR, a receptor tyrosine kinase, is involved in
many signaling pathways that modulate cell survival, proliferation
and apoptosis. Aberrant activation of EGFR has been shown to play a
critical role in cancer cell survival and development (18,21).
B-type PACs isolated from grape seeds have been shown to target and
downregulate EGFR expression in human head and neck squamous cell
carcinoma (HNSCC) cells and inhibited their invasiveness (22). Our study showed that treatment with
A-type PAC DP-9 decreased expression and activation and induced
nuclear translocation of EGFR in SKOV-3 cells dose-dependently.
Association of EGFR with DNA-PK, which is involved in DNA repair,
has been previously reported (23,24),
and its nuclear translocation has been confirmed to modulate DNA
repair caused by cisplatin or radiation in mouse fibroblast cell
lines (25). Although EGFR nuclear
translocation can be expected to activate DNA-PK as a counter
measure to DNA damage due to quercetin and PAC-9 treatment,
immunofluorescence microscopy analysis revealed downregulation of
DNA-PK in ovarian cancer cells exposed to quercetin aglycone or PAC
DP-9. The DNA-PK-mediated DNA repair that is induced by exposure to
cisplatin in cancer cells is believed to be an important factor in
reducing the efficacy of platinum-based chemotherapy (26,27).
Therefore, the inhibition of DNA-PK expression in quercetin
aglycone or PAC DP-9-treated ovarian cancer cells may partially
account for the increased efficacy of cisplatin in ovarian cancer
cells following quercetin aglycone or PAC DP-9 pre-treatment.
EGFR also regulates the extracellular
signal-regulated kinase ERK-MAPK pathway to maintain normal cell
growth, proliferation and differentiation (28). Aberrant activation of the
EGFR-Ras-Raf-MEK-ERK cascade is believed to contribute to cancer
development and progression (29).
Both quercetin aglycone and PAC DP-9 downregulated activated Raf,
ERK1/2 and MEK in OVCAR-8 cells, suggesting the ERK-MAPK pathway
may be one of the anti-proliferative mechanisms. The Raf-MEK-ERK
pathway is also known to control cell cycle progression through
induction of key cell cycle regulatory factors such as cyclins,
cyclin-dependent kinases (CDKs), and p21 (30). Phosphorylation of histone H3, which
is believed to play an important role in cell division and oncogene
induction, was shown to be stimulated by Ras-MAPK signaling pathway
(31,32). In our study, we report different
levels of upregulation of CDK inhibitors p18, p27, and most
significantly, p21 after treatment with quercetin aglycone or PAC
DP-9, as well as downregulation of cyclin D1 and phospho-histone
H3, indicating their effects on cell cycle regulation. Expression
of p21 can be induced by either p53-dependent or independent
pathways (33,34). In SKOV-3 cells, where the p53 gene
is mutated and loses its expression, PAC DP-9 induced p21
upregulation inversely correlated with the expression of ERK1/2,
suggesting that PAC DP-9 modulated a p53-independent ERK pathway
that mediates p21 regulation similar to a published mechanism in
rhabdosarcoma cells (34).
Upregulation of CDK inhibitors and downregulation of
cyclin D1 and phospho-histone H3 induced by the two cranberry
flavonoids reflected the ability of quercetin aglycone and PAC DP-9
to cause cell cycle arrest in SKOV-3 and OVCAR-8 cells. At
concentrations lower than IC50, both quercetin aglycone
and PAC DP-9 induced G1/S phase cell cycle arrest in OVCAR-8 cells,
consistent with the upregulation of cellular p21 that has been
shown to inhibit CDK regulation in G1/S phase progression (35). On the other hand, SKOV-3 cells
showed cell cycle arrest at G2/M phase within 24 h of treatment
with quercetin aglycone or PAC DP-9, similar as shown previously
(10). Interestingly, CDK2, which
is inhibited by p21 and facilitates G1/S phase transition (35), was upregulated after treatment with
quercetin aglycone or PAC DP-9 in both SKOV-3 and OVCAR-8 cells. We
confirmed direct protein-protein interaction between EGFR and CDK2
in SKOV-3 cells through Co-IP, suggesting that CDK-2 overexpression
could be induced by nuclearly localized EGFR to facilitate DNA
repair and synthesis after exposure to quercetin aglycone or PAC
DP-9. These observations suggest that cell division of the two
ovarian cancer cell lines was regulated through different
mechanisms by cranberry flavonoids. Targeting cell cycle
checkpoints has been proposed as a promising approach to cancer
treatment (36).
In conclusion, our study suggests that certain
cranberry flavonols and PACs possess cytotoxic properties against
ovarian cancer cells. The integration of quercetin and/or PAC DP-9
in ovarian cancer chemotherapy may provide for improved outcomes.
Quercetin aglycone and PAC DP-9 induced cellular apoptotic events
including cell cycle arrest and suppression of DNA repair pathways
that highlight their potential as dietarily available therapeutic
agents.
Acknowledgements
Y.W., A.P.S. and N.V. are grateful to National
Institutes of Health (NIH) for funding support via grant
5R01DE016139.
References
|
1
|
Jacobs IJ and Menon U: Progress and
challenges in screening for early detection of ovarian cancer. Mol
Cell Proteomics. 3:355–366. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Agarwal R and Kaye SB: Ovarian cancer:
Strategies for overcoming resistance to chemotherapy. Nat Rev
Cancer. 3:502–516. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
American Cancer Society. Ovarian Cancer
Key Statistics. http://www.cancer.org/cancer/ovariancancer/index.
2014
|
|
4
|
Foo LY, Lu Y, Howell AB and Vorsa N:
A-Type proanthocyanidin trimers from cranberry that inhibit
adherence of uropathogenic P-fimbriated Escherichia coli. J Nat
Prod. 63:1225–1228. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seeram NP, Adams LS, Hardy ML and Heber D:
Total cranberry extract versus its phytochemical constituents:
Antiproliferative and synergistic effects against human tumor cell
lines. J Agric Food Chem. 52:2512–2517. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Neto CC: Cranberry and its phytochemicals:
A review of in vitro anticancer studies. J Nutr. 137(Suppl):
S186–S193. 2007.
|
|
7
|
Neto CC, Krueger CG, Lamoureaux TL, et al:
MALDI-TOFMS characterization of proanthocyanidins from cranberry
fruit (Vaccinium macrocarpon) that inhibit tumor cell growth and
matrix metalloproteinase expression in vitro. J Sci Food Agric.
86:18–25. 2006. View Article : Google Scholar
|
|
8
|
Ferguson PJ, Kurowska E, Freeman DJ,
Chambers AF and Koropatnick DJ: A flavonoid fraction from cranberry
extract inhibits proliferation of human tumor cell lines. J Nutr.
134:1529–1535. 2004.PubMed/NCBI
|
|
9
|
Singh AP, Singh RK, Kim KK, Satyan KS,
Nussbaum R, Torres M, Brard L and Vorsa N: Cranberry
proanthocyanidins are cytotoxic to human cancer cells and sensitize
platinum-resistant ovarian cancer cells to paraplatin. Phytother
Res. 23:1066–1074. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim KK, Singh AP, Singh RK, Demartino A,
Brard L, Vorsa N, Lange TS and Moore RG: Anti-angiogenic activity
of cranberry proanthocyanidins and cytotoxic properties in ovarian
cancer cells. Int J Oncol. 40:227–235. 2012.
|
|
11
|
Singh AP, Lange TS, Kim KK, Brard L, Horan
T, Moore RG, Vorsa N and Singh RK: Purified cranberry
proanthocyanidines (PAC-1A) cause pro-apoptotic signaling, ROS
generation, cyclophosphamide retention and cytotoxicity in
high-risk neuroblastoma cells. Int J Oncol. 40:99–108. 2012.
|
|
12
|
Vvedenskaya IO, Rosen RT, Guido JE,
Russell DJ, Mills KA and Vorsa N: Characterization of flavonols in
cranberry (Vaccinium macrocarpon) powder. J Agric Food Chem.
52:188–195. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wilson T, Singh AP, Vorsa N, Goettl CD,
Kittleson KM, Roe CM, Kastello GM and Ragsdale FR: Human glycemic
response and phenolic content of unsweetened cranberry juice. J Med
Food. 11:46–54. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Singh AP, Wilson T, Kalk AJ, Cheong J and
Vorsa N: Isolation of specific cranberry flavonoids for biological
activity assessment. Food Chem. 116:963–968. 2009. View Article : Google Scholar :
|
|
15
|
Nicholson RI, Gee JMW and Harper ME: EGFR
and cancer prognosis. Eur J Cancer. 37(Suppl 4): S9–S15. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schilder RJ, Hall L, Monks A, Handel LM,
Fornace AJ Jr, Ozols RF, Fojo AT and Hamilton TC: Metallothionein
gene expression and resistance to cisplatin in human ovarian
cancer. Int J Cancer. 45:416–422. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bacchetti S and Graham F: Inhibition of
cell-proliferation by an adenovirus vector expressing the human
wild type-p53 protein. Int J Oncol. 3:781–788. 1993.PubMed/NCBI
|
|
18
|
Anderson NG, Ahmad T, Chan K, Dobson R and
Bundred NJ: ZD1839 (Iressa), a novel epidermal growth factor
receptor (EGFR) tyrosine kinase inhibitor, potently inhibits the
growth of EGFR-positive cancer cell lines with or without erbB2
overexpression. Int J Cancer. 94:774–782. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shen SC, Chen YC, Hsu FL and Lee WR:
Differential apoptosis-inducing effect of quercetin and its
glycosides in human promyeloleukemic HL-60 cells by alternative
activation of the caspase 3 cascade. J Cell Biochem. 89:1044–1055.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Murota K, Shimizu S, Chujo H, Moon JH and
Terao J: Efficiency of absorption and metabolic conversion of
quercetin and its glucosides in human intestinal cell line Caco-2.
Arch Biochem Biophys. 384:391–397. 2000. View Article : Google Scholar
|
|
21
|
Yarden Y: The EGFR family and its ligands
in human cancer. signalling mechanisms and therapeutic
opportunities. Eur J Cancer. 37(Suppl 4): S3–S8. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun Q, Prasad R, Rosenthal E and Katiyar
SK: Grape seed proanthocyanidins inhibit the invasiveness of human
HNSCC cells by targeting EGFR and reversing the
epithelial-to-mesenchymal transition. PLoS One. 7:e31093. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rodemann HP, Dittmann K and Toulany M:
Radiation-induced EGFR-signaling and control of DNA-damage repair.
Int J Radiat Biol. 83:781–791. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang SC and Hung MC: Nuclear translocation
of the epidermal growth factor receptor family membrane tyrosine
kinase receptors. Clin Cancer Res. 15:6484–6489. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liccardi G, Hartley JA and Hochhauser D:
EGFR nuclear translocation modulates DNA repair following cisplatin
and ionizing radiation treatment. Cancer Res. 71:1103–1114. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jamieson ER and Lippard SJ: Structure,
recognition, and processing of cisplatin-DNA adducts. Chem Rev.
99:2467–2498. 1999. View Article : Google Scholar
|
|
27
|
Jung Y and Lippard SJ: Direct cellular
responses to platinum-induced DNA damage. Chem Rev. 107:1387–1407.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stokoe D, Macdonald SG, Cadwallader K,
Symons M and Hancock JF: Activation of Raf as a result of
recruitment to the plasma membrane. Science. 264:1463–1467. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Roberts PJ and Der CJ: Targeting the
Raf-MEK-ERK mitogen-activated protein kinase cascade for the
treatment of cancer. Oncogene. 26:3291–3310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chang F, Steelman LS, Shelton JG, Lee JT,
Navolanic PM, Blalock WL, Franklin R and McCubrey JA: Regulation of
cell cycle progression and apoptosis by the Ras/Raf/MEK/ERK pathway
(Review). Int J Oncol. 22:469–480. 2003.PubMed/NCBI
|
|
31
|
Hans F and Dimitrov S: Histone H3
phosphorylation and cell division. Oncogene. 20:3021–3027. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chadee DN, Hendzel MJ, Tylipski CP, Allis
CD, Bazett-Jones DP, Wright JA and Davie JR: Increased Ser-10
phosphorylation of histone H3 in mitogen-stimulated and
oncogene-transformed mouse fibroblasts. J Biol Chem.
274:24914–24920. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Agarwal ML, Agarwal A, Taylor WR and Stark
GR: p53 controls both the G2/M and the G1 cell cycle checkpoints
and mediates reversible growth arrest in human fibroblasts. Proc
Natl Acad Sci USA. 92:8493–8497. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ciccarelli C, Marampon F, Scoglio A, Mauro
A, Giacinti C, De Cesaris P and Zani BM: p21WAF1
expression induced by MEK/ERK pathway activation or inhibition
correlates with growth arrest, myogenic differentiation and
onco-phenotype reversal in rhabdomyosarcoma cells. Mol Cancer.
4:41. 2005. View Article : Google Scholar
|
|
35
|
Harper JW, Adami GR, Wei N, Keyomarsi K
and Elledge SJ: The p21 Cdk-interacting protein Cip1 is a potent
inhibitor of G1 cyclin-dependent kinases. Cell. 75:805–816. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vermeulen K, Van Bockstaele DR and
Berneman ZN: The cell cycle: A review of regulation, deregulation
and therapeutic targets in cancer. Cell Prolif. 36:131–149. 2003.
View Article : Google Scholar : PubMed/NCBI
|