Introduction
Desmopressin (1-deamino-8-D-arginine vasopressin or
dDAVP) is a synthetic peptide derivative of the antidiuretic
hormone used to boost the levels of clotting factors in certain
haemostatic disorders (1). DDAVP
differs from the natural peptide by deamination of cystein in
position 1, which prolongs its half-life, and substitution of
L-arginine by D-arginine in position 8, which reduces the pressor
effect and confers selectivity for the vasopressin type 2 membrane
receptor (V2r) (2). This receptor
subtype is present in kidney collecting ducts and endothelium
(3,4). By acting on endothelial cells dDAVP
induces a strong haemostatic effect causing the release of
coagulation factor VIII, von Willebrand factor (VWF) and
plasminogen activators from microvascular stores into the
bloodstream (5). V2r expression
was also reported in transformed epithelial cells and several human
tumour cell lines, including breast cancer (6,7). V2r
stimulation in breast carcinoma is associated with
antiproliferative signalling, involving activation of adenylate
cyclase followed by intracellular cAMP elevation (8).
Preclinical studies in mice showed that intravenous
administration of dDAVP inhibited experimental lung metastases in a
dose-dependent manner (9,10) and dramatically decreased
locoregional and distant spread in a model of surgical manipulation
of aggressive breast tumours (11). Hermo et al confirmed the
beneficial effect of perioperative dDAVP on survival in dogs with
advanced mammary cancer (12,13).
As mentioned above, dDAVP drastically increases circulating levels
of VWF by acting on V2r in endothelial cells. Terraube and
collaborators showed that VWF plays a protective role against
cancer cell dissemination and absence of VWF leads to increased
metastatic potential (14).
Additionally, our group reported that dDAVP inhibited the early
angiogenic response and markedly decreased vascularisation of
growing subcutaneous tumours (15). Experimental evidence suggested that
dDAVP reduces angiogenesis by inducing the formation of
angiostatin, a potent inhibitor of angiogenesis that is generated
by cancer-mediated proteolysis of plasminogen (16,17).
Thus, dDAVP seems to produce a dual antimetastatic and
anti-angiogenic effect, breaking the cooperative interplay of
tumour and endothelial cells during disease progression (18). Taken together, dDAVP appears as a
promising lead compound for the development of novel peptide
analogues with enhanced anticancer efficacy. With this purpose,
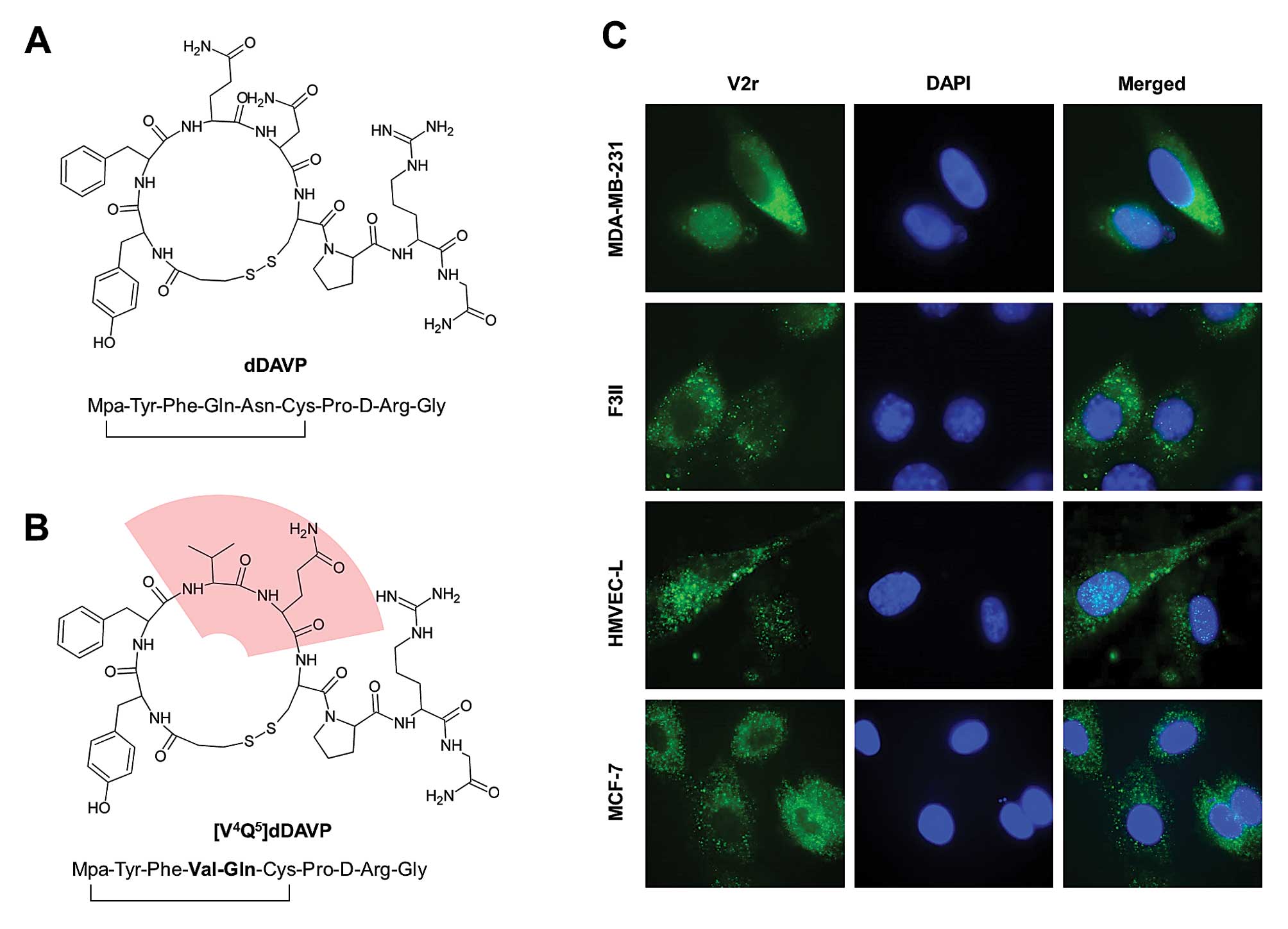
dDAVP (Fig. 1A) was rationally
modified, and the novel analogue [V4Q5]dDAVP
(1-deamino-4-valine-5-glutamine-8-D-arginine vasopressin) (Fig. 1B) was synthesized and assayed.
Amino acid positions 4 and 5 belong to the conformational peptide
loop which has a key role in ligand-receptor interaction and
antitumour activity (5,19–22).
In an initial evaluation, [V4Q5]dDAVP
exhibited a significantly higher cytostatic effect against breast
cancer cells than the parental compound dDAVP and compared to other
screened peptide derivatives (21). In the present study, we further
characterized the anticancer activity of the novel analogue
[V4Q5]dDAVP on V2r-expressing breast cancer
preclinical models. The effect of the compound on xenograft tumour
growth and angiogenesis was assessed. Additionally, we determined
the efficacy of the novel analogue on metastatic progression in
immunocompetent hosts.
Materials and methods
Cell lines and culture conditions
Human breast carcinoma cell lines MDA-MB-231 (ATCC
HTB-26) and MCF-7 (ATCC HTB-22) were obtained from the American
Type Culture Collection. MDA-MB-231 is a triple-negative breast
cancer (TNBC) cell line which lacks the oestrogen receptor (ER) and
progesterone receptor (PR), and expresses low levels of human
epidermal growth factor receptor 2 (HER2)/neu. It also belongs to
the claudin-low molecular subtype. MCF-7 is a
ER-positive/PR-positive luminal mammary carcinoma (23). The F3II mammary carcinoma cell line
is a highly invasive and metastatic variant derived from a clone of
a spontaneous BALB/c mouse mammary tumour. It is a
hormone-independent tumour cell line and express low levels of
HER2/neu. Tumour cells were grown in Dulbecco’s modified Eagle’s
medium (DMEM, Gibco, Rockville, MD, USA) plus 10% fetal bovine
serum (FBS), 2 mM glutamine and 80 μg/ml gentamycin in monolayer
culture, at 37°C in a humidified atmosphere of 5% CO2.
HMVEC-L human microvascular endothelial cell line was obtained from
Cascade Biologics and cultured in gelatin coated plates using
endothelial cell medium with specific growth factors (EGM-2 MV
Bullet Kit, Lonza, Milan, Italy). All cells were harvested using a
tripsin/EDTA solution (Gibco) diluted in phosphate-buffered saline
(PBS).
Immunofluorescence detection of V2r
Briefly, cells were seeded on glass coverslips, and
fixed with paraformaldehyde. After incubation with blocking agent,
cells were incubated with a goat polyclonal anti-V2r antibody for 1
h at 37°C (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Receptor-bound antibodies were detected with a secondary rabbit
polyclonal FITC-conjugated antibody (Chemicon International,
Temecula, CA, USA) and nuclei were labeled with DAPI (Vector
Laboratories, Peterborough, UK). Samples were examined using a
TE-2000 microscope (Nikon Inc., Tokyo, Japan). MCF-7 cells were
used as a positive control of V2r expression (6).
Peptide compounds and dosing
DDAVP and [V4Q5]dDAVP were
synthesized in the solid phase, using Nα-Fmoc protection and the
tea-bag strategy (21). Peptides
were purified by reversed-phase high-performance liquid
chromatography and quantified using a commercial dDAVP reference
standard (BCN Peptides, Barcelona, Spain).
Compounds were injected intravenously at 0.3 μg/kg,
this being a clinically relevant dose of dDAVP with widely
acknowledged haemostatic effects in humans (4), as well as antitumour properties in
mice (10). Since it is known that
peptides such as dDAVP can induce tachyphylaxis with daily
applications (3), compounds were
administered on a thrice-weekly basis when treatment lasted for
>5 days. In vitro experiments were performed using
nanomolar and low micromolar concentrations of the peptides, a
range consistent with the in vivo dosage (9,24).
Cell proliferation assay
Antiproliferative effect against rapidly growing
tumour cells was measured using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay (Sigma-Aldrich, St. Louis, MO, USA). Briefly, cells were
plated in 96-well flat bottom plates at a density of
2.5×103 per 200 μl in complete DMEM, allowed to attach
overnight, and then treated with dDAVP or
[V4Q5]dDAVP (100–1,500 nM) or vehicle for 72
h. Blockade of agonistic effect was achieved by incubation with the
selective and competitive V2r antagonist tolvaptan (Otsuka
Pharmaceutical Co., Tokyo, Japan). MTT reagent was added to each
well and the plate incubated for 4 h. After solubilisation using
dimethyl sulfoxide the absorbance of each well was measured at 570
nm. The optical density of untreated control cells was taken as
100% viability.
cAMP quantification
Briefly, carcinoma cells were stimulated with
[V4Q5]dDAVP (1,000 nM) or saline vehicle for
1 h and intracellular cAMP levels were measured using the Cyclic
AMP EIA kit (ACE, Cayman Chemical Co., Ann Arbor, MI, USA)
according to the manufacturer’s instructions.
PKA activity
The effect of [V4Q5]dDAVP on
PKA activity of MCF-7 total cell extract was determined by
measuring the incorporation of [32P] orthophosphate from
[32P]γ-ATP into PKA substrate histone H1. We tested the
activity of the enzyme present in cells incubated for 30 min or 1 h
with or without [V4Q5]dDAVP (1,000 nM) or
8-Br-cAMP (500 μM). 8-Br-cAMP is a membrane-permeable analogue of
cAMP and was used as a positive control of PKA activation. After
treatment, cell cultures were washed with PBS, scraped into a
buffer containing 25 mM Tris-HCl, pH 7.4, 0.5 mM EDTA, 0.5 mM EGTA,
1 μg/ml leupeptin and 1 μg/ml aprotinine, and homogenized with a
Pellet pestle motor homogenizer. This material was used for PKA
activity determinations. Histone H1 (0.5 μg/μl) was incubated with
35 μg of cellular protein in a reaction mixture consisting of 50 mM
Tris-HCl, 10 mM MgCl2, 100 μM ATP and 20 μCi of
[32P]-ATP (200 μl of total volume). After 30 min of
incubation, proteins were precipitated with 10% trichloroacetic
acid, after adding bovine serum albumin (0.3 μg/μl), and then were
centrifuged at 5,000 g for 10 min. Precipitated proteins were
spotted onto p81 Whatman paper, washed in 75 mM orthophosphoric
acid and dried before counting the radioactivity in a β counter.
Results are expressed by rate of PKA activity with or without cAMP
(1 μM).
Clonogenic assay
Cytostatic effects of
[V4Q5]dDAVP were also examined by the colony
formation assay (22). MDA-MB-231
cells were grown in complete medium with dDAVP or
[V4Q5]dDAVP (100–1,500 nM). Complete medium
with tested peptides was renewed after 72 h. Seven days after cell
seeding, colonies of >50 cells were counted. The concentration
producing 50% inhibition (IC50) was determined by
plotting a linear regression curve.
Determination of cell cycle
Cell cycle was evaluated by flow cytometry (25). After 48 h of starvation, MDA-MB-231
cells were treated for 24 h with dDAVP or
[V4Q5]dDAVP in complete medium and collected
by trypsinization. Cells were then fixed in 70% chilled methanol
for 30 min, treated with 1 μg/ml RNase A (Sigma-Aldrich) and
stained with 100 μg/ml propidium iodide. Cell cycle phase
distribution of nuclear DNA was carried out in a FACSCalibur
cytometer using WinMDI 2.9 software.
Endothelial cell morphogenesis assay
In vitro endothelial cell morphogenesis assay
was performed using Matrigel-coated 24-well plates (BD Biosciences,
San Jose, CA, USA) (15). Briefly,
1×105 HMVEC-L cells were incubated with dDAVP or
[V4Q5]dDAVP at a concentration of 100 nM.
After allowing capillary tube formation for 24 h, randomly chosen
fields were photographed at magnification ×100, and quantification
was conducted. The number of capillary-like tubes formed in control
cultures was taken as 100%.
Mice
Specific pathogen-free 8-week-old female BALB/c and
athymic BALB/c (nu/nu) mice were purchased from UNLP (Universidad
Nacional de La Plata, Buenos Aires, Argentina), and kept 5–8 mice
per cage in our animal house facility at the National University of
Quilmes. Food and water was provided ad libitum and general
health status of the animals was monitored daily. All protocols
were approved by the National University of Quilmes institutional
Animal Care Committee.
In vivo angiogenesis assays
To evaluate effects on MDA-MB-231-induced
angiogenesis, a modified Matrigel plug assay was conducted. A
mixture containing 500 μl of Matrigel, heparin (50 U/ml) and
4.5×106 tumour cells was injected subcutaneously into
BALB/c athymic mice. Treatment consisted of three weekly
intravenous doses of dDAVP or [V4Q5]dDAVP
(0.3 μg/kg).
Animals were sacrificed 14 days after cell
injection. Plugs were recovered and scanned at high resolution. The
extent of vascularisation was assessed by the amount of haemoglobin
detected in the implants using the Drabkin method (Sigma-Aldrich).
The mean optical density of plugs from control group was taken as 1
(relative haemoglobin content).
An intradermal angiogenesis assay was performed to
test [V4Q5]dDAVP effect on early F3II
tumour-induced vascularisation (15). F3II cells (2×105) per
site were inoculated in the flank of BALB/c mice in a DMEM and
trypan blue solution. After 5 days, animals were sacrificed and
skins were photographed. The vascular network around the tumour
cell implant was quantified using a millimeter grid. Daily
intravenous doses of dDAVP or [V4Q5]dDAVP
(0.3 μg/kg) were administered throughout the experiment.
Tumour progression
To generate breast cancer xenografts, a 300-μl
suspension containing 5×106 MDA-MB-231 cells in DMEM and
Matrigel (1:1 volume ratio) was injected subcutaneously in BALB/c
athymic mice. Tumours were measured periodically with a caliper and
tumour volume was calculated by the formula: 0.52 ×
width2 × length. Treatment started 14 days after
MDA-MB-231 cell inoculation, when tumours reached volumes of ~50
mm3. DDAVP or [V4Q5]dDAVP (0.3
μg/kg) were administered intravenously thrice weekly. When the
control group (saline vehicle) reached a mean tumour volume of 200
mm3 and exhibited signs of ulceration and necrosis,
animals were photographed and tumours from different experimental
groups were removed, fixed with formalin and routinely processed
for haematoxylin and eosin (H&E) staining in order to examine
tumour histology and vascularisation. The effect of
[V4Q5]dDAVP on survival was also evaluated.
Animals in saline vehicle or [V4Q5]dDAVP
treated groups were euthanized by cervical dislocation when the
humane tumour burden limits (>1,000 mm3) were reached
(26). To generate tumours in
immunocompetent hosts, 2×105 F3II cells were injected
subcutaneously in syngeneic BALB/c mice. Treatment started 7 days
later, when tumours reached volumes of ~50 mm3. DDAVP or
[V4Q5]dDAVP were administered as mentioned
above. On day 50, F3II tumour-bearing animals were sacrificed and
necropsied. To investigate the presence of spontaneous metastases,
lungs were removed, fixed in Bouin’s solution and macroscopic lung
nodules were counted under a dissecting microscope.
Experimental lung metastases assay
To evaluate [V4Q5]dDAVP effect
on blood-borne metastases, 2×105 F3II cells in DMEM were
injected into the tail vein of mice (9). On day 21, lungs were excised,
weighted, fixed in Bouin’s solution, photographed and lung nodules
were counted. DDAVP or [V4Q5]dDAVP were
administered at 0.3 μg/kg in two intravenous doses, the first at
time zero and the second 24 h after cell injection.
Toxicology studies
Acute toxicology studies were conducted at the
National University of Litoral (Argentina). All procedures were
approved by the Institutional Ethics and Security Committee and are
consistent with the Guide for the Care and Use of Laboratory
Animals (NRC 2011). Groups of 5 Wistar female rats received single
intravenous doses of 1, 10 or 100 μg/kg of
[V4Q5]dDAVP or dDAVP. A full clinical
evaluation, including heart and respiratory rates, nervous system,
motor activity, biochemical and haematological studies, was
conducted at 1, 3, 6, 12, 24 and 72 h after drug administration.
Body weight, food and water intake were monitored daily.
Statistical analysis
PRISM 6, Version 6.01 (GraphPad Software Inc., La
Jolla, CA, USA) was used to conduct all statistical analyses
(IC50 values, one-way and two-way ANOVA and Student’s
t-test). Tukey’s multiple comparisons test was used after ANOVA
analysis. In tumour progression protocols, growth rates represent
the slopes of the linear regressions of the tumour volumes over
time. In Kaplan-Meier plots, log-rank test and Cox regression
analysis was applied to establish the association of treatment with
survival. Differences were considered statistically significant at
a level of P<0.05. Data are presented as mean ± SD or SEM.
Results
V2r expression in breast cancer and
microvascular cells
Expression of V2r in MDA-MB-231 and F3II cells was
first confirmed by immunofluorescence (Fig. 1C). MCF-7, a cell line known to
display vasopressin membrane receptors (6), was used as a positive control of V2r
expression. HMVEC-L cells were also positive for the V2r, as
documented previously by reverse transcription-PCR (27).
Cytostatic effects of
[V4Q5]dDAVP on human breast cancer cells
We evaluated the cytostatic effect of the novel
analogue [V4Q5]dDAVP and the parental peptide
dDAVP on log-phase growing breast cancer cells (Fig. 2A). After a 72-h exposure, both
peptides caused a mild reduction of proliferation in MCF-7 cell
cultures (Fig. 2A, top). At
concentrations >500 nM, [V4Q5]dDAVP
treatment showed an enhanced cytostatic effect compared to dDAVP,
reducing cell proliferation by ≤26%. These results are consistent
with a previous study, where several dDAVP peptide analogues,
including [V4Q5] dDAVP, were screened using
MCF-7 cultures (21). Reduction of
tumour cell proliferation by [V4Q5]dDAVP was
associated with an activation of cAMP/PKA signalling axis. An
increase in intracellular cAMP levels (Fig. 2B) and PKA activation (Fig. 2C) of nearly 90 and 40%,
respectively, was observed after 60 min of incubation with
[V4Q5]dDAVP. The cytostatic effect of the
novel analogue was also evaluated in triple-negative MDA-MB-231
cells. The antiproliferative profile of
[V4Q5]dDAVP was similar to the one obtained
against MCF-7 cells (Fig. 2A,
bottom). Growth-modulating activity was completely abolished by the
selective V2r antagonist tolvaptan, indicating that reduction of
cell proliferation mainly results from V2r activation (Fig. 2A, bottom inset).
[V4Q5]dDAVP showed a much stronger effect on
low density breast cancer cell cultures. MDA-MB-231 clonogenic
growth was inhibited by 75% after 7-day treatment with 1,500 nM
[V4Q5]dDAVP. Compared to parental peptide,
novel analogue displayed an enhanced inhibitory effect on colony
formation, with IC50 values of 1,130 and 1,440 nM for
[V4Q5]dDAVP and dDAVP, respectively (Fig. 2D). Cell cycle distribution analysis
showed that a 24-h treatment with [V4Q5]dDAVP
(1,000 nM) resulted in partial arrest of MDA-MB-231 cells in G0/G1
phase (Fig. 2E).
 | Figure 2Effect of
[V4Q5]dDAVP on in vitro growth of
human breast cancer cells. (A) Antiproliferative effect of dDAVP or
[V4Q5]dDAVP on log-phase growing MCF-7 (upper
panel) and MDA-MB-231 (lower panel) human breast carcinoma cells.
Inset for MDA-MB-231, blockade of antiproliferative effect of
[V4Q5]dDAVP (1,500 nM) by the selective and
competitive V2r antagonist tolvaptan (1,500 nM). C, control; T,
tolvaptan; VQ, [V4Q5]dDAVP and VQ+T,
[V4Q5]dDAVP plus tolvaptan. (B) cAMP
concentration in MCF-7 cells after 1 h of
[V4Q5]dDAVP treatment. (C) PKA activity in
MCF-7 cells treated with [V4Q5]dDAVP (1,000
nM) or the membrane-permeable cAMP analogue 8-Br-cAMP (500 nM), an
activator of PKA. (D) Effect of dDAVP or
[V4Q5]dDAVP treatment on clonogenic growth of
MDA-MB-231 cells. Dotted line indicates 50% inhibition. (E)
DNA-cell cycle analysis of MDA-MB-231 cells treated with
[V4Q5]dDAVP (1,000 nM) for 24 h. In all
cases, data are presented as mean ± SEM. Results are representative
of at least three independent experiments. *P<0.05;
**P<0.01; ***P<0.001 versus control.
##P<0.01; ###P<0.001 dDAVP versus
[V4Q5]dDAVP. §§§P<0.001
[V4Q5]dDAVP plus tolvaptan versus
[V4Q5]dDAVP alone. |
[V4Q5]dDAVP
anticancer effects on human breast cancer xenografts
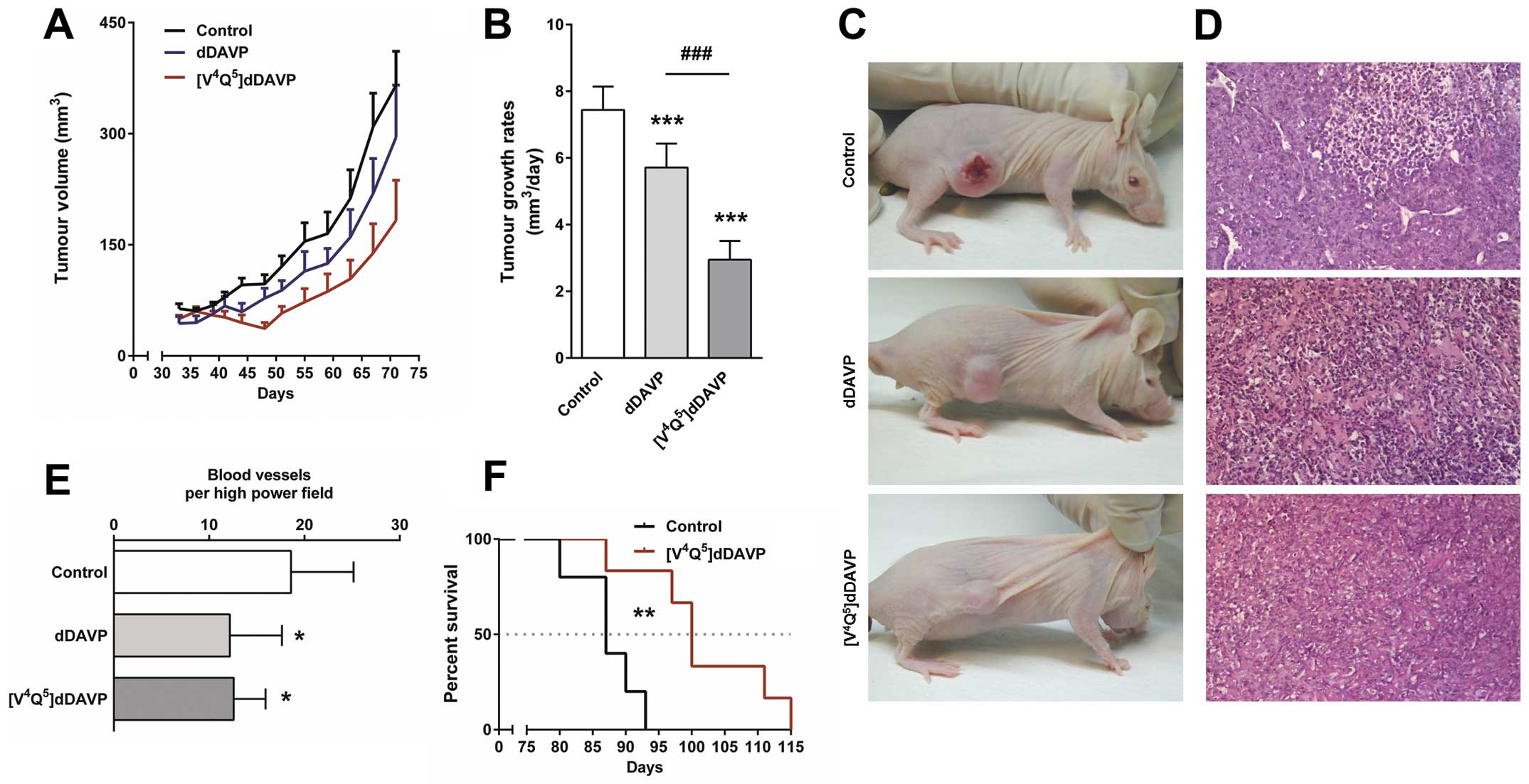
We next evaluated the novel analogue on MDA-MB-231
xenograft growth. Treatment with thrice weekly intravenous doses of
[V4Q5]dDAVP for 8 weeks reduced final tumour
load by 50% (Fig. 3A). Tumours
grew at rates of 2.95±0.56, 5.72±0.72 and 7.44±0.66
mm3/day (mean ± SD, P<0.001) in
[V4Q5]dDAVP-treated, dDAVP-treated and
control mice, respectively (Fig.
3B). In controls, xenografts grew by invading the subcutis and
dermis, causing visible skin ulceration and necrosis. On the other
hand, most animals treated with [V4Q5]dDAVP
or dDAVP displayed preservation of superficial layers of skin,
indicating inhibition of tumour infiltration and modulation of
tumour aggressiveness (Fig. 3C).
Histopathological studies of MDA-MB-231 xenografts from treated
mice showed a decrease in tumour vascularisation (Fig. 3D). Quantification of intratumoural
vascular density revealed a 30% reduction in the number of blood
vessels in [V4Q5]dDAVP- and dDAVP-treated
animals (Fig. 3E). As shown in the
Kaplan-Meier curve, [V4Q5]dDAVP treatment was
associated with increased survival (Fig. 3F).
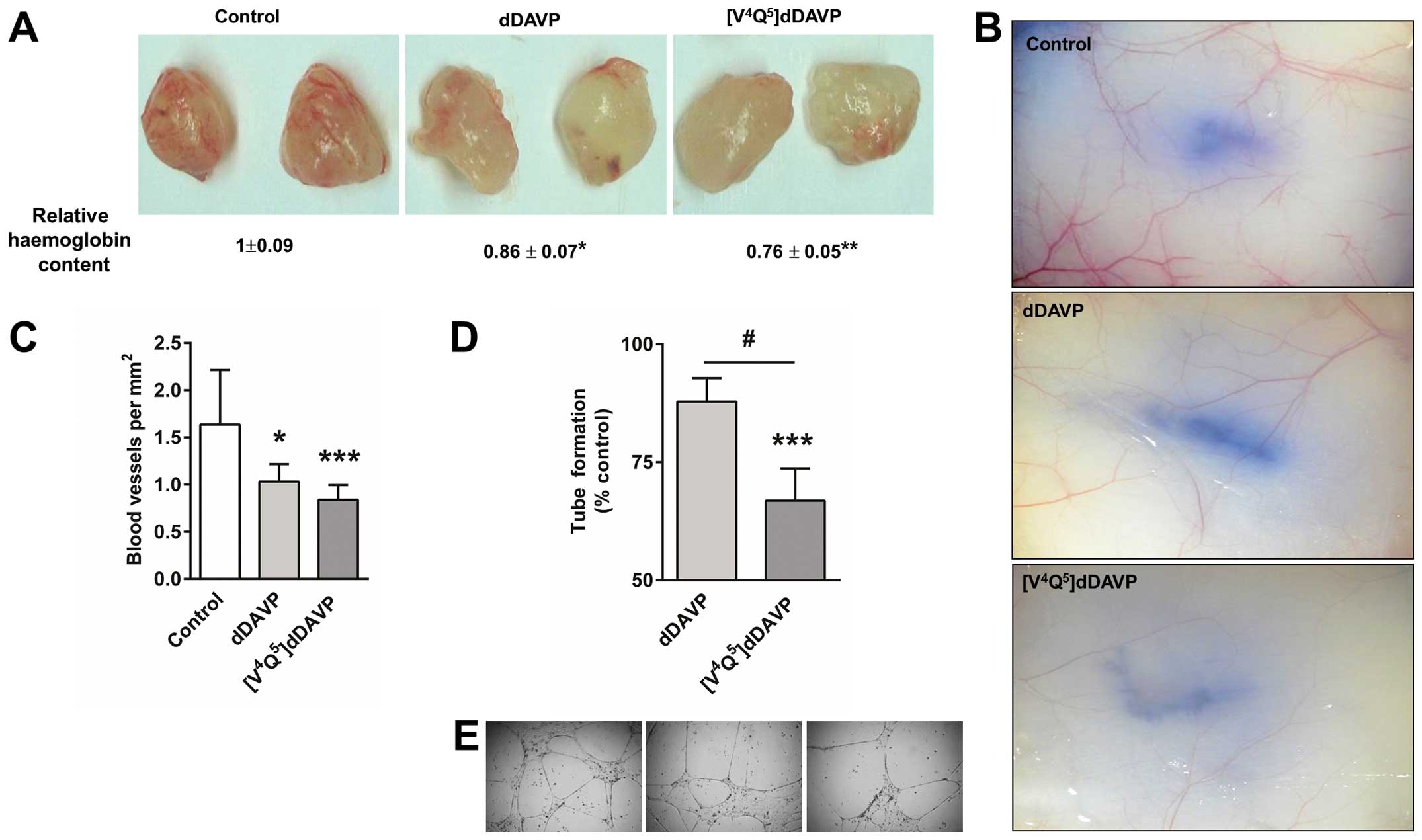
Reduction of angiogenesis by
[V4Q5]dDAVP
To further evaluate the efficacy on angiogenic
response, a modified Matrigel plug assay was used. Thrice weekly
intravenous doses of [V4Q5]dDAVP or dDAVP
resulted in a decrease in MDA-MB-231 cell-induced angiogenesis
(Fig. 4A). In addition, the highly
aggressive mammary carcinoma F3II cell line was intradermally
injected and used to assess the effect on early tumour-induced
vascular development. After 5 days, F3II cells generated highly
irregular and dense vascular networks around tumour cell implants
in control animals. In [V4Q5] dDAVP-treated
mice, tumour angiogenesis was drastically inhibited, showing a
vessel density reduction of ~50% (Fig.
4B and C). We also investigated direct effects of
[V4Q5]dDAVP on microvascular endothelial cell
morphogenesis (Fig. 4D and E).
Twenty-four-hour incubation with [V4Q5]dDAVP
reduced capillary-like tube formation by HMVEC-L cells. The
parental peptide dDAVP did not displayed any significant effects on
in vitro angiogenesis.
Effects of
[V4Q5]dDAVP on metastasic progression of F3II
mouse mammary tumours
We first evaluated the in vitro response to
[V4Q5]dDAVP and dDAVP in log-phase growing
hormone-independent F3II cells. Treatment during 72 h with both
peptidic compounds caused a mild cytostatic effect in a
concentration-dependent manner (Fig.
5A). We further tested the effects on progression of F3II
tumours in BALB/c mice. Both dDAVP and
[V4Q5]dDAVP treatments had limited impact on
final tumour volume (1194±240, 1574.8±466.1 or 1822.3±1185
mm3 in [V4Q5]dDAVP, dDAVP or
control group, respectively, mean ± SD, P>0.05).
Notwithstanding, tumours from
[V4Q5]dDAVP-treated mice grew at a lower rate
compared to tumours from animals administered with dDAVP or saline
vehicle (Fig. 5B). All control
animals displayed visible lung metastases, with a maximum of 6
macroscopic nodules per mouse. Remarkably, treatment with
intravenous doses of [V4Q5]dDAVP for 6 weeks
was able to completely abolish the formation of lung metastases
(Fig. 5C). On the contrary, the
effects of dDAVP on spontaneous metastases were not significant in
the present experimental conditions.
Inhibition of experimental lung
metastases by [V4Q5]dDAVP
F3II cells inoculated intravenously into BALB/c mice
induce multiple macroscopic lung lesions after 3 weeks.
Experimental lung colonisation by F3II cells was severely impaired
after either dDAVP or [V4Q5]dDAVP treatment.
However, whilst dDAVP reduced the number of lung nodules by 32%,
the novel analogue [V4Q5]dDAVP displayed
greater antimetastatic efficacy, reducing by 62% the formation of
metastases (Fig. 6A and B). In
addition, total lung weight was significantly reduced in
[V4Q5]dDAVP-treated animals (Fig. 6C), with a weight close to healthy
lung in BALB/c mice (28).
Toxicology studies
Preliminary acute toxicology studies conducted in
Wistar rats revealed that intravenous administration of
[V4Q5]dDAVP at doses of 1, 10 and 100 μg/kg
had no influence on general symptoms, body weight, food and water
consumption (Table I). These
observations suggest that individual injections are safe at doses
≥300-fold above that required for antiangiogenic/antimetastatic
effects. Mild transient increases of glycemia and bilirubin were
observed in treated groups. The other biochemical and
haematological parameters were not significantly altered. DDAVP was
administered as a reference standard, showing a safety profile
consistent with previous observations (13,15).
 | Table IAcute toxicology study in Wistar rats
after single intravenous doses of 1, 10 or 100 μg/kg of dDAVP or
[V4Q5]dDAVP. |
Table I
Acute toxicology study in Wistar rats
after single intravenous doses of 1, 10 or 100 μg/kg of dDAVP or
[V4Q5]dDAVP.
| Experimental
groups |
|---|
|
|
|---|
| Parametera | Control | dDAVP 1 μg/kg | dDAVP 10 μg/kg | dDAVP 100
μg/kg |
[V4Q5]dDAVP 1
μg/kg |
[V4Q5]dDAVP 10
μg/kg |
[V4Q5]dDAVP 100
μg/kg |
|---|
| Weightb (g) | 218.3±8.7 | 234.2±8.1 | 240.3±9.4 | 241.8±16.8 | 225.4±16.8 | 226.0±10.9 | 225.8±6.1 |
| Hematocritc (%) | 39.3±4.0 | 40.4±2.3 | 40.5±2.7 | 41.8±3.4 | 40.0±1.6 | 41.0±2.2 | 41.2±1.7 |
| RBC
(106/ml) | 5.1±0.5 | 5.2±0.3 | 5.5±0.3 | 5.7±0.3 | 5.6±0.2 | 5.4±0.5 | 5.5±0.2 |
| WBC
(103/ml) | 4.8±0.4 | 4.8±0.3 | 4.4±0.5 | 4.1±0.7 | 4.2±0.2 | 4.5±0.3 | 4.7±0.7 |
| Fibrinogenc (mg/dl) | 198.3±44.8 | 221.0±67.8 | 226.7±48.4 | 243.0±63.1 | 206.0±56.7 | 210.0±41.8 | 179.5±45.7 |
| Total
proteinc (g/dl) | 7.3±0.6 | 6.6±0.8 | 6.9±0.5 | 7.0±0.5 | 6.9±0.5 | 6.7±0.6 | 6.9±0.5 |
| Direct bilirubin
(mg/dl) | 0.08±0.02 | 0.13±0.04 | 0.14±0.02d | 0.17±0.03d | 0.11±0.01d | 0.12±0.02d | 0.16±0.05d |
| Glucose
(mg/dl) | 179.9±4.1 | 207.0±25.7d | 218.8±20.0d | 230.7±12.1d | 217.7±13.9d | 221.1±19.7d | 230.2±12.1d |
| Creatinine
(mg/ml) | 0.69±0.27 | 0.70±0.16 | 0.65±0.08 | 0.70±0.09 | 0.58±0.03 | 0.64±0.07 | 0.61±0.05 |
| GGT (IU/l) | 2.7±0.6 | 2.8±0.8 | 2.6±0.9 | 3.0±1.2 | 2.8±0.8 | 3.2±1.1 | 3.6±0.9 |
| AST (IU/l) | 34.7±6.4 | 36.6±11.6 | 30.0±5.6 | 47.5±17.8 | 39.2±6.7 | 51.2±30.6 | 46.6±20.1 |
| ALT (IU/l) | 19.0±1.0 | 22.8±3.4 | 22.6±3.4 | 21.3±2.1 | 20.6±2.7 | 19.6±3.1 | 20.4±3.1 |
Discussion
Vasopressin and its receptors were proposed as
attractive targets for breast cancer therapy almost two decades
ago, when vasopressin gene-related products were detected by
immunohistochemistry as a feature of all breast cancer subtypes
(29). Selective agonists of V2
vasopressin membrane receptor, such as dDAVP, seem to evoke dual
angiostatic and antimetastatic effects, breaking co-operative
interactions of tumour and endothelial cells during tumour
progression (18). Due to the
interesting anticancer activity of dDAVP in animal studies
(9,11,12,15),
as well as its known haemostatic properties (3), a prospective, open-label phase II
clinical trial is currently ongoing with the aim of assessing
safety and preliminary anticancer efficacy of perioperative use of
dDAVP in breast cancer patients (NCT01606072). Peptides such as
dDAVP are much appreciated as lead compounds for the development of
new drugs with enhanced biological activity. In the present study,
we characterized and compared the preclinical anticancer efficacy
of the novel analogue [V4Q5]dDAVP with its
parental peptide dDAVP. [V4Q5]dDAVP was
originally selected from a panel of peptidic analogues derivatized
from dDAVP with different sequence and structural modifications,
mainly aiming at the N-terminal loop of the molecule. This search
for more potent and selective V2r agonists included full-length
nonapeptides, tetrapeptides and chiral isomers (21).
In the present study, in vitro studies
exhibited moderate cytostatic activity of
[V4Q5]dDAVP on log-phase growing human breast
cancer cells, but showed a more potent inhibitory effect on low
density cell culture, with an IC50 value of 1.13 μM.
Chemical V2r blockade by tolvaptan completely abolished
[V4Q5]dDAVP effects in MDA-MB-231 cells.
These findings are in close agreement with the study by Keegan
et al (30), where mild cytostatic effects of dDAVP on breast
cancer cells were blocked by satavaptan, another non-peptidic V2r
antagonist. Action of [V4Q5]dDAVP was
associated with partial cell cycle arrest and normal V2r-activated
signal transduction, involving intracellular cAMP elevation and PKA
activation. Increases in cAMP intracellular levels using cAMP
analogues or cAMP elevating agents, such as hormones or forskolin,
can trigger cell cycle arrest or proapoptotic responses in numerous
cancer cell types, including breast cancer (31–33).
Consequently, several authors have postulated the adenylate
cyclase/cAMP/PKA axis as a growth suppressor system in breast
cancer that could be targeted to block tumour formation (33,34).
Triple-negative breast cancer is defined by a lack
of expression of both PR and ER, as well as a low expression of
(HER2)/neu (35). There have been
significant improvements in the outcome of other subtypes of breast
cancer, including ER-positive/HER2 overexpressed tumours,
attributed to the addition of targeted therapy, including hormonal
agents and trastuzumab/pertuzumab (36,37).
However, no targeted therapies are available for the treatment of
triple-negative breast cancer, and frontline treatments are limited
to surgical approaches and chemotherapeutics (38). In the present study we documented
the efficacy of [V4Q5]dDAVP on
triple-negative MDA-MB-231 breast cancer xenografts. Intravenous
injection of clinically-relevant doses of
[V4Q5]dDAVP caused a marked reduction in
tumour growth and an increase in survival. Histological examination
of xenografts also showed a significant decrease in tumour
angiogenesis in treated animals. In a previous study, our group
reported that i.v. administration of dDAVP at doses of 2 μg/kg was
able to significantly reduce intratumour vascularisation of F3II
mammary tumours (15). DDAVP seems
to modulate tumour angiogenesis by inducing the formation of
angiostatin, a potent angiogenesis inhibitor that is generated by
cancer-mediated proteolysis of plasminogen (16,17).
Interestingly, an enhanced production of angiostatin by human
mammary carcinoma cells was previously reported after incubation
with [V4Q5]dDAVP compared to dDAVP-treated
cells (39). Nevertheless, the
possibility that other underlying mechanisms account for the
antiangiogenic action of dDAVP or [V4Q5]dDAVP
cannot be ruled out. Systemic injection of dDAVP induces a rapid
release of VWF by stimulation of V2r present in microvasculature.
VWF is a large multimeric plasma glycoprotein that plays an
essential role in primary haemostasis. This factor acts as a
carrier for coagulation factor VIII and mediates platelet adhesion
to endothelial cells (27,40). Starke et al reported that
loss of endothelial VWF by short interfering RNA results in
increased in vitro angiogenesis. Additionally, VWF-deficient
mice displayed increased mature blood vessel density, suggesting a
potential role for VWF in the modulation of angiogenesis (41). Other possible mechanism involves
V2r-related signalling and actin. Stimulation of V2r in endothelial
cells leads to activation of cAMP-mediated signalling, which plays
a central role in actin cytoskeletal dynamics and cell migration
(27,42,43).
Interestingly, it has been reported that PKA activation suppresses
endothelial cell migration in vitro and angiogenesis in
vivo (44). The connection
between the cAMP signalling pathway and actin structures could
partially explain the direct effects of
[V4Q5]dDAVP on HMVEC-L tube formation.
However, the specific mechanisms responsible for
[V4Q5]dDAVP effects on microvascular
endothelial cells remain to be elucidated.
The F3II breast cancer cell line is invasive and
metastatic, characterized by an aggressive hormone-independent
growth and a low expression of (HER2)/neu, as revealed by
immunocytochemistry studies. In addition to angiostatic effects,
[V4Q5]dDAVP treatment of immunocompetent mice
bearing F3II tumours resulted in complete inhibition of metastatic
progression. [V4Q5]dDAVP also showed an
enhanced antimetastatic effect compared to dDAVP on experimental
metastases to lung. As mentioned above, dDAVP causes the release of
multimeric forms of VWF, reaching peak levels at 60–90 min after
i.v. injection (4). Using
VWF-deficient mice, Terraube et al demonstrated that the
absence of VWF leads to increased metastatic potential of
intravenously injected carcinoma cells. Furthermore, VWF was shown
to directly induce apoptosis of tumour cells in vitro and
caused death of metastatic cells arrested in the lungs (14,45).
By modulating the interaction between cancer cells and
subendothelial cells, VWF seems to reduce sustained adherence of
tumour cells in the microvasculature at the target organ, thus
inhibiting metastatic spread. More recently, it was found that
aggressive breast and lung cancer cells with high levels of ADAM28
(a disintegrin and metalloproteinase 28) are able to avoid
VWF-induced apoptosis at micrometastatic sites. ADAM28 binds and
cleaves VWF, thus favoring the survival of metastatic cells in the
tissue microenvironment (46).
Taken together, these results suggest that VWF released after V2r
stimulation plays a crucial role in resistance to blood-borne
metastases.
Hydrophobicity enhancement at position 4 (glutamine
by valine) and conservative substitution at position 5 (asparagine
by glutamine) in [V4Q5]dDAVP resulted in an
improved antitumour compound derived from dDAVP. The V2r is a
transmembrane receptor that belongs to the G protein-coupled
receptor family, having a deep cavity on the extracellular side
containing hydrophobic moieties (19,20).
Manning et al hypothesized that enhancing hydrophobicity at
position 4 improves the interaction of vasopressin-related ligands
with V2r (2). In a separate study,
Manning and collaborators reported that 4-valine-dDAVP has a
10-fold higher affinity for the human V2r than dDAVP, with Ki
values of 2.2 and 23.3 nM, respectevely (5). More recently, it was shown that,
unlike dDAVP, 4-valine-dDAVP administration was able to rescue the
function of a mutated V2r in a pathological setting, displaying an
enhanced agonistic potency on intracellular cAMP production without
cross-reacting with other vasopressin receptor subtypes (47). In order to improve the stability of
the analogue, we also introduced a conservative substitution at
position 5, replacing asparagine with glutamine, based on its
distinctive susceptibility to the deamidation process. Although
both asparagine and glutamine are susceptible to deamidation,
deamidation of glutamine proceeds at a much slower rate than
deamidation of asparagine at peptide level (48,49).
These rational modifications may favour
ligand-receptor affinity by promoting hydrophobic interactions
between the N-terminal conformational loop of
[V4Q5]dDAVP and the amino acids located at
the bottom of the V2r cavity. Enhancement of affinity between
[V4Q5]dDAVP and V2r present in cancer and
endothelial cells could result in augmented cAMP production and PKA
activation, angiostatin generation and VWF release, thus explaining
the increased biological activity of the peptidic analogue.
Nevertheless, further pharmacological experiments should be
performed to confirm stability, selectivity and potency of the
novel compound.
In conclusion, compared to dDAVP, the novel
analogue [V4Q5]dDAVP exhibited a
significantly higher inhibitory effect on breast cancer cell
proliferation and colony formation, as well as on MDA-MB-231 and
F3II tumour growth. [V4Q5]dDAVP also
displayed greater antimetastatic effects than dDAVP on spontaneous
and experimental lung colonization.
While treatment for localised tumours has generally
improved survival in the era of modern medicine, patients with
advanced stage metastatic disease still suffer from a lack of
effective therapies. Despite evident progress in overall mortality,
the efficacy of adjuvant chemotherapy in reducing metastatic risk
has reached a plateau (50). Given
that the benefit of chemotherapy is often mitigated by long-term
side effects linked to a lack of selectivity (51–53),
the potential combination with novel highly selective cytostatic
agents such as [V4Q5]dDAVP is highly
interesting. Preclinical efficacy of the compound without overt
toxicity supports further clinical development of
[V4Q5]dDAVP as a novel adjuvant or
maintenance therapy in aggressive and metastatic
hormone-independent breast cancer.
Acknowledgements
This study was supported by the National University
of Quilmes (grant 53/1004 to D.F. Alonso and D.E. Gomez), the
National Agency for the Promotion of Science and Technology
(ANPCYT, Argentina) (grant PICT2013/1772 to D.F. Alonso), the
National Institute of Cancer (grant INC2014 to D.F. Alonso) and
Chemo-Romikin. J. Garona and M.B. Pastrian are research fellows,
and U.D. Orlando, N.B. Iannucci, H.H. Ortega, E.J. Podesta, D.E.
Gomez, G.V. Ripoll and D.F. Alonso are members of the National
Research Council (CONICET, Argentina). The authors gratefully
acknowledge the generous assistance of Dr Alejandra Scursoni in
histopathological assessment.
References
|
1
|
Vande Walle J, Stockner M, Raes A and
Nørgaard JP: Desmopressin 30 years in clinical use: A safety
review. Curr Drug Saf. 2:232–238. 2007. View Article : Google Scholar
|
|
2
|
Manning M, Stoev S, Chini B, Durroux T,
Mouillac B and Guillon G: Peptide and non-peptide agonists and
antagonists for the vasopressin and oxytocin V1a, V1b, V2 and OT
receptors: Research tools and potential therapeutic agents. Prog
Brain Res. 170:473–512. 2008.PubMed/NCBI
|
|
3
|
Kaufmann JE and Vischer UM: Cellular
mechanisms of the hemostatic effects of desmopressin (DDAVP). J
Thromb Haemost. 1:682–689. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mannucci PM: Desmopressin (DDAVP) in the
treatment of bleeding disorders: The first 20 years. Blood.
90:2515–2521. 1997.PubMed/NCBI
|
|
5
|
Manning M, Misicka A, Olma A, Bankowski K,
Stoev S, Chini B, Durroux T, Mouillac B, Corbani M and Guillon G:
Oxytocin and vasopressin agonists and antagonists as research tools
and potential therapeutics. J Neuroendocrinol. 24:609–628. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
North WG, Fay MJ and Du J: MCF-7 breast
cancer cells express normal forms of all vasopressin receptors plus
an abnormal V2R. Peptides. 20:837–842. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Petit T, Davidson KK, Lawrence RA, von
Hoff DD and Izbicka E: Neuropeptide receptor status in human tumor
cell lines. Anticancer Drugs. 12:133–136. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Taylor AH, Ang VT, Jenkins JS, Silverlight
JJ, Coombes RC and Luqmani YA: Interaction of vasopressin and
oxytocin with human breast carcinoma cells. Cancer Res.
50:7882–7886. 1990.PubMed/NCBI
|
|
9
|
Alonso DF, Skilton G, Farías EF, Bal de
Kier Joffé E and Gomez DE: Antimetastatic effect of desmopressin in
a mouse mammary tumor model. Breast Cancer Res Treat. 57:271–275.
1999. View Article : Google Scholar
|
|
10
|
Garona J, Pifano M, Scursoni AM, Gomez DE,
Alonso DF and Ripoll GV: Insight into the effect of the vasopressin
analog desmopressin on lung colonization by mammary carcinoma cells
in BALB/c mice. Anticancer Res. 34:4761–4765. 2014.PubMed/NCBI
|
|
11
|
Giron S, Tejera AM, Ripoll GV, Gomez DE
and Alonso DF: Desmopressin inhibits lung and lymph node metastasis
in a mouse mammary carcinoma model of surgical manipulation. J Surg
Oncol. 81:38–44. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hermo GA, Torres P, Ripoll GV, Scursoni
AM, Gomez DE, Alonso DF and Gobello C: Perioperative desmopressin
prolongs survival in surgically treated bitches with mammary gland
tumours: A pilot study. Vet J. 178:103–108. 2008. View Article : Google Scholar
|
|
13
|
Hermo GA, Turic E, Angelico D, Scursoni
AM, Gomez DE, Gobello C and Alonso DF: Effect of adjuvant
perioperative desmopressin in locally advanced canine mammary
carcinoma and its relation to histologic grade. J Am Anim Hosp
Assoc. 47:21–27. 2011. View Article : Google Scholar
|
|
14
|
Terraube V, Pendu R, Baruch D, Gebbink MF,
Meyer D, Lenting PJ and Denis CV: Increased metastatic potential of
tumor cells in von Willebrand factor-deficient mice. J Thromb
Haemost. 4:519–526. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ripoll GV, Garona J, Pifano M, Farina HG,
Gomez DE and Alonso DF: Reduction of tumor angiogenesis induced by
desmopressin in a breast cancer model. Breast Cancer Res Treat.
142:9–18. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sakurai T and Kudo M: Signaling pathways
governing tumor angiogenesis. Oncology. 81(Suppl 1): 24–29. 2011.
View Article : Google Scholar
|
|
17
|
Westphal JR, Van’t Hullenaar R,
Geurts-Moespot A, Sweep FC, Verheijen JH, Bussemakers MM, Askaa J,
Clemmensen I, Eggermont AA, Ruiter DJ, et al: Angiostatin
generation by human tumor cell lines: involvement of plasminogen
activators. Int J Cancer. 86:760–767. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alonso DF, Ripoll GV, Garona J, Iannucci
NB and Gomez DE: Metastasis: Recent discoveries and novel
perioperative treatment strategies with particular interest in the
hemostatic compound desmopressin. Curr Pharm Biotechnol.
12:1974–1980. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Czaplewski C, Kaźmierkiewicz R and
Ciarkowski J: Molecular modeling of the human vasopressin V2
receptor/agonist complex. J Comput Aided Mol Des. 12:275–287. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Czaplewski C, Kaźmierkiewicz R and
Ciarkowski J: Molecular modelling of the vasopressin V2
receptor/antagonist interactions. Acta Biochim Pol. 45:19–26.
1998.PubMed/NCBI
|
|
21
|
Iannucci NB, Ripoll GV, Garona J, Cascone
O, Ciccia GN, Gomez DE and Alonso DF: Antiproliferative effect of
1-deamino-8-D-arginine vasopressin analogs on human breast cancer
cells. Future Med Chem. 3:1987–1993. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pastrian MB, Guzmán F, Garona J, Pifano M,
Ripoll GV, Cascone O, Ciccia GN, Albericio F, Gómez DE, Alonso DF,
et al: Structure-activity relationship of 1-desamino-8-D-arginine
vasopressin as an antiproliferative agent on human vasopressin V2
receptor-expressing cancer cells. Mol Med Rep. 9:2568–2572.
2014.PubMed/NCBI
|
|
23
|
Holliday DL and Speirs V: Choosing the
right cell line for breast cancer research. Breast Cancer Res.
13:2152011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ripoll GV, Giron S, Krzymuski MJ, Hermo
GA, Gomez DE and Alonso DF: Antitumor effects of desmopressin in
combination with chemotherapeutic agents in a mouse model of breast
cancer. Anticancer Res. 28A:2607–2611. 2008.
|
|
25
|
Cardama GA, Comin MJ, Hornos L, Gonzalez
N, Defelipe L, Turjanski AG, Alonso DF, Gomez DE and Menna PL:
Preclinical development of novel Rac1-GEF signaling inhibitors
using a rational design approach in highly aggressive breast cancer
cell lines. Anticancer Agents Med Chem. 14:840–851. 2014.
View Article : Google Scholar :
|
|
26
|
Jones LW, Eves ND, Courneya KS, Chiu BK,
Baracos VE, Hanson J, Johnson L and Mackey JR: Effects of exercise
training on antitumor efficacy of doxorubicin in MDA-MB-231 breast
cancer xenografts. Clin Cancer Res. 11:6695–6698. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaufmann JE, Oksche A, Wollheim CB,
Günther G, Rosenthal W and Vischer UM: Vasopressin-induced von
Willebrand factor secretion from endothelial cells involves V2
receptors and cAMP. J Clin Invest. 106:107–116. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Han SS, Cho CK, Lee YW and Yoo HS:
Antimetastatic and immunomodulating effect of water extracts from
various mushrooms. J Acupunct Meridian Stud. 2:218–227. 2009.
View Article : Google Scholar
|
|
29
|
North WG, Pai S, Friedmann A, Yu X, Fay M
and Memoli V: Vasopressin gene related products are markers of
human breast cancer. Breast Cancer Res Treat. 34:229–235. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Keegan BP, Akerman BL, Péqueux C and North
WG: Provasopressin expression by breast cancer cells: Implications
for growth and novel treatment strategies. Breast Cancer Res Treat.
95:265–277. 2006. View Article : Google Scholar
|
|
31
|
Carie AE and Sebti SM: A chemical biology
approach identifies a beta-2 adrenergic receptor agonist that
causes human tumor regression by blocking the Raf-1/Mek-1/Erk1/2
pathway. Oncogene. 26:3777–3788. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Insel PA, Zhang L, Murray F, Yokouchi H
and Zambon AC: Cyclic AMP is both a pro-apoptotic and
anti-apoptotic second messenger. Acta Physiol (Oxf). 204:277–287.
2012. View Article : Google Scholar
|
|
33
|
Naviglio S, Di Gesto D, Romano M,
Sorrentino A, Illiano F, Sorvillo L, Abbruzzese A, Marra M,
Caraglia M, Chiosi E, et al: Leptin enhances growth inhibition by
cAMP elevating agents through apoptosis of MDA-MB-231 breast cancer
cells. Cancer Biol Ther. 8:1183–1190. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Castoria G, Migliaccio A, D’Amato L, Di
Stasio R, Ciociola A, Lombardi M, Bilancio A, Di Domenico M, De
Falco A and Auricchio F: Integrating signals between cAMP and MAPK
pathways in breast cancer. Front Biosci. 13:1318–1327. 2008.
View Article : Google Scholar
|
|
35
|
Gluz O, Liedtke C, Gottschalk N, Pusztai
L, Nitz U and Harbeck N: Triple-negative breast cancer--current
status and future directions. Ann Oncol. 20:1913–1927. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bayraktar S and Glück S: Molecularly
targeted therapies for metastatic triple-negative breast cancer.
Breast Cancer Res Treat. 138:21–35. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Terrenato I, Arena V, Pizzamiglio S,
Pennacchia I, Perracchio L, Buglioni S, Ercolani C, Sperati F,
Costarelli L, Bonanno E, et al: External Quality Assessment (EQA)
program for the preanalytical and analytical immunohistochemical
determination of HER2 in breast cancer: An experience on a regional
scale. J Exp Clin Cancer Res. 32:582013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gangloff A, Hsueh WA, Kesner AL,
Kiesewetter DO, Pio BS, Pegram MD, Beryt M, Townsend A, Czernin J,
Phelps ME, et al: Estimation of paclitaxel biodistribution and
uptake in human-derived xenografts in vivo with
(18)F-fluoropaclitaxel. J Nucl Med. 46:1866–1871. 2005.PubMed/NCBI
|
|
39
|
Ripoll G, Iannucci N, Giron S, Cascone O,
Gomez D and Alonso D: Angiostatic activity of
1-Deamino-8-D-Arginine vasopressin and novel peptide analogues in
breast cancer cells. Proc AACR Abst. 295:2008.
|
|
40
|
Huang J, Roth R, Heuser JE and Sadler JE:
Integrin alpha(v) beta(3) on human endothelial cells binds von
Willebrand factor strings under fluid shear stress. Blood.
113:1589–1597. 2009. View Article : Google Scholar :
|
|
41
|
Starke RD, Ferraro F, Paschalaki KE,
Dryden NH, McKinnon TA, Sutton RE, Payne EM, Haskard DO, Hughes AD,
Cutler DF, et al: Endothelial von Willebrand factor regulates
angiogenesis. Blood. 117:1071–1080. 2011. View Article : Google Scholar :
|
|
42
|
Howe AK: Regulation of actin-based cell
migration by cAMP/ PKA. Biochim Biophys Acta. 1692:159–174. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Howe AK, Baldor LC and Hogan BP: Spatial
regulation of the cAMP-dependent protein kinase during chemotactic
cell migration. Proc Natl Acad Sci USA. 102:14320–14325. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kim S, Harris M and Varner JA: Regulation
of integrin alpha vbeta 3-mediated endothelial cell migration and
angiogenesis by integrin alpha5beta1 and protein kinase A. J Biol
Chem. 275:33920–33928. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Terraube V, Marx I and Denis CV: Role of
von Willebrand factor in tumor metastasis. Thromb Res. 120(Suppl
2): S64–S70. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mochizuki S, Soejima K, Shimoda M, Abe H,
Sasaki A, Okano HJ, Okano H and Okada Y: Effect of ADAM28 on
carcinoma cell metastasis by cleavage of von Willebrand factor. J
Natl Cancer Inst. 104:906–922. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Erdélyi LS, Balla A, Patócs A, Tóth M,
Várnai P and Hunyady L: Altered agonist sensitivity of a mutant v2
receptor suggests a novel therapeutic strategy for nephrogenic
diabetes insipidus. Mol Endocrinol. 28:634–643. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu H, Gaza-Bulseco G and Chumsae C:
Glutamine deamidation of a recombinant monoclonal antibody. Rapid
Commun Mass Spectrom. 22:4081–4088. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Robinson AB and Rudd CJ: Deamidation of
glutaminyl and asparaginyl residues in peptides and proteins. Curr
Top Cell Regul. 8:247–295. 1974.PubMed/NCBI
|
|
50
|
Lianos GD, Vlachos K, Zoras O, Katsios C,
Cho WC and Roukos DH: Potential of antibody-drug conjugates and
novel therapeutics in breast cancer management. Onco Targets Ther.
7:491–500. 2014.PubMed/NCBI
|
|
51
|
Bacic I, Druzijanic N, Karlo R, Skific I
and Jagic S: Efficacy of IP6 inositol in the treatment of breast
cancer patients receiving chemotherapy: Prospective, randomized,
pilot clinical study. J Exp Clin Cancer Res. 29:122010. View Article : Google Scholar
|
|
52
|
Fabbrocini G, Cameli N, Romano MC, Mariano
M, Panariello L, Bianca D and Monfrecola G: Chemotherapy and skin
reactions. J Exp Clin Cancer Res. 31:502012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang S, Noberini R, Stebbins JL, Das S,
Zhang Z, Wu B, Mitra S, Billet S, Fernandez A, Bhowmick NA, et al:
Targeted delivery of paclitaxel to EphA2-expressing cancer cells.
Clin Cancer Res. 19:128–137. 2013. View Article : Google Scholar :
|