Introduction
Apoptotic cells are morphologically characterized by
membrane blebbing, chromatin condensation, and the formation of
apoptotic bodies. Apoptosis is a cell suicide mechanism that is
regulated by two canonical programmed cellular signaling pathways;
the death receptor-mediated pathway (extrinsic) and the
mitochondrial pathway (intrinsic). Activation of caspases is
important in both pathways (1,2).
Interaction between ligands and death receptors initiates the
extrinsic pathway at the plasma membrane, subsequently activating
caspase-8, which is an initiator caspase. This protein, in turn,
directly activates downstream effector caspases, including
caspase-3 (3). Many physical and
chemical stimuli induce mitochondrial dysfunction and changes in
reactive oxygen species (ROS) production, triggering the intrinsic
pathway. Mitochondrial dysfunction induces the activation of
caspase-9 and subsequently activates effector caspases, such as
caspase-3. Following the activation of caspase-3, several specific
substrates are cleaved (4,5). Apoptosis is also associated with ROS,
mitochondrial membrane potential (MMP) and other relevant factors.
Apoptosis induction is a critical mechanism for numerous
anti-cancer compounds (6).
Selenium (Se) is an essential trace element
(7), and appropriate Se intake is
necessary for the body to synthesize selenoproteins. Several
studies have indicated that sodium selenite
(Na2SeO3) inhibits growth of a series of
cancer cell lines, including liver and prostate cancer, malignant
melanoma and various hematologic malignancies, by inducing
apoptosis via different mechanisms including mitochondria,
oxidative stress, p53-dependent signaling, and thioredoxin
reductase (8–11). Na2SeO3 in
particular exerts antitumor effects by inducing apoptosis (12–17).
Nasopharyngeal carcinoma (NPC) is the most common
epithelial malignancy of the nasopharynx. The pathogenesis of NPC
is yet not clear, and effective, low-toxicity therapies are not
available so far. Thus, research and development of potential drug
candidates for NPC are of utmost importance. In this study, we
investigated the anti-cancer effects and mechanisms of
Na2SeO3 in CNE-2 NPC cells. We found that
Na2SeO3 can inhibit cell proliferation and
induce cell apoptosis via cell cycle arresting and mitochondrial
pathways.
Materials and methods
Materials
Na2SeO3 was purchased from
Food and Drug Administration of China (Beijing, China) (lot no.
110713-200911) and dissolved in Milli-Q water to get a stock
concentration of 1 mM, then stored at −20°C until use. The Cell
Counting kit-8 (CCK-8) (cat. C0038), MMP assay kit with
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl
carbocyanine iodide (JC-1) (cat. C2006), ROS assay kit (cat.
S0033), DNA Ladder assay kit (cat. C0007), DAPI staining kit (cat.
C1005) and Hoechst 33258 staining kit (cat. C1018) were purchased
from Beyotime Institute of Biotechnology (Jiangsu, China). Annexin
V-FITC poptosis detection kit (cat. A211) and Cell Cycle assay kit
(cat. A411) were purchased from Vazyme Biotech Co., Ltd. (Jiangsu,
China). The primary antibodies for β-actin, Bcl-XL, Bax, Bak,
caspase-3 as well as c-caspase-3 were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). The horseradish peroxidase
(HPR)-linked goat anti-rabbit IgG secondary antibodies were
purchased from Bio-Rad (Hercules, CA, USA).
Cell culture
CNE-2 cell line (a human NPC cell line) was obtained
from the Institute of Biochemistry and Molecular Biology, Guangdong
Medical College (Guangdong, China). Cells were cultured in
RPMI-1640 medium (Gibco-BRL, Carlsbad, CA, USA) supplemented with
10% fetal bovine serum (FBS) (Gibco-BRL) and 100 μg/ml
penicillin-streptomycin (Beyotime Institute of Biotechnology), and
maintained at 37°C in a humidified atmosphere of 5%
CO2.
WST-8 conversion assay
The effect of Na2SeO3 on cell
viability/proliferation was determined using the WST-8 assay.
Briefly, CNE-2 cells were seeded into 96-well plates at a density
of 5,000 cells/well and incubated for 24 h. Then the cells were
treated with Na2SeO3 at different
concentrations (0, 2, 5, 10, 20, 50 and 100 μM) for 0, 24, 48, 72
or 96 h. WST-8 conversion was then assessed using a one-step CCK-8,
according to the manufacturer’s instructions. All tests were
carried out in triplicate. The absorbance was measured at 450 nm by
using a Synergy multifunctional microplate reader (Bio-Rad) with a
reference at 650 nm serving as blank. The inhibiting ability of
Na2SeO3-treated cells was calculated as the
percentage of inhibition compared to untreated cells, which were
arbitrarily assigned 100% viability. GraphPad Prism 4.0 was used to
analyze the IC50 of Na2SeO3 in
this case.
Cell cycle analysis
After treated with Na2SeO3 (0,
5, 10 and 20 μM) for 6 h, CNE-2 cells were collected and fixed
overnight in 75% cold ethanol at −20°C. The cells were then washed
twice with cold PBS (pH 7.4) and stained with Cell Cycle assay kit
according to manufacturer’s instructions. Cell cycle distribution
was determined using a flow cytometer (Beckman Coulter Epics
xL-MCL; Beckman Coulter, Miami, FL, USA) and analyzed using
CellQuest software (18).
DAPI staining
CNE-2 cells at a density of 1×105 were
grown overnight in a cell culture dish. Then the cells were
incubated with Na2SeO3 (0, 5, 10 and 20 μM)
for 3 h. After incubation, cells were washed with ice-cold PBS and
stained with DAPI staining fluid for 20 min in the dark at room
temperature. After that, the cells were washed twice with PBS. Cell
images were captured using a fluorescence microscope (Nikon Corp.,
Tokyo, Japan).
Hoechst 333258 staining
After treatment with Na2SeO3
at a series of concentration for 3 h, the CNE-2 cells were fixed
and washed twice with PBS, then incubated with Hoechst 333258 for
30 min at room temperature. Then the cells were washed with PBS for
three times, and the changes of nucleic morphologies were observed
under fluorescence microscopy (Inverted Biological Binocular
Microscope; Nikon Corp.).
DNA fragmentation analysis
CNE-2 cells (3×106 cells) were incubated
with Na2SeO3 (0, 5, 10 and 20 μM) for 6 or 12
h and then harvested. The characteristic ladder pattern of DNA
breakage was analyzed by agarose gel electrophoresis using an
Apoptosis DNA Ladder detection kit (Beyotime Institute of
Biotechnology) according to the manufacturer’s instructions. The
DNA preparations were electrophoresed in 1% agarose gel, then
stained with ethidium bromide and observed under UV
transilluminator (ChemiDoc XRS Syngene; Bio-Rad).
Annexin V-FITC apoptosis detection
Apoptosis was also analyzed by utilizing an Annexin
V-FITC apoptosis detection kit (Vazyme Biotech Co., Ltd.). Briefly,
cells were seeded in 100 mm-well plates and incubated for 24 h and
then treated with Na2SeO3 (0, 5, 10 and 20
μM) for 24 h. After treatment, ~1×106 cells were
harvested, washed twice with PBS, and stained with Annexin V-FITC
and PI according to the manufacturer’s instructions. The resulting
fluorescence was detected by flow cytometer (Beckman Coulter Epics
xL-MCL, Beckman Coulter) with CellQuest analysis software.
Measurement of intracellular active
oxygen
Formation of intracellular ROS was determined using
a fluorescent probe 2′,7-dichlorofluorescein diacetate (Beyotime
Institute of Biotechnology). DCFH-DA, a non-fluorescent substance,
can cross to cell membranes and be hydrolyzed by intracellular
esterase to DCFH, which can not cross the cell membranes, but
change to green fluorescent DCF in the presence of peroxides. CNE-2
cells were incubated with Na2SeO3 for 3 h,
followed by another 30-min incubation with 10 μM DCFH-DA. Then the
cells were washed with PBS three times and the changes of
fluorescence were observed using fluorescence microscopy.
Measurement of MMP
The MMP was determined using the
mitochondria-specific lipophilic cationic fluorescence dye JC-1
detection kit according to the manufacturer’s instructions
(Beyotime Institute of Biotechnology). In brief, CNE-2 cells, were
seeded in 6-well culture plates at a density of 3×105
cells/well and cultured with or without
Na2SeO3 (0, 5, 10 and 20 μM) for 3 h,
followed by a 30-min incubation with 10 μM JC-1 in the dark, then
the cells were washed twice with PBS, and the fluorescent intensity
was examined by a fluorescence microscope. In healthy cells with
high MMP, JC-1 gathers in mitochondrial matrix as J-aggregates,
which emits red fluorescence (normal membrane potential). When the
MMP collapses, the JC-1 cannot accumulate in mitochondria and are
converted to monomer which can emit green fluorescence (declined
membrane potential).
Western blotting
Cells were washed three times with ice-cold PBS.
Cell lysates were prepared with RIPA buffer (Beyotime Institute of
Biotechnology) containing 150 mM NaCl, 1% Triton X-100, 20 mM Tris
(pH 7.5) and 1% of two kinds of protein inhibitors including
protein phosphatase inhibitor and phenylmethanesulfonyl fluoride
(PMSF). After a forced vortex, cell lysates were incubated on ice
for 2 h, and centrifuged at 12,000 rpm for 15 min at 4°C to remove
insoluble debris. Protein concentrations were determined using the
BCA method (Beyotime Institute of Biotechnology). The whole cell
lysates were treated by boiling in loading buffer containing SDS
and electrophoresised in SDS-PAGE, then transferred onto membranes
(Immobilon-P; Millipore, Billerica, MA, USA). After blocking with
5% skim milk for 1 h, the membranes were incubated with specific
primary antibodies at 4°C overnight, followed by incubation with
enzyme-linked secondary antibodies for 2 h at room temperature. The
membranes were then visualized by enhanced chemiluminescence (ECL),
and the result was analyzed by ChemiDoc XRS transilluminator (both
from Bio-Rad). The gray analysis was carried out by ImageJ Gray
Analysis software.
Statistical analysis
Results are reported as mean ± SD of triplicate
independent experiments. Statistical analyses were performed with
the SPSS v.19.0 software and performed by one-way analysis of
variance (ANOVA). P<0.05 for each concentration versus control
was considered to indicate significance.
Results
Na2SeO3 inhibits
proliferation of CNE-2 cells
We first observed the effect of
Na2SeO3 on proliferation of CNE-2 cells. As
shown in Fig. 1A,
Na2SeO3 significantly inhibited proliferation
of CNE-2 cells in a time- and dose-dependent manner. At 20 μM,
Na2SeO3 inhibited proliferation of CNE-2
cells by 5.55, 29.50, 46.11 and 53.19% after treatment for 24, 48,
72 and 96 h, respectively. When the concentration of
Na2SeO3 increased to 100 μM, the inhibition
rate reached 77.20% after treatment for 96 h. The IC50
of Na2SeO3 for treating CNE-2 cells for
different time periods were analyzed by the curve fitting (Fig. 1B). As compared with the 24 and 72
h, the fitting degree of 48 and 96 h were more accurate, and the
IC50 was 19.86 and 11.9 μM, respectively. Based on these
results, Na2SeO3 was used at 5, 10 and 20 μM
in the following experiments. In addition, after treatment with
Na2SeO3, the cell morphology was observed
under a microscope. As shown in Fig.
1C, after 24 h treatment with Na2SeO3,
the cell density was significantly decreased in a dose-dependent
manner. The cells shrunk, retracted from neighboring cells, lost
their flat and polygonal shape, and ultimately detached from the
culture dish, indicative of a cell death induced by
Na2SeO3.
 | Figure 1The inhibition of sodium selenite
(Na2SeO3) on CNE-2 cells at different times.
(A) The effects of different concentrations of
Na2SeO3 at different times on CNE-2 cell
proliferation. *P<0.05,**p<0.01 vs.
control. The S2, S5, S10, S20, S50 and S100 mean was 2, 5, 10, 20,
50 and 100 μM Na2SeO3, respectively. (B)
Curve fitting analyzed the IC50 of different
concentrations of Na2SeO3 on CNE-2 at
different times. (C) Growth inhibition and morphologic changes of
CNE-2 cells treated with Na2SeO3 (a: control,
b: 5 μM, c: 10 μM, d: 20 μM) for 24 h compared with control cells
(non-Na2SeO3-treated). Cells were
photographed with inverted contrast microscopy (magnification,
×200). |
Na2SeO3 induces
cell cycle arrest in CNE-2 cells
We next observed the effect of
Na2SeO3 on cell cycle of CNE-2 cells. CNE-2
cells were treated with Na2SeO3 at a series
of concentrations for 6 h followed by PI staining and analyzed by a
flow cytometer. As shown in Fig. 2A
and B, treatment of CNE-2 cells with
Na2SeO3 at relatively higher concentrations
(10 and 20 μM) resulted in a significant accumulation of cells at
G0/G1 phase (p<0.05); a sub-G1
apoptotic peak was also observed, while at a relatively lower
concentration (5 μM), Na2SeO3 induced S phase
arrest (p<0.05). Together, these findings suggest that
Na2SeO3 could induce cell cycle arrest in
CNE-2 cells.
Na2SeO3 induces
apoptosis in CNE-2 cells
The cell cycle arrest as well as the apoptotic peak
induced by Na2SeO3 led us to further
determine if Na2SeO3 could induce apoptosis
of CNE-2 cells using an Annexin V-FITC apoptosis detection kit.
Na2SeO3 treated or untreated cells were
analyzed by flow cytometry using Annexin V-FITC/PI double staining
assay. The apoptotic cells could be divided into early-stage
apoptosis (Annexin V+ and PI−) and late-stage
apoptosis (Annexin V+ and PI+), which are
shown in the lower right (LR) and upper right (UR) quadrants of the
FACS histograms, respectively (Fig.
3A, left panel). As shown in Fig.
3B, the percentage of total apoptotic cells in CNE-2 cells was
5.2% in control cells (early-stage: 1.3% and late-stage: 3.9%),
9.3% in cells treated with 5 μM Na2SeO3
(early-stage: 6.0% and late-stage: 3.3%), 51.2% in cells treated
with 10 μM Na2SeO3 (early-stage: 50.3% and
late-stage: 0.9%) and 60.6% in cells treated with 20 μM
Na2SeO3 (early-stage: 60.0% and late-stage:
0.6%). These results indicate that Na2SeO3
significantly induced apoptosis (including early- and late-stage
apoptosis, p<0.01) in CNE-2 cells.
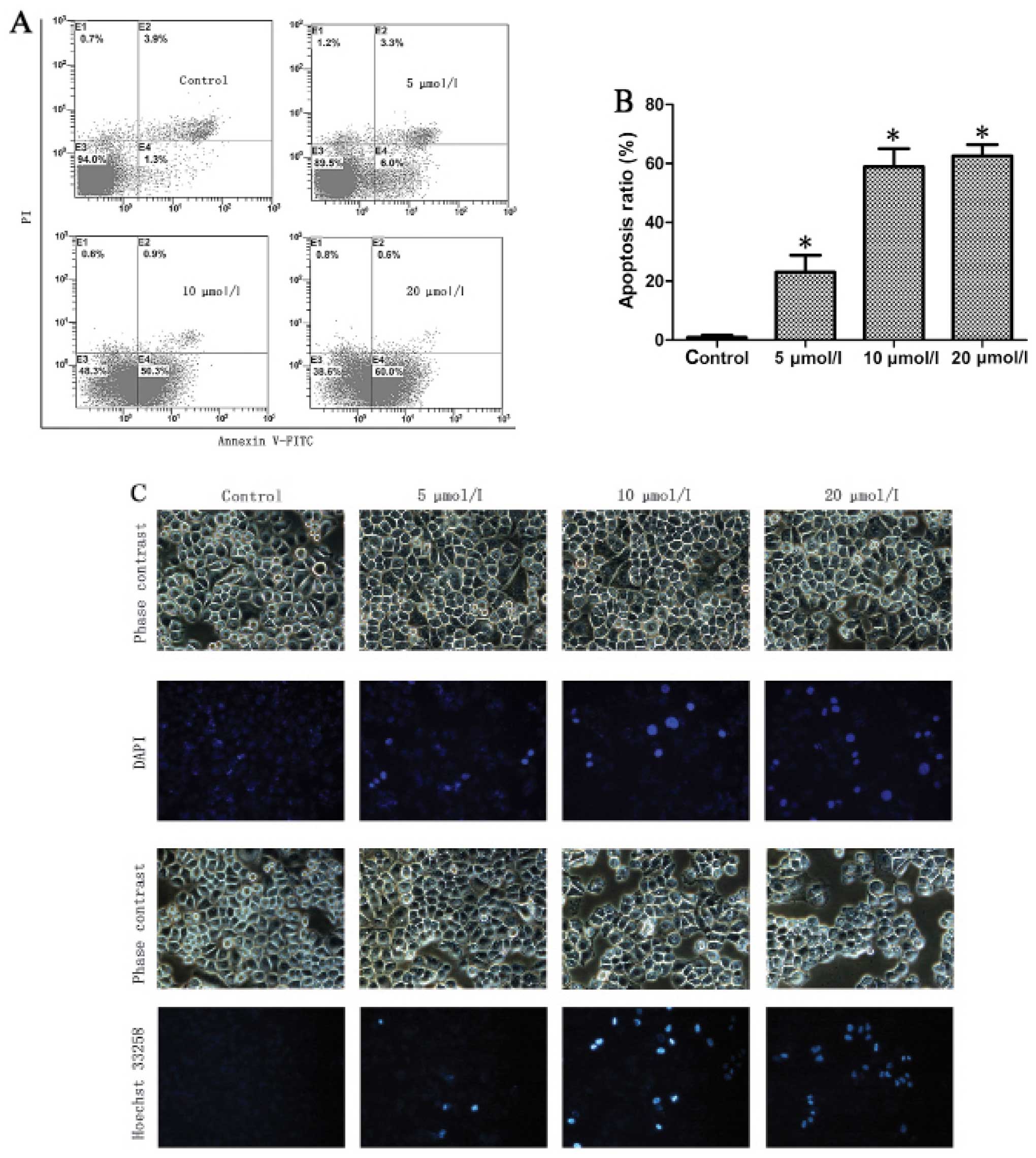 | Figure 3Sodium selenite
(Na2SeO3) induces apoptosis in CNE-2 cells.
(A) Flow cytometry analysis of Annexin V-FITC/PI double-stained
CNE-2 cells. The treatment of CNE-2 cells with
Na2SeO3 (24 h) results in significant
increases in the percentages of apoptotic cells. (B) Values are
expressed as the mean ± SD of three experiments in duplicate,
*p<0.05 vs. control. (C) DAPI and Hoechst 33258
stained nucleus of control and Na2SeO3 (5, 10
and 20 μmol/l; 3 h)-treated cells (magnification, ×200). (D) DNA
ladder of control and Na2SeO3 (5, 10 and 20
μmol/l; 6 or 12 h)-treated cells. Lane 1, 1,000 bp marker; lanes
2–5, Na2SeO3 treated cells 6 h (2: control,
3: 5 μmol/l, 4: 10 μmol/l, 5: 20 μmol/l); lane 6, 15,000 bp marker;
lanes 7–10, Na2SeO3 treated cells for 12 h
(7: control, 8: 5 μmol/l, 9: 10 μmol/l, 10: 20 μmol/l). |
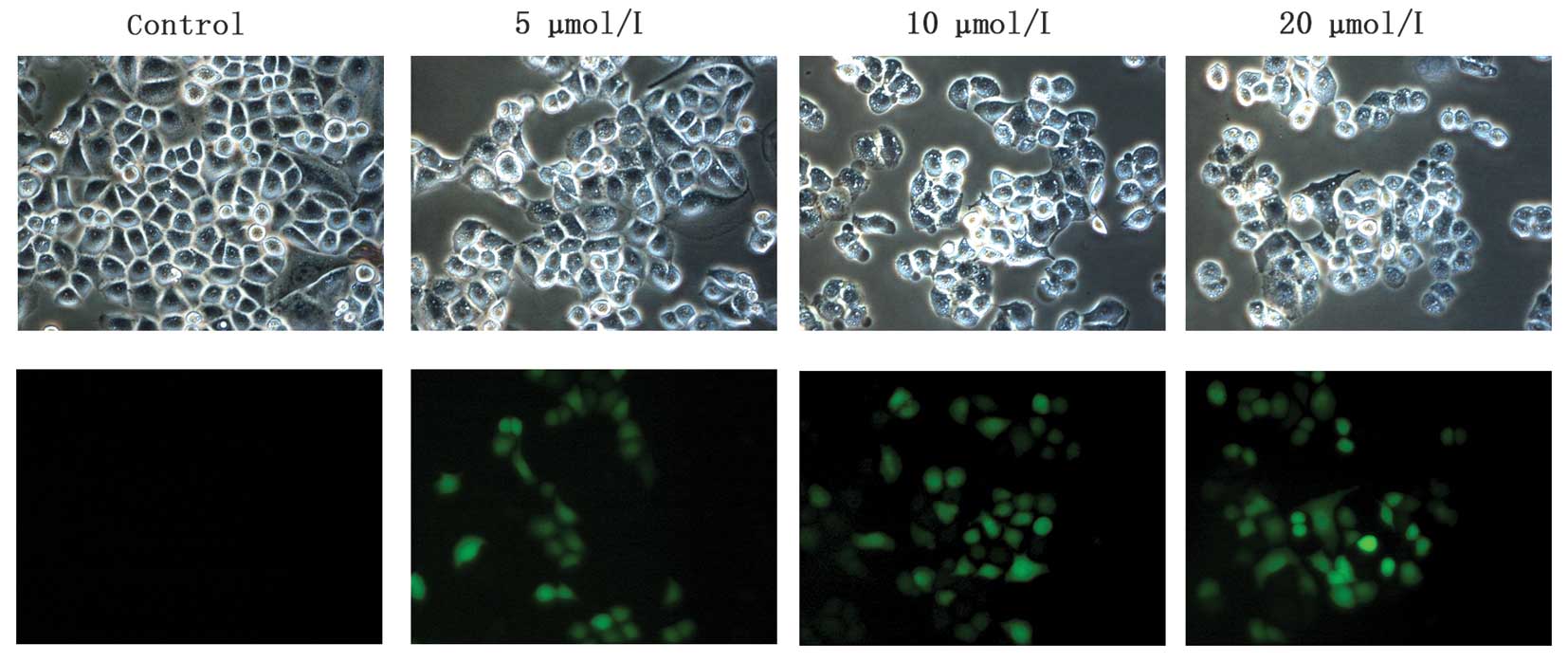
To further confirm
Na2SeO3-induced apoptosis, we next observed
the morphological change of cell nuclei induced by
Na2SeO3 via staining cell nuclei with DAPI
and Hoechst 333258. As shown in Fig.
3C, chromatic agglutination and karyopyknosis were observed
after treatment with Na2SeO3 (5, 10 and 20
μM) for 3 h, and fragmented nuclei were observed after treatment
for 6 and 12 h. In contrast, cells in control group exhibited
normal intact nuclei. Furthermore, after treatment with
Na2SeO3, a series of DNA ladders were
observed (Fig. 3D), indicative of
late-stage apoptosis in CNE-2 cells. These results further
demonstrated that Na2SeO3 can induce
significant apoptosis in CNE-2 cells.
Na2SeO3
downregulates Bcl-XL and upregulates Bak and Bax
The Bcl-XL, Bak and Bax proteins play crucial roles
in the regulation of apoptosis (19,20).
We examined the changes in the expression of Bcl-XL, Bak and Bax by
western blotting in CNE-2 cells in response to
Na2SeO3 treatment. The representative blots
for CNE-2 cells are shown in Fig.
4A, and the relative expression of these proteins was also
calculated through normalization to β-actin expression and is
summarized in Fig. 4B. The results
showed that after treatment with Na2SeO3 (5,
10 and 20 μM) for 6 h, the expression of Bak and Bax was increased
and the expression of Bcl-XL was decreased in a dose-dependent
manner in CNE-2 cells (Fig. 4).
These data suggest that Na2SeO3 might induce
CNE-2 cell apoptosis through downregulation of Bcl-XL and
upregulation of Bak and Bax.
Na2SeO3 induces
disruption of MMP in CNE-2 cells
Mitochondria play a key role in cell apoptosis and
depletion of MMP is one of the early and key events that occur
following induction of cellular apoptosis. To determine the changes
of MMP in CNE-2 cells after Na2SeO3
treatment, JC-1 staining was carried out. The fluorescence
microscopy observation confirmed that Na2SeO3
(5, 10 and 20 μM)-treated cells showed a progressive loss of red
J-aggregates fluorescence and appearance of green monomer
fluorescence in the cytoplasm, and this decrease obviously occurred
in a dose-dependent manner (Fig.
5). These data suggest that the intrinsic mitochondrial pathway
of apoptosis might be one of the mechanisms involved in cell death
of CNE-2 cells induced by Na2SeO3.
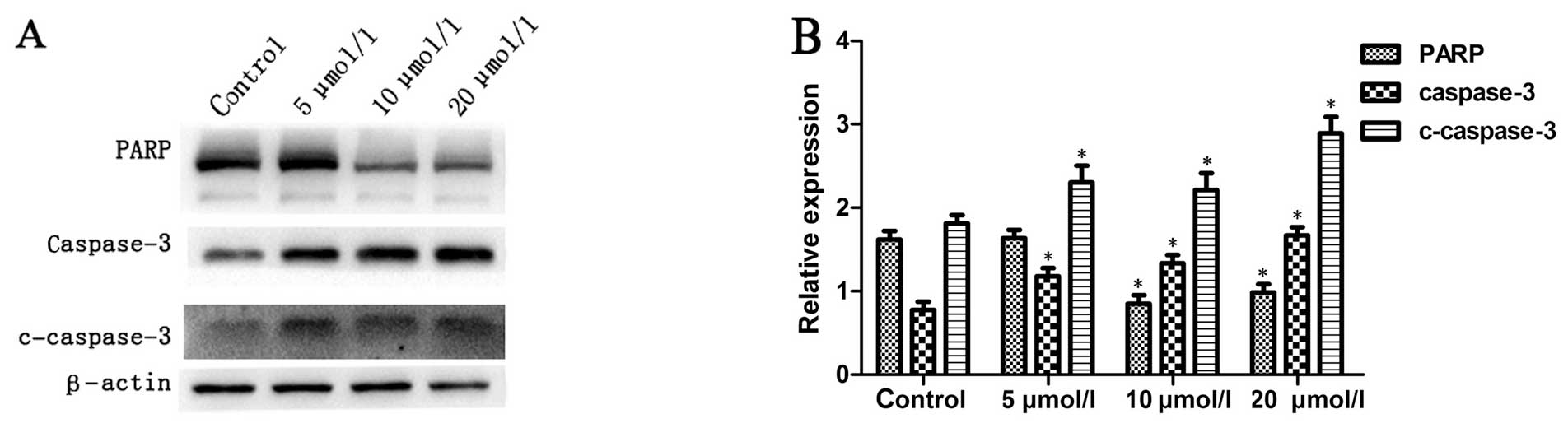
Na2SeO3 increases
caspase-3 activity in CNE-2 cells
It is well known that the intracellular
translocation of Bak and Bax can induce the loss of MMP, which is
linked to the initiation and activation of the apoptotic process in
cells (21,22). Due to the loss of MMP, cytochrome
c is released into the cytosol from mitochondria, which
activates pro-caspase-9 in the apoptosome and leads to the cleavage
of caspase-3 (1). Subsequently,
active cleaved caspase-3 can cleave a broad spectrum of target
proteins and finally result in apoptotic cell death. To examine
whether apoptosis induced by Na2SeO3 involves
caspase activation, the total and cleaved caspase-3 were examined
by western blotting. The results showed that treatment with
Na2SeO3 for 6 h resulted in significant
increase in caspase-3 activity in a dose-dependent manner (Fig. 6) as indicated by increase of
cleaved caspase-3 and decrease of total PARP, a substrate of
caspase-3, suggesting that apoptosis of CNE-2 cells induced by
Na2SeO3 might involve the activation of
caspase-3 pathway.
Na2SeO3 induces ROS
production in CNE-2 cells
Previous reports have shown that ROS generation
plays a critical role in apoptosis induction (23–25).
Therefore, we next investigated the changes of ROS level in
Na2SeO3-treated CNE-2 cells. As shown in
Fig. 7,
Na2SeO3 (5, 10 and 20 μM) enhanced the levels
of ROS in CNE-2 cells in a dose-dependent manner, which might also
contribute to Na2SeO3-induced apoptosis in
CNE-2 cells.
Discussion
Compared to other types of head and neck cancer, NPC
is a highly metastatic disease. Despite advances in diagnosis and
treatment, the 5-year survival of this malignant disease remains
disappointing due to the local recurrence/metastasis and resistance
to chemo- and radiotherapies. Therefore, an increasing
understanding of the complex metastases/recurrence mechanisms of
NPC is imperative to the development of more effective
mechanism-based therapeutic modalities for this malignancy.
Se is an essential trace element because of its role
in glutathione peroxidase (GSH-Px), the daily dietary supply of Se
in human body must reach 50 μg according to the Chinese Nutrition
Society, a standard that has been adopted by the World Health
Organization (Geneva, Switzerland) (26). In addition to its nutritional
functions, accumulating evidence has shown that super-nutritional
selenite intake has antitumor activity both in vitro and
in vivo (14,27–32).
Mechanistically, Na2SeO3 can induce cell
apoptosis through mitochondrial apoptotic pathway in a variety of
cancer cell lines including ovarian carcinoma, lung carcinoma,
colon cancer and breast cancer cells (15,33–36).
In the present study, we demonstrated for the first time to our
knowledge that Na2SeO3 can inhibit
proliferation and induce cell cycle arrest in CNE-2 NPC cells.
Interestingly, treatment of CNE-2 cells with higher concentrations
of Na2SeO3 resulted in a significant
accumulation of cells at G 0/G1 phase, while
treatment with the agent at lower concentrations arrested the cell
cycle at S phase. Therefore, further studies are warranted to
elucidate the detailed underlying mechanisms.
Mitochondrial pathway is critical in cell apoptosis.
Mitochondrial dysfunction can induce the activation of caspase-9
and the subsequent caspase-3, followed by cleavage of its
substrates such as PARP, eventually inducing cell apoptosis
(4,5). Increase of ROS production could
induce mitochondrial dysfunction. Our studies show that
Na2SeO3 could increase ROS production, induce
disruption of MMP and activate the caspase-3 pathway. All these
data suggest that Na2SeO3-induced apoptosis
in CNE-2 cells might be associated with the mitochondrial apoptotic
pathway. This was further confirmed by the results that
Na2SeO3 could downregulate Bcl-XL and
upregulate Bak and Bax, which are apoptosis-related proteins
located in mitochondria. Therefore, we speculated that
Na2SeO3 inhibited CNE-2 cell growth through
its effects on mitochondria.
In summary, our studies demonstrate that
Na2SeO3 has significant anti-proliferation-
and apoptosis-induction effects in CNE-2 cells by cell cycle
arresting and regulation of mitochondria-mediated intrinsic caspase
pathway, suggesting that Na2SeO3 might have
potent therapeutic potentials in the treatment of NPC.
Acknowledgements
This study was financially supported by grants from
the National Natural Science Foundation of China (no. 81272434),
the Science and Technological Program of Dongguan’s Higher
Education, Science and Research, and Health Care Institutions (no.
2011108102044), the Doctor Initial Funding of Guangdong Medical
College (no. B2011022), and the Science and Technology Innovation
Fund of Guangdong Medical College (no. STIF201105).
References
|
1
|
Wolf BB and Green DR: Suicidal tendencies:
Apoptotic cell death by caspase family proteinases. J Biol Chem.
274:20049–20052. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Susin SA, Daugas E, Ravagnan L, Samejima
K, Zamzami N, Loeffler M, Costantini P, Ferri KF, Irinopoulou T,
Prévost MC, et al: Two distinct pathways leading to nuclear
apoptosis. J Exp Med. 192:571–580. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Elrod HA and Sun SY: Modulation of death
receptors by cancer therapeutic agents. Cancer Biol Ther.
7:163–173. 2008. View Article : Google Scholar
|
|
4
|
Kim BG, Kwon HY, Sohn EJ, Hwang S, Kwon OS
and Kim SH: Activation of caspases and inhibition of ribosome
biogenesis mediate antitumor activity of Chijongdan in A549
non-small lung cancer cells. BMC Complement Altern Med. 14:4202014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zorofchian Moghadamtousi S, Karimian H,
Rouhollahi E, Paydar M, Fadaeinasab M and Abdul Kadir H: Annona
muricata leaves induce G1 cell cycle arrest and
apoptosis through mitochondria-mediated pathway in human HCT-116
and HT-29 colon cancer cells. J Ethnopharmacol. 156:277–289. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qu L, Liu FX, Cao XC, Xiao Q, Yang X and
Ren KQ: Activation of the apoptosis signal-regulating kinase
1/c-Jun N-terminal kinase pathway is involved in the
casticin-induced apoptosis of colon cancer cells. Exp Ther Med.
8:1494–1500. 2014.PubMed/NCBI
|
|
7
|
Chen Y, Mo HZ, Hu LB, Li YQ, Chen J and
Yang LF: The endogenous nitric oxide mediates selenium-induced
phytotoxicity by promoting ROS generation in Brassica rapa. PLoS
One. 9:e1109012014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li Z, Meng J, Xu TJ, Qin XY and Zhou XD:
Sodium selenite induces apoptosis in colon cancer cells via
Bax-dependent mitochondrial pathway. Eur Rev Med Pharmacol Sci.
17:2166–2171. 2013.PubMed/NCBI
|
|
9
|
Li XL, Wong YS, Xu G and Chan JC:
Selenium-enriched Spirulina protects INS-1E pancreatic beta cells
from human islet amyloid polypeptide-induced apoptosis through
suppression of ROS-mediated mitochondrial dysfunction and PI3/AKT
pathway. Eur J Nutr. Aug 12–2014.(Epub ahead of print).
|
|
10
|
Sarveswaran S, Liroff J, Zhou Z, Nikitin
AY and Ghosh J: Selenite triggers rapid transcriptional activation
of p53, and p53-mediated apoptosis in prostate cancer cells:
Implication for the treatment of early-stage prostate cancer. Int J
Oncol. 36:1419–1428. 2010.PubMed/NCBI
|
|
11
|
Huang F, Huang J, Lv Q, Yang Y, Wu G and
Xu C: Selenite induces apoptosis in colorectal cancer cells through
interaction with thioredoxin reductase. BMB Rep. pii: 2370.
2013.
|
|
12
|
Cherukuri DP and Nelson MA: Role of
reactive oxygen species (ROS) and JNKs in selenite-induced
apoptosis in HepG2 cells. Cancer Biol Ther. 7:697–698. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen XJ, Duan FD, Zhang HH, Xiong Y and
Wang J: Sodium selenite-induced apoptosis mediated by ROS attack in
human osteosarcoma U2OS cells. Biol Trace Elem Res. 145:1–9. 2012.
View Article : Google Scholar
|
|
14
|
Huang F, Nie C, Yang Y, Yue W, Ren Y,
Shang Y, Wang X, Jin H, Xu C and Chen Q: Selenite induces
redox-dependent Bax activation and apoptosis in colorectal cancer
cells. Free Radic Biol Med. 46:1186–1196. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang W, Xiao H and Parkin KL: Apoptosis
in MCF-7 breast cancer cells induced by S-alkenylmercaptocysteine
(CySSR) species derived from Allium tissues in combination with
sodium selenite. Food Chem Toxicol. 68:1–10. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu S, Bao Y, Ma D, Zi Y, Yang C, Yang M,
Xing M and Yang W: Sodium selenite inhibits leukemia HL-60 cell
proliferation and induces cell apoptosis by enhancing the
phosphorylation of JNK1 and increasing the expression of p21 and
p27. Int J Mol Med. 34:1175–1179. 2014.PubMed/NCBI
|
|
17
|
Suzuki M, Endo M, Shinohara F, Echigo S
and Rikiishi H: Rapamycin suppresses ROS-dependent apoptosis caused
by selenomethionine in A549 lung carcinoma cells. Cancer Chemother
Pharmacol. 67:1129–1136. 2011. View Article : Google Scholar
|
|
18
|
Shi X, Jin Y, Cheng C, Zhang H, Zou W,
Zheng Q, Lu Z, Chen Q, Lai Y and Pan J: Triptolide inhibits Bcr-Abl
transcription and induces apoptosis in STI571-resistant chronic
myelogenous leukemia cells harboring T315I mutation. Clin Cancer
Res. 15:1686–1697. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oltvai ZN, Milliman CL and Korsmeyer SJ:
Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that
accelerates programmed cell death. Cell. 74:609–619. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kitamura Y, Shimohama S, Kamoshima W, Ota
T, Matsuoka Y, Nomura Y, Smith MA, Perry G, Whitehouse PJ and
Taniguchi T: Alteration of proteins regulating apoptosis, Bcl-2,
Bcl-x, Bax, Bak, Bad, ICH-1 and CPP32, in Alzheimer’s disease.
Brain Res. 780:260–269. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hockenbery D, Nuñez G, Milliman C,
Schreiber RD and Korsmeyer SJ: Bcl-2 is an inner mitochondrial
membrane protein that blocks programmed cell death. Nature.
348:334–336. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gross A, McDonnell JM and Korsmeyer SJ:
BCL-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma Q, Fang H, Shang W, Liu L, Xu Z, Ye T,
Wang X, Zheng M, Chen Q and Cheng H: Superoxide flashes: Early
mitochondrial signals for oxidative stress-induced apoptosis. J
Biol Chem. 286:27573–27581. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Said RS, Badr AM, Nada AS and El-Demerdash
E: Sodium selenite treatment restores long-lasting ovarian damage
induced by irradiation in rats: Impact on oxidative stress and
apoptosis. Reprod Toxicol. 43:85–93. 2014. View Article : Google Scholar
|
|
25
|
Weekley CM, Jeong G, Tierney ME, Hossain
F, Maw AM, Shanu A, Harris HH and Witting PK: Selenite-mediated
production of superoxide radical anions in A549 cancer cells is
accompanied by a selective increase in SOD1 concentration, enhanced
apoptosis and Se-Cu bonding. J Biol Inorg Chem. 19:813–828. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang GQ and Gu LZ: The requirements and
acceptable daily intake of trace element selenium in human body.
Prog Physiol Sci. 23:184–186. 1992.
|
|
27
|
Guan L, Han B, Li J, Li Z, Huang F, Yang Y
and Xu C: Exposure of human leukemia NB4 cells to increasing
concentrations of selenite switches the signaling from pro-survival
to pro-apoptosis. Ann Hematol. 88:733–742. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Z, Shi K, Guan L, Cao T, Jiang Q, Yang
Y and Xu C: ROS leads to MnSOD upregulation through ERK2
translocation and p53 activation in selenite-induced apoptosis of
NB4 cells. FEBS Lett. 584:2291–2297. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guan L, Han B, Li Z, Hua F, Huang F, Wei
W, Yang Y and Xu C: Sodium selenite induces apoptosis by
ROS-mediated endoplasmic reticulum stress and mitochondrial
dysfunction in human acute promyelocytic leukemia NB4 cells.
Apoptosis. 14:218–225. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mi L, Xiao Z, Hood BL, Dakshanamurthy S,
Wang X, Govind S, Conrads TP, Veenstra TD and Chung FL: Covalent
binding to tubulin by isothiocyanates. A mechanism of cell growth
arrest and apoptosis. J Biol Chem. 283:22136–22146. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiang Q, Wang Y, Li T, Shi K, Li Z, Ma Y,
Li F, Luo H, Yang Y and Xu C: Heat shock protein 90-mediated
inactivation of nuclear factor-κB switches autophagy to apoptosis
through BECN1 transcriptional inhibition in selenite-induced NB4
cells. Mol Biol Cell. 22:1167–1180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang HY and Ou BX: Experimental study on
selenium preventing nasopharyngeal carcinoma. Zhonghua Yu Fang Yi
Xue Za Zhi. 26:281–283. 1992.(In Chinese). PubMed/NCBI
|
|
33
|
Park JS, Ryu JY, Jeon HK, Cho YJ, Park YA,
Choi JJ, Lee JW, Kim BG and Bae DS: The effects of selenium on
tumor growth in epithelial ovarian carcinoma. J Gynecol Oncol.
23:190–196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Park SH, Kim JH, Chi GY, Kim GY, Chang YC,
Moon SK, Nam SW, Kim WJ, Yoo YH and Choi YH: Induction of apoptosis
and autophagy by sodium selenite in A549 human lung carcinoma cells
through generation of reactive oxygen species. Toxicol Lett.
212:252–261. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Králová V, Benešová S, Cervinka M and
Rudolf E: Selenite-induced apoptosis and autophagy in colon cancer
cells. Toxicol In Vitro. 26:258–268. 2012. View Article : Google Scholar
|
|
36
|
Sharma G, Park J, Sharma AR, Jung JS, Kim
H, Chakraborty C, Song DK, Lee SS and Nam JS: Methoxy
poly(ethyleneglycol)-poly(lactide) nanoparticles encapsulating
quercetin act as an effective anticancer agent by inducing
apoptosis in breast cancer. Pharm Res. 32:723–735. 2015. View Article : Google Scholar
|