Introduction
Metastases of primary tumors are the main cause of
cancer-related death, such that the mechanism of their development
is an important area of investigation (1). Metastasis has been shown to involve
several distinct steps: detachment and migration from the primary
tumor, penetration of the basement membrane, entry into the blood
or lymphatic stream, exit from the blood or lymphatic stream, and,
finally, the formation of a metastatic nodule (2). The first of these steps, detachment
and migration from the primary tumor, involves
epithelial-mesenchymal transition (EMT), which is controlled by
multiple molecules and signaling pathways (1,3).
High-mobility-group A2 (HMGA2) is a small nonhistone
chromosomal protein that binds through its three AT-hook
DNA-binding motifs to AT-rich sequences in the minor groove of DNA
strands (4). Although HMGA2 has no
intrinsic transcriptional activity, it modulates transcription by
altering the architecture of chromatin (5,6).
Since HMGA2 is highly expressed during embryogenesis but is absent
or present only at low levels in normal adult tissues, it seems to
play a critical role in cell proliferation and differentiation
during embryonic development (7,8).
However, according to several studies, HMGA2 is also expressed in
the development of malignancy and, through its relationship to EMT,
participates in tumor metastasis (9,10).
Yet, while a correlation between HMGA2 overexpression and
malignancy has been reported (11,12),
the exact mechanism is not fully explained. In gastric cancer, for
example, there are few studies on the consequences of HMAG2
overexpression in these tumors.
The aim of the present study was to use surgical
gastric cancer specimens and patient clinicopathological data to
investigate the clinical significance of HMGA2 overexpression in
gastric cancer. In addition, the mechanism by which HMGA2
expression acts on the gastric cancer and the association between
that mechanism and EMT were analyzed in an in vitro study
using a gastric cancer cell line.
Materials and methods
Patients and samples
A series of 170 consecutive gastric cancer patients
who underwent gastrectomy at Uijeongbu St. Mary’s Hospital
(Uijeongbu, Gyeonggi-Do, Korea) from 2001 to 2005 were enrolled in
the study. Clinicopathological parameters including operative
details were collected retrospectively from the hospital’s Gastric
Cancer Patients Registry. Cancer stage was determined based on the
TNM classification of the Seventh American Joint Cancer Committee
(AJCC).
The 123 male and 47 female patients ranged in age
from 29 to 89 years (median 61.5 years). In this group, 90%
underwent lymph node dissection level D2 and greater. Distal and
total gastrectomy was performed in 110 and 60 patients,
respectively. According to the final pathological diagnosis, 23
patients had stage I disease, 51 stage II, 94 stage III, and 2
stage IV (Table I). Adjuvant
chemotherapy was administered to stage II and stage III patients
according to institutional guidelines. Fluorouracil (5-FU)- or
cisplatin-based systemic chemotherapy was administered with
adjuvant intention to all patients. None of the patients received
neoadjuvant chemotherapy.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Variable | n=170 |
|---|
| Gender |
| Male | 123 |
| Female | 47 |
| Age in years |
| Median (range) | 61.5 (29–89) |
| Extent of
resection | |
| Subtotal | 110 |
| Total | 60 |
| Lymph node
dissection |
| D1+ | 17 |
| D2 | 88 |
| More than D2 | 65 |
| Retrieved lymph
nodes | |
| Mean ± SD
(range) | 29.2±13.9 (3–76) |
| Reconstruction |
| Billroth-I | 12 |
| Billroth-II | 98 |
| Roux-en-Y | 60 |
| Pathological stage
(7th AJCC) |
| I | 23 |
| II | 51 |
| III | 94 |
| IV | 2 |
Regular follow-up was conducted according to our
standard protocol (every 3 and 6 months in advanced and early
gastric cancer, respectively, for the first 3 years, every 12
months thereafter) and included an evaluation of tumor markers,
abdominal CT, and endoscopic examination. The mean follow-up period
of the 170 enrolled patients was 71.4±50.5 months (range, 0–152
months). The survival results were confirmed by using the
registration data of the Korea National Statistical Office (KNSO)
and the patients’ medical records.
From the 170 participants in the study, 76 gastric
cancer samples and the corresponding control samples were used for
reverse transcription-polymerase chain reaction (RT-PCR) and
western blot. All samples had been frozen immediately after
surgical resection and stored in liquid nitrogen of −90°C.
Immunohistochemistry was performed on 170 tissue samples, obtained
as paraffin-embedded resected gastric specimens, after
histopathological diagnosis.
This study was approved by the Institutional Review
Board of the Ethics Committee of the College of Medicine, Catholic
University of Korea (UC13SISI0008).
Immunohistochemistry
Formalin-fixed and paraffin-embedded human gastric
tumor tissues were sectioned at a thickness of 4 μm and then
immunohistochemically stained using a standard avidin-biotin
peroxidase complex method (13).
The slides were deparaffinized in xylene three times for 10 min,
rehydrated through a graded ethanol series to distilled water, and
then incubated for 10 min with 3% hydrogen peroxidase-methanol to
inhibit endogenous peroxidase activity. For antigen retrieval, the
slides were treated with 10 mM citrate buffer (pH 6.0) at 98°C for
15 min in a microwave oven and allowed to cool for 1 h at room
temperature. After incubation of the sections for 10 min in a
blocking solution (Histo-Plus kit, Zymed, San Francisco, CA, USA)
containing 10% normal serum in phosphate-buffered saline (PBS),
they were treated with a primary rabbit polyclonal anti-HMGA2
antibody (Abcom, Cambridge, MA, USA) diluted 1:100 in blocking
solution and then incubated overnight at 4°C in a humidified
chamber. The primary antibodies were detected with a secondary
antibody (Histo-Plus, Zymed) used together with biotin, incubating
the slides for 10 min at 45°C. The sections were rinsed three times
in PBS and then incubated for 10 min with streptavidin-horseradish
peroxidase complex (Histo-Plus, Zymed). Antigens were localized
using 3,3′-diaminobenzidine tetrahydrochloride as the chromogen
followed by counterstaining with hematoxylin.
Evaluation of immunoscores
The HMGA2 positivity of gastric tumor glands was
defined as nuclear staining with the corresponding antigen. The
additional presence of HMGA2 deposits in the cytoplasm was also
considered positive. The extent and the intensity of
immunopositivity were considered when scoring the expression of
HMGA2 protein. The intensity of positive staining was scored as
follows: 0, negative; 1, weak; 2, moderate; and 3, strong, and the
extent of positive staining according to the percentage of positive
cells in the respective lesions: 0, 0%; 1, 1–10%; 2, 11–25%; 3,
26–50%; 4, 51–75%; 5, 76–90%; and 6, >90%. The final score was
obtained by multiplying the positivity and intensity scores,
yielding a range from 0 to 18. HMGA2 expression was considered
positive when the final score was ≥9.
Cell culture and transfection
MKN28 cells, obtained from the Korean Cell Line
Bank, were maintained in RPMI-1640 (Hyclone, Logan, UT, USA)
supplemented with 10% calf serum (Hyclone) and incubated at 37°C in
a 5% CO2 humidified atmosphere. Two pairs of
oligonucleotides encoding the pre-micro-RNA (miRNA) were
synthesized, annealed, and then cloned into plasmid
pcDNATM6.2-GW/EmGFP-miR (Invitrogen, Carlsbad, CA, USA), carrying a
gene encoding the selectable marker blasticidin. MKN28 cells were
seeded in six-well plates in RPMI-1640 containing 10% fetal bovine
serum (FBS) without antibiotics for 24 h, then transfected with 400
ng of either the purified pcDNATM6.2-GW/EmGFP-miR expression
vector, containing the HMGA2 insert HMGA2i-1 or HMGA2i-2, or the
negative control (HMGA2i-NC) obtained from Invitrogen. The inserts
were designed to different coding regions of human HMGA2 mRNA
sequence (NM_003484.1) using the BLOCK-iT RNAi express search
engine (http://rnaidesigner.lifetechnologies.com/rnaiexpress/rnaiExpress.jsp).
The sequence information for the HMGA2 RNAi duplexes is provided in
Table II. Effectin transfection
reagents (Qiagen, Hilden, Germany) were used according to the
manufacturer’s instructions. The efficiency of transfection was
determined by fluorescence microscopy after 24 h. Stable cell lines
were selected using blasticidin, a translational inhibitor, and
were harvested for the evaluation of gene expression or for use in
functional assays.
 | Table IIPrecursor sequences of
miRNA-HMGA2. |
Table II
Precursor sequences of
miRNA-HMGA2.
| No. | miRNA-HMGA2
precursor sequence |
|---|
| HMGA2i-1 |
| Sense |
5′-TGCTGCTTGGTAGTAGATTGTCCCATGTTTTGGCCACTGACTGACATGGGACACTACTACCAAG-3′ |
| Antisense |
5′-CCTGCTTGGTAGTAGTGTCCCATGTCAGTCAGTGGCCAAAACATGGGACAATCTACTACCAAGC-3′ |
| HMGA2i-2 |
| Sense |
5′-TGCTGTTTGGTACTGTTTCCCAGAGAGTTTTGGCCACTGACTGACTCTCTGGGACAGTACCAAA-3′ |
| Antisense |
5′-CCTGTTTGGTACTGTCCCAGAGAGTCAGTCAGTGGCCAAAACTCTCTGGGAAACAGTACCAAAC-3′ |
Quantitative real-time reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated from cells using the RNeasey
mini kit (Qiagen) according to the manufacturer’s protocol. Total
RNA (3 μg) were reverse-transcribed into cDNA using the Superscript
III first-strand synthesis system (Invitrogen). The resultant cDNA
was used for PCR amplification with Quick Taq HS dye mix (Toyobo,
Osaka, Japan). The primers for HMGA2 were: sense 5′-CAG
CCGTCCACTTCAGC-3′ and antisense 5′-TGCCTTTGG GTCTTCC-3′. The
primers for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were:
sense 5′-GAAGGTGA AGGTCGGAGTC-3′ and antisense 5′-GAAGATGGTGA
TGGGATTTC-3′. GAPDH was amplified in parallel as the internal
control. PCR was performed at 94°C for 2 min, followed by 35 cycles
of 94°C for 30 sec, 58°C for 30 sec, and 72°C for 30 sec, and
finally one cycle of 72°C for 10 min. The PCR products were then
separated on 2% agarose gels and visualized by ethidium bromide
staining. For the detection of HMGA2 mRNA expression, real-time
quantitative PCR (QPCR) analysis was performed using iQ SYBR Green
supermix (Bio-Rad, Hercules, CA, USA). cDNA (2 μl) was combined
with the HMGA2 or GAPDH primers and the SYBR Green master mix in a
final volume of 20 μl in a 96-well RT-PCR plate (iCycleriQ PCR
plates; Bio-Rad). QPCR was carried out in an iCycler (Bio-Rad) for
35 cycles. The experiments were performed in duplicate for each set
of primers.
Western blotting
The cells were collected and washed with PBS.
Proteins were extracted using the NE-PER nuclear and cytoplasmic
protein extraction kit (Thermo Scientific, Rockford, IL, USA)
according to the manufacturer’s protocol. Approximately 10 mg of
frozen tissue were homogenized in 600 μl of Pro-Prep protein
extraction solution (iNtRON Biotechnology, Korea) after which cell
lysis was induced by incubation of the homogenates for 30 min on
ice. The protein concentration was determined using the Pierce BCA
protein assay kit (Thermo Scientific). Equal amounts of proteins
were separated on 8% or 15% SDS-polyacrylamide gels and transferred
onto nitrocellulose membranes (Whatman, Dassel, Germany). The
membranes were blocked with 5% skim milk in Tris-buffered saline
Tween-20 (TBST) for 1 h, incubated overnight at 4°C with primary
antibodies against HMGA2 (1:1000; Abcom), N-cadherin (1:1000; Cell
Signaling Technology, Beverly, MA, USA), E-cadherin (1:1000; Thermo
Scientific), Snail (1:1000; Abcam), β-catenin (1:1000; Invitrogen),
and Zeb1 (1:1000, Abcam), and washed extensively with TBST. Lamin
B1 (1:10000; Abcam) antibody served as the loading control. The
immune complexes were detected autoradiographically using the
appropriate horseradish-peroxidase-labeled secondary antibodies
(Bio-Rad) at 1:2000 dilutions and the enhanced chemiluminescence
detection reagent ECL (Thermo Scientific).
Cell migration and invasion assay
The Transwell migration assay was performed in
6.5-mm diameter Boyden chambers with a pore size of 8.0 μm (Corning
Inc., Corning, NY, USA). Cells (2×105) were resuspended
in migration medium (serum-free RPMI-1640 containing 5% bovine
serum albumin, BSA) and placed in the upper compartment of the
Transwell chambers. The lower compartment was filled with 600 μl of
RPMI-1640 medium containing 10% FBS. After incubation of the
chambers for 24 h at 37°C, cells on the lower surface of the filter
were stained with 0.1% crystal violet, rinsed with distilled water,
and eluted with 10% acetic acid for 15 min. Optical densities were
measured on a VersaMax spectrophotometer (Molecular Devices,
Silicon Valley, CA, USA) at 590 nM. For invasion assays, the cells
were plated in 24-well Matrigel-coated invasion chambers, in which
the lower chambers contained 600 μl of RPMI-1640 and 10% FBS as a
chemoattractant. A suspension of 2×105 cells in 200 μl
of serum-free RPMI-1640 containing 5% BSA was added to the upper
chamber. The cells were incubated for 38 h at 37°C in a humidified
incubator with 5% CO2, during which time the invasive
cells attached to the lower surface of the membrane insert. The
staining of the invasive cell and measuring of the optical
densities procedure for the invasion assay were similar to the
migration assay. The migration and invasion assays were repeated
three times.
Statistical analysis
Differences between groups were analyzed using the
t-test for continuous variables and the χ2 test or
Fisher’s exact test for proportions. Survival was analyzed using
Kaplan-Meier methods with a log-rank test for univariate analysis.
Multivariate analysis for survival was carried out using the Cox
proportional hazards model with the ‘Backward LR’ method.
Statistical analyses were performed with SPSS (Statistical Package
for Social Science, Chicago, IL, USA), version 13.0. P<0.05
indicated statistical significance.
Results
HMGA2 expression is increased in human
gastric cancer tissues
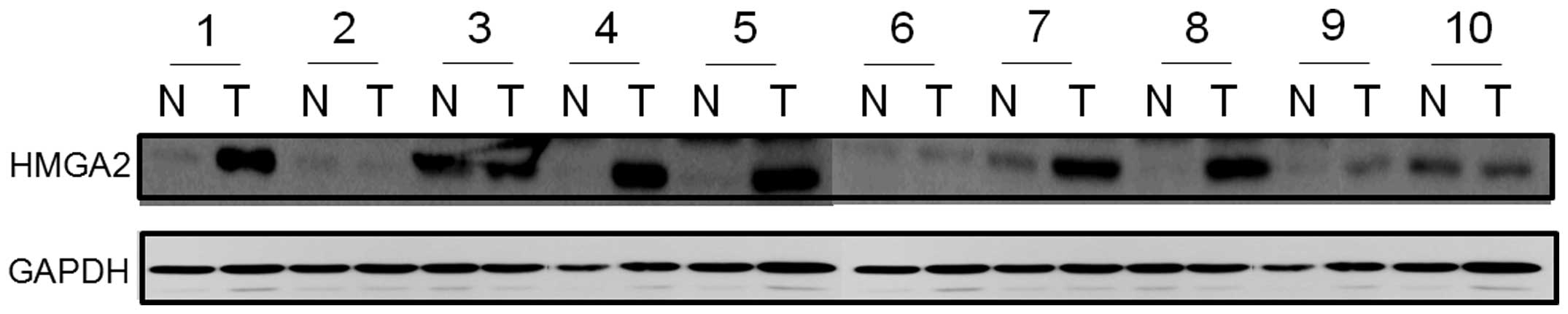
HMGA2 protein expression was evaluated by western
blotting in 10 paired human gastric cancer tissues and
corresponding normal gastric mucosa. In six of the cancer tissues,
HMGA2 protein levels were higher than in normal tissues whereas in
the remaining four samples the expression levels were similar
(Fig. 1). Similarly, HMGA2 mRNA
expression levels, examined in the paired human gastric cancer
tissue and corresponding normal tissue of 76 patients using
real-time PCR, were significantly higher in malignant than in
normal tissues (P<0.001) (Fig.
2).
Correlation between HMGA2 expression and
clinicopathological features in gastric cancer patients
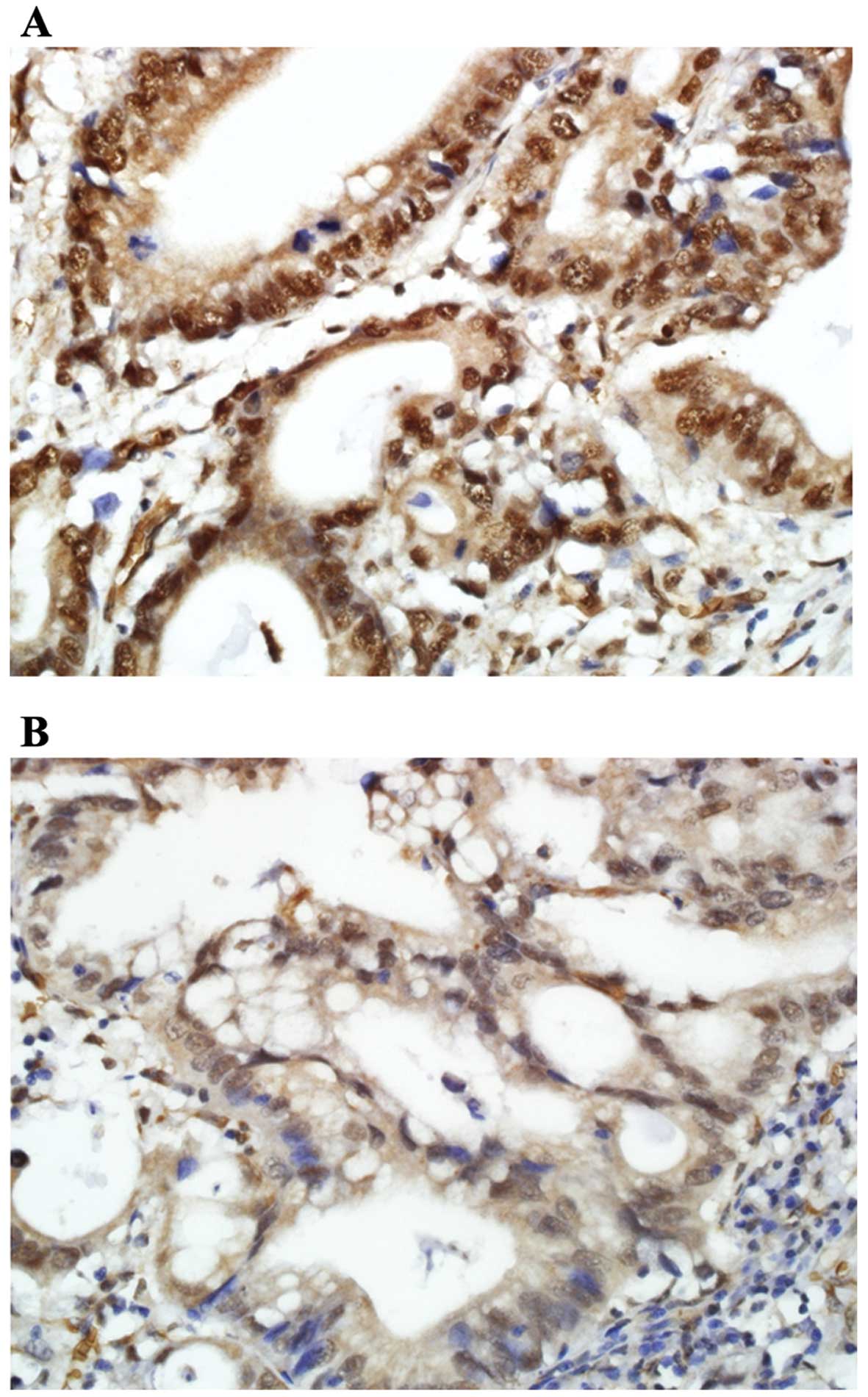
Immunohistochemical staining revealed HMGA2
expression mainly in the nucleus and only partly in the cytoplasm
of tumor cells (Fig. 3). Among 170
gastric cancer patients, 39 (22.9%) had high expression of HMGA2 in
their tumor tissues. HMGA2 expression in gastric cancer was
significantly correlated with the depth of invasion (P=0.015) and
lymph node metastasis (P=0.047). In addition, a significant
association was determined between pathological stage, evaluated
according to the seventh AJCC system, and HMGA2 expression
(P=0.003) (Table III).
 | Table IIICorrelation between HMGA2 expression
and clinicopathological characteristics. |
Table III
Correlation between HMGA2 expression
and clinicopathological characteristics.
| HMGA2
expression | |
|---|
|
| |
|---|
| Variable | Positive n=39 | Negative n=131 | P-value |
|---|
| Age, years (mean ±
SD) | 59.3±10.6 | 59.3±11.6 | 0.967 |
| Gender, n (%) | | | 0.467 |
| Male | 30 (24.4) | 93 (75.6) | |
| Female | 9 (19.1) | 38 (80.9) | |
| Size, cm (mean ±
SD) | 5.48±2.7 | 5.18±2.7 | 0.546 |
| Histological type,
n (%) | | | 0.499 |
|
Differentiated | 10 (19.6) | 41 (80.4) | |
|
Undifferentiated | 29 (24.4) | 90 (75.6) | |
| Lauren
classification, n (%) | | | 0.232 |
| Intestinal
type | 12 (20.7) | 46 (79.3) | |
| Diffuse type | 24 (27.6) | 63 (72.4) | |
| Mixed type | 3 (12.0) | 22 (88.0) | |
| Lymphatic invasion,
n (%) | | | 0.083 |
| Present | 35 (25.7) | 101 (74.3) | |
| Absent | 4 (11.8) | 30 (88.2) | |
| Vascular invasion,
n (%) | | | 0.773 |
| Present | 5 (26.3) | 14 (73.7) | |
| Absent | 34 (22.5) | 117 (77.5) | |
| Perineural
invasion, n (%) | | | 0.073 |
| Present | 26 (28.3) | 66 (71.7) | |
| Absent | 13 (16.7) | 65 (83.3) | |
| Depth of invasion,
n (%) | | | 0.015 |
| T1 | 1 (33.3) | 2 (66.7) | |
| T2 | 2 (6.3) | 30 (93.8) | |
| T3 | 1 (7.7) | 12 (92.3) | |
| T4 | 35 (28.7) | 87 (71.3) | |
| Lymph node
metastasis, n (%) | | | 0.047 |
| N0 | 11 (16.9) | 54 (83.1) | |
| N1 | 9 (32.1) | 19 (67.9) | |
| N2 | 4 (12.1) | 29 (87.9) | |
| N3 | 15 (34.1) | 29 (65.9) | |
| Pathological stage,
n (%) | | | 0.003 |
| I | 1 (4.3) | 22 (95.7) | |
| II | 9 (17.6) | 42 (82.4) | |
| III | 27 (28.7) | 67 (71.3) | |
| IV | 2 (100.0) | 0 (0.0) | |
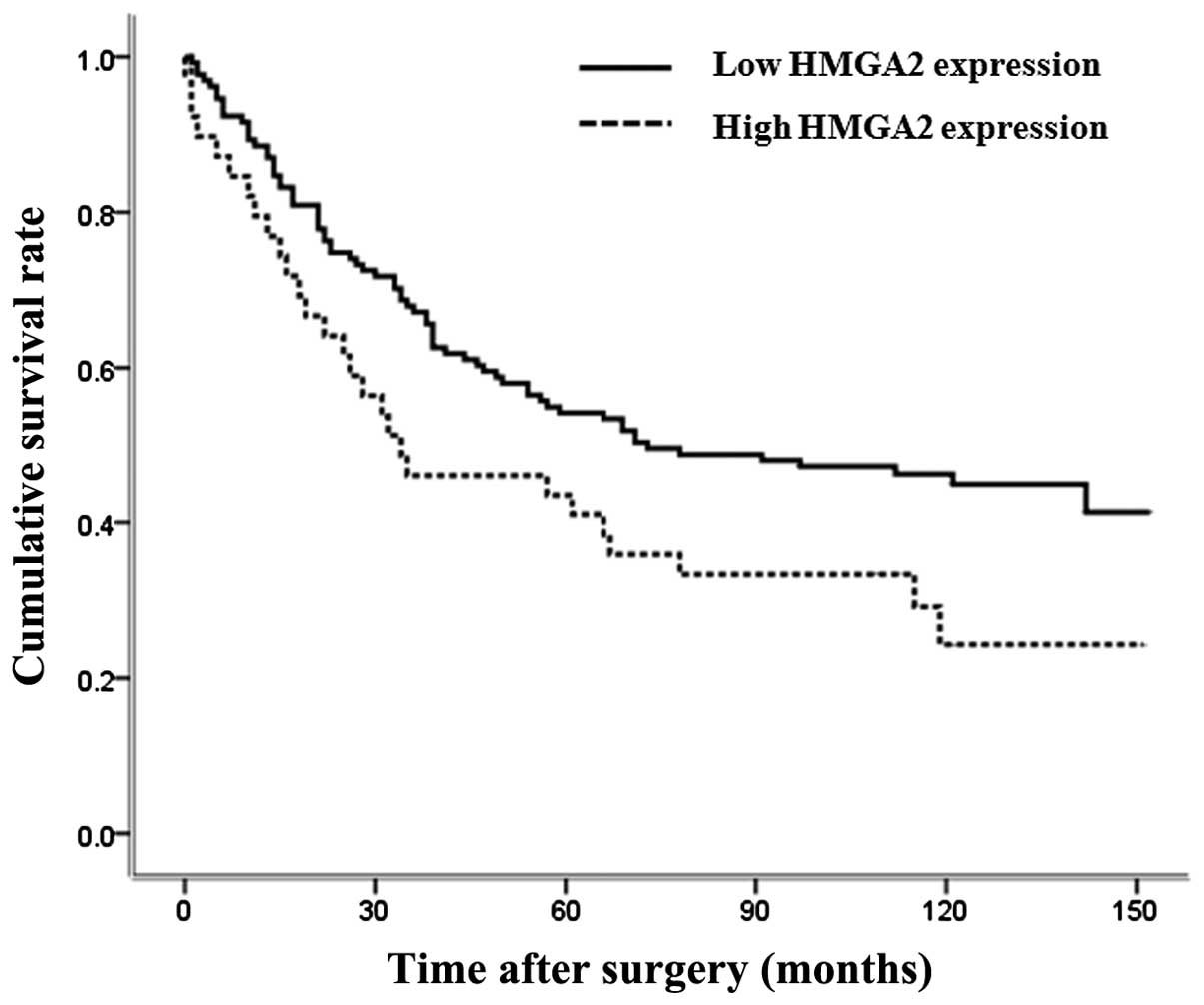
Patients with high HMGA2 expression had a
significantly worse overall survival outcome than those with low
expression of the protein (P=0.028, Fig. 4). However, in multivariate survival
analysis using a Cox proportional hazards regression model, HMGA2
expression was not an independent prognostic factor for gastric
cancer.
HMGA2 knockdown in gastric cancer cells
by short-hairpin RNA (shRNA)
Based on a literature search, MKN28 was selected as
the HMGA2-expressing gastric cancer cell line to investigate the
functional role of HMGA2 in gastric cancer. In these cells,
knockdown of HMGA2 was achieved using shRNA. The expression levels
of HMGA2 in the knockdown cells were measured by QPCR and western
blotting. These methods confirmed HMGA2 knockdown by shRNA at the
mRNA and protein levels, respectively, in MKN28 cells (Fig. 5).
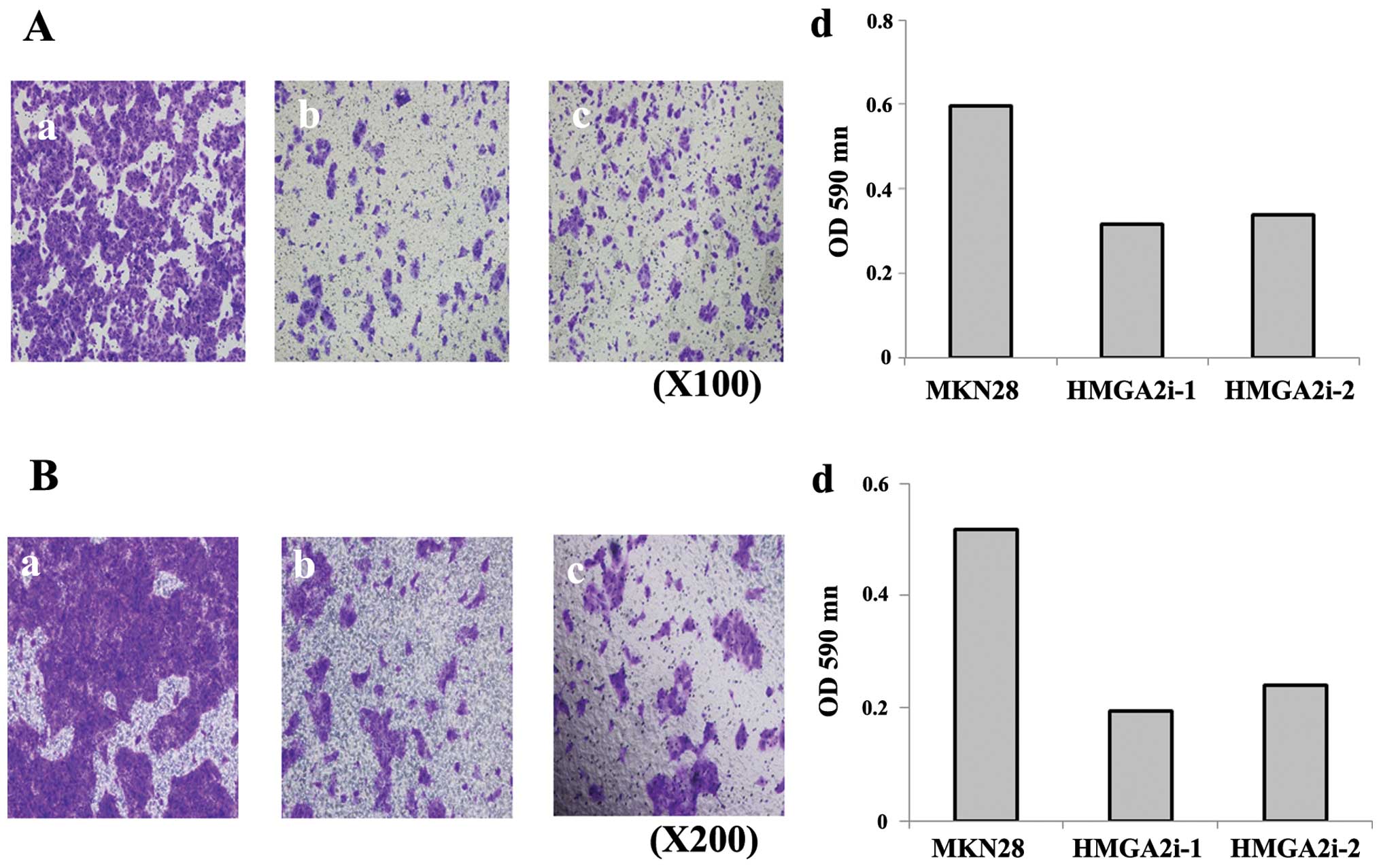
Migration and invasion of gastric cancer
cells are inhibited by HMGA2 knockdown
To assess the effect of HMGA2 expression on cancer
cell migration and invasion, in vitro migration and invasion
assays were conducted with the HMGA2 knockdown cells. Expression of
either HMGA2i-1 or HMGA2i-2 inhibited MKN28 cell migration
(Fig. 6a) and invasion (Fig. 6b).
HMGA2 knockdown represses EMT
Inhibition of cell migration and invasion in MKN28
HMGA2 knockdown cells suggested that HMGA2 participates in EMT and
thus in tumor metastasis. To confirm the relevance of HMGA2 in EMT,
the expression of established EMT-related proteins in HMGA2
knockdown MKN28 cells was evaluated by western blotting. The
results showed increased expression of the epithelial marker
E-cadherin and decreased expression of the mesenchymal marker
N-cadherin. In addition, HMGA2 knockdown induced downregulation of
Snail and Zeb1, two transcriptional markers of EMT, and of
β-catenin, a key molecule in the Wnt/β-catenin signaling pathway,
which is known to be involved in this metastasis-related process
(Fig. 7).
Discussion
In the metastasis of a primary tumor, and thus in
cancer-related death, EMT is regarded as a key event. Signaling
molecules, such as smad and β-catenin, and transcriptional factors,
such as Zeb and Snail, have been shown to participate in EMT
(14,15), but the mechanism of their
involvement has yet to be fully explained. HMGA2 is among the newly
identified factors involved in the EMT of malignancies of
epithelial origin. HMGA2 is overexpressed in many epithelial-type
malignancies, such as breast cancer (16), lung cancer (17), oral squamous cell carcinoma
(18), and pancreatic carcinoma
(19). In addition, overexpression
is a predictor of poor prognosis in patients with lung cancer
(20), oral squamous cell
carcinoma (18), ovarian cancer
(21), metastatic breast cancer
(22), and colorectal cancer
(23). In gastric cancer, there is
one previous study suggesting that HMGA2 overexpression might be
related to the patient prognosis (24). However, the mechanism of the
relationship between poor prognosis and HMGA2 overexpression was
not ascertained. In another study, although a mechanism for the
association between HMGA2 and EMT in gastric cancer was suggested,
the clinical role and prognostic value of HMGA2 were poorly
explained (12). Thus, the present
study was designed to determine the clinical impact of HMGA2
expression and its correlation with EMT in gastric cancer.
Our results showed that HMGA2 was overexpressed in
gastric cancer tissue compared to normal epithelium. As determined
by western blotting, the expression of HMGA2 protein in gastric
cancer tissues was much higher than in corresponding normal gastric
mucosa. This finding was supported by the 5-fold increase in HMGA2
mRNA in gastric cancer tissues compared to normal tissues and
together with the protein data suggested an association between
HMGA2 expression and clinical outcome in gastric cancer. Indeed,
the high-level overexpression of HMGA2 was related to a poor
prognosis and significantly lower overall survival rates. These
findings are consistent with those presented in a previous study
(24) and suggested that HMGA2
levels are a significant prognostic factor for poor clinical
outcome in gastric cancer.
Several studies have demonstrated a crucial role for
EMT in the progression of gastric cancer (25–27).
In other epithelial malignancies, HMGA2 was shown to participate in
tumor metastasis and disease progression by inducing EMT (28–30),
but only one study found evidence of the involvement of HMGA2 in
the EMT of gastric cancer (12).
Therefore, in the present work we investigated the relationship
between HMGA2 and EMT by examining the expression patterns of
molecules known to be involved in EMT, including E- and N-cadherin
(13,14) and several other transcriptional
factors and signal molecules (31–33).
The downregulation of E-cadherin and the upregulation of N-cadherin
are considered as hallmarks of EMT (27), with a loss of E-cadherin associated
with a poor prognosis in gastric cancer (25). To further elucidate the role of
HMGA2 during EMT, we conducted an in vitro study in which
MKN28 cells expressing HMGA2 were transfected with two different
shRNAs, resulting in two knockdown models, and the levels of
several EMT-related factors were then determined.
The transcriptional factor Zeb1 induces EMT by
repressing the expression of E-cadherin, via binding to its E-box.
Snail, another transcriptional regulator, also represses E-cadherin
transcription directly during EMT (14,15).
The present study was based on the hypothesis that the expression
of EMT-related factors is altered following the knockdown of HMGA2.
As expected, the knockdown of HMGA2 increased the expression of
E-cadherin and repressed the expression of N-cadherin, Zeb1, and
Snail. Furthermore, in Transwell migration and invasion assays, the
expression of HMGA2 significantly influenced the degree of tumor
cell migration and invasion, two properties of oncogenesis related
to EMT. Specifically, compared with the control group, the number
of migrating and invading MKN28 cells was significantly higher in
the absence of HMGA2 knockdown. These results provide strong
evidence for a correlation between HMGA2 overexpression and EMT. In
addition, they support the ability of HMGA2 to promote the
metastatic properties of tumor cells and thus, at least in part,
explain the poor prognosis of gastric cancer patients with
high-level HMGA2 expression.
Additional evidence for a role of HMGA2 in EMT is
the nuclear expression of β-catenin. In EMT, β-catenin has been
described as the ultimate downregulator of E-cadherin, via the
upregulation of Snail (34). To
determine whether HMGA2 affects the nuclear expression of
β-catenin, we compared control cells with HMGA2 knockdown cells.
The results showed significantly lower β-catenin expressions in the
two types of knockdown cells than in control cells and the
concurrent repression of Snail expression. A possible mechanism
underlying this relationship is that HMGA2 overexpression increases
the nuclear expression of β-catenin, which leads to an increase in
Snail expression and the subsequent repression of E-cadherin. This
would explain the stimulation by HMGA2 overexpression of EMT in
gastric cancer.
To our knowledge, this is the first study to clarify
both the clinical impact of HMGA2 overexpression; i.e., as an
indicator of a poor prognosis in gastric cancer, and the molecular
mechanism of the association between HMGA2 expression and EMT in
these tumors. Thus, in gastric cancer patients with HMGA2
overexpression, an aggressive treatment strategy should be
considered. The development of therapies that interfere with HMGA2
expression, thereby reducing its pro-metastatic function via EMT,
would initiate a new era of treatment of gastric cancer.
Acknowledgements
This study was supported by a grant from the
National Research Foundation of Korea (no. 2012R1A1A1043576) and
the Catholic Medical Center Research Foundation, awarded in the
2012 program year.
References
|
1
|
Fidler IJ: Critical determinants of
metastasis. Semin Cancer Biol. 12:89–96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Steeg PS: Tumor metastasis: Mechanistic
insights and clinical challenges. Nat Med. 12:895–904. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yoon JH, Choi WS, Kim O and Park WS: The
role of gastrokine 1 in gastric cancer. J Gastric Cancer.
14:147–155. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reeves R and Nissen MS: The
A.T-DNA-binding domain of mammalian high mobility group I
chromosomal proteins. A novel peptide motif for recognizing DNA
structure. J Biol Chem. 265:8573–8582. 1990.PubMed/NCBI
|
|
5
|
Sgarra R, Rustighi A, Tessari MA, Di
Bernardo J, Altamura S, Fusco A, Manfioletti G and Giancotti V:
Nuclear phospho-proteins HMGA and their relationship with chromatin
structure and cancer. FEBS Lett. 574:1–8. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reeves R: Structure and function of the
HMGI(Y) family of architectural transcription factors. Environ
Health Perspect. 108(Suppl 5): 803–809. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rogalla P, Drechsler K, Frey G, Hennig Y,
Helmke B, Bonk U and Bullerdiek J: HMGI-C expression patterns in
human tissues. Implications for the genesis of frequent mesenchymal
tumors. Am J Pathol. 149:775–779. 1996.PubMed/NCBI
|
|
8
|
Chiappetta G, Avantaggiato V, Visconti R,
Fedele M, Battista S, Trapasso F, Merciai BM, Fidanza V, Giancotti
V, Santoro M, et al: High level expression of the HMGI (Y) gene
during embryonic development. Oncogene. 13:2439–2446.
1996.PubMed/NCBI
|
|
9
|
Thuault S, Valcourt U, Petersen M,
Manfioletti G, Heldin CH and Moustakas A: Transforming growth
factor-beta employs HMGA2 to elicit epithelial-mesenchymal
transition. J Cell Biol. 174:175–183. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Watanabe S, Ueda Y, Akaboshi S, Hino Y,
Sekita Y and Nakao M: HMGA2 maintains oncogenic RAS-induced
epithelial-mesen-chymal transition in human pancreatic cancer
cells. Am J Pathol. 174:854–868. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wei CH, Wei LX, Lai MY, Chen JZ and Mo XJ:
Effect of silencing of high mobility group A2 gene on gastric
cancer MKN-45 cells. World J Gastroenterol. 19:1239–1246. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zha L, Zhang J, Tang W, Zhang N, He M, Guo
Y and Wang Z: HMGA2 elicits EMT by activating the Wnt/β-catenin
pathway in gastric cancer. Dig Dis Sci. 58:724–733. 2013.
View Article : Google Scholar
|
|
13
|
Joo YE, Chung IJ, Park YK, Koh YS, Lee JH,
Park CH, Lee WS, Kim HS, Choi SK, Rew JS, et al: Expression of
cyclooxygenase-2, p53 and Ki-67 in gastric cancer. J Korean Med
Sci. 21:871–876. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jing Y, Han Z, Zhang S, Liu Y and Wei L:
Epithelial-mesenchymal transition in tumor microenvironment. Cell
Biosci. 1:292011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rogalla P, Drechsler K, Kazmierczak B,
Rippe V, Bonk U and Bullerdiek J: Expression of HMGI-C, a member of
the high mobility group protein family, in a subset of breast
cancers: Relationship to histologic grade. Mol Carcinog.
19:153–156. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Meyer B, Loeschke S, Schultze A, Weigel T,
Sandkamp M, Goldmann T, Vollmer E and Bullerdiek J: HMGA2
overexpression in non-small cell lung cancer. Mol Carcinog.
46:503–511. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miyazawa J, Mitoro A, Kawashiri S, Chada
KK and Imai K: Expression of mesenchyme-specific gene HMGA2 in
squamous cell carcinomas of the oral cavity. Cancer Res.
64:2024–2029. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abe N, Watanabe T, Suzuki Y, Matsumoto N,
Masaki T, Mori T, Sugiyama M, Chiappetta G, Fusco A and Atomi Y: An
increased high-mobility group A2 expression level is associated
with malignant phenotype in pancreatic exocrine tissue. Br J
Cancer. 89:2104–2109. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sarhadi VK, Wikman H, Salmenkivi K, Kuosma
E, Sioris T, Salo J, Karjalainen A, Knuutila S and Anttila S:
Increased expression of high mobility group A proteins in lung
cancer. J Pathol. 209:206–212. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shell S, Park SM, Radjabi AR, Schickel R,
Kistner EO, Jewell DA, Feig C, Lengyel E and Peter ME: Let-7
expression defines two differentiation stages of cancer. Proc Natl
Acad Sci USA. 104:11400–11405. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Langelotz C, Schmid P, Jakob C, Heider U,
Wernecke KD, Possinger K and Sezer O: Expression of
high-mobility-group-protein HMGI-C mRNA in the peripheral blood is
an independent poor prognostic indicator for survival in metastatic
breast cancer. Br J Cancer. 88:1406–1410. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang X, Liu X, Li AY, Chen L, Lai L, Lin
HH, Hu S, Yao L, Peng J, Loera S, et al: Overexpression of HMGA2
promotes metastasis and impacts survival of colorectal cancers.
Clin Cancer Res. 17:2570–2580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Motoyama K, Inoue H, Nakamura Y, Uetake H,
Sugihara K and Mori M: Clinical significance of high mobility group
A2 in human gastric cancer and its relationship to let-7 microRNA
family. Clin Cancer Res. 14:2334–2340. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Murai T, Yamada S, Fuchs BC, Fujii T,
Nakayama G, Sugimoto H, Koike M, Fujiwara M, Tanabe KK and Kodera
Y: Epithelial-to-mesenchymal transition predicts prognosis in
clinical gastric cancer. J Surg Oncol. 109:684–689. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ryu HS, Park J, Kim HH, Kim WH and Lee HS:
Combination of epithelial-mesenchymal transition and cancer stem
cell-like phenotypes has independent prognostic value in gastric
cancer. Hum Pathol. 43:520–528. 2012. View Article : Google Scholar
|
|
27
|
Peng Z, Wang CX, Fang EH, Wang GB and Tong
Q: Role of epithelial-mesenchymal transition in gastric cancer
initiation and progression. World J Gastroenterol. 20:5403–5410.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu J, Liu Z, Shao C, Gong Y, Hernando E,
Lee P, Narita M, Muller W, Liu J and Wei JJ: HMGA2
overexpression-induced ovarian surface epithelial transformation is
mediated through regulation of EMT genes. Cancer Res. 71:349–359.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Morishita A, Zaidi MR, Mitoro A,
Sankarasharma D, Szabolcs M, Okada Y, D’Armiento J and Chada K:
HMGA2 is a driver of tumor metastasis. Cancer Res. 73:4289–4299.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu X, Lai L, Wang X, Xue L, Leora S, Wu
J, Hu S, Zhang K, Kuo ML, Zhou L, et al: Ribonucleotide reductase
small subunit M2B prognoses better survival in colorectal cancer.
Cancer Res. 71:3202–3213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sánchez-Tilló E, Liu Y, de Barrios O,
Siles L, Fanlo L, Cuatrecasas M, Darling DS, Dean DC, Castells A
and Postigo A: EMT-activating transcription factors in cancer:
Beyond EMT and tumor invasiveness. Cell Mol Life Sci. 69:3429–3456.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rosivatz E, Becker I, Specht K, Fricke E,
Luber B, Busch R, Höfler H and Becker KF: Differential expression
of the epithelial-mesenchymal transition regulators snail, SIP1,
and twist in gastric cancer. Am J Pathol. 161:1881–1891. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Castro Alves C, Rosivatz E, Schott C,
Hollweck R, Becker I, Sarbia M, Carneiro F and Becker KF: Slug is
overexpressed in gastric carcinomas and may act synergistically
with SIP1 and Snail in the down-regulation of E-cadherin. J Pathol.
211:507–515. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stemmer V, de Craene B, Berx G and Behrens
J: Snail promotes Wnt target gene expression and interacts with
beta-catenin. Oncogene. 27:5075–5080. 2008. View Article : Google Scholar : PubMed/NCBI
|