Introduction
Clinical staging for malignancies provides a useful
guidance for predicting survival and for deciding optimal treatment
strategies (1). Design of a cancer
staging system depends on the identification of individual
prognostic factors that can predict survival of cancer patients
(1–3). Unlike other solid tumors, the
prognosis and treatment strategies for subjects with hepatocellular
carcinoma (HCC) depend not only on the tumor characteristics but
also on the degree of liver function (2–9).
Based on the identification of relevant predictors for both the
tumor burden and liver functional reserve, several staging systems
for HCC including both aspects had been proposed.
In 1998, the Cancer of the Liver Italian Program
(CLIP) proposed a new scoring system (CLIP scoring system) that
accounts for both tumor characteristics and liver function relevant
to prognostic evaluation for HCC patients. This score consisted of
four variables of Child-Pugh classification, α-fetoprotein (AFP)
value, tumor morphology and portal vein invasion and its prognostic
ability has been validated in several countries (2–5). On
the other hand, Llovet et al (6) proposed Barcelona Clinic Liver Cancer
(BCLC) classification system for HCC consisting of tumor
characteristics, associated liver disease and the Eastern
Cooperative Oncology Group (ECOG) performance status (ECOG-PS) in
1999. This is the only system that provides treatment
recommendations for each HCC stage based on the best treatment
strategies currently available and has been externally validated in
the United States and Europe and endorsed by both the European
Association for the Study of the Liver (EASL) and the American
Association for the Study of Liver Diseases (AASLD). (7–9) In
Japan, in 2003, Kudo et al proposed the Japan Integrated
Staging (JIS) system consisting of Child-Pugh classification and
HCC stage as defined by TNM classification by the Liver Cancer
Study Group of Japan (LCSGJ) as a prognostic system and they
demonstrated that this system was a better prognostic system than
CLIP scoring system using a large cohort (n=4525) (10–12).
Currently, more than ten staging classification for HCC are
available (13).
The major difference between CLIP scoring system,
BCLC classification system and JIS system is that only BCLC
classification system included ECOG-PS as a variable. The PS scale
is a major survival determinant in patients with HCC (14,15).
Especially in HCC patients complicated with liver cirrhosis (LC),
those with deteriorated PS are encountered in the daily clinical
practice. This is probably due to the fact that LC related
complications such as ascites, encephalopathy and muscle wasting
lead to deterioration of PS (16)
Furthermore, in Japan, the proportion of aged HCC patients with
potentially poorer PS has been increasing (17).
Currently, there are two modified JIS system:
biomarker combined JIS system and the model for end stage liver
disease-based JIS system (18,19).
In the present study, on the basis of above, we herein propose a PS
combined JIS (PS-JIS) system for HCC patients with LC. The aims of
the present study were to examine the prognostic ability of our
proposed PS-JIS system in HCC patients with LC comparing with other
prognostic systems.
Patients and methods
Patients
A total of 1,170 consecutive treatment-naïve
patients diagnosed with HCC complicated with LC were admitted to
the Department of Gastroenterology and Hepatology, Osaka Red Cross
Hospital, Japan, between March 2004 and June 2014. LC was
determined based on radiologic findings including typical computed
tomography (CT) or ultrasound findings, laboratory parameters
and/or histological findings obtained by surgical specimens or
liver biopsy. PS was evaluated by using the ECOG performance scale
ranging from 0 (asymptomatic) to 4 (confined to bed).
As reported by Kudo et al JIS score was
calculated by summation of TNM stage score by the LCSGJ (stage I,
0; stage I, 1; stage III, 2; and stage IV, 3) and Child-Pugh
classification (A, 0; B, 1; and C, 2) (10,11).
Our proposed PS-JIS system was calculated by summation of TNM stage
score by the LCSGJ (stage I, 0; stage II, 1; stage III, 2; and
stage IV, 3), Child-Pugh classification (A, 0; B, 1; and C,2) and
PS (PS 0, 0; PS 1, 1; and PS >2, 2). Thus, scores of our
proposed PS-JIS system ranged from 0 to 7 (Table I). The disease was staged for all
analysed patients by means of five staging systems including JIS
system, our proposed PS-JIS system, BCLC classification system, TNM
classification system and CLIP scoring system. We examined the
prognostic ability in each prognostic system using concordance
index (c-index) as described later. Furthermore, we examined
prognostic factors associated with overall survival (OS) using
univariate and multivariate analyses. The following data were used
for the current analyses: gender, age, tumor number, maximum tumor
size, Child-Pugh classification, ECOG-PS, initial treatment
modality, cause of liver disease, aspartate aminotransferase (AST),
alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma
glutamyl transpeptidase (GGT), platelet count and tumor
markers.
 | Table IDefinition of the proposed performance
status combined Japan Integrated Staging system. |
Table I
Definition of the proposed performance
status combined Japan Integrated Staging system.
| Score |
|---|
|
|
|---|
| Variables | 0 | 1 | 2 | 3 |
|---|
| Child-Pugh stage | A | B | C | |
| TNM stage
(LCSGJ) | I | II | III | IV |
| Performance
status | 0 | 1 | >2 | |
Prior to therapy for HCC, written informed consent
for HCC therapy was obtained from all subjects. The ethics
committee of our department approved the protocol for this study.
The present study comprised a retrospective analysis of patients’
medical records in our database and all treatments were performed
in an open-label manner.
Diagnosis of HCC and HCC therapy
HCC was diagnosed based on the results from
abdominal ultrasound and dynamic CT scan (hyper-attenuation during
the arterial phase in the entire or part of the tumor, and
hypo-attenuation in the portal-venous phase) and/or magnetic
resonance imaging (MRI) mainly as recommended by the AASLD
(14). Arterial and portal phase
dynamic CT images were obtained ~30 and 120 sec after injection of
contrast material. In our hospital, abdominal angiography combined
with CT (angio-CT) was routinely performed before therapy for HCC
after obtaining informed consent for performing abdominal
angiography. This was performed based on the fact that this
technique was useful for detecting small satellite nodules as
reported by Yamasaki et al (20). Then, we confirmed HCC using CT
during hepatic arteriography (CTHA) and CT during
arterial-portography (CTAP). Vascular invasion was determined by
dynamic CT and/or angio-CT. During initial evaluation for HCC, a
chest X-ray was performed, and if abnormal, a chest CT scan was
done. Bone scintigraphy or brain CT scan or MRI was done if there
was any suggesting symptoms or clinical indication. As for HCC
therapy, the most appropriate treatment modality for each HCC
patient was selected through discussion with surgeons,
hepatologists and radiologists (21,22).
Best supportive care was provided when treatment efficacy was
considered limited or patients refused therapy for HCC. In the
present analysis, there was no patient treated with liver
transplantation.
Follow-up after initial therapy for
HCC
Follow-up observation consisted of regularly blood
tests and monitoring of tumor markers, including AFP and
des-γ-carboxy prothrombin (DCP), which was measured using a
chemiluminescent enzyme immunoassay (Lumipulse PIVKAII Eisai; Eisai
Co., Ltd., Tokyo, Japan). Dynamic CT scan was performed every 3–4
months after initial therapy for HCC. When HCC recurrence or
disease progression was detected based on radiologic findings, most
appropriate therapy was performed in each patient.
Statistical analysis
In the present study, OS was the only end point.
Data were analyzed using univariate and multivariate analyses. To
analyze the significance of prognostic predictors, continuous
variables were divided by the median values for all cases (n=1,170)
and treated as dichotomous covariates. The cumulative OS rate was
calculated by Kaplan-Meier method and tested by log-rank test. A
Cox proportional hazard model via a stepwise forward method was
used for multivariate analyses of factors with P-value <0.05 in
univariate analyses. These statistical methods were used to
estimate the interval from the date of diagnosis for HCC until the
date of death or last follow-up date.
To evaluate the discriminatory ability for
predicting survival, we assessed the accuracy of prediction of
death at 1, 3 and 5 years for each scoring system. This score was
assessed by calculating the area under the receiver operating
characteristic (ROC) curve for each score [which is equivalent to
the concordance index (c-index)] (23). To perform this test, subjects
censored before 1, 3 and 5 years were excluded from the analysis.
C-index of 0.5 indicates that the model is no better than chance at
making a prediction of membership in a group and a value of 1.0
indicates that the model perfectly identifies those within a group
and those not. Models are typically considered reasonable when the
c-index is >0.70 (24).
Data were analyzed using SPSS software (SPSS, Inc.,
Chicago, IL, USA) for Microsoft Windows. Data are expressed as
median value (range). A P-value <0.05 were considered to be
statistically significant.
Results
Patient demographic characteristics
Baseline demographic characteristics of analysed
patients (n=1,170) are shown in Table
II. They included 742 males and 446 female. The median age was
70 (range, 32–91). There were 804 patients in Child-Pugh A, 303 in
Child-Pugh B and 63 in Child-Pugh C. In terms of ECOG-PS, they
included 885 subjects in PS 0, 148 in PS 1, 93 in PS 2, 29 in PS 3
and 15 in PS 4, respectively. The median maximum tumor diameter was
2.5 cm (range, 0.5–18 cm). The proportion of hepatitis
virus-related (hepatitis B, C or B and C) was 81.6% (955/1170). In
the present analyses, AFP values were missing from two subjects and
DCP values were missing from 15 subjects.
 | Table IIBaseline characteristics
(n=1,170). |
Table II
Baseline characteristics
(n=1,170).
| Variables | No. or median value
(range) |
|---|
| Age (years) | 70 (32–91) |
| Gender,
male/female | 724/446 |
| Causes of liver
disease, B/C/non-B non-C/B and C | 120/816/215/19 |
| Child-Pugh,
A/B/C | 804/303/63 |
| ECOG performance
status, 0/1/2/3/4 | 885/148/93/29/15 |
| Maximum tumor size
(cm) | 2.5 (0.5–18) |
| Tumor number,
single/multiple | 632/538 |
| AST (IU/l) | 57 (9–536) |
| ALT (IU/l) | 44 (3–438) |
| Total bilirubin
(mg/dl) | 0.9 (0.2–19.6) |
| Serum albumin
(g/dl) | 3.7 (1.1–5.1) |
| ALP (IU/l) | 348 (87–3344) |
| GGT (IU/l) | 64 (10–1460) |
| Prothrombin time
(%) | 80 (32–145) |
| Platelets
(×104/mm3) | 9.2 (1.6–37.3) |
| AFP (ng/ml)a | 29.2
(1.4–843700) |
| DCP
(mAU/ml)b | 55 (1–328340) |
Initial treatment for HCC, overall
survival and causes of death for all cases
As an initial therapy for HCC, surgical resection
(SR) was performed in 205 patients, percutaneous ablative therapies
(PATs) such as radiofrequency ablation (RFA) or percutaneous
ethanol injection in 632, trancatheter arterial chemotherapy with
or without embolization (trans-catheter arterial therapies, TATs)
in 281, molecular targeted therapy such as sorafenib in four,
radiation therapy in two and no specific therapy in 13.
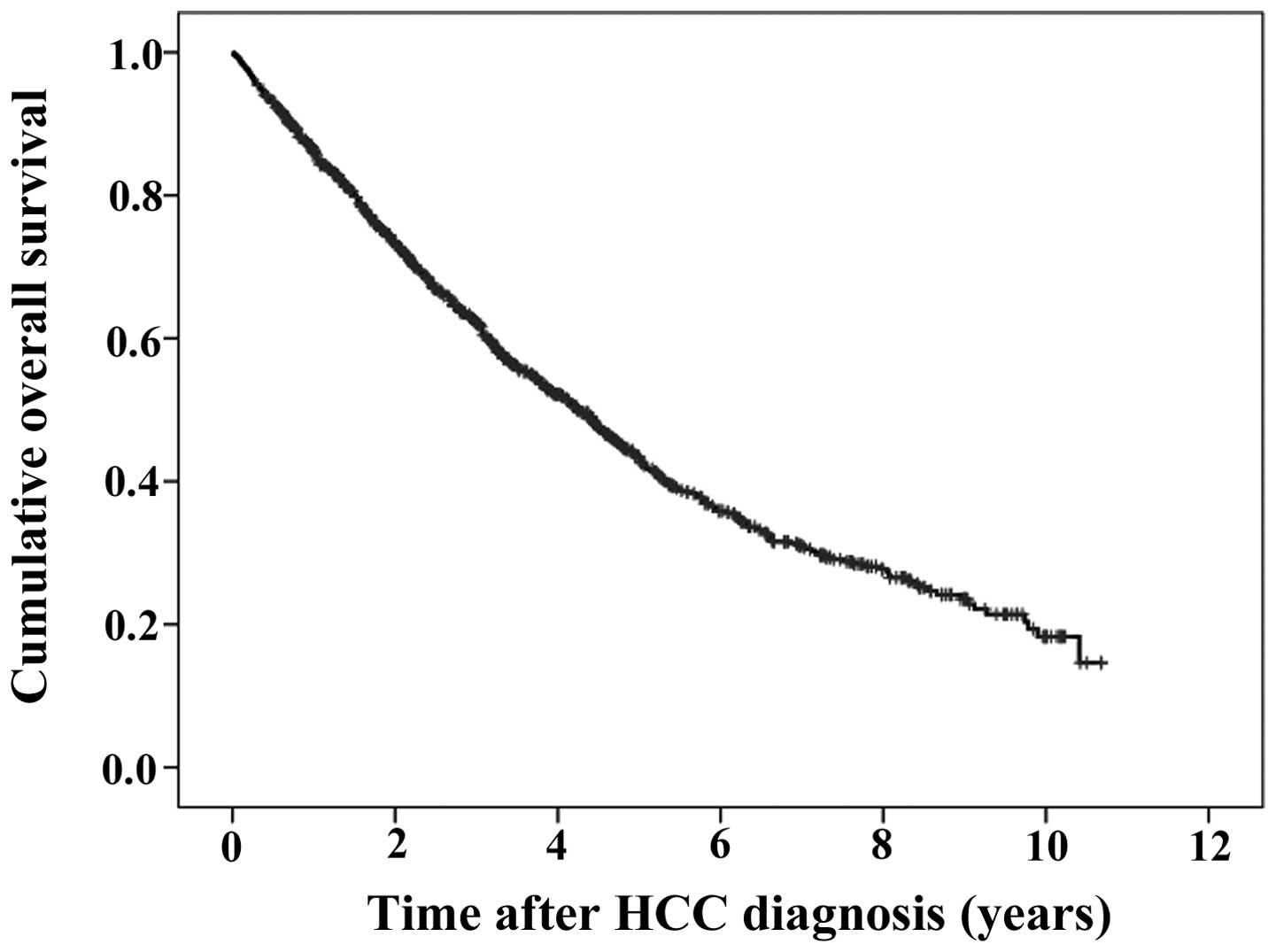
The median follow-up period was 2.8 years. The 1-,
3- and 5-year cumulative OS rates were 86.3, 62.3 and 43.5%,
respectively (Fig. 1). During
follow-up period, there were 625 (53.4%) deaths. The causes of
death were HCC recurrence in 346 patients, liver failure in 204
patients and miscellaneous causes in 75 patients, respectively.
Univariate and multivariate analyses of
factors contributing to OS
Using univariate analyses of factors contributing to
OS, tumor number (P<0.001), maximum tumor size >2.5 cm
(P<0.001), Child-Pugh classification (P<0.001), PS
(P<0.001), initial treatment modality (P<0.001), AST >57
IU/l (P<0.001), ALP >348 IU/l (P<0.001), GGT >64 IU/l
(P<0.001), AFP >29.2 ng/ml (P<0.001) and DCP >55 mAU/ml
(P<0.001) were found to be significant factors associated with
OS (Table III). The multivariate
analyses involving ten factors with P<0.05 in the univariate
analysis demonstrated that tumor number, Child-Pugh classification
(P<0.001 for B and P<0.001 for C as reference of A), PS
(P=0.044 for PS 1 and P<0.001 for PS >2 as reference of PS
0), initial treatment modality (P=0.001 for other treatments than
SR or PATs and P<0.001 for no specific therapy as reference of
SR), AST >57 IU/l (P<0.001), ALP >348 IU/l (P<0.001),
AFP >29.2 ng/ml (P=0.003) and DCP >55 mAU/ml (P<0.001)
were significant independent predictors linked to OS. The hazard
ratios (HRs), 95% confidence intervals (CIs) and P-values for these
factors are detailed in Table
III.
 | Table IIIUnivariate and multivariate analyses
of factors contributing to overall survival (n=1,170). |
Table III
Univariate and multivariate analyses
of factors contributing to overall survival (n=1,170).
| | | Multivariate
analysis |
|---|
| | |
|
|---|
| Variables | n | Univariate
analysis | Hazard ratio (95%
CI) | P-valuea |
|---|
| Gender, male vs.
female | 724/446 | 0.081 | | |
| Age (years), >70
vs. <70 | 615/555 | 0.175 | | |
| Tumor number,
single vs. multiple | 632/538 | <0.001 | 0.587
(0.493–0.698) | <0.001 |
| Maximum tumor size
(cm), >2.5 vs. <2.5 | 599/571 | <0.001 | | |
| Child-Pugh, A vs. B
vs. C | 804/303/63 | <0.001 | | |
| Child-Pugh A | | | 1.000
(reference) | |
| Child-Pugh B | | | 0.537
(0.380–0.759) | <0.001 |
| Child-Pugh C | | | 0.300
(0.212–0.425) | <0.001 |
| ECOG-PS, 0 vs. 1
vs. >2 | 885/148/137 | <0.001 | | |
| PS 0 | | | 1.000
(reference) | |
| PS 1 | | | 0.731
(0.539–0.992) | 0.044 |
| PS >2 | | | 0.437
(0.339–0.563) | <0.001 |
| Initial treatment
modality, SR/PATs/others/none | 205/632/287/46 | <0.001 | | |
| SR | | | 1.000
(reference) | |
| PATs | | | 0.742
(0.479–1.147) | 0.179 |
| Others | | | 0.468
(0.304–0.723) | 0.001 |
| None | | | 0.392
(0.243–0.631) | <0.001 |
| Cause of liver
disease, virus related vs. NBNC | 955/215 | 0.511 | | |
| AST (IU/l), >57
vs. <57 | 593/577 | <0.001 | 0.710
(0.601–0.839) | <0.001 |
| ALT (IU/l), >44
vs. <44 | 587/583 | 0.132 | | |
| ALP (IU/l), >348
vs. <348 | 586/584 | <0.001 | 0.726
(0.614–0.858) | <0.001 |
| GGT (IU/l), >64
vs. <64 | 590/580 | <0.001 | | |
| Platelet count
(×104/mm3), >9.2 vs. <9.2 | 590/580 | 0.783 | | |
| AFP (ng/ml),
>29.2 vs. <29.2b | 584/584 | <0.001 | 0.832
(0.704–0.983) | 0.030 |
| DCP (mAU/ml),
>55 vs. <55c | 580/575 | <0.001 | 0.465
(0.391–0.553) | <0.001 |
Comparison of PS-JIS score and existing
criteria for HCC for all cases using c-index
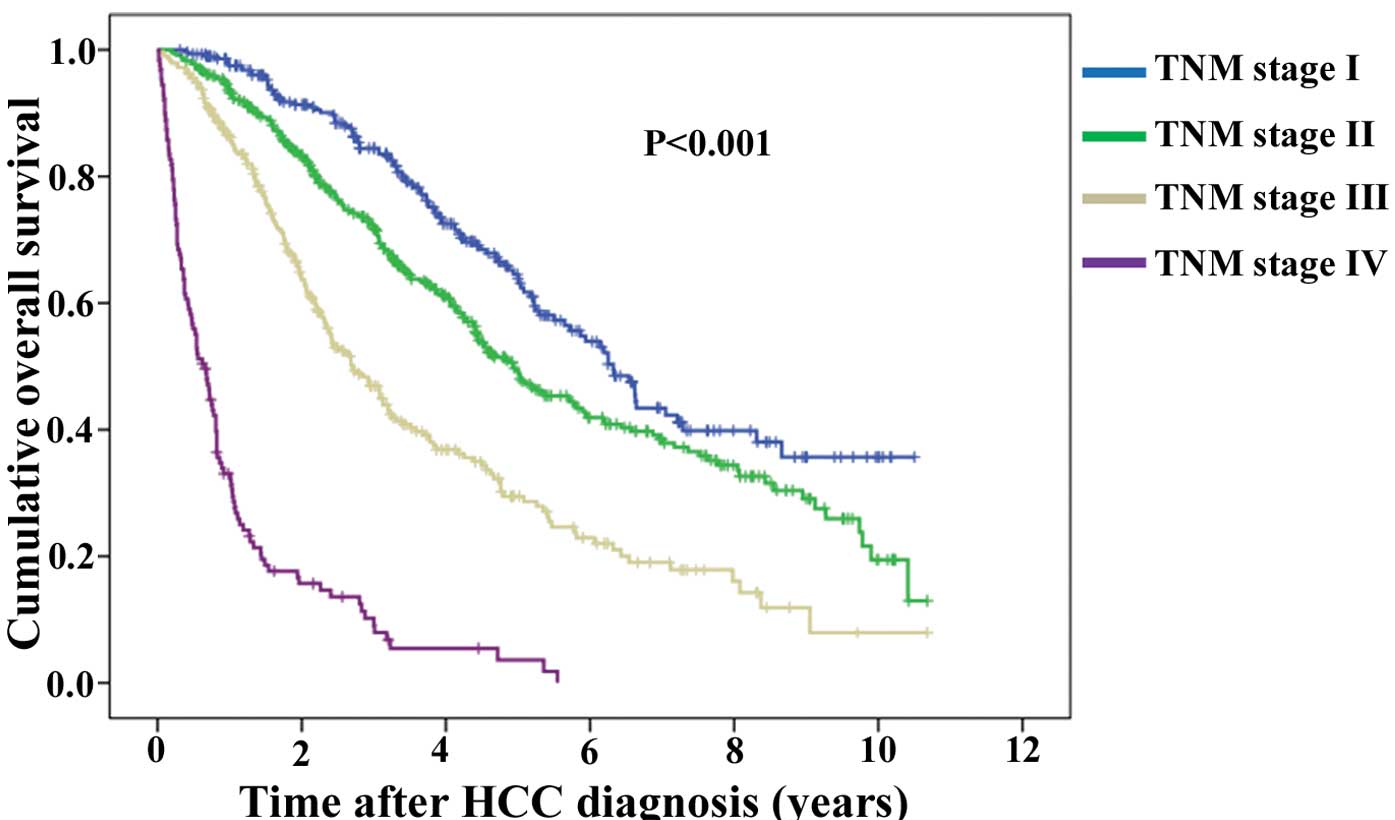
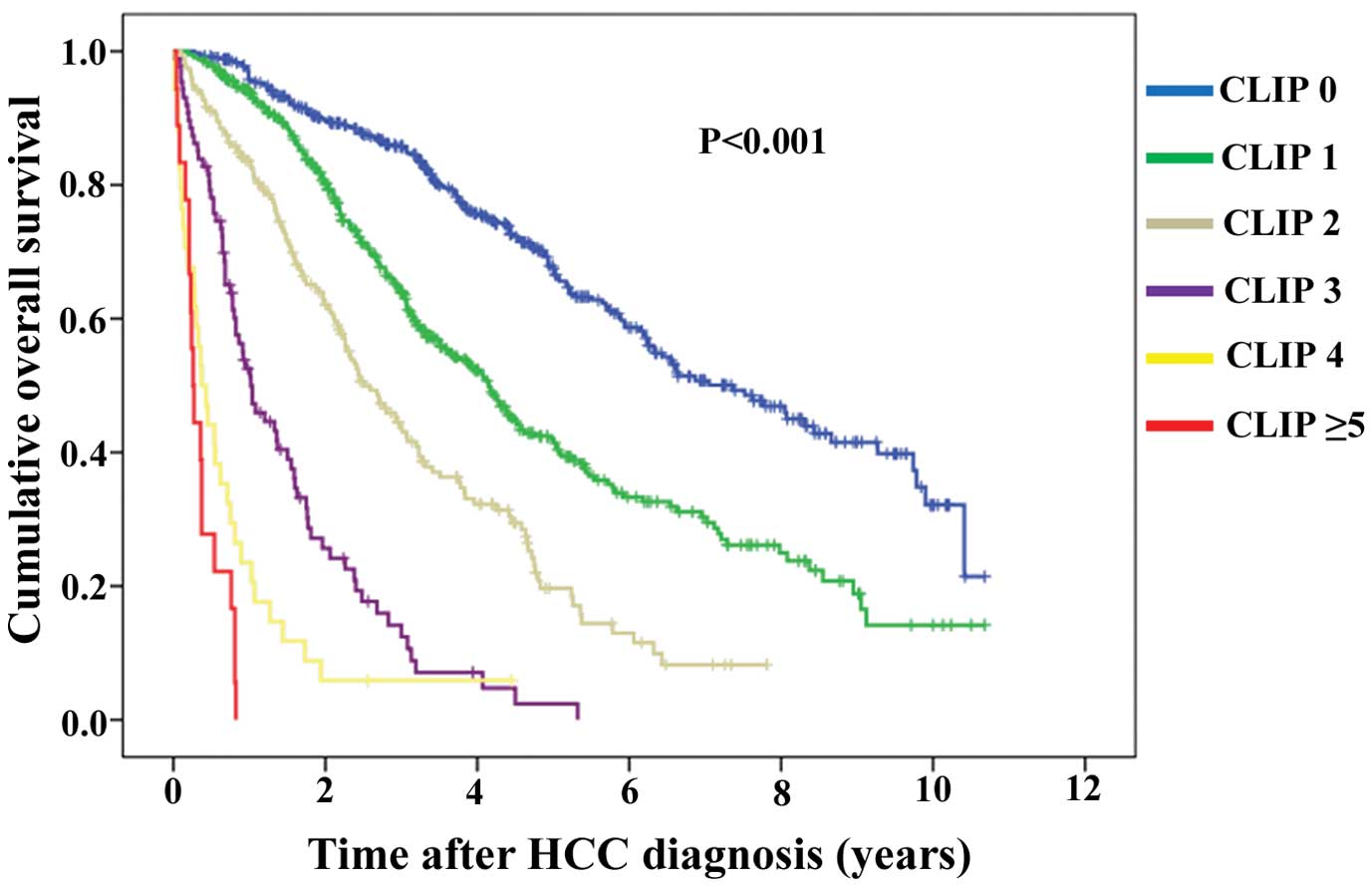
Kaplan-Meier curves of OS according to five criteria
are demonstrated: JIS system, PS-JIS system, BCLC classification
system, TNM classification system and CLIP scoring system (Figs. 2Figure 3Figure 4Figure 5–6). Number and median OS of patients with
each score are demonstrated in Table
IV. P-values between adjacent groups in each system are shown
in Table IV. Overall significance
in each prognostic system was P<0.001. All P-values between
adjacent groups in each system reached significance except for
differences in PS-JIS score 4 and 5 (P=0.873), PS-JIS score 6 and 7
(P=0.199) and CLIP score 4 and 5 or 6 (P=0.082).
 | Table IVPatient survival according to
different staging system. |
Table IV
Patient survival according to
different staging system.
| Staging system | MST (years) | 95% CI | P-value
(overall) | P-value in each
adjacent group |
|---|
| JIS system | | | <0.001 | |
| 0 (n=222) | 6.64 | 4.87–8.41 | | 0 vs. 1, 0.001 |
| 1 (n=408) | 5.72 | 4.62–6.82 | | 1 vs. 2,
<0.001 |
| 2 (n=297) | 3.15 | 2.57–3.73 | | 2 vs. 3,
<0.001 |
| 3 (n=139) | 1.71 | 1.48–1.94 | | 3 vs. 4,
<0.001 |
| 4 (n=86) | 0.75 | 0.63–0.87 | | 4 vs. 5, 0.001 |
| 5 (n=18) | 0.23 | 0.17–0.29 | | |
| PS-JIS system | | | <0.001 | |
| 0 (n=187) | 8.31 | 5.90–10.72 | | 0 vs. 1, 0.015 |
| 1 (n=348) | 6.64 | 5.60–7.68 | | 1 vs. 2,
<0.001 |
| 2 (n=288) | 3.59 | 2.96–4.22 | | 2 vs. 3,
<0.001 |
| 3 (n=170) | 2.38 | 1.94–2.82 | | 3 vs. 4,
<0.001 |
| 4 (n=98) | 1.41 | 0.99–1.83 | | 4 vs. 5, 0.873 |
| 5 (n=35) | 1.54 | 0.87–2.21 | | 5 vs. 6, 0.003 |
| 6 (n=34) | 0.65 | 0.02–1.28 | | 6 vs. 7, 0.199 |
| 7 (n=10) | 0.21 | 0.01–0.48 | | |
| BCLC classification
system | | | <0.001 | |
| 0 (very early
stage, n=187) | 8.31 | 5.90–10.72 | | 0 vs. A,
<0.001 |
| A (early stage,
n=427) | 5.47 | 4.61–6.33 | | A vs. B,
<0.001 |
| B (intermediate
stage, n=194) | 3.07 | 2.61–3.53 | | B vs. C,
<0.001 |
| C (advanced stage,
n=265) | 2.10 | 1.48–2.72 | | C vs. D,
<0.001 |
| D (end stage,
n=97) | 1.07 | 0.66–1.49 | | |
| TNM classification
system | | | <0.001 | |
| Stage I
(n=290) | 6.32 | 5.68–6.96 | | I vs. II,
0.001 |
| Stage II
(n=463) | 4.93 | 4.26–5.60 | | II vs. III,
<0.001 |
| Stage III
(n=290) | 2.71 | 2.28–3.14 | | III vs. IV,
<0.001 |
| Stage IV
(n=127) | 0.66 | 0.49–0.83 | | |
| CLIP scoring
systema | | | <0.001 | |
| 0 (n=415) | 7.36 | 6.25–8.47 | | 0 vs. 1,
<0.001 |
| 1 (n=422) | 4.15 | 3.69–4.61 | | 1 vs. 2,
<0.001 |
| 2 (n=192) | 2.51 | 2.04–2.98 | | 2 vs. 3,
<0.001 |
| 3 (n=87) | 1.02 | 0.72–1.32 | | 3 vs. 4, 0.001 |
| 4 (n=34) | 0.37 | 0.20–0.54 | | 4 vs. 5 or 6,
0.082 |
| 5 or 6 (n=18) | 0.26 | 0.20–0.32 | | |
To examine predictability of each staging system, we
compared them using the c-index. The 1-year c-indexes of JIS
system, PS-JIS system, BCLC classification system, TNM
classification system and CLIP scoring system were 0.841, 0.847,
0.815, 0.819 and 0.817, respectively. The 3-year c-indexes of JIS
system, PS-JIS sytem, BCLC classification system, TNM
classification system and CLIP scoring system were 0.797, 0.816,
0.778, 0.754 and 0.777, respectively. The 5-year c-indexes of JIS
system, PS-JIS system, BCLC classification system, TNM
classification system and CLIP scoring system were 0.775, 0.808,
0.775, 0.723 and 0.776, respectively. Collectively, in each
time-point, the c-index of PS-JIS score was the highest in these
staging systems, indicating that PS-JIS score had the best
predictability among these staging systems (Table V).
 | Table VComparison of discriminative ability
using 1-, 3- and 5-year concordance index (c-index) among five
prognostic systems. |
Table V
Comparison of discriminative ability
using 1-, 3- and 5-year concordance index (c-index) among five
prognostic systems.
| 1-year | 3-year | 5-year |
|---|
|
|
|
|
|---|
| c-index | 95% CI | c-index | 95% CI | c-index | 95% CI |
|---|
| All cases
(n=1170) |
| JIS | 0.841 | 0.804–0.878 | 0.797 | 0.768–0.826 | 0.775 | 0.742–0.808 |
| PS-JIS | 0.847 | 0.814–0.880 | 0.816 | 0.788–0.843 | 0.808 | 0.778–0.838 |
| BCLC | 0.815 | 0.781–0.848 | 0.778 | 0.748–0.807 | 0.775 | 0.741–0.808 |
| TNM | 0.819 | 0.780–0.859 | 0.754 | 0.723–0.786 | 0.723 | 0.687–0.759 |
| CLIP | 0.817 | 0.775–0.859 | 0.777 | 0.747–0.808 | 0.776 | 0.743–0.809 |
| SR (n=205) |
| JIS | 0.706 | 0.587–0.825 | 0.717 | 0.631–0.803 | 0.641 | 0.546–0.737 |
| PS-JIS | 0.718 | 0.603–0.832 | 0.711 | 0.626–0.796 | 0.661 | 0.567–0.755 |
| BCLC | 0.675 | 0.547–0.802 | 0.657 | 0.566–0.748 | 0.615 | 0.518–0.712 |
| TNM | 0.685 | 0.557–0.813 | 0.674 | 0.583–0.765 | 0.590 | 0.492–0.689 |
| CLIP | 0.739 | 0.618–0.859 | 0.722 | 0.637–0.808 | 0.681 | 0.589–0.773 |
| PATs (n=632) |
| JIS | 0.642 | 0.539–0.744 | 0.700 | 0.651–0.749 | 0.706 | 0.657–0.756 |
| PS-JIS | 0.714 | 0.623–0.805 | 0.736 | 0.690–0.782 | 0.753 | 0.706–0.799 |
| BCLC | 0.740 | 0.647–0.834 | 0.701 | 0.653–0.749 | 0.716 | 0.667–0.765 |
| TNM | 0.621 | 0.517–0.725 | 0.655 | 0.603–0.707 | 0.646 | 0.593–0.700 |
| CLIP | 0.544 | 0.423–0.664 | 0.662 | 0.610–0.713 | 0.697 | 0.647–0.747 |
| TATs (n=281) |
| JIS | 0.834 | 0.784–0.883 | 0.825 | 0.764–0.887 | 0.827 | 0.737–0.917 |
| PS-JIS | 0.842 | 0.793–0.892 | 0.843 | 0.780–0.903 | 0.861 | 0.785–0.938 |
| BCLC | 0.772 | 0.716–0.829 | 0.809 | 0.741–0.878 | 0.841 | 0.758–0.923 |
| TNM | 0.820 | 0.764–0.876 | 0.775 | 0.706–0.843 | 0.791 | 0.701–0.881 |
| CLIP | 0.838 | 0.786–0.889 | 0.820 | 0.760–0.880 | 0.837 | 0.754–0.921 |
Comparison of PS-JIS system and existing
criteria for HCC according to initial treatment modality
We also performed subgroup analyses according to
initial treatment modality using c-index. In patients treated with
SR (n=205), in 1-, 3- and 5-year c-index, CLIP scoring system had
the highest value among five staging systems (c-index, 0.739, 0.722
and 0.681, respectively). In patients treated with PATs (n=632), in
1-year c-index, BCLC classification system had the highest value
(c-index, 0.740), whereas in 3- and 5-year c-index, PS-JIS system
had the highest value (c-index, 0.736 and 0.753). In patients
treated with TATs (n=281), in 1-, 3- and 5-year c-index, PS-JIS
system had the highest value (c-index, 0.842, 0.843 and 0.861,
respectively) (Table V).
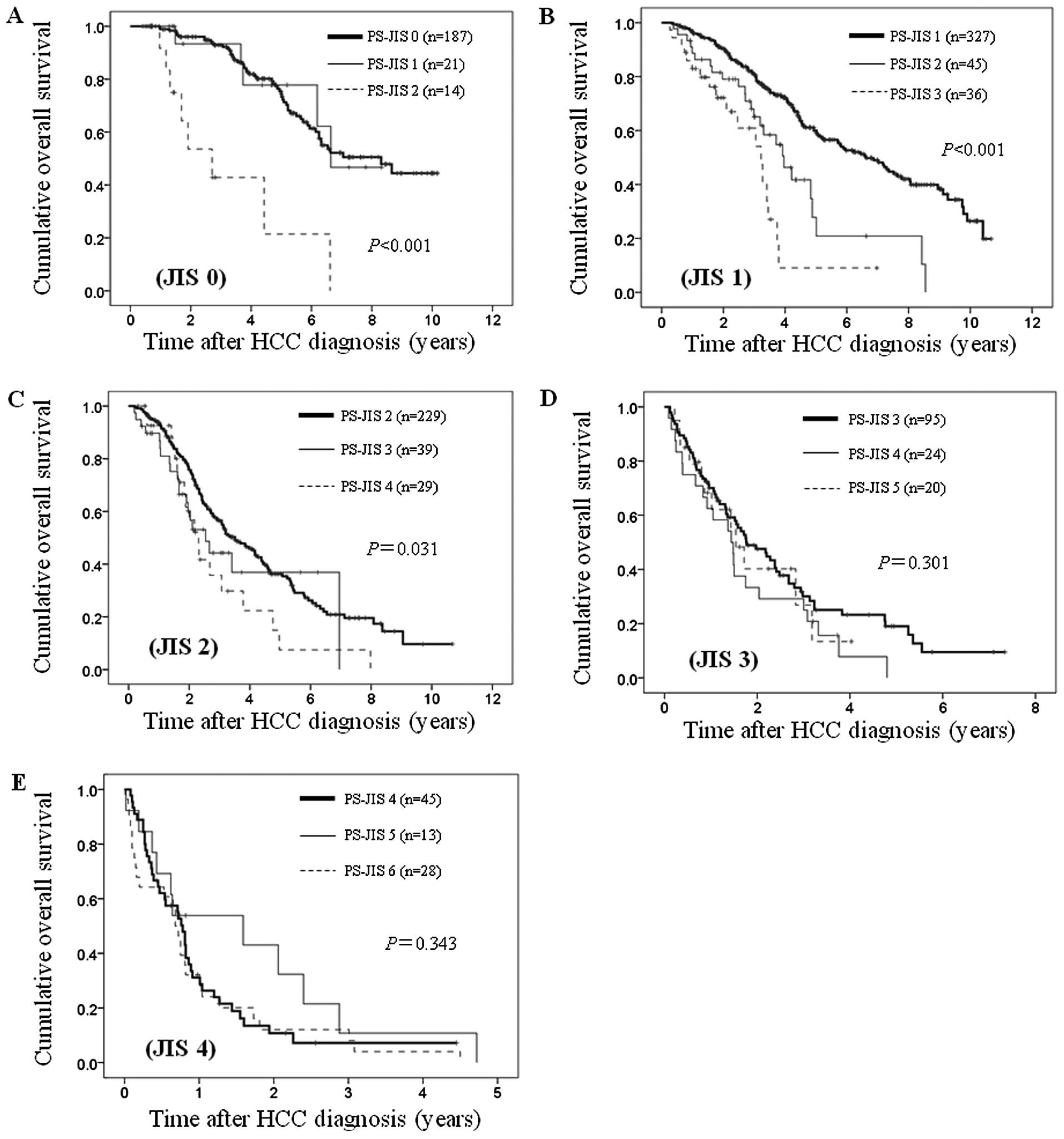
Subgroups analyses with regard to the
effect of PS-JIS score stratified by JIS system
With the purpose of investigating the effect of
PS-JIS, we performed subgroup analyses according to JIS system.
In patients with JIS 0 [n=222: PS-JIS 0 (n=187),
PS-JIS 1 (n=21) and PS-JIS 2 (n=14)], JIS 1 [n=408: PS-JIS 1
(n=327), PS-JIS 2 (n=45) and PS-JIS 3 (n=36)] and JIS 2 [n=297:
PS-JIS 2 (n=229), PS-JIS 3 (n=39) and PS-JIS 4 (n=29)], the
differences in the three groups reached significance (P<0.001,
P<0.001 and P=0.031, respectively) (Fig. 7A–C). While in patients with JIS 3
[n=139: PS-JIS 3 (n=95), PS-JIS 4 (n=24) and PS-JIS 5 (n=20)] and
JIS 4 [n=86: PS-JIS 4 (n=45), PS-JIS 5 (n=13) and PS-JIS 6 (n=28)],
the differences in the three groups did not reach significance
(P=0.301 and P=0.343, respectively). (Fig. 7D and E). Due to the small number of
patients with JIS 5 (n=18), we did not perform subgroup analysis in
this group.
Discussion
The major difference among CLIP scoring system, BCLC
classification system, TNM classification system and JIS system is
that only BCLC classification included the ECOG-PS as a variable,
although PS is a major prognostic factor for HCC patients (15). This factor is clinically important
for deciding treatment strategy for HCC and we believe that
examining the effect of PS combined well known existing prognostic
system on survival is worth reporting. Thus, we conducted the
current analysis.
In our results, tumor number, Child-Pugh
classification, PS, initial treatment modality, AST, ALP, AFP value
and DCP value were significant predictors linked to OS in the
multivariate analyses and c-index of PS-JIS was the highest at
every time-point (1-, 3- and 5-year) for all cases. These results
suggest that our proposed PS-JIS system can be a better prognostic
system than the other existing prognostic systems. On the other
hand, all P-values between adjacent groups in each system reached
significance except for differences in PS-JIS score 4 and 5
(P=0.873), PS-JIS score 6 and 7 (P=0.199) and CLIP score 4 and 5 or
6 (P=0.082). This is probably due to the small sample sizes of
these subgroups. Another possible reason is that PS-JIS (score
range, 0–7) and CLIP score (score range, 0–6) are more complex
scoring systems than the other prognostic systems.
In patients treated with SR, CLIP scoring system had
the highest c-index among five prognostic systems at every
time-point in our analyses. On the other hand, Zhao et al
(25) demonstrated that TNM
staging system is a better staging model for HCC of Chinese
population who received SR among seven currently applied staging
systems including TNM, CLIP, BCLC, Okuda, CUPI, Tokyo score and
CLIP score. As any staging system is constructed from selected
prognostic factors of certain stage of HCC in a specific
population, the predictive ability of the staging system could be
considerably impaired if it is applied to another patient
population (7,26,27).
The clinical outcome is closely associated with patient
characteristics and subsequent therapeutic strategy (7,26,27).
As for etiology of liver disease, hepatitis C virus is in the
majority in Japan, while hepatitis B virus is in the majority in
China. In addition, treatment strategies for HCC are slightly
different between Japan and China (22,28).
Discrepancies of our and their study results may be attributed to
differences of the backgrounds between countries. Furthermore, in
our results, in patients treated with SR, only 6.3% (13/205) had
poorer PS (PS >2) compared with the proportion of PS >2 of
11.7% (137/1170) for all cases. Thus, the effect of PS on survival
may be diminished in this population as compared with other
subgroups.
A previous study reported that the BCLC
classification system shows a superior discriminatory power in
their HCC patients who underwent RFA (n=112) among seven prognostic
system, however, in the present study, in patients treated with
PATs, in 1-year c-index, BCLC classification system had the highest
value, while in 3- and 5-year c-index, PS-JIS system had the
highest value (29). Likewise, Cho
et al (18) demonstrated
that CLIP system provided the best prognostic stratification for
HCC patients who underwent transarterial chemoembolization (n=131),
whereas in our analysis, in patients treated with TATs, in 1-, 3-
and 5-year c-index, PS-JIS system had the highest value. As well as
in patients treated with SR, these discrepancies can probably be
explained in part by the difference of the baseline patient
characteristics in the investigated populations.
According to sub-analyses stratified by JIS score,
in early stages (JIS score 0, 1 and 2), there was overall
significance among three groups of PS 0, 1 and >2 in terms of
OS, whereas in advanced stages (JIS score 3 and 4), such
significance was not found among three groups of PS 0, 1 and >2.
Our results indicate that especially in patients with early stage
of HCC or less advanced LC, our proposed PS-JIS system can be a
better prognostic system than the original JIS scoring system. In
Japan, new emerging diagnostic imagings and the adequate selection
of high-risk groups for HCC occurrence could enable detection of
early stage HCC, potentially improving outcome (17). In that sense, our proposed PS-JIS
system can be a promising scoring system.
We acknowledge several limitations in the current
analyses. First, this is a single center retrospective study which
included only Japanese HCC patients. Second, inter-observer bias
for evaluating PS could exist although the PS scale was determined
at the time of HCC diagnosis. Third, pathologic confirmation of HCC
was not routinely performed except for cases who underwent SR.
Caution should therefore be exercised in interpretation of our
results, and our proposed staging system should be validated in
another independent population. There are several missing values in
the present study study. However, the number of patients with
missing data was very small considering large sample of our study
(n=1,170), which may not effect on interpretation of our
results.
In conclusion, our proposed PS-JIS score can be a
useful prognostic system for HCC patients complicated with LC.
Acknowledgements
We would like to thank Haruko Takada for the data
collection.
References
|
1
|
Poon D, Anderson BO, Chen LT, Tanaka K,
Lau WY, Van Cutsem E, Singh H, Chow WC, Ooi LL, Chow P, et al;
Asian Oncology Summit. Management of hepatocellular carcinoma in
Asia: Consensus statement from the Asian Oncology Summit 2009.
Lancet Oncol. 10:1111–1118. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
No authors listed. A new prognostic system
for hepatocellular carcinoma: a retrospective study of 435
patients: the Cancer of the Liver Italian Program (CLIP)
investigators. Hepatology. 28:751–755. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huitzil-Melendez FD, Capanu M, O’Reilly
EM, Duffy A, Gansukh B, Saltz LL and Abou-Alfa GK: Advanced
hepatocellular carcinoma: Which staging systems best predict
prognosis? J Clin Oncol. 28:2889–2895. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takanishi DM Jr, Severino R and Wong LL:
The Cancer of the Liver Italian Program (CLIP) score: Validation of
a new prognostic system for hepatocellular carcinoma. Hawaii Med J.
66:209–212. 2007.PubMed/NCBI
|
|
5
|
Levy I and Sherman M; Liver Cancer Study
Group of the University of Toronto. Staging of hepatocellular
carcinoma: Assessment of the CLIP, Okuda, and Child-Pugh staging
systems in a cohort of 257 patients in Toronto. Gut. 50:881–885.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Llovet JM, Brú C and Bruix J: Prognosis of
hepatocellular carcinoma: The BCLC staging classification. Semin
Liver Dis. 19:329–338. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marrero JA, Fontana RJ, Barrat A, Askari
F, Conjeevaram HS, Su GL and Lok AS: Prognosis of hepatocellular
carcinoma: Comparison of 7 staging systems in an American cohort.
Hepatology. 41:707–716. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cillo U, Vitale A, Grigoletto F, Farinati
F, Brolese A, Zanus G, Neri D, Boccagni P, Srsen N, D’Amico F, et
al: Prospective validation of the Barcelona Clinic Liver Cancer
staging system. J Hepatol. 44:723–731. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bruix J, Sherman M, Llovet JM, Beaugrand
M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M
and Rodés J; EASL Panel of Experts on HCC; European Association for
the Study of the Liver. Clinical management of hepatocellular
carcinoma. Conclusions of the Barcelona-2000 EASL conference. J
Hepatol. 35:421–430. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kudo M, Chung H and Osaki Y: Prognostic
staging system for hepatocellular carcinoma (CLIP score): Its value
and limitations, and a proposal for a new staging system, the Japan
Integrated Staging Score (JIS score). J Gastroenterol. 38:207–215.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
No authors listed. Liver Cancer Study
Group of Japan: The general rules for the clinical and pathological
study of primary liver cancer. Jpn J Surg. 19:98–129. 1989.
View Article : Google Scholar
|
|
12
|
Kudo M, Chung H, Haji S, Osaki Y, Oka H,
Seki T, Kasugai H, Sasaki Y and Matsunaga T: Validation of a new
prognostic staging system for hepatocellular carcinoma: The JIS
score compared with the CLIP score. Hepatology. 40:1396–1405. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gomaa AI, Hashim MS and Waked I: Comparing
staging systems for predicting prognosis and survival in patients
with hepatocellular carcinoma in Egypt. PLoS One. 9:e909292014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bruix J and Sherman M; Practice Guidelines
Committee, American Association for the Study of Liver Diseases.
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hsu CY, Lee YH, Hsia CY, Huang YH, Su CW,
Lin HC, Lee RC, Chiou YY, Lee FY and Huo TI: Performance status in
patients with hepatocellular carcinoma: Determinants, prognostic
impact, and ability to improve the Barcelona Clinic Liver Cancer
system. Hepatology. 57:112–119. 2013. View Article : Google Scholar
|
|
16
|
Montano-Loza AJ: Clinical relevance of
sarcopenia in patients with cirrhosis. World J Gastroenterol.
20:8061–8071. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Osaki Y and Nishikawa H: Treatment for
hepatocellular carcinoma in Japan over the last three decades: Our
experience and published work review. Hepatol Res. 45:59–74. 2015.
View Article : Google Scholar :
|
|
18
|
Cho YK, Chung JW, Kim JK, Ahn YS, Kim MY,
Park YO, Kim WT and Byun JH: Comparison of 7 staging systems for
patients with hepatocellular carcinoma undergoing transarterial
chemoembolization. Cancer. 112:352–361. 2008. View Article : Google Scholar
|
|
19
|
Kitai S, Kudo M, Minami Y, Ueshima K,
Chung H, Hagiwara S, Inoue T, Ishikawa E, Takahashi S, Asakuma Y,
et al: A new prognostic staging system for hepatocellular
carcinoma: Value of the biomarker combined Japan integrated staging
score. Intervirology. 51(Suppl 1): 86–94. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamasaki T, Kurokawa F, Shirahashi H,
Kusano N, Hironaka K and Okita K: Percutaneous radiofrequency
ablation therapy with combined angiography and computed tomography
assistance for patients with hepatocellular carcinoma. Cancer.
91:1342–1348. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park JW, Amarapurkar D, Chao Y, Chen PJ,
Geschwind JF, Goh KL, Han KH, Kudo M, Lee HC, Lee RC, et al:
Consensus recommendations and review by an International Expert
Panel on Interventions in Hepatocellular Carcinoma (EPOIHCC). Liver
Int. 33:327–337. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kudo M, Izumi N, Kokudo N, Matsui O,
Sakamoto M, Nakashima O, Kojiro M and Makuuchi M; HCC Expert Panel
of Japan Society of Hepatology. Management of hepatocellular
carcinoma in Japan: Consensus-Based Clinical Practice Guidelines
proposed by the Japan Society of Hepatology (JSH) 2010 updated
version. Dig Dis. 29:339–364. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hanley JA and McNeil BJ: The meaning and
use of the area under a receiver operating characteristic (ROC)
curve. Radiology. 143:29–36. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pencina MJ and D’Agostino RB: Overall C as
a measure of discrimination in survival analysis: Model specific
population value and confidence interval estimation. Stat Med.
23:2109–2123. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao J, Yan T, Huang Z, Bi X, Zhao H, Zhou
J, Li Z, Li Y, Li C and Cai J: Evaluations of seven different
clinical staging systems for Chinese patients with hepatocellular
carcinoma undergoing curative resection. Zhonghua Yi Xue Za Zhi.
94:903–907. 2014.(In Chinese). PubMed/NCBI
|
|
26
|
Cillo U, Bassanello M, Vitale A,
Grigoletto FA, Burra P, Fagiuoli S, D’Amico F, Ciarleglio FA,
Boccagni P, Brolese A, et al: The critical issue of hepatocellular
carcinoma prognostic classification: Which is the best tool
available? J Hepatol. 40:124–131. 2004. View Article : Google Scholar
|
|
27
|
Collette S, Bonnetain F, Paoletti X,
Doffoel M, Bouché O, Raoul JL, Rougier P, Masskouri F, Bedenne L
and Barbare JC: Prognosis of advanced hepatocellular carcinoma:
Comparison of three staging systems in two French clinical trials.
Ann Oncol. 19:1117–1126. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Omata M, Lesmana LA, Tateishi R, Chen PJ,
Lin SM, Yoshida H, Kudo M, Lee JM, Choi BI, Poon RT, et al: Asian
Pacific Association for the Study of the Liver consensus
recommendations on hepatocellular carcinoma. Hepatol Int.
4:439–474. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guglielmi A, Ruzzenente A, Pachera S,
Valdegamberi A, Sandri M, D’Onofrio M and Iacono C: Comparison of
seven staging systems in cirrhotic patients with hepatocellular
carcinoma in a cohort of patients who underwent radiofrequency
ablation with complete response. Am J Gastroenterol. 103:597–604.
2008. View Article : Google Scholar
|