Introduction
Ovarian cancer is one of the most serious
malignancies for women in the world, ranking as the fifth leading
cause of cancer-related deaths (1)
and the death rate of ovarian cancer has not seen any remarkable
changes for over five decades in the USA (2). Due to a lack of effective biomarkers
for screening (3,4), nearly 60–70% of ovarian cancers are
diagnosed at advanced stages (5),
with a poor prognosis of ~30% for a 5-year survival rate (6). These facts emphasize the need for
novel therapies to prevent and treat ovarian cancer. Several lines
of evidence indicate that nutritional compounds display potent
anticancer activity in many human cancers (5). The identification of nutritional
agents that can suppress the growth and progression of ovarian
cancer could lead to new treatment modalities and improved patient
outcomes for this lethal malignancy.
Recent studies have focused on the anticancer
activity of flavonoids isolated from plants and animals. Flavonoids
are natural polyphenols present in a wide variety of fruits and
vegetables (7). Some flavonoids,
such as apigenin, genistein and catechin, have been shown to
inhibit the growth of ovarian, breast, colon, prostate and leukemia
cancer cells (8–17). Nobiletin
(5,6,7,8,30,40-hexamethoxyflavone) is a polymethoxyflavonoid found
in citrus fruits such as Citrus depressa and Citrus
reticulate (18). Previous
mammalian in vivo studies show that nobiletin can suppress
inflammation-associated tumorigenesis aberrant cell proliferation
and colon carcinogenesis (19–21).
Nobiletin has been also shown to suppress angiogenesis in
vitro in human umbilical vein endothelial cells. The
anti-angiogenic activity of nobiletin has been shown in chicken
chorioallantoic membranes and in zebrafish models (22,23).
It has been revealed that nobiletin exhibited a cell
differentiation-modulating activity (24,25)
and inhibited the phosphorylation of MEK (26,27).
Nobiletin is a decreases metastasis of human fibrosarcoma HT-1080
cells and gastric cancers (27).
The mitogen-activated protein kinase (MAPK) signaling pathway is a
key regulator of cell proliferation, survival and differentiation.
The MAPK pathway is constitutively activated in ovarian cancers via
gain of function mutations in Ras or Raf. In addition, mutations in
PI-3 kinase pathway have been implicated in the progression of
ovarian cancers. The hyperactivation of the MAP kinase pathway
facilitates the neoplastic transformation of ovarian tumors.
Selective MEK1 inhibitors have been shown to suppress the growth of
estrogen-responsive ovarian cancers. Since nobiletin also functions
as a MEK1 inhibitor we conjectured that perhaps it could suppress
the growth of human ovarian cancers. The growth-inhibitory activity
of nobiletin has yet to be studied in ovarian cancer. Our report
fills this void of knowledge and describes the anti-neoplastic
activity of nobiletin in human ovarian cancer. In this report, we
show that nobiletin decreases the viability of the human ovarian
cancer cell lines OVCAR-3 and CP-70. The growth-inhibitory effects
of nobiletin were observed at concentration as low as 5 μM.
Nobiletin had no effect on the viability of normal ovarian
epithelial cells at <40 μM. Therefore, it displayed a strong
selectivity for human ovarian cancer cells over normal ovarian
cells. Nobiletin potently decreased the growth rate of human
ovarian tumors xenografted in athymic mouse models and chicken CAM
models. The anticancer activity of nobiletin was correlated with
its anti-angiogenic and anti-apoptosis activity in ovarian cancers.
We also analyzed the signaling pathways underlying the
anti-angiogenic activity of nobiletin. The anti-angiogenic activity
of nobiletin was correlated with decreased levels of Akt, HIF-1α,
NF-κB and vascular epithelial growth factor (VEGF) in ovarian
cancer cells. Taken together, our data suggest that nobiletin may
have applications in the therapy of human ovarian cancer.
Materials and methods
Ethics statement
Male four-week-old athymic mice were obtained from
Charles River Laboratories and acclimatized for one week. They were
housed in autoclaved cages with ad libitum access to food
and water in HEPA-filtered racks and closely monitored by animal
facility staff. All procedures involving nude mice were conducted
according to the Animal Care and Use guidelines in a facility
accredited by the Association for Assessment and Accreditation of
Laboratory Animal Care (AAALAC) International and were approved by
the Institutional Animal Care and Use Committee (IACUC) of Joan C.
Edwards School of Medicine, Marshall University (protocol no.
560).
Reagents, antibodies and constructs
Nobiletin was prepared from a polymethoxyflavonoid
mixture, which was provided by Zhejiang Quzhou Tiansheng Plant
Extraction Co. Ltd. in China, containing ~60% nobiletin and
tangeretin. The polymethoxyflavonoid mixture was dissolved in
methanol-dimethyl sulfoxide (1:1), its concentration was 50 mg/ml,
then chromatographed with high-performance liquid chromatography
(HPLC) (Waters) eluted with methanol-H2O (70:30) in 8
ml/min at room temperature, separated into two fractions, collected
individually, evaporated, obtained fraction I and fraction II.
Fraction I was identified as nobiletin (Fig. 1A) by HPLC-MS, UV-vis chromatography
and comparing peak time with that of nobiletin sample from Sigma
and previous reports (data not shown). Its purity was >98%.
Monoclonal antibodies against HIF-1α, NF-κB (p50), PTEN, c-Myc,
GAPDH, p-AKT, total AKT, p-mTOR and total mTOR were purchased from
Santa Cruz Biotechnology (Santa Cruz, CA, USA). The secondary
antibodies of anti-rabbit and anti-mouse were purchased from Thermo
Scientific (Pierce, Rockford, IL, USA). The Hif -1α and mAkt
plasmid constructs were obtained from Addgene (www.addgene.org) (28).
Cell culture and treatment
Human ovarian cancer cell lines, OVCAR-3 and
A2780/CP70, were provided by Dr B. Jiang, Department of
Microbiology, Immunology, and Cell Biology, West Virginia
University. IOSE-364, normal ovarian surface epithelial cells from
healthy women, but immortalized with SV40 T/t, were courtesy of Dr
N. Auersperg at University of British Columbia, Canada. All cells
were maintained in RPMI-1640 medium supplemented with 100 U/ml
penicillin, 100 μg/ml streptomycin and 10% US-qualified fetal
bovine serum (Invitrogen, Grand Island, NY, USA) in a humidified
incubator with 5% CO2 at 37°C. Nobiletin was dissolved
in dimethyl sulfoxide (DMSO) to make stock solutions of 100 mM and
equal amount of DMSO was included in controls for every
experiment.
Cell proliferation
The effect of nobiletin on the viability of ovarian
cancer cells (OVCAR-3 and A2780/CP70) colorimetrically determined
with a CellTiter 96 Aqueous One Solution Cell Proliferation Assay
kit from Promega (Madison, WI, USA). The cells were seeded into
96-well plates at a density of 5×103/well and incubated
for 24 h at 37°C. Subsequently, the cells were treated with vehicle
or varying concentrations of nobiletin for another 24 h at 37°C.
After 24 h, the medium was removed and cell viability was measured
according to the manufacturer’s instructions. Each sample was
measured in triplicate. Cell viability was expressed as percentage
of control from three independent experiments.
Apoptosis assay
The apoptotic effects of nobiletin on ovarian cancer
cells were determined by FITC Annexin V Apoptosis Detection Kit I
from BD Biosciences. Cells were washed with cold PBS twice and then
were resuspended in binding buffer at a concentration of
1×106/ml. An aliquot of 100 μl of the cell solution
(1×105 cells) was transferred to a 5-ml tissue culture
tube. Subsequently, 5 μl of FITC Annexin V and 5 μl propidium
iodide (PI) was added to the cells. The cells were gently vortexed
and incubated for 15 min at room temperature in the dark. The next
step involved the addition of 400 μl of 1X binding buffer to each
tube. The samples were analyzed by flow cytometry (Cytomic FC
500MCL) within 1 h. Three independent experiments were assayed.
Data represent mean ± SE from 3 independent experiments.
ELISA for VEGF
The levels of VEGF in cell culture supernatants were
analyzed by a Quantikine Human VEGF Immunoassay kit (R&D
Systems, Minneapolis, MN, USA). Cells (1×104/well) were
seeded into 96-well plates and incubated overnight. Subsequently,
the cells were treated with nobiletin for 16 h in serum-free
medium. Culture supernatants were collected and spun down at 10,000
g at 4°C. The supernatant was collected and stored at −70°C. The
amounts of VEGF were measured following the manufacturer’s
instructions, and normalized to cell numbers for each treatment. A
total of three independent experiments, each in triplicates, were
assayed, and the mean VEGF protein level from each triplicate was
used for statistical analysis.
Western blot analysis
Ovarian cancer cells (106) were seeded in
60-mm dishes and incubated for 16 h before treated with nobiletin
for 24 h. The cells were washed with PBS, lysed in 100 μl mammalian
protein extraction reagent including 1 l halt protease, 1 μl
phosphatase inhibitor and 2 μl EDTA (M-PER, Pierce), according to
the manufacturer’s instructions. Total protein levels were assayed
with a BCA Protein Assay kit (Pierce). Forty microgram of protein
lysates was separated by 10% SDS-PAGE and transferred into
nitrocellulose membrane with a Mini-Protean 3 system (Bio-Rad
Laboratories, Hercules, CA, USA). The membranes were blocked in 5%
milk in PBS containing 0.1% Tween-20 for 1 h at room temperature.
The membranes were incubated with the appropriate dilutions of the
primary antibodies and secondary antibodies. The signal obtained in
the western blot experiments was detected by the SuperSignal West
Dura Extended Duration Substrate (Pierce Biotechnologies). Protein
bands were quantitated with NIH ImageJ software, normalized by
corresponding GAPDH or total AKT, total mTOR bands, and expressed
as percentages of control. A total of three independent experiments
were carried out for statistical analysis.
Transient transfection and reporter
assay
Transient transfection and reporter assay were
modified from our published report (28,29).
Ovarian cancer cells were seeded in 96-well plate at 10,000
cells/well and incubated overnight. For transfection with
HIF-1α/mAkt plasmids, cells were then transfected with 0.05 μg VEGF
luciferase reporter, 0–0.25 μg HIF-1α/mAkt or SR-α (as vehicle)
plasmids by 0.6 μl jetPRIME reagent (VWR) for 4 h and removed the
medium. Followed by 16-h treatment with 0- or 40-μM nobiletin. The
cells were harvested and analyzed for luciferase activities with
ONE-Glo Luciferase Assay system (Promega) and detected by Lumat
LB9507 (Berthold Technologies). Total protein levels with a BCA
Protein Assay kit (Pierce), and the activities of VEGF reporter
were normalized by corresponding total protein levels for
statistical analysis. The experiments were conducted three
times.
Chicken embryo chorioallantoic membrane
(CAM) assay
The A2780 cells at grown to 70% confluence, were
harvested, washed with PBS and re-suspended in serum-free medium.
Aliquots of the cells (0.1 ml, 2×107/ml) were mixed with
0.1 ml of Matrigel (BD Bioscience, San Jose, CA, USA), supplemented
with 0 or 20 μM nobiletin, pre-gelled on an autoclaved silicone mat
for 30–40 min, and implanted onto the CAM of 9-day-old chicken
embryo. Chicken embryos were incubated for 4–5 days, photographed
for Matrigel implant, and counted for branching blood vessels.
Angiogenesis was evaluated by normalizing number of branching
vessels to that of control CAM. A total of 10 eggs were assayed for
each group.
Antitumor studies in athymic mice
Twenty-four week-old male nude mice were obtained
from Charles River Laboratories and acclimatized for one week. They
were housed in autoclaved cages with ad libitum access to
food and water in HEPA-filtered racks and closely monitored by
animal facility staff. All procedures were conducted according to
the Animal Care and Use guidelines in a facility accredited by the
Association for Assessment and Accreditation of Laboratory Animal
Care (AAALAC) International and were approved by the Institutional
Animal Care and Use Committee (IACUC) of Joan C. Edwards School of
Medicine, Marshall University (protocol no. 560).
CP70 cells were harvested and re-suspended in a 1:1
(v/v) solution of serum-free media and Matrigel matrix (BD
Biosciences). Two million cells in 100 μl were injected
subcutaneously between the scapulae of each mouse (30). After the tumors reached 100
mm3, the mice were randomized and divided into two
groups comprising of ten mice each. The treatment group (N=10) was
fed AIN-76A diet with 5% lipid level containing 100 mg nobiletin/kg
food. The control group (N=10) was fed AIN76A diet containing
vehicle. Mice were weighed once per week. Their food consumption
was monitored daily. Tumor volumes were calculated as (l ×
w2)/2 (31,32).
Statistical analysis
Results are expressed as mean ± standard error of
mean (SEM) using Microsoft Excel (Windows 8). Statistical
assessment was carried out with the program system of SPSS (Version
16.0 for Windows). The results were analyzed using one-way analysis
of variance (ANOVA) and post hoc test (2-sided Dunnett’s
test) to test both overall differences and specific differences
between each treatment and control. A P-value of <0.05 was
considered statistically significant.
Results
Effect of nobiletin on ovarian cancer
cell viability
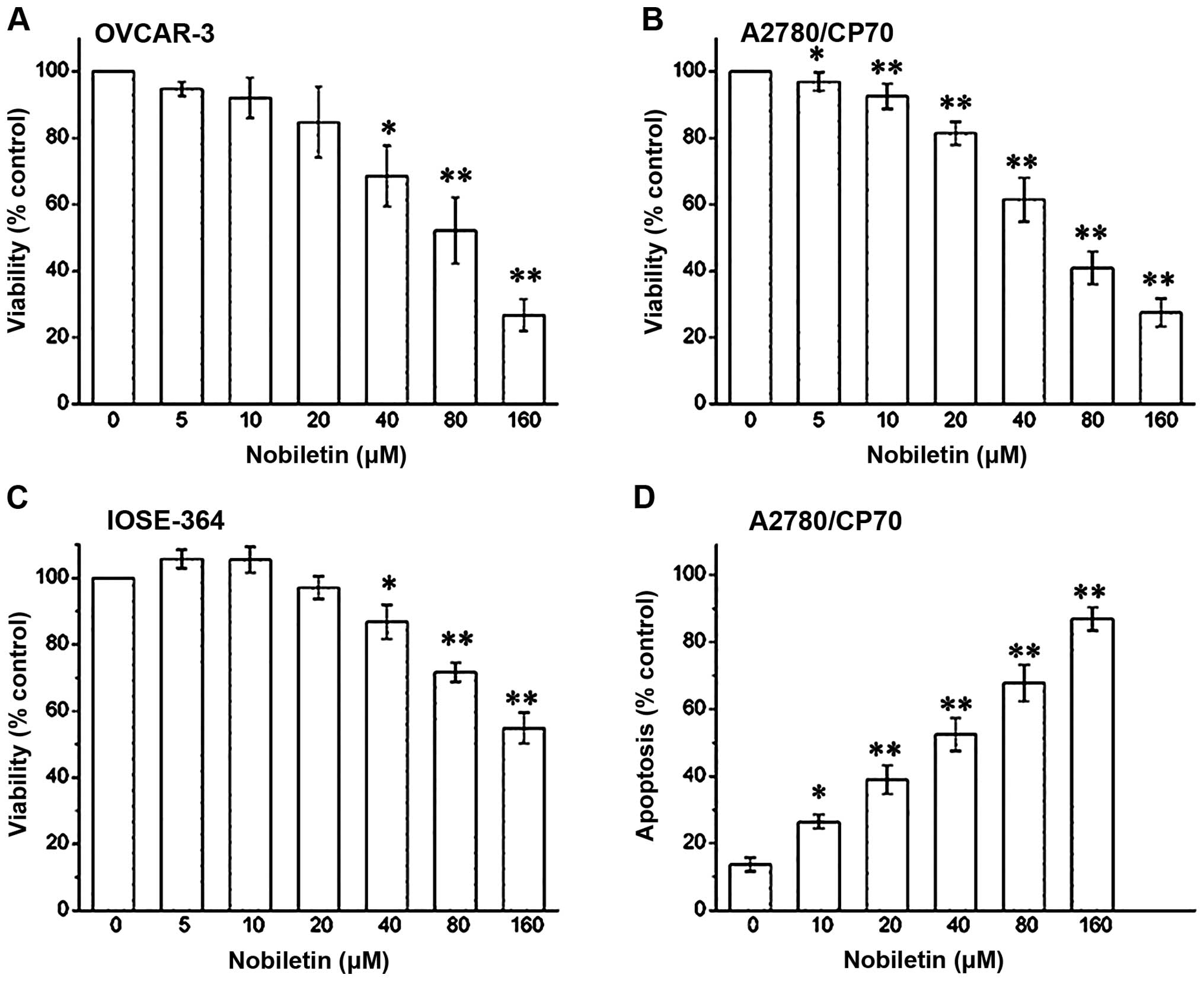
The treatment of OVCAR-3 and CP70 ovarian cancer
cells with nobiletin caused a concentration-dependent decrease in
cell viability over 24 h. Beginning at a concentration of 5 μM
nobiletin, OVCAR-3 cell viability consistently decreased from 95±1
to 28±4% at a concentration of 160 μM nobiletin (P<0.01)
(Fig. 2A). Similarly, CP70 cell
viability was also suppressed with different concentration. At a
concentration of 5 μM nobiletin cell viability was 96±1%
(P<0.05), that was gradually inhibited to 26±3% by a 160-μM
nobiletin treatment (P<0.01) (Fig.
2B). We also examined the growth inhibitory activity of
nobiletin on IOSE-364 normal ovarian cells (Fig. 2C). We observed that nobiletin had a
lower growth-inhibitory activity in IOSE-364 cells than in A2780
ovarian cancer cells. Therefore, our data suggest that nobiletin is
somewhat more selective towards ovarian cancer cells than normal
cells.
Effect of nobiletin on ovarian cancer
cell apoptosis
We ascertained that the growth inhibitory effects of
nobeletin were due to cellular apoptosis. Annexin FITC assays
revealed that nobiletin induced apoptosis in CP70 ovarian cancer
cells in a concentration-dependent manner (Fig. 2D). CP70 cell apoptosis was 13±1%
when it was not treated with nobiletin, which was significant
increased at a concentration of 10 μM nobiletin (26±2%). CP70 cell
apoptosis was gradually increased to 88±2% by a 160-μM nobiletin
treatment (P<0.01).
Effect of nobiletin on angiogenesis and
growth of the tumor
To assess whether nobiletin inhibits the growth of
human ovarian cancers in vivo, we used the CAM and athymic
mouse models to study the effect of nobiletin on the growth rate of
human ovarian cancer cells in vivo. The administration of 20
μM nobiletin significantly attenuated (P<0.01) the growth of
OVCAR human ovarian tumors implanted on CAM (Fig. 3A and B). We counted the number of
blood vessels to assess whether nobiletin was suppressing the
growth of ovarian tumors by inhibition of angiogenesis. The chicken
CAM experiment was repeated using A2780 cells and similar results
were obtained. We observed that the treatment of OVCAR and A2780
cells with 20 μM nobiletin was able to inhibit angiogenesis. The
implanted cancer cells grow to a tumor weight of 58±5 mg, with 29±4
blood vessels counted. Inclusion of 20 μM nobiletin in this
implant, however, reduced tumor growth down to 34±3 mg (P<0.01)
and inhibited blood vessel development to 19±2 (P<0.05). We
speculated that the effect of nobiletin on the promotion of cancer
cell apoptosis and inhibition of angiogesis lead to the growth
attenuation of tumors implanted on CAM. A typical image (Fig. 3C) is shown to contrast the tumors
with or without nobiletin in terms of both tumor size and
angiogenesis. The great effect of nobiletin on inhibiting tumor
growth was conformed in vivo using an athymic mouse model,
where they exhibited smaller tumor size (Fig. 3D). The tumors implanted on mice
grow to a volume of 2450 mm3 at the 15th day. However,
the treatment of nobiletin (100 mg/kg food) inhibited tumor growth
to 300 mm3.
Effect of nobiletin on VEGF
expression
Our results showed that nobiletin significantly
inhibited the expression of vascular endothelial grow factor (VEGF)
in ovarian cancer cells, and this inhibition effect was enhanced
with the increase of nobiletin concentration (Fig. 4). In both OVCAR-3 and A2780/CP-70
cells, the inhibitory effect of nobiletin on VEGF secretion reached
a significant level when its concentration was >20 μM. The
levels of secreted VEGF protein in OVCAR-3 cell culture supernates
were downregulated to 77±2% at a concentration of 10 μM nobiletin
(P<0.01) and to 28±1% at a concentration of 160 μM nobiletin
(P<0.01) (Fig. 4A). Similarly
the levels of secreted VEGF protein in CP70 cells ranged from 66±2%
(20 μM) to 16±2% (160 μM) with respect to different nobiletin
concentration (Fig. 4B).
Signaling pathways underline the growth
inhibiting activity of nobiletin
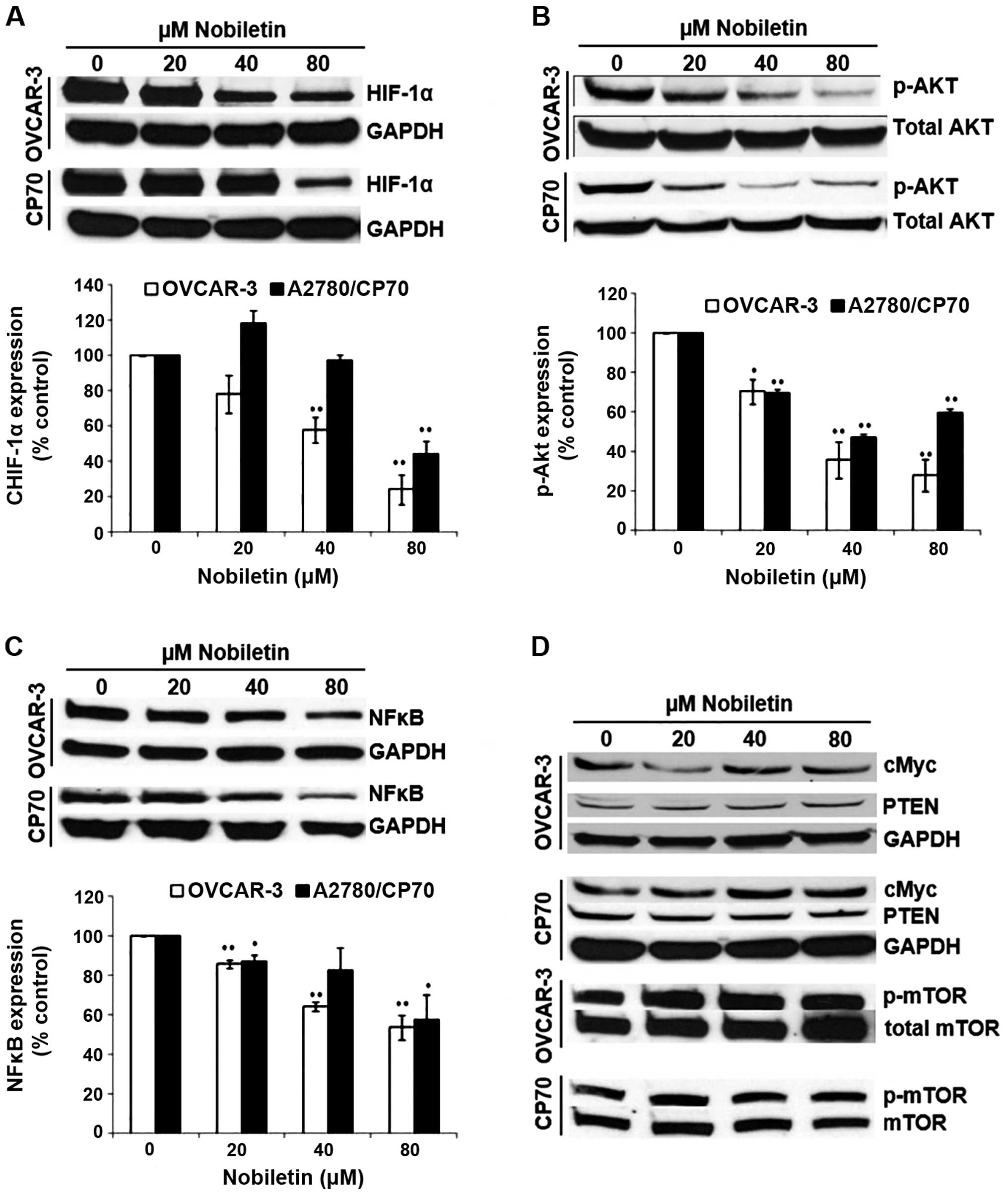
HIF-1α is one of the key factors for the regulation
of VEGF expression. As shown in Fig.
5A, nobiletin had a certain impact on HIF-1α expression of
ovarian cancer cells. For OVCAR-3, 20-μM nobiletin treatment led to
inhibition of HIF-1α protein to 78±6% by 24 h of treatment. Higher
concentrations of nobiletin resulted in greater inhibition, with
the levels of HIF1-α protein down to 57±3% by 40-μM nobiletin
treatment (P<0.01) and 23±3% by 80-μM nobiletin treatment
(P<0.01). However, for CP70, HIF-1α expression was slightly
enhanced (118±2%) when the concentration of nobiletin was <20 μM
and was significantly inhibited (47±2%) when its concentration was
80 μM (P<0.01). It seems CP70 cells were more resistant to the
effect of nobiletin than OVCAR-3 cells on HIF-1α expression.
Nobiletin inhibited phosphorylation of AKT, which is
known to be the major signal for cell survival and proliferation
(33). As shown in Fig. 5B, nobiletin decreased AKT
phosphorylation for both OVCAR-3 and CP70 ovarian cancer cells.
P-AKT level was downregulated from 70±2% by 20-μM nobiletin
treatment to 29±4% by 80-μM nobiletin treatment in OVCAR-3 cells.
The phosphorylation of AKT was also inhibited from 69±1% by the
20-μM nobiletin treatment to 49±1% by 40-μM nobiletin treatment in
CP70 cells. However, the effect of nobiletin on the inhibition of
AKT phosphorylation in C70 cells was decreased when treated with
160-μM nobiletin. Again, it seems CP70 cells were more resistant to
the effect of nobiletin than OVCAR-3 cells on AKT
phosphorylation.
NF-κB is a common transcription factor that is
related to many signal transduction pathways of cancer cell
proliferation and angiogenesis (34,35).
The effect of nobiletin on NF-κB expression is shown in Fig. 5C. Results showed that nobiletin had
an impact on NF-κB (p50) expression. The level of NF-κB (p50)
decreased with the increase of nobiletin concentration in OVCAR-3
and CP-70 cells. For OVCAR-3, the inhibitory effect reached a
significant level (87±1%) when the concentration of nobiletin was
20 μM (P<0.01). In CP70, NF-κB (p50) level reduced with the
increase of nobiletin concentration, and it reached a significant
level when the nobiletin concentration was 20 (88±1%) and 80 μM
(57±6%) (P<0.05).
c-Myc is a transcription factor that plays a role in
cell cycle progression, apoptosis and cellular transformation.
Unlike kaempferol, which inhibited c-Myc protein expression
(35), our results indicated that
nobiletin did not inhibit c-Myc expression in ovarian cancer cells
(Fig. 5D). Its mechanism needs to
be further investigated. PTEN acts as a tumor suppressor gene which
is involved in the regulation of the cell cycle, preventing cells
from growing and dividing too rapidly (36). The protein of mTOR is a
serine/threonine protein kinase that regulates cell growth, cell
proliferation, cell motility, cell survival, protein synthesis, and
transcription (37). Results also
showed that nobiletin had no significant effect on PTEN and p-mTOR
expression in ovarian cancer OVCAR-3 and CP-70 (Fig. 5D).
Nobiletin inhibits VEGF by regulating
HIF-1α expression and Akt signaling
To see that HIF-1 is not only regulated by nobiletin
treatment, but also plays a role in the nobiletin inhibition on
VEGF expression, ovarian cancer cells were transfected with the
VEGF-promoter reporter together with HIF-1 plasmids. While
nobiletin treatment significantly inhibited VEGF transcriptional
activation, this inhibition was concentration-dependent and
significantly reversed by forced expression of HIF-1α protein
(Fig. 6A).
The role played by Akt signaling in the nobiletin
regulation of VEGF expression was investigated in both ovarian cell
lines. It was found that the phosphorylation of Akt was
significantly inhibited by 2-h nobiletin treatment (Fig. 6B). After transfecting with
VEGF-promoter reporter and mAkt plasmids, VEGF transcriptional
activation was significantly reduced by nobiletin treatment in both
ovarian cancer cell types, and this effect was significantly
reversed by forced expression of Akt protein (Fig. 6B).
Discussion
Angiogenesis is a necessary condition for sustained
tumor growth. Tumor cells take in nutrition and oxygen through the
generated blood vessels, which produce substances needed for growth
(38). Angiogenesis plays a
central role in the development and progression of ovarian cancer
(39). Histological studies
demonstrated that ovarian tumors are richly vascularized. A
correlation between microvascular count and biological
aggressiveness was also found (40,41).
Vascular endothelial growth factor (VEGF), as a key regulator of
angiogenesis in ovarian cancer, is involved in various steps of
ovarian carcinogenesis (42,43).
VEGF plays a central role in tumor vasculature development and
maintenance. VEGF expression promotes angiogenesis, thereby
stimulating tumor growth and metastasis. Hypoxia-inducible factor
1α (HIF-1α) is a heterodimeric basic helix-loop-helix protein that
directly activates transcription of VEGF gene by binding to a HRE
(44). Previous studies showed
that nobiletin inhibited angiogenesis in human umbilical vein
endothelial cells (HUVECs) and zebrafish models (22,23,45).
Similarly, the results showed that nobiletin effectively inhibited
angiogenesis in ovarian cancer cells planted on chicken embryos
models, indicating its potential to inhibit tumor growth in
vivo.
While VEGF expression is an important factor in
tumor growth and metastasis, HIF-1α is one of the key factors for
VEGF expression. Previous studies (46,47)
showed that the anticancer flavonoid compound kaempferol inhibited
angiogenesis in ovarian cancer cells by downregulating HIF-1α
expression. This is consistent with our study, indicating that the
nobiletin inhibitory effects on angiogenesis can be traced back to
suppression of HIF-1α expression. However, contradictory results
were found in zebrafish models which showed that nobiletin
increased mRNA levels of VEGF (22). This could be because nobiletin
induced anti-angiogenesis through different mechanism in the two
kinds of cells. PI3 kinase/Akt pathways could attenuate ovarian
carcinoma through mediating angiogenesis and vascular permeability
(48). In addition, taking into
consideration previous studies that HIF-1α is related to the PI3
kinase/Akt signaling pathways (30–32),
we tested if nobiletin inhibited expression of VEGF through
PI3K/AKT pathways. Our results revealed that nobiletin
significantly inhibited the phosphorylation of Akt. The inhibitory
effect of nobiletin on Akt phosphorylation was more pronounced than
on HIF-1α expression shown above. Previous studies showed that
nobiletin suppressed PI3K/Akt pathways in human HepG2 (49). It was similar to our findings that
nobiletin inhibited angiogenesis mainly through Akt pathways which
results in the downregulation of VEGF expression (Fig. 6B). The mechanism by which nobiletin
inhibits the PI3K/Akt signaling pathway is not fully understood.
However, it has been shown that nobiletin suppressed invasion and
migration of human gastric adenocarcinoma AGS cells through
FAK/PI3K/Akt pathways (50).
Some researchers found that Akt pathways regulates
the expression, activation and translocation of NF-κB (51–53).
Suppression of NF-κB in tumor samples also inhibits proliferation,
causes apoptosis, indicating the crucial role of NF-κB in cell
proliferation and survival. We consider that it plays an important
role on the nobiletin-induced apoptosis in A2780/CP70 cells. It was
also found that Hif -1α promoter is responsive to selective NF-κB
subunits, indicating that NF-κB is a direct modulator of Hif -1α
expression (54). NF-κB, which is
related to many signal transduction pathways of cancer cells
(34), has been identified in
tumors of epithelial origin such as breast, colon, lung and ovarian
cancers (55). Recent research
suggested the importance of NF-κB in the propagation of ovarian
cancer cell lines (56). It was
also found that NF-κB had a relationship with angiogenesis; that
is, NF-κB regulates c-Myc expression. Overexpression of NF-κB
removed the kaempferol inhibitory effect on c-Myc expression
(35). Our results showed that the
effect of nobiletin on NF-κB in ovarian cancer cells varies with
cancer cells. Similar to the results found in AKT phosphorylation,
NF-κB levels in the OVCAR-3 cells were more sensitive than those in
the CP70 line. Nevertheless, nobiletin treatment significantly
reduced NF-κB expression in both cell lines in a
concentration-dependent manner. As previous study showed NF-κB have
been linked to regulation of VEGF production (57), we conclude nobiletin antagonizes
VEGF expression through NF-κB (Fig.
7).
The proposed mechanism by which nobiletin hampers
angiogenesis involves lowering concentrations of VEGF regulators,
namely HIF-1α and NF-κB (Fig. 7).
We found nobiletin to have little effect on c-Myc, PTEN, and p-mTOR
expression, which indicates that nobiletin does not inhibit
expression of VEGF through PTEN/mTOR pathways, neither is c-Myc the
key protein that is affected by nobiletin treatment in ovarian
cells. Noteworthy, both HIF-1α and Akt were demonstrated to play
direct roles in VEGF secretion. Overexpression of either protein in
the presence of nobiletin neutralized its VEGF diminishing effects.
Since Akt phosphorylation is intimately linked to HIF-1α
activation, it is likely nobiletin exerts its cancer fighting
properties through blocking Akt phosphorylation. Impeding Akt
activity likewise reduces HIF-1α and NF-κB levels, subsequently
dropping VEGF production and obstructing angiogenesis. Considering
all the evidence, we believe this is the central pathway through
which nobiletin mediates its tumor limiting effects.
Importantly, our in vitro research conducted
agrees with both of our in vivo models. In our CAM model,
nobiletin treatment significantly reduced not only the tumor size
but also the number of blood vessels, confirming its potency in
countering angiogenesis. Furthermore, nobiletin also suppressed
tumor growth rates of CP-70 human ovarian cancer cells in the nude
mouse model. The administration of nobiletin did not cause any
discomfort in mice. The weights of mice, their food intake, water
intake were unaffected by the administration of nobiletin (data not
shown).
The challenge of conventional chemotherapy in
ovarian cancer is that chemotherapeutic drugs are also toxic to
normal ovarian epithelial cells. We find that nobiletin potently
inhibits the viability of human ovarian cancer cells, and has
minimal effects of the viability of normal ovarian cells. The
selective inhibitory effect might be at least partly attributed to
the apoptosis and anti-angiogenesis induced by nobiletin.
Overexpression and activation of Akt, which results in the survival
of cancer cells that normally undergo apoptosis (58), occurs in different kinds of
cancers, such as gastric, lung, panacreatic and ovarian cancer
(59). Nobiletin inhibits overian
cancer cells selectively, possibly due to its effect on the
phosphorylation of Akt.
Platinum drugs have been most frequently applied for
the treatment of cancers including ovarian cancer. However,
acquired resistance to conventional platinum based chemotherapy has
become a major impediment in cancer treatment. Novel therapies that
can reverse drug resistance or kill drug resistant ovarian cancer
cells directly are highly desired. Flavonoid compounds like
tangeretin (60) and kaempferol
(61) have been shown to sensitize
ovarian cancer cells to the apoptotic effects of cisplatin. Since
nobiletin selectively inhibits ovarian cancer cells, it is expected
that nobiletin may be useful both as a single agent as well as in
combination therapies in the treatment of ovarian cancers.
Acknowledgements
This study was supported by a grant of the West
Virginia Experimental Program to Stimulate Competitive Research and
NIH grants (5P20RR016477 and 8P20GM103434) from the National
Institutes of Health awarded to the West Virginia IDeA Network of
Biomedical Research Excellence.
References
|
1
|
Jemal A, Tiwari RC, Murray T, Ghafoor A,
Samuels A, Ward E, Feuer EJ and Thun MJ: American Cancer Society:
Cancer statistics, 2004. CA Cancer J Clin. 54:8–29. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bast RC Jr, Xu FJ, Yu YH, Barnhill S,
Zhang Z and Mills GB: CA 125: The past and the future. Int J Biol
Markers. 13:179–187. 1998.
|
|
4
|
Winstead ER: Ovarian cancer study raises
questions about developing markers for early detection. NCI cancer
bulletin [On-line serial]. 8(5): Available simplencicancerbulletin@mail.nih.gov
Message: NCI Cancer Bulletin. March 8–2011
|
|
5
|
Fishman DA and Schwartz PE: Current
approaches to diagnosis and treatment of ovarian germ cell
malignancies. Curr Opin Obstet Gynecol. 6:98–104. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Greenlee RT, Hill-Harmon MB, Murray T and
Thun M: Cancer statistics, 2001. CA Cancer J Clin. 51:15–36. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bosetti C, Rossi M, McLaughlin JK, Negri
E, Talamini R, Lagiou P, Montella M, Ramazzotti V, Franceschi S and
LaVecchia C: Flavonoids and the risk of renal cell carcinoma.
Cancer Epidemiol Biomarkers Prev. 16:98–101. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bosetti C, Bravi F, Talamini R, Parpinel
M, Gnagnarella P, Negri E, Montella M, Lagiou P, Franceschi S and
La Vecchia C: Flavonoids and prostate cancer risk: A study in
Italy. Nutr Cancer. 56:123–127. 2006. View Article : Google Scholar
|
|
9
|
Theodoratou E, Kyle J, Cetnarskyj R,
Farrington SM, Tenesa A, Barnetson R, Porteous M, Dunlop M and
Campbell H: Dietary flavonoids and the risk of colorectal cancer.
Cancer Epidemiol Biomarkers Prev. 16:684–693. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Adhami VM, Malik A, Zaman N, Sarfaraz S,
Siddiqui IA, Syed DN, Afaq F, Pasha FS, Saleem M and Mukhtar H:
Combined inhibitory effects of green tea polyphenols and selective
cyclooxygenase-2 inhibitors on the growth of human prostate cancer
cells both in vitro and in vivo. Clin Cancer Res. 13:1611–1619.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Choi EJ, Kim T and Lee MS: Pro-apoptotic
effect and cytotoxicity of genistein and genistin in human ovarian
cancer SK-OV-3 cells. Life Sci. 80:1403–1408. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Seo HS, DeNardo DG, Jacquot Y, Laïos I,
Vidal DS, Zambrana CR, Leclercq G and Brown PH: Stimulatory effect
of genistein and apigenin on the growth of breast cancer cells
correlates with their ability to activate ER alpha. Breast Cancer
Res Treat. 99:121–134. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fang J, Zhou Q, Liu LZ, Xia C, Hu X, Shi X
and Jiang BH: Apigenin inhibits tumor angiogenesis through
decreasing HIF-1alpha and VEGF expression. Carcinogenesis.
28:858–864. 2007. View Article : Google Scholar
|
|
14
|
Birt DF, Hendrich S and Wang W: Dietary
agents in cancer prevention: Flavonoids and isoflavonoids.
Pharmacol Ther. 90:157–177. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gossner G, Choi M, Tan L, Fogoros S,
Griffith KA, Kuenker M and Liu JR: Genistein-induced apoptosis and
autophagocytosis in ovarian cancer cells. Gynecol Oncol. 105:23–30.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Spinella F, Rosanò L, Di Castro V,
Decandia S, Albini A, Nicotra MR, Natali PG and Bagnato A: Green
tea polyphenol epigallocatechin-3-gallate inhibits the endothelin
axis and downstream signaling pathways in ovarian carcinoma. Mol
Cancer Ther. 5:1483–1492. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brusselmans K, Vrolix R, Verhoeven G and
Swinnen JV: Induction of cancer cell apoptosis by flavonoids is
associated with their ability to inhibit fatty acid synthase
activity. J Biol Chem. 280:5636–5645. 2005. View Article : Google Scholar
|
|
18
|
Nogata Y, Sakamoto K, Shiratsuchi H, Ishii
T, Yano M and Ohta H: Flavonoid composition of fruit tissues of
citrus species. Biosci Biotechnol Biochem. 70:178–192. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Murakami A, Nakamura Y, Torikai K, Tanaka
T, Koshiba T, Koshimizu K, Kuwahara S, Takahashi Y, Ogawa K, Yano
M, et al: Inhibitory effect of citrus nobiletin on phorbol
ester-induced skin inflammation, oxidative stress, and tumor
promotion in mice. Cancer Res. 60:5059–5066. 2000.PubMed/NCBI
|
|
20
|
Kohno H, Yoshitani S, Tsukio Y, Murakami
A, Koshimizu K, Yano M, Tokuda H, Nishino H, Ohigashi H and Tanaka
T: Dietary administration of citrus nobiletin inhibits
azoxymethane-induced colonic aberrant crypt foci in rats. Life Sci.
69:901–913. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Suzuki R, Kohno H, Murakami A, Koshimizu
K, Ohigashi H, Yano M, Tokuda H, Nishino H and Tanaka T: Citrus
nobiletin inhibits azoxymethane-induced large bowel carcinogenesis
in rats. Biofactors. 22:111–114. 2004. View Article : Google Scholar
|
|
22
|
Lam KH, Alex D, Lam IK, Tsui SK, Yang ZF
and Lee SM: Nobiletin, a polymethoxylated flavonoid from citrus,
shows anti-angiogenic activity in a zebrafish in vivo model and
HUVEC in vitro model. J Cell Biochem. 112:3313–3321. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kunimasa K, Ikekita M, Sato M, Ohta T,
Yamori Y, Ikeda M, Kuranuki S and Oikawa T: Nobiletin, a citrus
polymethoxyflavonoid, suppresses multiple angiogenesis-related
endothelial cell functions and angiogenesis in vivo. Cancer Sci.
101:2462–2469. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kunimasa K, Kuranuki S, Matsuura N,
Iwasaki N, Ikeda M, Ito A, Sashida Y, Mimaki Y, Yano M, Sato M, et
al: Identification of nobiletin, a polymethoxyflavonoid, as an
enhancer of adiponectin secretion. Bioorg Med Chem Lett.
19:2062–2064. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saito T, Abe D and Sekiya K: Nobiletin
enhances differentiation and lipolysis of 3T3-L1 adipocytes.
Biochem Biophys Res Commun. 357:371–376. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miyata Y, Sato T, Yano M and Ito A:
Activation of protein kinase C βII/ɛ-c-Jun NH2-terminal kinase
pathway and inhibition of mitogen-activated protein/extracellular
signal-regulated kinase 1/2 phosphorylation in antitumor invasive
activity induced by the polymethoxy flavonoid, nobiletin. Mol
Cancer Ther. 3:839–847. 2004.PubMed/NCBI
|
|
27
|
Miyata Y, Sato T, Imada K, Dobashi A, Yano
M and Ito A: A citrus polymethoxyflavonoid, nobiletin, is a novel
MEK inhibitor that exhibits antitumor metastasis in human
fibrosarcoma HT-1080 cells. Biochem Biophys Res Commun.
366:168–173. 2008. View Article : Google Scholar
|
|
28
|
Luo H, Li B, Li Z, Cutler SJ, Rankin GO
and Chen YC: Chaetoglobosin K inhibits tumor angiogenesis through
downregulation of vascular epithelial growth factor-binding
hypoxia-inducible factor 1α. Anticancer Drugs. 24:715–724. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fang J, Cao Z, Chen YC, Reed E and Jiang
BH: 9-β-D-Arabinofuranosyl-2-fluoroadenine inhibits expression of
vascular endothelial growth factor through hypoxia-inducible
factor-1 in human ovarian cancer cells. Mol Pharmacol. 66:178–186.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Blancher C, Moore JW, Robertson N and
Harris AL: Effects of ras and von Hippel-Lindau (VHL) gene
mutations on hypoxia-inducible factor (HIF)-1α, HIF-2α, and
vascular endothelial growth factor expression and their regulation
by the phosphatidylinositol 3′-kinase/Akt signaling pathway. Cancer
Res. 61:7349–7355. 2001.PubMed/NCBI
|
|
31
|
Laughner E, Taghavi P, Chiles K, Mahon PC
and Semenza GL: HER2 (neu) signaling increases the rate of
hypoxia-inducible factor 1α (HIF-1α) synthesis: Novel mechanism for
HIF-1-mediated vascular endothelial growth factor expression. Mol
Cell Biol. 21:3995–4004. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stiehl DP, Jelkmann W, Wenger RH and
Hellwig-Bürgel T: Normoxic induction of the hypoxia-inducible
factor 1alpha by insulin and interleukin-1beta involves the
phosphatidylinositol 3-kinase pathway. FEBS Lett. 512:157–162.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Miura T, Chiba M, Kasai K, Nozaka H,
Nakamura T, Shoji T, Kanda T, Ohtake Y and Sato T: Apple
procyanidins induce tumor cell apoptosis through mitochondrial
pathway activation of caspase-3. Carcinogenesis. 29:585–593. 2008.
View Article : Google Scholar
|
|
34
|
Jeong WS and Kong ANT: Biological
properties of monomeric and polymeric catechins: Green tea
catechins and procyanidins. Pharm Biol. 42(s1): 84–93. 2004.
View Article : Google Scholar
|
|
35
|
Luo H, Rankin GO, Juliano N, Jiang BH and
Chen YC: Kaempferol inhibits VEGF expression and in vitro
angiogenesis through a novel ERK-NFκB-c-Myc-p21 pathway. Food Chem.
130:321–328. 2012. View Article : Google Scholar
|
|
36
|
Chu EC and Tarnawski AS: PTEN regulatory
functions in tumor suppression and cell biology. Med Sci Monit.
10:RA235–RA241. 2004.PubMed/NCBI
|
|
37
|
Hay N and Sonenberg N: Upstream and
downstream of mTOR. Genes Dev. 18:1926–1945. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ferrara N: Vascular endothelial growth
factor as a target for anticancer therapy. Oncologist. 9(Suppl 1):
2–10. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ramakrishnan S, Subramanian IV, Yokoyama Y
and Geller M: Angiogenesis in normal and neoplastic ovaries.
Angiogenesis. 8:169–182. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hazelton D, Nicosia RF and Nicosia SV:
Vascular endothelial growth factor levels in ovarian cyst fluid
correlate with malignancy. Clin Cancer Res. 5:823–829.
1999.PubMed/NCBI
|
|
41
|
Alvarez AA, Krigman HR, Whitaker RS, Dodge
RK and Rodriguez GC: The prognostic significance of angiogenesis in
epithelial ovarian carcinoma. Clin Cancer Res. 5:587–591.
1999.PubMed/NCBI
|
|
42
|
Duyndam MC, Hilhorst MC, Schlüper HM,
Verheul HM, van Diest PJ, Kraal G, Pinedo HM and Boven E: Vascular
endothelial growth factor-165 overexpression stimulates
angiogenesis and induces cyst formation and macrophage infiltration
in human ovarian cancer xenografts. Am J Pathol. 160:537–548. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hefler LA, Mustea A, Könsgen D, Concin N,
Tanner B, Strick R, Heinze G, Grimm C, Schuster E, Tempfer C, et
al: Vascular endothelial growth factor gene polymorphisms are
associated with prognosis in ovarian cancer. Clin Cancer Res.
13:898–901. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Forsythe JA, Jiang BH, Iyer NV, Agani F,
Leung SW, Koos RD and Semenza GL: Activation of vascular
endothelial growth factor gene transcription by hypoxia-inducible
factor 1. Mol Cell Biol. 16:4604–4613. 1996.PubMed/NCBI
|
|
45
|
Lam IK, Alex D, Wang YH, Liu P, Liu AL, Du
GH and Lee SM: In vitro and in vivo structure and activity
relationship analysis of polymethoxylated flavonoids: Identifying
sinensetin as a novel antiangiogenesis agent. Mol Nutr Food Res.
56:945–956. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Luo H, Rankin GO, Liu L, Daddysman MK,
Jiang BH and Chen YC: Kaempferol inhibits angiogenesis and VEGF
expression through both HIF dependent and independent pathways in
human ovarian cancer cells. Nutr Cancer. 61:554–563. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jiang BH and Liu LZ: AKT signaling in
regulating angiogenesis. Curr Cancer Drug Targets. 8:19–26. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hu L, Hofmann J and Jaffe RB:
Phosphatidylinositol 3-kinase mediates angiogenesis and vascular
permeability associated with ovarian carcinoma. Clin Cancer Res.
11:8208–8212. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Shi MD, Liao YC, Shih YW and Tsai LY:
Nobiletin attenuates metastasis via both ERK and PI3K/Akt pathways
in HGF-treated liver cancer HepG2 cells. Phytomedicine. 20:743–752.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lee YC, Cheng TH, Lee JS, Chen JH, Liao
YC, Fong Y, Wu CH and Shih YW: Nobiletin, a citrus flavonoid,
suppresses invasion and migration involving FAK/PI3K/Akt and small
GTPase signals in human gastric adenocarcinoma AGS cells. Mol Cell
Biochem. 347:103–115. 2011. View Article : Google Scholar
|
|
51
|
Kar S, Palit S, Ball WB and Das PK:
Carnosic acid modulates Akt/IKK/NF-κB signaling by PP2A and induces
intrinsic and extrinsic pathway mediated apoptosis in human
prostate carcinoma PC-3 cells. Apoptosis. 17:735–747. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kim MO, Moon DO, Heo MS, Lee JD, Jung JH,
Kim SK, Choi YH and Kim GY: Pectenotoxin-2 abolishes constitutively
activated NF-κB, leading to suppression of NF-κB related gene
products and potentiation of apoptosis. Cancer Lett. 271:25–33.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ozes ON, Mayo LD, Gustin JA, Pfeffer SR,
Pfeffer LM and Donner DB: NF-κB activation by tumour necrosis
factor requires the Akt serine-threonine kinase. Nature. 401:82–85.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
van Uden P, Kenneth NS and Rocha S:
Regulation of hypoxia-inducible factor-1α by NF-kappaB. Biochem J.
412:477–484. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Karin M: Nuclear factor-kappaB in cancer
development and progression. Nature. 441:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lin YG, Kunnumakkara AB, Nair A, Merritt
WM, Han LY, Armaiz-Pena GN, Kamat AA, Spannuth WA, Gershenson DM,
Lutgendorf SK, et al: Curcumin inhibits tumor growth and
angiogenesis in ovarian carcinoma by targeting the nuclear
factor-kappaB pathway. Clin Cancer Res. 13:3423–3430. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Gonzalez-Perez RR, Xu Y, Guo S, Watters A,
Zhou W and Leibovich SJ: Leptin upregulates VEGF in breast cancer
via canonic and non-canonical signalling pathways and
NFkappaB/HIF-1alpha activation. Cell Signal. 22:1350–1362. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Testa JR and Bellacosa A: AKT plays a
central role in tumorigenesis. Proc Natl Acad Sci USA.
98:10983–10985. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Cain K, Langlais C, Sun XM, Brown DG and
Cohen GM: Physiological concentrations of K+ inhibit
cytochrome c-dependent formation of the apoptosome. J Biol Chem.
276:41985–41990. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Arafa SA, Zhu Q, Barakat BM, Wani G, Zhao
Q, El-Mahdy MA and Wani AA: Tangeretin sensitizes
cisplatin-resistant human ovarian cancer cells through
downregulation of phosphoinositide 3-kinase/Akt signaling pathway.
Cancer Res. 69:8910–8917. 2009. View Article : Google Scholar
|
|
61
|
Luo H, Daddysman MK, Rankin GO, Jiang BH
and Chen YC: Kaempferol enhances cisplatin’s effect on ovarian
cancer cells through promoting apoptosis caused by down regulation
of c-Myc. Cancer Cell Int. 10:162010. View Article : Google Scholar
|