Introduction
Hepatocellular carcinoma (HCC) is the sixth most
common cancer worldwide and the second leading cause of
cancer-related deaths (1). The
application of potentially curative interventions such as liver
transplantation, resection, and thermal ablation is limited to ~30%
of patients with tumors or liver function that meets defined
criteria (1,2). HCCs resist conventional systemic
therapies. Although the standard systemic chemotherapy for patients
with advanced HCC depends on the kinase inhibitor sorafenib,
therapy with sorafenib resulted in a mean survival benefit of only
3 months (3,4). Alternative agents for systemic
therapy are urgently needed.
Galectin-9 is one of the soluble
β-galactoside-binding animal lectins. It has been reported to
trigger the death of T-cell lines and various T-cell subsets,
resulting in immunomodulation with negative selection during T-cell
development, in studies by us and others (5–9).
Recent reports revealed the possibility that galectin-9 can be
applied to cancer therapy (10,11).
Galectin-9 induces apoptosis of chronic myelogenous leukemia cells
and myeloma cells (12,13). In addition to its induction of
apoptosis in malignant melanoma cells, galectin-9 also arrested the
cell cycle (14). Regarding the
effects of galectin-9 on HCC, the cell surface expression of
galectin-9 in HCC is considered to suppress metastasis and to
improve the prognosis of cancer-bearing patients (15).
The interaction of galectin-9 and Tim-3, a specific
ligand of galectin-9, was shown to mediate T-cell senescence in
hepatitis B virus (HBV)-associated HCC (16), but the antitumor effect of
galectin-9 on HCC was not determined. The purpose of the present
study was to determine whether galectin-9 can suppress the growth
of HCC by inducing apoptosis and cell cycle arrest in vitro
and in vivo. Another goal of the present study was to
identify any microRNAs (miRNAs) associated with the antitumor
effect of galectin-9.
Materials and methods
Antibodies and reagents
Recombinant mutant forms of human galectin-9 that
lack the linker peptides were expressed and purified as described
(17). Alexa Fluor® 647
Annexin V, propidium iodide (PI), PE anti-human Tim-3 conjugated
antibody (clone: F38-2E2) and its isotype, Human TruStain FcX IgG
and Annexin V binding buffer were obtained from Biolegend (San
Diego, CA, USA). Anti-DYRK1A antibody (ab65220) was provided by
Abcam (Cambridge, UK). Anti-β-actin monoclonal antibody (clone:
AC-15) was supplied from Sigma-Aldrich (St. Louis, MO, USA).
Cell lines and culture
The HCC cell line HLE was obtained from the Japanese
Cancer Research Resources Bank (Osaka, Japan). Li-7 and Huh7 were
supplied by Riken Cell Bank (Tsukuba, Japan). The two Chinese
hamster ovary (CHO) cell lines, CHO-K1 (Tim-3 knocked out cells)
and CHO-Tim-3 (Tim-3 overexpressed cells), were gifts from Dr Vijay
Kuchroo (Harvard Medical School). HLE, CHO-K1 and CHO-Tim-3 cells
were grown in DMEM, Li-7 in RPMI-1640, and Huh7 in MEM,
supplemented with 10% fetal bovine serum (FBS) and 100 mg/l of
penicillin-streptomycin in a humidified atmosphere with 5% of
CO2 at 37°C.
Cell proliferation assay
Cell proliferation assays were conducted in HLE,
Li-7 and Huh7 cells with WST-8 (18). Each cell type (5×103)
was seeded into 96-well plates and cultured in 100 μl of culture
medium for 24 h. Cells were treated with 0.01, 0.03, 0.1, 0.3 or 1
μM galectin-9. In addition, 30 mM of lactose was added to inhibit
the binding of galectin-9, and sucrose was used as a control
(19).
FACS analysis
We conducted a flow cytometric analysis with Annexin
V and PI to clarify whether galectin-9 has apoptotic and necrotic
effects on HCC (18,20). Briefly, we treated HLE, Li-7 and
Huh7 cells (1×105 cells in 200 μl of culture medium)
with 0.3 μM galectin-9 for 12 h at 37°C. The cells were analyzed
with the Mofro Astrios flow cytometer (Beckman Coulter,
Indianapolis, IN, USA). The results were analyzed by Kaluza
software (Beckman Coulter).
The expression of Tim-3 on the cell surface was
characterized by a single-color flow cytometric analysis (7). The CHO-K1 cell line was used as the
negative control and the CHO-Tim-3 cells were used as the positive
control. HLE, CHO-K1 and CHO-Tim-3 cells were dissociated from a
monolayer culture with enzyme-free cell dissociation buffer. Li-7
and Huh7 cells were dissociated with 0.05% of trypsin and incubated
for 12 h in a non-adhesive 96-well microplate. The flow cytometric
analysis was conducted using the Cytomics FC 500 flow cytometer
(Beckman Coulter), and the results were analyzed by Kaluza
software.
We analyzed the effects of galectin-9 on the
cell-cycle profile of HCC cells by using the Cell Cycle Phase
Determination Kit (Cayman Chemical, Ann Arbor, MI, USA). The assays
were conducted as described (21,22).
The flow cytometric analysis was conducted using the Cytomics FC
500 flow cytometer, and the percentage of cells in different phases
of the cell cycle was analyzed using the Kaluza software.
ELISA assay for apoptosis
We evaluated the amounts of caspase-cleaved
keratin18 (CCK18) by using the M30 Apoptosense ELISA kit (Peviva,
Bromma, Sweden) (23). Each cell
line (5×103) was seeded into 96-well plates and cultured
in 100 μl of culture medium for 24 h. The cells were then treated
with 0.3 μM galectin-9. The rest of the assay procedures were
performed according to the manufacturer’s instructions. The amounts
of antigen in the controls and samples were calculated by
interpolation into a standard curve.
Xenograft model analysis
Animal experiments were performed according to the
guidelines of the Kagawa University Committee on Experimental
Animals and the guidelines regarding the use of animals tissued by
the UK National Cancer Research Institute (24).
We purchased female athymic mice (BALB/c-nu/nu;
8-week-old; 20–25 g) from Japan SLC (Shizuoka, Japan). The animals
were maintained under specific pathogen-free conditions using a
laminar air flow rack and had continuous free access to food
sterilized by irradiation with γ-rays, which was purchased from
CLEA Japan (Tokyo, Japan) and autoclaved water.
We used tissues instead of cell suspensions for the
xenograft model of Li-7 cells because a 90-day period would be
necessary for the tumors to grow to palpable size in mice injected
with a cell suspension of Li-7 cells. Li-7 tissues were
transplanted subcutaneously (s.c.) in the flank of nude mice for
initial passage. The tumor tissues were harvested when the tumors
grew to 15 mm in diameter, and the tumor tissue was cut into pieces
3 mm in diameter.
Mice were inoculated with a tumor fragment of Li-7
(25). Huh7 cells
(2×106 cells per animal) were also injected s.c. in nude
mice. When the xenografts were palpable as a mass of >6 mm in
diameter in all recipients, the mice were randomly assigned to the
control group or a treated group. Treated mice (n=6) were injected
s.c. with galectin-9 (90 μg) three times a week. Only
phosphate-buffered saline (PBS) was administered to the control
group (n=6). The tumor growth was monitored by the same
investigators (K. Fujita and T. Masaki). The tumor volume was
calculated as follows: tumor volume (mm3) = [tumor
length (mm) × tumor width (mm2)]/2. The animals bearing
an excessive tumor volume (>2,000 mm3) were
euthanized for ethical reasons (24).
TUNEL assay
The tumor tissues were fixed in 4% buffered
paraformaldehyde. We performed a terminal deoxynucleotidyl
transferase dUTP nick end labeling (TUNEL) assay to detect
apoptotic cells using the TACS2 TdT-DAB In Situ Apoptosis
Detection kit (Trevigen, Gaithersburg, MD, USA) according to the
manufacturer’s instructions (18).
Colorimetric assay for caspase-4, -8 and
-9
We determined the amount of caspase-4, -8 and -9 by
using the caspase-4, FLICE/caspase-8 and caspase-9 colorimetric
assay kits (BioVision, Milpitas, CA, USA), respectively (18). HLE and Li-7 cells were cultured
with or without 0.3 μM galectin-9, and the subsequent procedures
were performed according to the manufacturer’s instructions.
Analysis of miRNA microarray
We extracted the total RNA of cultured cells or
tumor tissues using the miRNeasy Mini kit (Qiagen, Venlo, The
Netherlands) as described (21,22,26).
Cells were cultured with or without galectin-9 for 24 h in
pentaplicate. The samples were labeled using a miRCURY Hy3 Power
Labeling kit (Exiqon, Vedbaek, Denmark) and hybridized on a human
miRNA Oligo chip, version 14.0 (Toray, Tokyo, Japan). Scanning was
conducted with the 3D-Gene Scanner 3000 (Toray). We used 3D-Gene
extraction software (ver. 1.2, Toray) to read the raw intensity of
the image. The raw data were analyzed with GeneSpringGX (ver. 10.0,
Agilent Technologies, Santa Clara, CA, USA) and quantile normalized
(27). We calculated the fold
changes in miRNA expression level between the treated groups and
control group. Hierarchical clustering was accomplished using the
furthest neighbor method and Pearson’s product-moment correlation
coefficient as a metric.
Transfection of miRNA
Li-7 cells were transfected with miR-1246 mimic
(mirVana miRNA mimic, Ambion, Carlsbad, CA, USA), miR-6131 mimic,
and Lipofectamine 2000 according to the manufacturer’s protocol.
HCC cells were transfected with miR-1246 mimic, miR-6131 mimic, or
negative control #1 (Ambion) under galectin-9. The WST-8 assay and
ELISA of CCK18 were conducted at the indicated time-points.
Western blot analysis
The cell lysate was processed according to the
methods described in our previous reports (21,22).
All the steps were carried out at 4°C. Protein concentrations were
measured with a spectrophotometer NanoDrop 2000 (Thermo Fisher
Scientific, Wilmington, DE, USA). Samples were electrophoresed
through 10% SDS-PAGE, and the proteins were transferred to
nitrocellulose membranes. The membranes were incubated with primary
antibodies after blocking and then incubated with horseradish
peroxidase (HRP)-conjugated secondary antibodies. Immunoreactive
proteins were visualized with an enhanced chemiluminescence
detection system (Perkin-Elmer, San Jose, CA, USA) on X-ray
film.
Bioinformatics
Target sites for miR-1246 found in DYRK1A 3′UTR are
aligned among 16 mammals by TargetScan.
Statistical analysis
Data were analyzed by paired t-test for tumor
volumes in vivo, which were analyzed by two-way
repeated-measures analysis of variance (ANOVA). P-values <0.05
were recognized as significant. All statistical analyses were
performed with Prism 6 software (Graph Pad Software, La Jolla, CA,
USA).
Results
Galectin-9 inhibits the cell
proliferation of HLE and Li-7 cells in vitro by inducing
apoptosis
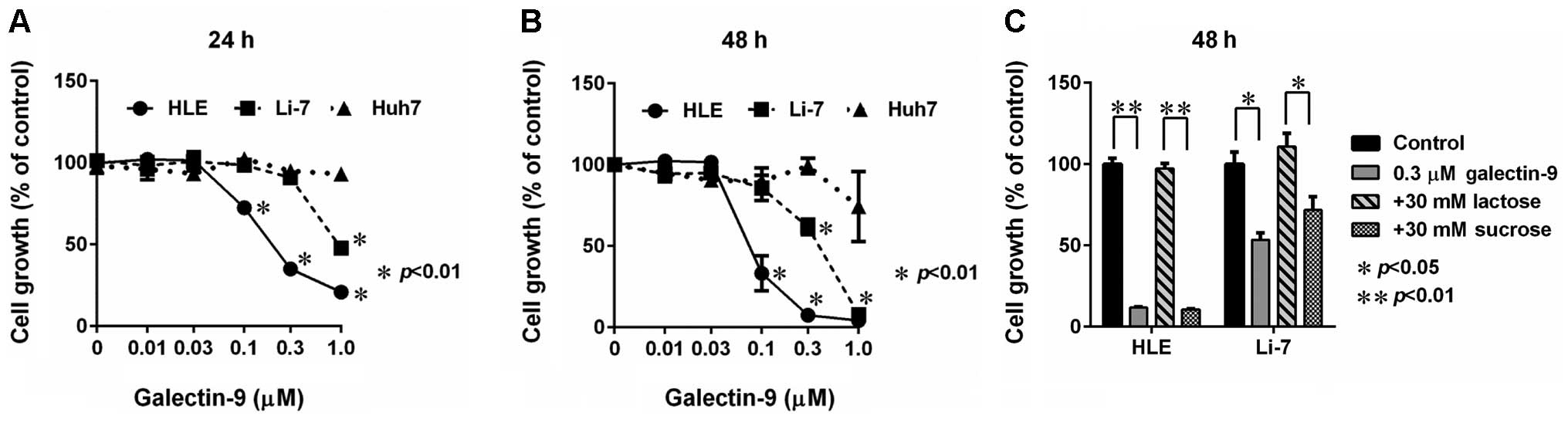
We screened the inhibitory effect of galectin-9 on
HCC by performing a WST-8 assay, and we found that galectin-9
inhibited the cell proliferation of the HLE and Li-7 cell lines in
a dose- and time-dependent manner. The inhibition of cell
proliferation was more evident in the HLE cells than the Li-7
cells. However, no anti-proliferative effect of galectin-9 was
detected in Huh7 cells (Fig. 1A and
B). The effect of galectin-9 was antagonized by 30 mM lactose,
suggesting that the β-galactoside binding nature of galectin-9 is
essential for the activity (Fig.
1C).
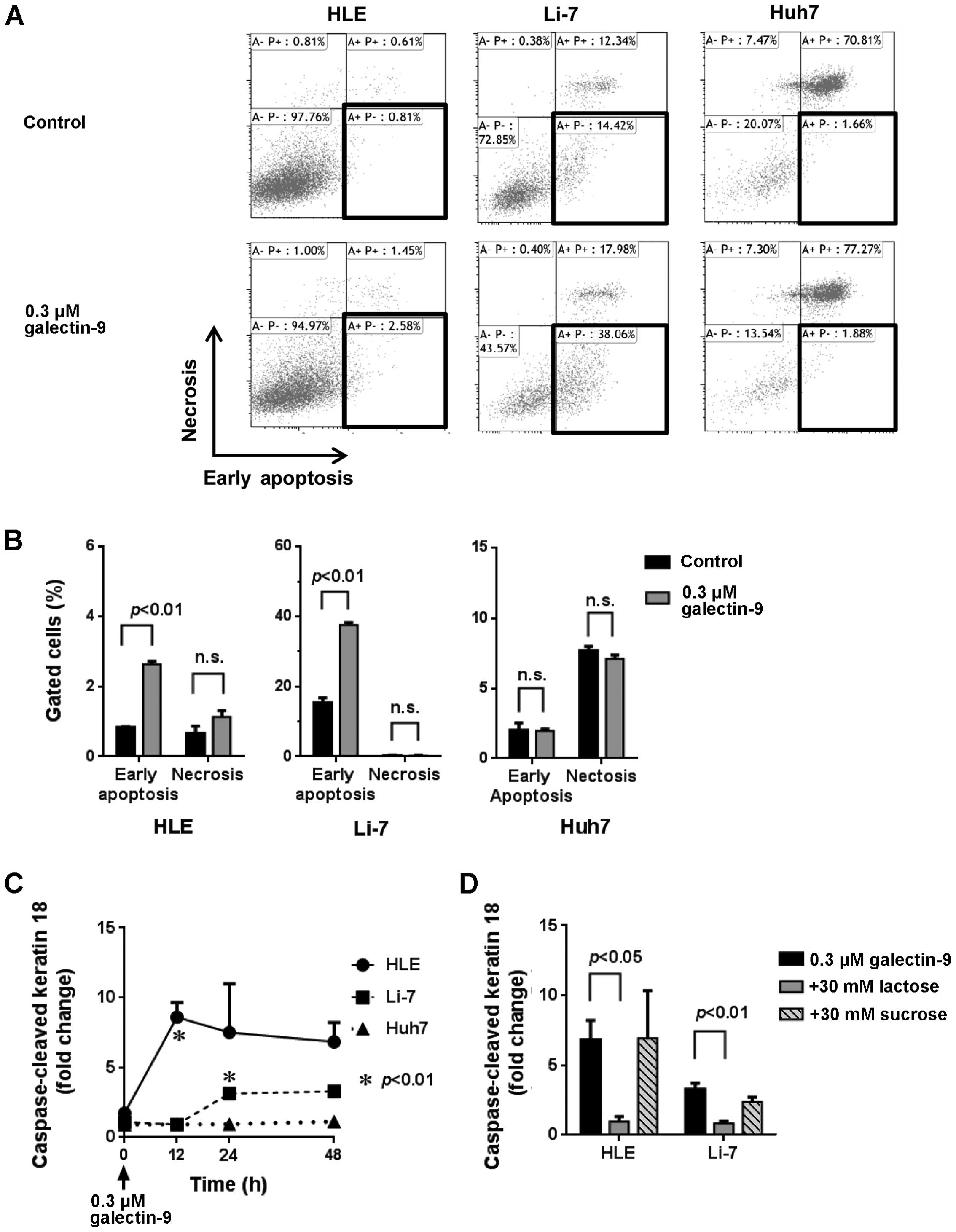
We then performed flow cytometric analysis to assess
the contribution of apoptosis and necrosis to the suppression of
tumor growth. As shown in Fig. 2A and
B, the antiproliferative effects of galectin-9 on the HLE and
Li-7 cells was not due to necrosis, but rather to the induction of
an early apoptotic change that was Annexin V-positive and
PI-negative (18,20). The Huh7 cells did not exhibit early
apoptotic or necrotic changes after treatment with galectin-9.
These results resemble the data of WST-8 assay (Fig. 1A and B).
We determined the levels of CCK18 by ELISA to
establish whether apoptosis is involved in galectin-9-induced cell
death, and we found that galectin-9 increased the levels of CCK18
in HLE and Li-7 cells as illustrated in Fig. 2C, suggesting that the apoptotic
process following phosphatidylserine exposure proceeds to cut
intermediate filaments of cells (23). A galectin-9-induced increase in
CCK18 was also inhibited by one of the antagonists of galectin-9,
30 mM lactose, compared to 30 mM sucrose (Fig. 2D).
Effects of galectin-9 on the cell cycle
in HLE, Li-7 and Huh7 cells
We further performed a flow cytometric analysis of
the cell cycle to evaluate the contribution of cell cycle arrest to
the suppression of HCC cell lines by galectin-9. HLE, Li7, and Huh7
cells were treated with 0.3 μM galectin-9, and we observed that the
proportion of cells in S phase was very slightly increased in the
HLE and Li-7 cells with the treatment by galectin-9 (Fig. 3A and B). These results suggested
that galectin-9 inhibits tumor growth predominently through cancer
cell apoptosis.
Galectin-9 induces apoptosis in HCC cell
lines independently of Tim-3
The cell surface expression of Tim-3, a specific
ligand of galectin-9, was assessed by flow cytometric analysis
because we speculated that a Tim-3/galectin-9 pathway is also
involved in apoptosis of HCC cells, similarly to T lymphocytes
(7). Intriguingly, we were not
able to detect evident Tim-3 on HLE or Li-7 cells, suggesting that
galectin-9 induces apoptosis of HCC cells through binding with an
unknown ligand other than Tim-3 (Fig.
3C).
Galectin-9 inhibits the growth of Li-7
cells in vivo by inducing apoptosis
To determine whether galectin-9 affects tumor growth
in vivo, we performed experiments with a xenograft animal
model. As shown in Fig. 4A,
galectin-9 significantly inhibited the tumor growth of Li-7 cells
on day 7. On the contrary, the tumor growth of Huh7 cells was not
suppressed in vivo with the treatment of galectin-9
(Fig. 4A). The antitumor effect
in vivo resembled that observed in our in vitro
experiments. The groups of mice did not show any significant
differences in body weight or water consumption.
Influence of apoptosis in xenografts
treated with and without galectin-9
Apoptotic cells were detected by TUNEL assay in the
tissues of Li-7 treated with galectin-9 as presented in Fig. 4B-b. However, apoptotic cells were
not detected in control tissues (Fig.
4B-a). The results of the xenograft model analysis matched
those observed in vitro.
Galectin-9 increases the levels of
caspase-9 and -4
We assessed the levels of caspase-4, -8 and -9 to
clarify whether endoplasmic reticulum stress (ER stress), death
receptor or mitochondrial pathway contributes to apoptosis induced
by galectin-9 (28). The results
of our in vitro and in vivo experiments (Fig. 4C) showed that the levels of
caspase-4 and -9 were upregulated with the galectin-9 treatment,
whereas caspase-8 was not changed by the galectin-9 treatment,
suggesting that ER stress and mitochondrial pathway were involved
in galectin-9-induced apoptosis.
miRNA profiling of Li-7 in vitro and in
vivo
To investigate the intracellular pathway of
apoptosis, we screened the expression levels of miRNAs in Li-7
cells and xenografts, and we compared the miRNA profiles obtained
with or without galectin-9. We used cells treated with galectin-9
for 24 h as in vitro samples and xenografts as the in
vivo samples. The unsupervised hierarchical clustering analysis
showed that the treated group clustered together and separately
from the controls both in vitro and in vivo (Fig. 5).
We identified 14 miRNAs that were differently
expressed (12 upregulated and two downregulated miRNAs) in culture
(Table I) and 30 miRNAs
differently expressed (14 upregulated and 16 downregulated) in
xenograft tumor tissues (Table
II). We found that two miRNAs, miR-1246 and miR-6131, were
upregulated in both cultured cells and tumor tissues treated with
galectin-9.
 | Table IStatistical results and chromosomal
locations of miRNAs in Li-7 cells treated with galectin-9, compared
to control cells (p<0.05). |
Table I
Statistical results and chromosomal
locations of miRNAs in Li-7 cells treated with galectin-9, compared
to control cells (p<0.05).
| Name | Fold
(treated/control) mean ± SD | p-value | Chromosomal
localization |
|---|
| Upregulated |
| miR-4294 | 3.04±1.22 | 0.023 | 10 |
| miR-1227-5p | 2.96±1.33 | 0.008 | 19 |
| miR-642b-3p | 2.41±0.86 | 0.020 | |
| miR-6131a | 2.22±0.66 | 0.001 | 5 |
| miR-1246a | 1.83±0.46 | 0.003 | 2q31.1 |
| miR-4289 | 1.82±0.58 | 0.016 | 9 |
| miR-3621 | 1.78±0.45 | 0.010 | 9 |
| miR-3960 | 1.69±0.38 | 0.004 | 9 |
| miR-1237-5p | 1.64±0.33 | 0.001 | 11 |
| miR-4257 | 1.62±0.40 | 0.025 | 1 |
| miR-4485 | 1.61±0.40 | 0.050 | 11 |
| miR-4463 | 1.50±0.31 | 0.034 | 6 |
| Downregulated |
| miR-142-5p | 0.45±0.39 | 0.040 | 17 |
| miR-489 | 0.42±0.43 | 0.044 | 7 |
 | Table IIStatistical results and chromosomal
locations of miRNAs in xenografts from the treated group, compared
to those from the control group (p<0.005). |
Table II
Statistical results and chromosomal
locations of miRNAs in xenografts from the treated group, compared
to those from the control group (p<0.005).
| Name | Fold
(treated/control) mean ± SD | p-value | Chromosomal
localization |
|---|
| Upregulated |
| miR-1246a | 2.76±0.75 | 0.0027 | 2q31.1 |
| miR-6075 | 2.39±0.59 | 0.0040 | 5 |
| miR-3180 | 2.34±0.69 | 0.0031 | |
| miR-6076 | 2.23±0.40 | 0.0024 | 14 |
| miR-4459 | 2.19±0.46 | 0.0033 | 5 |
| miR-6131a | 1.98±0.44 | 0.0031 | 5 |
| miR-1908 | 1.93±0.28 | 0.0016 | 11 |
| miR-4749-5p | 1.86±0.28 | 0.0005 | 19 |
| miR-1909-3p | 1.74±0.44 | 0.0007 | 19p13.3 |
| miR-3940-5p | 1.71±0.21 | 0.0033 | 19 |
| miR-4763-3p | 1.68±0.32 | 0.0021 | 22 |
| miR-4530 | 1.65±0.21 | 0.0016 | 19 |
| miR-3196 | 1.60±0.21 | 0.0012 | 20 |
| miR-4730 | 1.50±0.22 | 0.0045 | 17 |
| Downregulated |
| miR-140-5p | 0.67±0.14 | 0.0015 | 16q22.1 |
| let-7g-5p | 0.67±0.11 | 0.0011 | 3p21.1 |
| miR-660-5p | 0.66±0.10 | 0.0008 | Xp11.23 |
| miR-23a-3p | 0.66±0.09 | 0.0035 | 19p13.13 |
| miR-708-5p | 0.65±0.14 | 0.0019 | 11q14.1 |
| miR-30e-5p | 0.65±0.16 | 0.0024 | 1p34.2 |
| miR-378g | 0.64±0.09 | 0.0017 | |
| miR-92a-3p | 0.64±0.11 | 0.0021 | |
| miR-17-3p | 0.62±0.21 | 0.0021 | 13q31.3 |
| miR-339-5p | 0.61±0.12 | 0.0036 | 7p22.3 |
| miR-4664-5p | 0.60±0.11 | 0.0025 | 6 |
| miR-532-5p | 0.59±0.10 | 0.0021 | Xp11.23 |
| miR-4443 | 0.58±0.08 | 0.0007 | 3 |
| miR-30c-5p | 0.57±0.10 | 0.0004 | |
| miR-10a-5p | 0.53±0.09 | 0.0026 | 17q21.32 |
| miR-152 | 0.53±0.17 | 0.0020 | 17q21.32 |
The microarray data obtained in this study are
registered at the NCBI Gene expression Omnibus (GEO). The accession
numbers are GSE55665 for the in vitro data and GSE55666 for
the in vivo data.
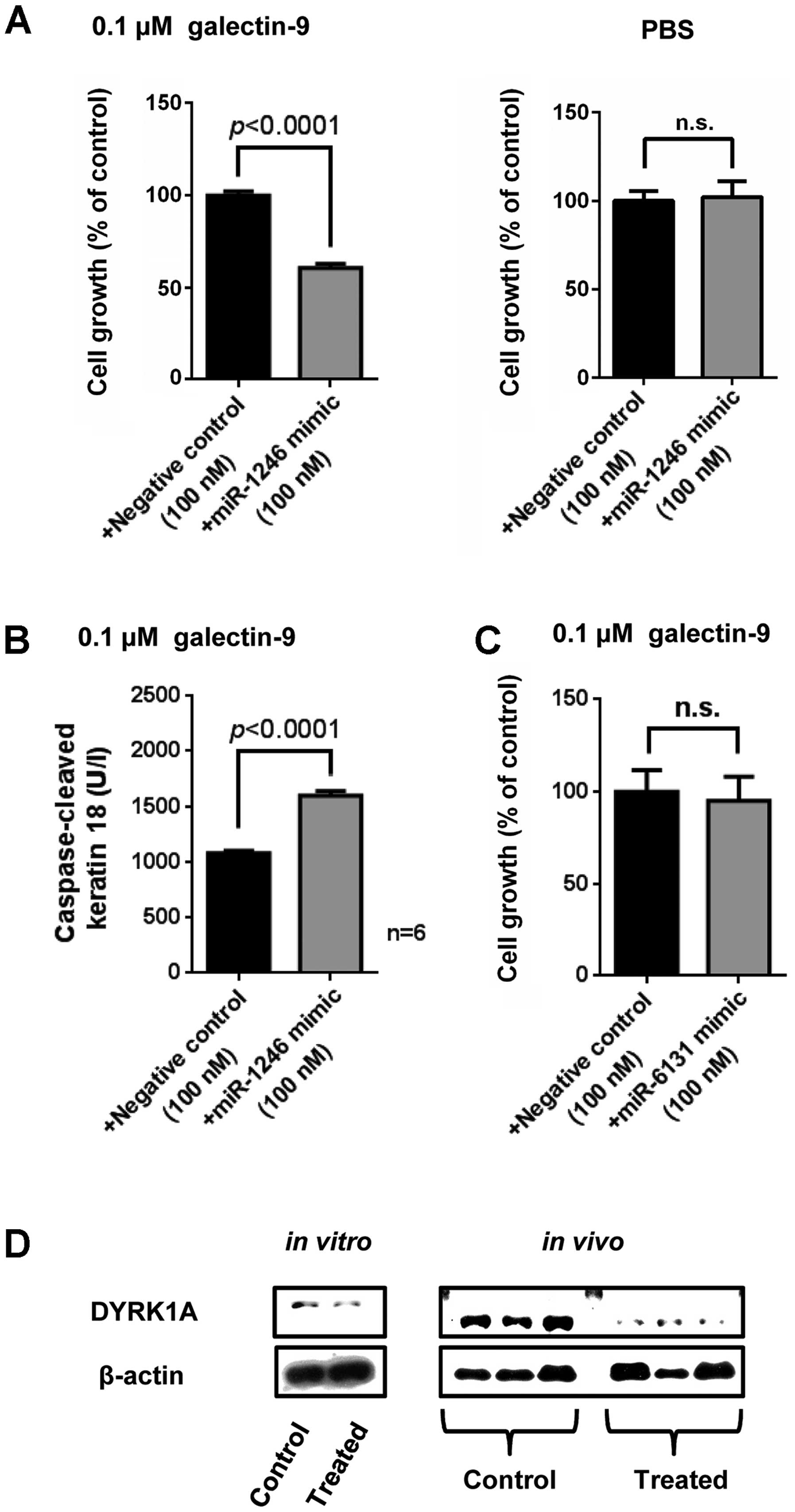
miR-1246 enhances the apoptosis of Li-7
cells when galectin-9 is administered
We performed a WST-8 assay to determine whether the
miR-1246 upregulated with galectin-9 treatment contributes to
galectin-9-induced apoptosis. As shown in Fig. 6A, the tumor growth was inhibited in
the transfection of miR-1246 in Li-7 cells under 0.1 μM galectin-9
treatment, but the suppression of tumor growth was not detected in
the transfection of miR-1246 in Li-7 cells without galectin-9
treatment. miR-1246 also induced apoptosis with galectin-9
treatment in Li-7 cells (Fig. 6B).
These data suggest that miR-1246 acted as the inhibitor molecule of
the cell proliferation through apoptosis in Li-7 cells treated with
galectin-9. The miR-6131, showed no additional inhibitory effect on
the cell growth of Li-7 (Fig.
6C).
Galectin-9 downregulates the expression
of DYRK1A, resulting in sensitized caspase-9 activation
We speculated that galectin-9 suppresses the
expression level of oncogene DYRK1A through the upregulation of
miR-1246 in Li-7 cells and tissues (29). We used cells treated with
galectin-9 for 24 h for in vitro samples. The western blot
analysis showed that DYRK1A was downregulated by galectin-9 both
in vitro and in vivo (Fig. 6D), suggesting that the
miR-1246-DYRK1A-caspase-9 axis was involved in the
galectin-9-evoked apoptosis. Target sites for miR-1246 found in
DYRK1A 3′UTR are conserved among 16 mammals (Fig. 7A).
Discussion
HCC is the sixth most frequent malignancy and the
third most common cause of cancer-related death worldwide (1,30).
Conventional chemotherapy does not always provide significant
clinical benefits or prolonged survival for patients with advanced
HCC (30,31). It is thus necessary to develop new
drugs for the treatment of HCC.
It is evident from several reports that galectin-9,
one of the soluble β-galactoside-binding animal lectins, has a role
as an anticancer agent. Accumulated data suggest that recombinant
protease-resistant galectin-9 has an antiproliferative effect on
cancers such as myeloma (13),
melanoma (14), chronic myeloid
leukemia (12), B-cell lymphoma
(19) and colon cancer (32). However, the antitumor effect of
recombinant galectin-9 for HCC remains unknown, and the antitumor
mechanisms of galectin-9 in various cancers including HCC have not
been fully clarified. Here we found that recombinant galectin-9
inhibited the growth of HCC cells and tumorigenesis both in
vitro and in vivo. We also identified miRNAs that lead
to apoptosis associated with the antitumor effect of galectin-9 in
HCC.
In the present study, galectin-9 suppressed the cell
proliferation in two HCC cell lines (Li-7 and HLE) in vitro
and in vivo by inducing cell apoptosis. In both the in
vitro and in vivo experiments, miR-1246 was upregulated
in cells and tissues of Li-7 treated with galectin-9, and the
transfection of miR-1246 increased the apoptosis under the
administration of galectin-9.
The recombinant galectin-9 led to a strong,
dose-dependent inhibition of cell proliferation in the HCC cell
lines HLE and Li-7, but not in the HCC cell line Huh-7. Although
these data suggest that galectin-9 would be a very effective
treatment for some HCCs, a question remains as to why was there a
difference in the inhibition of cell growth among HCC cell lines.
The HLF and Li-7 cells showed the induction of caspase-4 and
-9-dependent apoptosis, but it was not independent of Tim-3, a
specific ligand of galectin-9. The induction of apoptosis by
galectin-9 did not occur in the Huh-7 cells. These data suggest
that the main thrust of the antitumor effect of galectin-9 is to
bring about apoptosis.
Our in vivo experiment using subcutaneous
HCC-bearing athymic nude mice also demonstrated that galectin-9
suppressed the growth of Li-7 cells, as demonstrated in the in
vitro study. The data obtained from the TUNEL staining also
indicated that the antitumor effect of galectin-9 was due to
apoptosis. These findings suggest that the antitumor effect of
galectin-9 may be related to the induction of apoptosis in HCC
cells.
MicroRNAs are evolutionarily endogenous noncoding
RNAs that have been identified as post-transcriptional regulators
of gene expression. The miRNAs bind mainly the 3′ untranslated
regions (UTRs) of target mRNAs, resulting in mRNA degradation or
the blockade of mRNA translation (31,33).
miRNAs thus play crucial roles in the cell cycle, differentiation,
maturation, functioning and apoptosis of cells. In addition, it has
become clear that aberrant miRNA expression is a common feature of
various human malignancies (34).
In terms of the relationship between miRNAs and HCC, several
studies have shown that specific miRNAs are expressed aberrantly in
malignant HCC cells or tissues compared to nonmalignant hepatocytes
and tissues (31).
However, although miRNAs are becoming increasingly
recognized as regulatory molecules in HCC, their involvement in the
responses to environmental changes (such as exposure to drugs)
remains largely unknown. In the present study, using miRNA
expression arrays, we determined the variations in miRNA profiles
in HCC cell lines both in culture and in xenograft tumor tissues
treated with galectin-9 compared to those not treated with
galectin-9. The cluster analyses that we conducted clearly showed
that galectin-9 treatment affected the expression of numerous
miRNAs in cultured cells and in tumor tissues. In the analysis, we
selected sets of miRNAs that altered their expression levels
significantly before and after galectin-9 treatment. We identified
14 miRNAs that were differentially expressed (12 upregulated and
two downregulated) in culture and 30 miRNAs that were
differentially expressed (14 downregulated and 16 upregulated) in
xenograft tumor tissues. These miRNA are candidates for
investigations of the effectiveness of galectin-9 treatment and for
the search for clues regarding the molecular basis of the
anticancer effects of galectin-9.
We found that miR-1246 and miR-6131 are upregulated
in both cultured cells and tumor tissues treated with galectin-9.
Zhang et al (29)
demonstrated that miR-1246 is associated with apoptosis, targeting
and degrading mRNA of oncogene DYRK1A, and making cells more
fragile to apoptosis. DYRK1A is a member of the family of
therine-threonine kinases, which phosphorylate caspase-9 and
inactivate it, preventing cells from undergoing apoptosis. Our
present findings indicate that the upregulation of miR-1246 was
accompanied by a downregulation of DYRK1A and upregulation of
caspase-9. We thus speculate that the anticancer effect of
galectin-9 is due not only to ER stress, but also apoptosis via a
mitochondrial pathway through the miR-1246-DYRK1A-caspase-9 axis
(Fig. 7B). In previous studies,
and in our present study, miR-1246 was upregulated in cancer cells
after treatment with an anticancer agent (21,22,35,36).
Cancers originated from various organs might owe their
proliferation to regulating miR-1246-DYRK1A-caspase-9 axis because
this pathway is highly conserved among species (Fig. 7A). The mechanism of cooperation
between miR-1246 and DYRK1A remains to be further investigated. The
role of miR-6131 remains unknown, and it is possible that miR-6131
has no role in the suppression of cancer progression.
Our data suggest that the mechanism of the
anticancer molecule galectin-9 is unique. The main role of
antitumor effects of galcetin-9 appears to depend on the induction
of the apoptosis of cancer cells, not the arrest of the cell cycle.
To date, several anticancer drugs used for HCC, i.e., sorafenib,
cisplatin and fluorouracil (5-FU) were shown to induce the arrest
of the cell cycle (37). We thus
propose the possibility of using galectin-9 to treat HCC patients
in combination with other anticancer drugs that induce cell cycle
arrest. In addition, because galectin-9 is stabilized against
protease in sera, intravenous galectin-9 treatment is expected to
have antitumor effects for patients with HCC (17).
In conclusion, our main findings are that galectin-9
inhibited the growth of two HCC cell lines and primary human HCCs
both in vitro and in vivo through apoptosis, and that
miR-1246 mediated the signals of galectin-9, possibly through the
downregulation of DYRK1A and upregulation of caspase-9. Recombinant
galctin-9 is a new potential therapeutic target for HCC that may
overcome resistance to conventional chemotherapy as an adjunct to
conventional chemotherapy. Clinical studies of galcetin-9 as a new
anticancer agent thus seem appropriate.
Acknowledgements
Toshiro Niki and Mitsuomi Hirashima are board
members of GalPharma Co., Ltd. The two authors have the following
patents related to material pertinent to this article: ‘Novel
modified galectin 9 proteins and use thereof’ which is applied by
GalPharma and issued in Japan (4792390), the USA (8,268,324), EPC
(1736541), Canada (2,561,696), India (239130), and Korea
[(10-1222281) as of 2013.12.2]. The two authors have the following
products related to material pertinent to this article: stable-form
Gal-9.
Abbreviations:
|
DMEM
|
Dulbecco’s modified Eagle’s medium
|
|
DYRK1A
|
dual specificity tyrosine (Y)
phosphorylation-regulated kinase 1A
|
|
ELISA
|
enzyme linked immunosorbent assay
|
|
ER stress
|
endoplasmic reticulum stress
|
|
FACS
|
flow cytometric analysis
|
|
FBS
|
fetal bovine serum
|
|
HCC
|
hepatocellular carcinoma
|
|
PCR
|
polymerase chain reaction
|
|
PI
|
propidium iodide
|
|
SDS-PAGE
|
sodium dodecyl sulfate-polyacrylamide
gel electrophoresis
|
References
|
1
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meza-Junco J, Montano-Loza AJ, Liu DM,
Sawyer MB, Bain VG, Ma M and Owen R: Locoregional radiological
treatment for hepatocellular carcinoma; Which, when and how? Cancer
Treat Rev. 38:54–62. 2012. View Article : Google Scholar
|
|
3
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: SHARP Investigators Study Group: Sorafenib in advanced
hepatocellular carcinoma. N Engl J Med. 359:378–390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bruix J, Raoul JL, Sherman M, Mazzaferro
V, Bolondi L, Craxi A, Galle PR, Santoro A, Beaugrand M,
Sangiovanni A, et al: Efficacy and safety of sorafenib in patients
with advanced hepatocellular carcinoma: Subanalyses of a phase III
trial. J Hepatol. 57:821–829. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wada J, Ota K, Kumar A, Wallner EI and
Kanwar YS: Developmental regulation, expression, and apoptotic
potential of galectin-9, a beta-galactoside binding lectin. J Clin
Invest. 99:2452–2461. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matsumoto R, Matsumoto H, Seki M, Hata M,
Asano Y, Kanegasaki S, Stevens RL and Hirashima M: Human ecalectin,
a variant of human galectin-9, is a novel eosinophil
chemoattractant produced by T lymphocytes. J Biol Chem.
273:16976–16984. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu C, Anderson AC, Schubart A, Xiong H,
Imitola J, Khoury SJ, Zheng XX, Strom TB and Kuchroo VK: The Tim-3
ligand galectin-9 negatively regulates T helper type 1 immunity.
Nat Immunol. 6:1245–1252. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rabinovich GA, Liu FT, Hirashima M and
Anderson A: An emerging role for galectins in tuning the immune
response: Lessons from experimental models of inflammatory disease,
autoimmunity and cancer. Scand J Immunol. 66:143–158. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nagahara K, Arikawa T, Oomizu S, Kontani
K, Nobumoto A, Tateno H, Watanabe K, Niki T, Katoh S, Miyake M, et
al: Galectin-9 increases Tim-3+ dendritic cells and
CD8+ T cells and enhances antitumor immunity via
galectin-9-Tim-3 interactions. J Immunol. 181:7660–7669. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fujihara S, Mori H, Kobara H, Rafiq K,
Niki T, Hirashima M and Masaki T: Galectin-9 in cancer therapy.
Recent Pat Endocr Metab Immune Drug Discov. 7:130–137. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wiersma VR, de Bruyn M, Helfrich W and
Bremer E: Therapeutic potential of Galectin-9 in human disease. Med
Res Rev. 33(Suppl 1): E102–E126. 2013. View Article : Google Scholar
|
|
12
|
Kuroda J, Yamamoto M, Nagoshi H, Kobayashi
T, Sasaki N, Shimura Y, Horiike S, Kimura S, Yamauchi A, Hirashima
M, et al: Targeting activating transcription factor 3 by Galectin-9
induces apoptosis and overcomes various types of treatment
resistance in chronic myelogenous leukemia. Mol Cancer Res.
8:994–1001. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kobayashi T, Kuroda J, Ashihara E, Oomizu
S, Terui Y, Taniyama A, Adachi S, Takagi T, Yamamoto M, Sasaki N,
et al: Galectin-9 exhibits anti-myeloma activity through JNK and
p38 MAP kinase pathways. Leukemia. 24:843–850. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kageshita T, Kashio Y, Yamauchi A, Seki M,
Abedin MJ, Nishi N, Shoji H, Nakamura T, Ono T and Hirashima M:
Possible role of galectin-9 in cell aggregation and apoptosis of
human melanoma cell lines and its clinical significance. Int J
Cancer. 99:809–816. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang ZY, Dong JH, Chen YW, Wang XQ, Li
CH, Wang J, Wang GQ, Li HL and Wang XD: Galectin-9 acts as a
prognostic factor with antimetastatic potential in hepatocellular
carcinoma. Asian Pac J Cancer Prev. 13:2503–2509. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X,
Liu J, Shi L, Liu C, Wang G, et al: Tim-3/galectin-9 signaling
pathway mediates T-cell dysfunction and predicts poor prognosis in
patients with hepatitis B virus-associated hepatocellular
carcinoma. Hepatology. 56:1342–1351. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nishi N, Itoh A, Fujiyama A, Yoshida N,
Araya S, Hirashima M, Shoji H and Nakamura T: Development of highly
stable galectins: Truncation of the linker peptide confers
protease-resistance on tandem-repeat type galectins. FEBS Lett.
579:2058–2064. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nishikawa T, Nakajima T, Moriguchi M, Jo
M, Sekoguchi S, Ishii M, Takashima H, Katagishi T, Kimura H, Minami
M, et al: A green tea polyphenol, epigalocatechin-3-gallate,
induces apoptosis of human hepatocellular carcinoma, possibly
through inhibition of Bcl-2 family proteins. J Hepatol.
44:1074–1082. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kashio Y, Nakamura K, Abedin MJ, Seki M,
Nishi N, Yoshida N, Nakamura T and Hirashima M: Galectin-9 induces
apoptosis through the calcium-calpain-caspase-1 pathway. J Immunol.
170:3631–3636. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Martin SJ, Reutelingsperger CP, McGahon
AJ, Rader JA, van Schie RC, LaFace DM and Green DR: Early
redistribution of plasma membrane phosphatidylserine is a general
feature of apoptosis regardless of the initiating stimulus:
Inhibition by overexpression of Bcl-2 and Abl. J Exp Med.
182:1545–1556. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kato K, Gong J, Iwama H, Kitanaka A, Tani
J, Miyoshi H, Nomura K, Mimura S, Kobayashi M, Aritomo Y, et al:
The anti-diabetic drug metformin inhibits gastric cancer cell
proliferation in vitro and in vivo. Mol Cancer Ther. 11:549–560.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kobayashi M, Kato K, Iwama H, Fujihara S,
Nishiyama N, Mimura S, Toyota Y, Nomura T, Nomura K, Tani J, et al:
Antitumor effect of metformin in esophageal cancer: In vitro study.
Int J Oncol. 42:517–524. 2013.
|
|
23
|
Schutte B, Henfling M, Kölgen W, Bouman M,
Meex S, Leers MP, Nap M, Björklund V, Björklund P, Björklund B, et
al: Keratin 8/18 breakdown and reorganization during apoptosis. Exp
Cell Res. 297:11–26. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Workman P, Aboagye EO, Balkwill F, Balmain
A, Bruder G, Chaplin DJ, Double JA, Everitt J, Farningham DA,
Glennie MJ, et al: Committee of the National Cancer Research
Institute: Guidelines for the welfare and use of animals in cancer
research. Br J Cancer. 102:1555–1577. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tamura T, Fujita F, Tanimoto M, Koike M,
Suzuki A, Fujita M, Horikiri Y, Sakamoto Y, Suzuki T and Yoshino H:
Anti-tumor effect of intraperitoneal administration of
cisplatin-loaded microspheres to human tumor xenografted nude mice.
J Control Release. 80:295–307. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miyoshi H, Kato K, Iwama H, Maeda E,
Sakamoto T, Fujita K, Toyota Y, Tani J, Nomura T, Mimura S, et al:
Effect of the anti-diabetic drug metformin in hepatocellular
carcinoma in vitro and in vivo. Int J Oncol. 45:322–332.
2014.PubMed/NCBI
|
|
27
|
Bolstad BM, Irizarry RA, Astrand M and
Speed TP: A comparison of normalization methods for high density
oligonucleotide array data based on variance and bias.
Bioinformatics. 19:185–193. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rao RV, Ellerby HM and Bredesen DE:
Coupling endoplasmic reticulum stress to the cell death program.
Cell Death Differ. 11:372–380. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Y, Liao JM, Zeng SX and Lu H: p53
downregulates Down syndrome-associated DYRK1A through miR-1246.
EMBO Rep. 12:811–817. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Asghar U and Meyer T: Are there
opportunities for chemotherapy in the treatment of hepatocellular
cancer? J Hepatol. 56:686–695. 2012. View Article : Google Scholar
|
|
31
|
Callegari E, Elamin BK, Sabbioni S,
Gramantieri L and Negrini M: Role of microRNAs in hepatocellular
carcinoma: A clinical perspective. Onco Targets Ther. 6:1167–1178.
2013.PubMed/NCBI
|
|
32
|
Nobumoto A, Nagahara K, Oomizu S, Katoh S,
Nishi N, Takeshita K, Niki T, Tominaga A, Yamauchi A and Hirashima
M: Galectin-9 suppresses tumor metastasis by blocking adhesion to
endothelium and extracellular matrices. Glycobiology. 18:735–744.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kerr TA, Korenblat KM and Davidson NO:
MicroRNAs and liver disease. Transl Res. 157:241–252. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kushibiki T, Hirasawa T, Okawa S and
Ishihara M: Regulation of miRNA expression by low-level laser
therapy (LLLT) and photodynamic therapy (PDT). Int J Mol Sci.
14:13542–13558. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bach D, Fuereder J, Karbiener M,
Scheideler M, Ress AL, Neureiter D, Kemmerling R, Dietze O,
Wiederstein M, Berr F, et al: Comprehensive analysis of alterations
in the miRNome in response to photodynamic treatment. J Photochem
Photobiol B. 120:74–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Deng L, Ren Z, Jia Q, Wu W, Shen H and
Wang Y: Schedule-dependent antitumor effects of 5-fluorouracil
combined with sorafenib in hepatocellular carcinoma. BMC Cancer.
13:3632013. View Article : Google Scholar : PubMed/NCBI
|