Introduction
Mantle cell lymphoma (MCL), a heterogeneous subtype
of B-cell non-Hodgkin lymphoma (NHL), accounts for ~7% of NHL cases
in the USA and Europe and has one of the worst outcomes of all the
lymphomas (1,2). It is characterized by the t(11;14)
(q13;q32) translocation, which results in overexpression of cyclin
D1 and deregulation of the cell cycle (2). At initial diagnosis, most patients
with the median age ~68–70 years have advanced stage disease, and
the median overall survival is 3–5 years (3). Although several novel agents have
proven to be effective, MCL remains a largely incurable disease and
the following relapse is still challenging. Therefore,
understanding the molecular mechanisms of MCL pathogenesis and drug
resistance will aid in the development of highly active targeted
therapies for the disease.
B7-H3, a new member of B7 immunoregulatory family
with immunoglobulin-like structure (4), is induced in activated dendritic
cells, monocytes and T cells (5).
Aberrant expression of B7-H3 has been reported and associated with
poor prognosis in patients with neuroblastoma (6), lung cancer (7), pancreatic cancer (8), colorectal cancer (9), hepatocellular carcinoma (10) and breast cancer (11). In hematologic malignancy, the
overexpression of B7-H3 also has been described, including acute
leukemia (12), multiple myeloma
(13) and several types of
lymphoma (14).
The physiological and pathological role of B7-H3
remains contentious, with stimulatory (4,15,16)
and inhibitory (17–19) immunoregulatory functions in
cellular and antitumor immune response described in early studies.
Currently, the non-immunological functions of B7-H3 in cancer
progression and chemoresistance have received increasing attention.
Tekle et al demonstrated that the B7-H3 silencing reduced
metastatic capacity of MDA-MB-435 melanoma cells and significantly
increased the survival of nude mice (20). Other studies also reported that
B7-H3 was important in regulating the adhesive, migratory, and
invasive capacity in breast cancer (21), glioblastoma (22), pancreatic cancer (23), prostate cancer (24) and osteosarcoma (25). Liu et al discovered that
silencing of B7-H3 sensitized the breast cancer cell lines to
paclitaxel by abrogating Jak2/Stat3 phosphorylation (26). In pancreatic carcinoma cells, B7-H3
was demonstrated to induce gemcitabine resistance as well (27). The above research indicated that
B7-H3 may be a potential therapeutic target.
However, little is known about the direct impact of
B7-H3 on tumor progression and B7-H3 RNAi-based targeting therapy
in MCL. In this study, we investigated the role of B7-H3 in mantle
cell lymphoma Maver and Z138 cell proliferation, the cell cycle,
migration, invasion and in the chemosensitivity in vitro and
in vivo.
Materials and methods
Cell line and cell culture
Human mantle cell lymphoma Maver and Z138 cell lines
were obtained from the American Type Culture Collection (ATCC,
Rockville, MD, USA). The cells were, respectively cultured in
Iscove’s modified Dulbecco’s medium (IMDM) and RPMI-1640 (Gibco
Invitrogen, Grand Island, NY, USA) medium containing 2 mM
L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin and 10%
heat-inactivated fetal bovine serum (FBS) (Thermo Scientific
HyClone, South Logan, UT, USA), at 37°C in a 5% CO2
incubator.
Lentivirus-based RNA interference
transfection and generation of stable cell lines
The human B7-H3 (Gene ID: 80381) targeting small
hairpin RNA (shRNA) sequence 5′-TCGTG TGCTGGAGAAAGATCAAACAGAGC-3′
and a negative non-targeted control sequence 5′-GCACTACCAGAGCTAA
CTCAGATAGTACT-3′ were used to generate recombinant lentiviral
particles (20). These recombinant
lentivirus were prepared and titered to 5×109 TU/ml
(transfection unit), and the multiplicity of infection (MOI) was
10. Antibiotic-resistant clones were isolated and maintained in
medium containing 200 μg/ml puromycin (Sigma-Aldrich, St. Louis,
MO, USA). The B7-H3 knockdown was confirmed by RT-PCR, western
blotting and fluorescence activating cell sorter (FACS). The
infected cells comprised the B7-H3 shRNA (KD) and negative
non-targeted control (NC) groups, and the non-infected cells were
the control (CON) groups. These three groups of each cell line were
used for the following experiments.
Gene expression of B7-H3, RT-PCR, western
blotting and flow cytometry
Total RNA was extracted and reverse transcribed for
cDNA. The primers used for PCR were as follows: 5′-CTC
TGCCTTCTCACCTCTTTG-3′ (forward) and 5′-CCTTGAG GGAGGAACTTTATC-3′
(reverse) for B7-H3 (134 bp) (23); 5′-TTGACGGTAAGGACGGACTC-3′ (forward)
and 5′-ACTT GCAGTACTCCCCATCG-3′ (reverse) for matrix
metalloproteinase-2 (MMP-2, 153 bp); 5′-TTGACAGCGACAAGAAG TGG-3′
(forward) and 5′-CCCTCAGTGAAGCGGTACAT-3′ (reverse) for matrix
metalloproteinase-9 (MMP-9, 148 bp) (28); and 5′-TGACGTGGACATCCGCAAAG-3′
(forward) and 5′-CTGGAAGGTGGACAGCGAGG-3′ (reverse) for β-actin (205
bp). The PCR conditions were 94°C for 2 min, then 28–36 cycles
(B7-H3/MMP-2/MMP-9: 36 cycles; β-actin: 28 cycles) at 94°C for 30
sec, 56–59°C (B7-H3: 56°C; MMP-2/MMP-9/β-actin: 59°C) for 30 sec,
72°C for 30 sec, and finally 72°C for 2 min.
The cells were lysed on ice, and the concentration
of protein was determined using the BCA method. A total of 30 μg
proteins were transferred onto nitrocellulose filter (NC) membranes
after 10% SDS-PAGE. The membranes were blocked and incubated with
primary antibodies, including rabbit anti-B7-H3 (1:500 dilution,
110 kDa) (clone EPNCIR122; Epitomics, Burlingame, CA, USA) or mouse
anti-β-actin (1:1,000 dilution, 43 kDa) (clone C-2; Santa Cruz, CA,
USA) monoclonal antibodies overnight at 4°C. After incubation with
IRDye 800CW conjugated goat (polyclonal) anti-rabbit/antimouse IgG
secondary antibody (1:10,000 dilution) (LI-COR, NE, USA) for 1 h,
the fluorescent bands was visualized with an Odyssey infrared
imaging system (LI-COR), and the gray values were analyzed using
Odyssey V3.0 software.
Single cell suspensions were stained with antibodies
on ice for 30 min. After three washes in PBS, the cells were
analyzed using a flow cytometry system (FACSCalibur; BD
Biosciences, San Jose, CA, USA). The monoclonal antibodies used to
measure the expression of the cell surface markers by flow
cytometry included B7-H3 (CD276)-APC (clone 7-517) from eBioscience
(San Diego, CA, USA) and CD3-APC, CD19-APC, CD117-APC, CD20-FITC,
CD10-PE, CD5-PE, CD38-FITC, CD56-PE, CD138-APC, CD34-PE,
CD45-PerCP, and appropriate isotype controls from BD
Biosciences.
CCK-8 assay
The Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Tokyo, Japan) was used to study the effects of B7-H3
shRNA on cell proliferation. Resuspended cells were plated at
5×104 cells/well in a 96-well plate for 24-, 48- or 72-h
inoculation. A volume of 10 μl/well of CCK-8 solution was added
into the plate. After incubation at 37°C for 5 h, the absorbance
was measured at 450 nm using a microplate reader. The assay was
performed in sextuplicate for each group.
Colony forming assay
A 5×103 single-cell suspension was
resuspended in 1 ml medium with 20% FBS and 0.9% methyl cellulose
(Sigma, St. Louis, MO, USA). The samples were plated in 24-well
plates and incubated for 14 days. A colony with >50 cells was
counted as one positive colony. The colony-forming ability (CFA) =
(colonies counts in experiment group/ control group) × 100%. Each
experiment was repeated three times.
Subcutaneous xenograft model and
tumorigenicity assay
Female BALB/c nude mice, 5–6 weeks of age, 16–18 g
of weight (Experimental Animal Center, Peking University Health
Science Center, Beijing, China), were bred under specified
pathogen-free (SPF) conditions. All of the care, experimental
procedures and handling of animals were performed with approval of
the Institutional Authority for Laboratory Animal Care of Peking
University. Mice were pretreated by intraperitoneal (i.p.)
injection, with 100 mg/kg cyclophosphamide (Jiangsu Hengrui
Medicine Co. Ltd., China) once daily for two consecutive days. The
next day, 2×107 cells (the Maver and Z138 cells in CON,
NC or KD group respectively, 6 mice per group) in 100 μl normal
saline (NS) were injected subcutaneously (s.c.) into right axilla
region of the nude mice.
The xenografted tumor volume (V) was measured every
other day and calculated as: V (mm3) = length ×
width2 × 0.5. The inhibition rate of tumor growth was
calculated using the formula: (1 - average tumor weight of treated
group/average tumor weight of control group) × 100%.
Immunohistochemical analysis
At the end of observation (20 days
post-inoculation), the xenograft tumors of Maver and Z138 mice were
excised and fixed in 10% neutrally buffered formalin for 24 h.
After embedded in paraffin, deparaffinized and rehydrated, the
histologic sections were subjected to heat-induced epitope
retrieval using microwave in 1 mmol/l EDTA buffer (pH 8.0) and
quenched for endogenous peroxidase activity with 3% hydrogen
peroxide. Then the sections were stained with an rabbit polyclonal
antibody to the cell proliferation-associated antigen Ki-67 (1:500
dilution) or a mouse monoclonal antibody to proliferating cell
nuclear antigen (PCNA) (1:300 dilution) (Dako Corp., Copenhagen,
Denmark), respectively, followed by incubation with the secondary
antibody (Dako Corp.) according to the avidin-biotin-peroxidase
method. Phosphate-buffered saline (PBS) were used as negative
controls. After incubation with 3,3′-diaminobenzidine (DAB) and
counterstained with hematoxylin, the sections were analyzed in 5
randomly selected microscopic fields (400x). The Ki-67 labeling
index or the positive expression rate of PCNA = (positively stained
cells counts / the total number of nucleated cells) × 100%.
Cell cycle analysis
The Maver and Z138 cells in each group were
collected, washed with PBS and fixed in 70% ethanol overnight at
−20°C. The cellular DNA was stained with propidium iodide (PI) (500
μg/ml) (Biosea, China) (10 μl/105 cells) for 10 min at
room temperature. The DNA content and cell number were determined
by FACS analysis, and the cell cycle profiles were analyzed using
the ModFit program (Verify Software House, Inc.). The proliferation
index (PI) was calculated using the following equation: PI =
[(S+G2M) / (G0/G1+S+G2M)] × 100%. Each experiment was repeated
three times.
Cell migration and invasion assay
For the in vitro migration and invasion
assays, 4×105 or 2×105 cells were resuspended
in serum-free medium and placed on the top of an 8-μm pore size
Transwell chamber (8.0 μm PC, Corning-Costar, Corning, NY, USA) or
Matrigel (1:5 dilution) (BD Biosciences, San Jose, CA, USA)
invasion chambers. The lower chambers contained medium with 10%
FBS. After 24 h of incubation, the migrating cells in the lower
chamber or invading cells on the bottom of each well were stained
with 4′,6-diamidino-2-phenylindole (DAPI) (1 mg/ml, Solarbio,
China) or 0.1% crystal violet following by fixation in methyl
alcohol for 30 min, respectively. Then, the number of cells in 6
randomly selected microscopic fields (200x) was counted with a BX51
fluorescence microscope (Olympus, Japan) or a DMIL inverted phase
microscope (Leica, German). The migration rate = (cells counts in
the lower chamber / total number on the top of Transwell chamber) ×
100%. MMP-2 and MMP-9 were detected by RT-PCR to further determine
the abilities of tumor cells to penetrate the cell matrix. Each
experiment was repeated three times.
Analysis of drug-induced cytotoxicity and
apoptosis
Rituximab (R, 500, 1,000, 1,500, 2,000, 2,500,
3,000, 3,500 and 4,000 μg/ml) [MabThera, Roche Pharma Ltd.,
Germany] or bendamustine (Ben, 1, 2, 4, 8, 10, 16, 20 and 40 μg/ml)
(Ribomustin, Ribosepharm GmbH, Germany) was added to three groups
of Maver and Z138 cells for 24, 48 or 72 h respectively, and then
the absorbance was detected using the CCK-8 reagent to evaluate the
effects of cell proliferation inhibition by the chemotherapy
drugs.
A total of 4×105 Maver cells in each
group were grown in triplicate in 6-well plates with 3,500 μg/ml R
and/or 4 μg/ml Ben for 12 and 24 h, while the same counts of Z138
cells with 2,500 μg/ml R and/or 4 μg/ml Ben. A volume of 10 μl
Annexin V-FITC (20 μg/ml) (Biosea, China) was added to the
collected cells. After incubation for 15 min at room temperature,
300 μl binding buffer was added. Then, we added 10 μl PI (50 μg/ml)
(Biosea, China) to the mixtures. The cells were examined by flow
cytometry within 1 h to determine the cell apoptosis rates induced
by the chemotherapy drugs. CellQuest software (Becton-Dickinson)
was used for data acquisition and analysis.
Caspase-3 assay
The Maver cells (8×106 cells/dish) were
treated with 3,500 μg/ml R and/or 4 μg/ml Ben for 0 and 24 h, while
the same counts of Z138 cells were treated with 2,500 μg/ml R
and/or 4 μg/ml Ben. The cells were then collected and lysed on ice
for 1 h. The concentration of protein was detected using the BCA
method. A total of 150 μg protein was detected using the caspase-3
Colorimetric Assay kit (Keygen, China). The activity of caspase-3 =
OD405 in the experiment group/OD405 in the
control group.
The effect of B7-H3 RNAi on
chemosensitivity in vivo
Mice bearing non-infected Maver and Z138 cell
xenografts were allocated randomly into 10 groups respectively (6
mice per group), including: a) an equal volume of NS, b) R (50
mg/kg), c) negative non-targeted control plasmid (pNC) + R, d)
short hairpin RNAs targeting B7-H3 plasmid (pKD) + R, e) Ben (25
mg/kg), f) pNC + Ben, g) pKD + Ben, h) R+ Ben, i) pNC + R+ Ben, j)
pKD + R + Ben. The treatments began at day 8 and day 10 after
inoculation, respectively, for Maver and Z138 mice, when tumors
reached an average volume of 100 mm3. The chemotherapy
drugs and NS were i.p. administered, while the mixture of plasmid
DNA (10 μg) and Lipofectamine 2000 (30 μl; Invitrogen) in 50 μl NS
were intratumorally injected. All of the treatments were performed
every other day, for a total of three times.
Statistical analysis
The data are shown as the mean ± standard deviation
(SD) of triplicate values for each experiment. Statistical
comparisons were performed using Student’s t-test. A value of
p<0.05 was considered statistically significant. The statistical
analysis was performed using SPSS 18.0 software (Chicago, IL,
USA).
Results
B7-H3 stably silences MCL cell line
generation
B7-H3 knockdown in Maver and Z138 cells was
performed using lentivirus transduction to stably express shRNA
targeting B7-H3. There was no significant difference of B7-H3
expression between the parental non-infection (CON) cells and the
transfection negative non-targeted control cells (NC) in each cell
line by RT-PCR, western blotting and FACS (p>0.05). The B7-H3
expression in the shB7-H3/Maver and shB7-H3/Z138 cells (KD groups)
was decreased compared to the relative NC groups (p<0.05). The
inhibition rates of mRNA expression in Maver and Z138 cells were
84.5 and 81.2%, whereas the nuclear and cytoplasmic proteins were
reduced by 80.3 and 74.5%, and the membrane proteins were the most
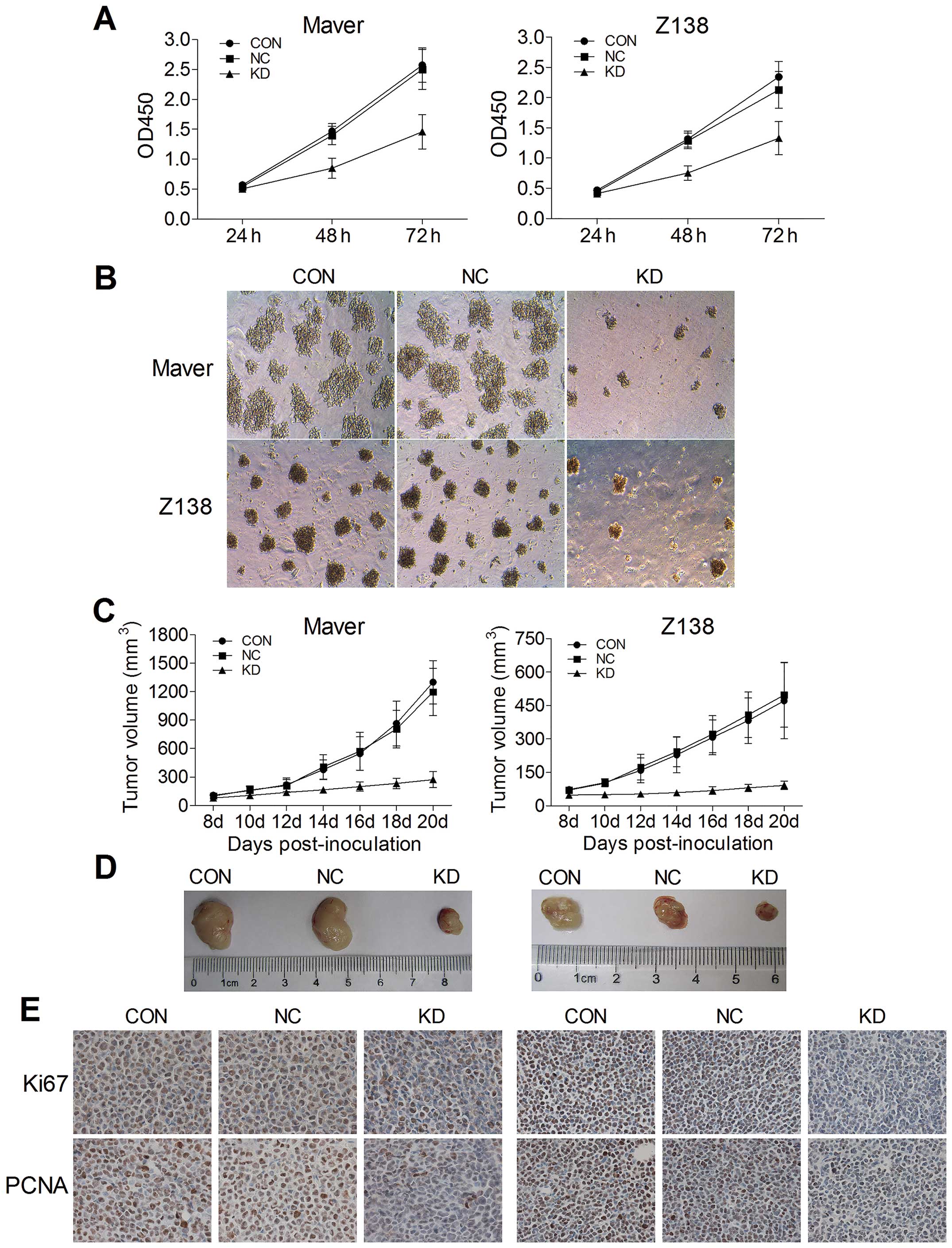
significantly inhibited by 86.9 and 82.4%, respectively (Fig. 1).
B7-H3 knockdown inhibits tumor
proliferation in vitro and in vivo
To evaluate the effects of B7-H3 knockdown on tumor
cell proliferation in vitro, we used CCK-8 and colony
formation assays. The stable knock-down of B7-H3 in Maver and Z138
cells significantly reduced cell growth. Compared to the relative
NC groups, the growth of shB7-H3/Maver and shB7-H3/Z138 cells was
decreased by 41.7 and 37.5% after 72 h of incubation, respectively
(p=0.015 and 0.028) (Fig. 2A). The
colony formation assay further confirmed that B7-H3 silencing
inhibits Maver and Z138 cell proliferation. At day 14 of
incubation, the colony-forming ability (CFA) of shB7-H3/Maver and
shB7-H3/Z138 cells was significantly decreased by 71.2 and 77.2%,
respectively compared with the NC groups (Fig. 2B).
To detect the in vivo effects of B7-H3
knockdown of tumorigenicity, we established xenograft models by
subcutaneously injecting Maver and Z138 cells into right axilla
region of BALB/c nude mice. The increases of tumor volumes in B7-H3
silencing Maver and Z138 mice were slowed down comparing to their
NC groups injected with non-targeted sequence transfected cells,
while no significant differences were observed between their NC
groups and the non-infection groups (p>0.05) (Fig. 2C). At day 20 after inoculation, the
tumors were excised from the mice and weighed, and the inhibition
rates of tumor growth with B7-H3 knockdown were 59.1 and 65.0% in
Maver and Z138 xenograft models (p=0.010 and 0.003) (Fig. 2D). Furthermore, we assessed two
markers reflecting tumor cell proliferation activity, Ki-67 and
PCNA, through immunohistochemical staining. There were apparently
fewer positively Ki-67 and PCNA stained cells in the B7-H3
knockdown groups than in the relative NC groups of Maver and Z138
xenograft models. The Ki-67 labeling indexes of the excised
xenografts in B7-H3 knockdown groups were 34.1±5.2 and 42.3±4.1%,
respectively, significantly lower than in the relative NC groups in
Maver and Z138 mice (p=0.001 and 0.001). The B7-H3 knockdown also
decreased the positive expression rates of PCNA compared with the
NC groups in the two types of MCL xenograft models (38.9±3.9 vs.
76.4±5.9 and 39.9±2.4 vs. 80.3±5.5%) (Fig. 2E).
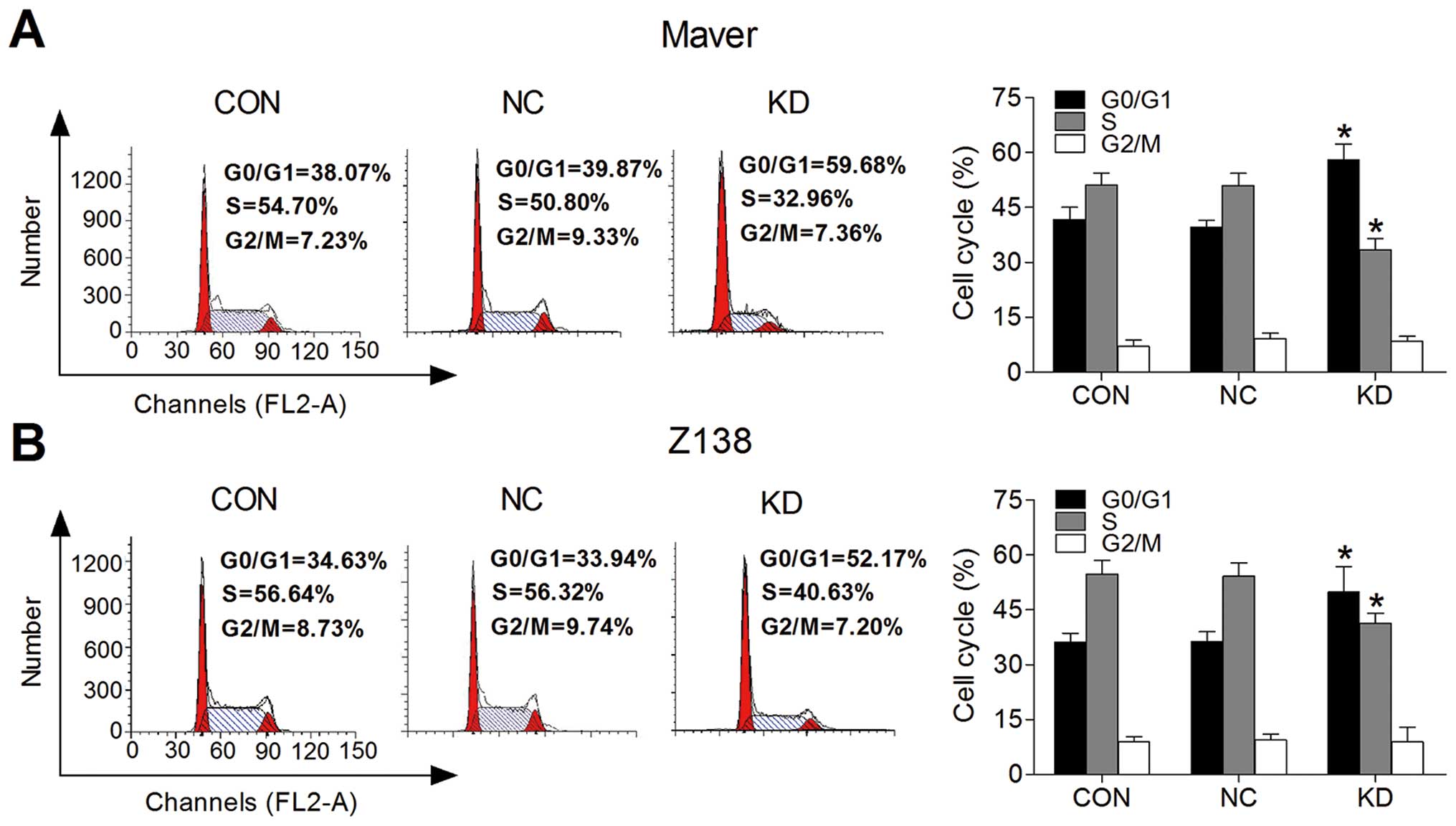
B7-H3 knockdown arrests MCL cell cycle at
the G0/G1 phase
We used flow cytometry comparing the G0/G1, S and
G2/M phases to determine whether B7-H3 expression affects cell
cycle progression. Fig. 3A and B,
respectively, show that the Maver and Z138 cell cycle progression
was inhibited after B7-H3 silencing. The proliferation index (PI)
was decreased by 18.46% in the KD group comparing to the NC group
of Maver cells (p=0.002); while it was reduced by 13.49% in B7-H3
knockdown Z138 cells (p=0.034). This suggests that the knockdown of
B7-H3 arrests MCL cell cycle at the G0/G1 phase to inhibit cell
proliferation.
B7-H3 knockdown inhibits MCL cell
migration and invasion
We used Transwell migration and invasion assays to
compare the cell migration rate and invasive capacity in each group
to determine whether B7-H3 acts as a tumor migration and invasion
regulator. Fig. 4A shows that the
migration rates of the shB7-H3/Maver and shB7-H3/Z138 cells to the
lower chamber were significantly reduced after 24-h incubation
compared with the NC groups, with a 6.9 vs. 34.4% and a 7.4 vs.
63.8% reduction, respectively. In addition, the invasive capacity
of shB7-H3/Maver and shB7-H3/Z138 cells was reduced by 85.5 and
80.1% compared to the NC groups (Fig.
4B). Both the cell migratory and invasive potential in the CON
and NC groups of Maver and Z138 cells were similar (p>0.05).
Furthermore, we measured the invasion-related proteins by RT-PCR,
and found that MMP-2 and MMP-9 were lower in shB7-H3/Maver and
shB7-H3/Z138 cells than in the NC groups (Fig. 4C). These results indicate that
silencing B7-H3 can impede cell migration and inhibit cell invasion
via downregulating the expression of MMP-2 and MMP-9.
B7-H3 knockdown enhances drug-induced
cytotoxicity and apoptosis in vitro
To determine whether B7-H3 knockdown affects
drug-induced cytotoxicity and apoptosis, we selected R and Ben,
which is increasingly being used for the frontline treatment of
mantle cell lymphoma (29).
Following treatment with various concentrations of R or Ben for 24,
48 or 72 h, a dose-dependent and time-dependent inhibition of cell
growth was observed in each group of Maver and Z138 cells using the
CCK-8 assay (data not shown). Concentrations of 3,500 μg/ml R
and/or 4 μg/ml Ben were treated in Maver cell groups, while 2,500
μg/ml R and/or 4 μg/ml Ben were selected for the Z138 cell groups,
and then the absorbance was compared (Fig. 5). The cell survival rates in the KD
groups of Maver and Z138 cells were significantly decreased
compared with their NC groups (p<0.05), and these two drug
combinations synergistically inhibited MCL cell proliferation.
Exposure to 3,500 μg/ml R and/or 4 μg/ml Ben for 12
and 24 h in Maver cells or 2,500 μg/ml R and/or 4 μg/ml Ben in Z138
cells suggested that B7-H3 silencing promoted apoptosis in a
time-dependent manner. The apoptosis rates of shB7-H3/Maver cells
in the drug combination groups were 63.84±7.07% for 12 h and
82.43±4.68% for 24 h, which were the most significantly increased
(p=0.008 and 0.001). The same conclusion can be drawn for Z138
cells (68.06±7.01% for 12 h and 95.99±3.84% for 24 h) (p=0.006 and
0.002) (Fig. 6A–D). After exposure
to R and/or Ben for 24 h, we measured the activity of the
apoptosis-related protein caspase-3. The results demonstrated that
the activity of caspase-3 in the KD group of Maver cells compared
to the NC group was significantly increased treated with R, Ben,
and R+Ben (3.32±0.37, 3.36±0.41, and 4.57±0.50 vs. 2.01±0.35,
2.33±0.26, and 2.93±0.61, respectively) (p=0.011, 0.022, and 0.023)
(Fig. 6E). Also, the same
conclusion can be drawn for Z138 cells (3.45±0.43, 3.79±0.31, and
4.65±0.31 vs. 2.42±0.34, 2.68±0.52 and 3.40±0.35, respectively)
(p=0.032, 0.034, and 0.010) (Fig.
6F). These results indicate that silencing B7-H3 increases
drug-induced cytotoxicity and promotes drug-mediated apoptosis by
increasing the caspase-3 activity in vitro.
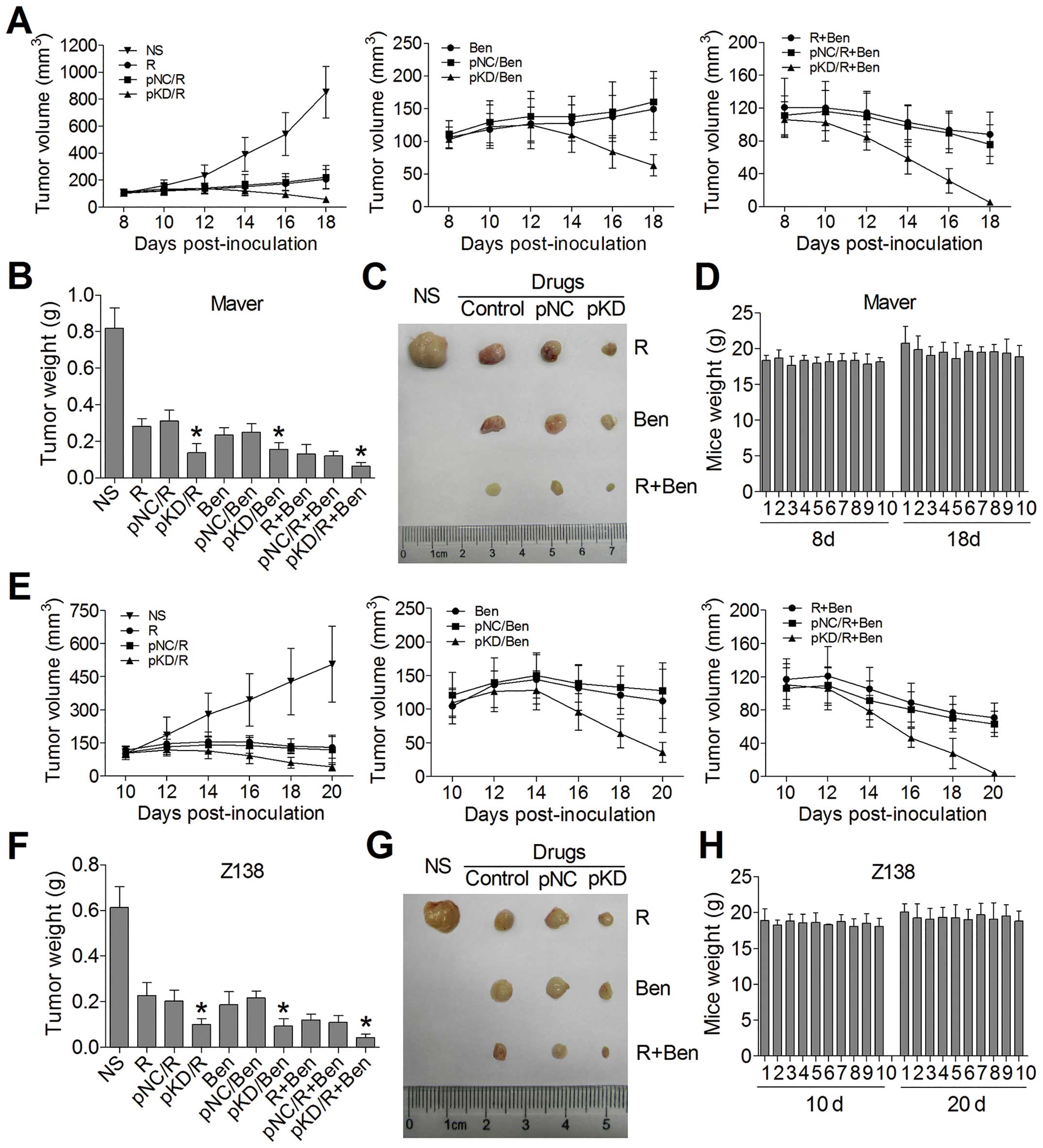
B7-H3 knockdown increases
chemosensitivity in xenograft model
In order to explore the impact of B7-H3 knockdown on
the antitumor activity of chemotherapy drugs in vivo, we
constructed tumor-bearing mouse models injecting into non-infected
Maver and Z138 cells, respectively. The treatments began on day 8
or day 10 in Maver or Z138 xenograft model, when the average tumor
volumes reached 100 mm3. As shown in Fig. 6A and E, the plasmids of B7-H3 shRNA
(pKD) combined with R and/or Ben were more effective in reducing
the established Maver and Z138 tumor growth comparing to the groups
of non-targeted control plasmid (pNC) combining chemotherapy, while
there were no significant differences in the tumor volumes between
the pNC combined with chemotherapy groups and the chemotherapy
groups alone. At the end of observation, the inhibition rates of
tumor growth in pKD combined with R, Ben, and R+Ben groups in Maver
xenograft model were 83.3, 80.9 and 92.3% respectively, which were
higher than in the pNC combined with chemotherapy groups with 62.2,
69.5 and 85.4% (p=0.019, 0.049 and 0.043) (Fig. 7B and C). The same conclusion can be
drawn for Z138 xenograft model, and the inhibition rates of tumor
growth were 83.7, 84.8 and 92.9% vs. 66.8, 64.7 and 82.1% (p=0.030,
0.009 and 0.027) (Fig. 7F and G).
Both the Maver and Z138 groups of B7-H3 shRNA combined with two
drugs received the best antitumor activity. Besides, all of the
mice treated with B7-H3 shRNA exhibited no body weight loss at the
end of our experiment (Fig. 7D and
H). These results indicate that the B7-H3 silencing can
apparently enhance chemosensitivity to mantle cell lymphoma in the
xenograft model.
Discussion
In the present study, we first generated and
confirmed the Maver and Z138 mantle cell lymphoma cells with
targeting B7-H3 knockdown using lentivirus transduction. B7-H3
expression abundance was decreased at the mRNA and protein level in
both MCL cell lines. The membrane proteins were the most
significantly inhibited by 86.9 and 82.4%, respectively. Therefore,
the B7-H3 knockdown was specific and efficient, and the MCL cell
models may be used for subsequent assays.
Previous studies found that the silencing of B7-H3
did not affect pancreatic (23)
and prostate cancer (24) cell
proliferation and moderately reduced (20–30%) the growth of
melanoma cells (20) in
vitro. Interestingly, we found that artificial silencing of
B7-H3 significantly inhibited Maver and Z138 cell growth by 41.7
and 37.5% in 72 h, compared with the relative NC groups. After 14
days of culture, the colony-forming ability in these two B7-H3
knockdown cell lines were both inhibited by 71.2 and 77.2%,
respectively, in contrast to no significant effect on colony
formation performing by B7-H3 silence (30). The different effects of B7-H3 may
depend on the various tumor types. Then, we established xenograft
models to study the in vivo effect of B7-H3 knockdown in
tumorigenicity. The growth of established B7-H3 knockdown Maver and
Z138 xenografts slowed down compared with their NC groups by 59.1
and 65.0% at the end of observation, respectively. The similar
growth inhibition of B7-H3-knockdown xenografts in glioma (22), breast cancer (26) and pancreatic cancer (23) has been observed in other reseach.
Furthermore, we found that the expressions of Ki-67 and PCNA were
significantly decreased in the B7-H3 silenced MCL xenografts. To
determine whether B7-H3 knockdown affects the cell cycle, we
compared the proliferation index (PI) in shB7-H3/Maver and
shB7-H3/Z138 cells with the NC groups, and found that the B7-H3
silencing arrested the cell cycle at the G0/G1 phase. Clearly, the
in vivo results confirmed our in vitro observations,
and the above findings indicate that B7-H3 knockdown can inhibit
the mantle cell lymphoma proliferation through suppressing cell
cycle progression and reducing the expression of Ki-67 and
PCNA.
Several studies reported that B7-H3 promoted tumor
invasion and metastasis in cutaneous melanoma (30), osteosarcoma (25), and non-small cell lung cancer
(31). In this study, we found
that both the cell migratory and invasive potential in the B7-H3
knockdown groups of Maver and Z138 cells was reduced compared to
the NC groups. Since MMP-2 and MMP-9 are proteolytic enzymes
involved in tumor cell migration, invasion, and metastasis
(32), we measured the mRNA level
of both MMPs by RT-PCR and found that the expressions of MMP-2 and
MMP-9 were apparently decreased in shB7-H3/Maver and shB7-H3/Z138
cells. Tekle et al also showed a similar change of MMP-2 in
B7-H3 knockdown melanoma cells (20). It indicates that silencing B7-H3
can impede cell migration and inhibit cell invasion via
downregulating the expression of MMP-2 and MMP-9.
In recent years, the prognosis of mantle cell
lymphoma has improved likely due to two important factors: the
incorporation of high-dose cytarabine in the induction treatment,
followed by autologous hematopoietic cell transplantation in first
remission, and the addition of the anti-CD20 monoclonal antibody
rituximab (R) to chemotherapy regimens (33). However, the management of
relapsed/refractory disease represents a challenge, and a series of
novel agents have entered clinical trials. Among these new
strategies, bendamustine (Ben), a bifunctional alkylating agent, in
combination with rituximab could be recommended as a first-line
therapy for MCL (34). In our
study, we first observed the cytotoxic effect and apoptosis induced
by R and/or Ben in Maver and Z138 cells, and the results confirmed
that silencing of B7-H3 increases chemosensitivity in both the
single drug and two drug combination groups. The synergistic cell
apoptotic effects in Maver and Z138 cells induced by R and Ben were
promoted by B7-H3 knockdown in a time-dependent manner. It
indicates that B7-H3 knockdown enhances drug-induced cytotoxicity
and apoptosis, and a similar conclusion was drawn in B7-H3 silenced
breast cancer cells treated with paclitaxel (26). Furthermore, we detected the
activity of caspase-3 in Maver and Z138 cells incubated with R
and/or Ben for 24 h, which is the mitochondrial downstream effector
caspase in apoptosis signaling cascades. It showed that the
blockade of B7-H3 significantly increased the caspase-3 activity
induced by R and/or Ben in Maver and Z138 cells, compared with
their NC groups.
With the development of B7-H3 targeted therapeutics,
a range of anti-B7-H3 antibodies are under research and some have
entered clinical trial for B7-H3-expressing cancers, such as MGA271
(35). In our study, we treated
the Maver and Z138 xenograft models with the plasmid of B7-H3 shRNA
combined with R and/or Ben, and found that the inhibition rates of
tumor growth were dramatically higher than in the relative
non-targeted control plasmid combining chemotherapy groups. At the
end of observation, the inhibition rates were even up to 92.3 and
92.9% in the groups of B7-H3 shRNA combined with two drugs in Maver
and Z138 mice, respectively. Our findings in vivo
demonstrated that the B7-H3 silence apparently enhanced the
chemosensitivity of rituximab and bendamustine to mantle cell
lymphoma in the xenograft model. Similar promoting effects of B7-H3
on cancer resistance to drug treatments in breast cancer (26) and pancreatic carcinoma (27) xenograft models have been
reported.
In this study, we used RNA interference technology
to reduce B7-H3 expression in mantle cell lymphoma cells and
xenografts, and found that the knockdown of B7-H3 inhibited tumor
proliferation, cell cycle progression, migration and invasion. The
silencing of B7-H3 increased drug-induced apoptosis and enhanced
therapeutic efficacy. Moreover, further investigations should be
performed to explore the exact signaling pathways of B7-H3
contributing to oncogenesis and chemoresistance.
Acknowledgements
The authors are very grateful to Dr Chen Huang of
Key Laboratory of Peking University Third Hospital for assisting
with a preparation of this manuscript. This study was supported by
a grant from the National Natural Science Foundation of China
(81172245).
Abbreviations:
|
MCL
|
mantle cell lymphoma
|
|
NHL
|
non-Hodgkin lymphoma
|
|
FBS
|
fetal bovine serum
|
References
|
1
|
Campo E and Rule S: Mantle cell lymphoma:
Evolving management strategies. Blood. 125:48–55. 2015. View Article : Google Scholar
|
|
2
|
Chen Y, Wang M and Romaguera J: Current
regimens and novel agents for mantle cell lymphoma. Br J Haematol.
167:3–18. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ghielmini M and Zucca E: How I treat
mantle cell lymphoma. Blood. 114:1469–1476. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chapoval AI, Ni J, Lau JS, Wilcox RA,
Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K, et al: B7-H3: A
costimulatory molecule for T cell activation and IFN-gamma
production. Nat Immunol. 2:269–274. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Steinberger P, Majdic O, Derdak SV,
Pfistershammer K, Kirchberger S, Klauser C, Zlabinger G, Pickl WF,
Stöckl J and Knapp W: Molecular characterization of human
4Ig-B7-H3, a member of the B7 family with four Ig-like domains. J
Immunol. 172:2352–2359. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu H, Cheung IY, Guo HF and Cheung NK:
MicroRNA miR-29 modulates expression of immunoinhibitory molecule
B7-H3: Potential implications for immune based therapy of human
solid tumors. Cancer Res. 69:6275–6281. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen C, Shen Y, Qu QX, Chen XQ, Zhang XG
and Huang JA: Induced expression of B7-H3 on the lung cancer cells
and macrophages suppresses T-cell mediating anti-tumor immune
response. Exp Cell Res. 319:96–102. 2013. View Article : Google Scholar
|
|
8
|
Yamato I, Sho M, Nomi T, Akahori T,
Shimada K, Hotta K, Kanehiro H, Konishi N, Yagita H and Nakajima Y:
Clinical importance of B7-H3 expression in human pancreatic cancer.
Br J Cancer. 101:1709–1716. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ingebrigtsen VA, Boye K, Tekle C, Nesland
JM, Flatmark K and Fodstad O: B7-H3 expression in colorectal
cancer: Nuclear localization strongly predicts poor outcome in
colon cancer. Int J Cancer. 131:2528–2536. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun TW, Gao Q, Qiu SJ, Zhou J, Wang XY, Yi
Y, Shi JY, Xu YF, Shi YH, Song K, et al: B7-H3 is expressed in
human hepatocellular carcinoma and is associated with tumor
aggressiveness and postoperative recurrence. Cancer Immunol
Immunother. 61:2171–2182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arigami T, Narita N, Mizuno R, Nguyen L,
Ye X, Chung A, Giuliano AE and Hoon DS: B7-h3 ligand expression by
primary breast cancer and associated with regional nodal
metastasis. Ann Surg. 252:1044–1051. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu Y, Lv X, Wu Y, Xu J, Wang L, Chen W,
Zhang W, Li J, Zhang S and Qiu H: Expression of costimulatory
molecule B7-H3 and its prognostic implications in human acute
leukemia. Hematology. Aug 16–2014.(Epub ahead of print). PubMed/NCBI
|
|
13
|
Zhao D, Lin L, Ge Q, et al: Relation of
B7-H3 molecule expression in multiple myeloma with poor prognosis
and bone destruction. Zhongguo Shi Yan Xue Ye Xue Za Zhi.
21:637–642. 2013.(In Chinese). PubMed/NCBI
|
|
14
|
Wilcox RA, Ansell SM, Lim MS, Zou W and
Chen L: The B7 homologues and their receptors in hematologic
malignancies. Eur J Haematol. 88:465–475. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Luo L, Chapoval AI, Flies DB, Zhu G,
Hirano F, Wang S, Lau JS, Dong H, Tamada K, Flies AS, et al: B7-H3
enhances tumor immunity in vivo by costimulating rapid clonal
expansion of antigen-specific CD8+ cytolytic T cells. J
Immunol. 173:5445–5450. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun X, Vale M, Leung E, Kanwar JR, Gupta R
and Krissansen GW: Mouse B7-H3 induces antitumor immunity. Gene
Ther. 10:1728–1734. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brunner A, Hinterholzer S, Riss P, Heinze
G and Brustmann H: Immunoexpression of B7-H3 in endometrial cancer:
Relation to tumor T-cell infiltration and prognosis. Gynecol Oncol.
124:105–111. 2012. View Article : Google Scholar
|
|
18
|
Leitner J, Klauser C, Pickl WF, Stöckl J,
Majdic O, Bardet AF, Kreil DP, Dong C, Yamazaki T, Zlabinger G, et
al: B7-H3 is a potent inhibitor of human T-cell activation: No
evidence for B7-H3 and TREML2 interaction. Eur J Immunol.
39:1754–1764. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Suh WK, Gajewska BU, Okada H, Gronski MA,
Bertram EM, Dawicki W, Duncan GS, Bukczynski J, Plyte S, Elia A, et
al: The B7 family member B7-H3 preferentially down-regulates T
helper type 1-mediated immune responses. Nat Immunol. 4:899–906.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tekle C, Nygren MK, Chen YW, Dybsjord I,
Nesland JM, Maelandsmo GM and Fodstad O: B7-H3 contributes to the
metastatic capacity of melanoma cells by modulation of known
metastasis-associated genes. Int J Cancer. 130:2282–2290. 2012.
View Article : Google Scholar
|
|
21
|
Chen YW, Tekle C and Fodstad O: The
immunoregulatory protein human B7H3 is a tumor-associated antigen
that regulates tumor cell migration and invasion. Curr Cancer Drug
Targets. 8:404–413. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lemke D, Pfenning PN, Sahm F, Klein AC,
Kempf T, Warnken U, Schnölzer M, Tudoran R, Weller M, Platten M, et
al: Costimulatory protein 4IgB7H3 drives the malignant phenotype of
glioblastoma by mediating immune escape and invasiveness. Clin
Cancer Res. 18:105–117. 2012. View Article : Google Scholar
|
|
23
|
Zhao X, Li DC, Zhu XG, Gan WJ, Li Z, Xiong
F, Zhang ZX, Zhang GB, Zhang XG and Zhao H: B7-H3 overexpression in
pancreatic cancer promotes tumor progression. Int J Mol Med.
31:283–291. 2013.
|
|
24
|
Yuan H, Wei X, Zhang G, Li C, Zhang X and
Hou J: B7-H3 over expression in prostate cancer promotes tumor cell
progression. J Urol. 186:1093–1099. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang L, Zhang Q, Chen W, Shan B, Ding Y,
Zhang G, Cao N, Liu L and Zhang Y: B7-H3 is overexpressed in
patients suffering osteosarcoma and associated with tumor
aggressiveness and metastasis. PLoS One. 8:e706892013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu H, Tekle C, Chen YW, Kristian A, Zhao
Y, Zhou M, Liu Z, Ding Y, Wang B, Mælandsmo GM, et al: B7-H3
silencing increases paclitaxel sensitivity by abrogating Jak2/Stat3
phosphorylation. Mol Cancer Ther. 10:960–971. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao X, Zhang GB, Gan WJ, Xiong F, Li Z,
Zhao H, Zhu DM, Zhang B, Zhang XG and Li DC: Silencing of B7-H3
increases gemcitabine sensitivity by promoting apoptosis in
pancreatic carcinoma. Oncol Lett. 5:805–812. 2013.PubMed/NCBI
|
|
28
|
Li Y, Wang J, Li C and Ke XY: Contribution
of PD-L1 to oncogenesis of lymphoma and its RNAi-based targeting
therapy. Leuk Lymphoma. 53:2015–2023. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rummel MJ, Niederle N, Maschmeyer G, Banat
GA, von Grünhagen U, Losem C, Kofahl-Krause D, Heil G, Welslau M,
Balser C, et al; Study group indolent Lymphomas (StiL).
Bendamustine plus rituximab versus CHOP plus rituximab as
first-line treatment for patients with indolent and mantle-cell
lymphomas: An open-label, multicentre, randomised, phase 3
non-inferiority trial. Lancet. 381:1203–1210. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang J, Chong KK, Nakamura Y, Nguyen L,
Huang SK, Kuo C, Zhang W, Yu H, Morton DL and Hoon DS: B7-H3
associated with tumor progression and epigenetic regulatory
activity in cutaneous melanoma. J Invest Dermatol. 133:2050–2058.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun Y, Wang Y, Zhao J, Gu M, Giscombe R,
Lefvert AK and Wang X: B7-H3 and B7-H4 expression in non-small-cell
lung cancer. Lung Cancer. 53:143–151. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shapiro SD: Matrix metalloproteinase
degradation of extracellular matrix: Biological consequences. Curr
Opin Cell Biol. 10:602–608. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mussetti A, Kumar A, Dahi PB, Perales MA
and Sauter CS: Lifting the mantle: Unveiling new treatment
approaches in relapsed or refractory mantle cell lymphoma. Blood.
Nov 1–2014.(Epub ahead of print). pii: S0268-960X(14)00083-6.
View Article : Google Scholar
|
|
34
|
Gil L, Kazmierczak M, Kroll-Balcerzak R
and Komarnicki M: Bendamustine-based therapy as first-line
treatment for non-Hodgkin lymphoma. Med Oncol. 31:9442014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Loo D, Alderson RF, Chen FZ, Huang L,
Zhang W, Gorlatov S, Burke S, Ciccarone V, Li H, Yang Y, et al:
Development of an Fc-enhanced anti-B7-H3 monoclonal antibody with
potent antitumor activity. Clin Cancer Res. 18:3834–3845. 2012.
View Article : Google Scholar : PubMed/NCBI
|