Introduction
Malignant melanoma is one of the most common causes
of brain metastases (1–3) which is reported in 10–40% of melanoma
patients (4–6). Lifetime incidence of central nervous
system (CNS) involvement in patients with malignant melanoma is
reported with ~10% in the literature (7,8) and
drastically limits prognosis: survival of untreated patients is
only weeks, treated patients will live <1 year despite a small
number of long-term survivors with a 10-year survival <10%
(9–12). Patients with multiple brain
metastases have a reported OS of 3–4 months (10,13).
Brain metastasis is the main cause of mortality and
morbidity among patients with metastatic melanoma (13,14)
where 73% of patients who died from malignant melanoma showed
subclinical brain involvement as evidenced by autopsy (14–16).
Few studies elucidated potential risk factors for
the development of brain metastases in malignant melanoma including
gender, Breslow thickness, ulceration, melanoma location,
histological type, certain genetic alterations (i.e., BRAF
mutation) and positive sentinel node (7,17–19).
There are data from randomized trials comparing
whole-brain radiotherapy (WBRT) and chemotherapy in patients with
melanoma metastatic to the brain where treatment decision depends
on clinical factors such as size, location, number of metastases,
disease extent but also performance status and the age of the
patients (20–22). Treatment approaches to metastatic
malignant melanoma include systemic therapy (23,24)
as well as local treatment such as stereotactic radiotherapy
(25) and surgery (26,27)
for patients with solitary brain metastasis and absent or stable
extracranial disease (28).
Patients with inoperable or multiple brain metastases may be
candidates for WBRT (29) which is
also part of the multimodal treatment concept of metastatic
malignant melanoma (21,30,31)
with an expected median survival of 2.5–4 months after
hypofractionated radiotherapy (32) as opposed to steroid therapy alone
(33,34). However, both the treatment sequence
and the decision for a validated standard approach of combined
therapy in metastasized melanoma can be challenging (35,36).
The objective of this single-center retrospective
clinical study was to identify potential prognostic factors for OS
and LC and to investigate the influence of different treatment
modalities in patients with malignant melanoma metastatic to the
brain.
Materials and methods
We retrospectively analyzed 100 consecutive patients
with histologically confirmed malignant melanoma who presented with
metastases at Martin Luther University Halle-Wittenberg, Department
of Radiation Oncology or Department of Dermatology between April
1992 and October 2011. A positive vote was given and the study was
approved by the ethics committee of the Medical Faculty of the
Martin Luther University Halle-Wittenberg. Sociodemographic and
clinical patient data were collected from the patients’ charts, the
intracranial course of disease was evaluated with CT or MRI and
survival status was obtained for each patient via local citizen
registration offices.
Endpoints in this study were overall survival (OS,
from initial diagnosis of brain metastasis until death or last
seen), local tumor control per single lesion (LC, i.e., no size
increase of present metastases, absence of recurrence of treated
metastases and of hemorrhage) and intracranial tumor control
(absence of recurrence of treated metastases, absence of new
metastases, absence from size increase and hemorrhage of present
brain metastases). Tumor control was based on the time until local
progression occurred or absence of local progression was last
documented at follow-up.
OS and intracranial tumor control per patient were
evaluated for the entire patient cohort (n=100) and LC per single
lesion (n=72 lesions) was assessed in n=37 patients with any
available follow-up imaging.
Statistical analyses were performed using the
Statistica software (version 10, StatSoft, Tulsa, OK, USA). The
Kaplan-Meier method was used in the univariate evaluation of
potential prognostic factors and the log-rank test compared
survival between subgroups. Significant factors from the univariate
analysis were included in the multivariate analysis using a
multiple Cox regression. Statistical significance was accepted with
two-sided p-values <0.05.
Results
Patient, tumor and treatment
characteristics
Sixty-five percent of patients were male, 35%
female. Median age at the time of initial diagnosis of malignant
melanoma was 57 (27–81) years and median age at the time of
diagnosis of brain metastasis was 62 (28–81) years. The median time
from first diagnosis of malignant melanoma to occurrence of brain
metastasis was 2.5 years (50 days - 17.3 years). Clinical melanoma
characteristics are presented in Table
I, and Table II shows
characteristics of brain metastasis.
 | Table IClinical melanoma characteristics
(n=100). |
Table I
Clinical melanoma characteristics
(n=100).
| Characteristic | Patients n (%) |
|---|
| Histology |
| SSMa | 29 (29) |
| NMb | 42 (42) |
| ALMc | 5 (5) |
| UCMd | 11 (11) |
| Unkown
primary | 13 (13) |
| LMMe | 0 (0) |
| Breslowf |
| <2 mm | 30 (30) |
| >2 mm | 51 (51) |
| Unknown | 19 (19) |
| Ulceration |
| Yes | 39 (39) |
| No | 36 (36) |
| Unknown | 25 (25) |
| Stageg |
| 1A | 6 (6) |
| 1B | 14 (14) |
| 2A | 19 (19) |
| 2B | 0 (0) |
| 2C | 10 (10) |
| 3A | 2 (2) |
| 3B | 18 (18) |
| 3C | 0 (0) |
| 4 | 23 (23) |
| Unknown | 8 (8) |
| Clark-Level |
| I | 0 (0) |
| II | 11 (11) |
| III | 29 (29) |
| IV | 27 (27) |
| V | 12 (12) |
| Unknown | 21 (21) |
| Initial lymph node
metastasesg |
| Yes | 22 (22) |
| No | 56 (56) |
| N/A | 22 (22) |
| Lymph node
metastasesi |
| Yes | 61 (61) |
| No | 39 (39) |
| Extracranial
metastasesj |
| Yes | 71 (71) |
| No | 29 (29) |
| Organ systems
affected by metastasesj |
| 0 | 29 (29) |
| 1 | 25 (25) |
| 2 | 22 (22) |
| 3 | 18 (18) |
| 4 | 6 (6) |
 | Table IICharacteristics of brain metastases
(n=100). |
Table II
Characteristics of brain metastases
(n=100).
| Characteristic | Patients n (%) |
|---|
| Initial
imaging |
| CT | 77 (77) |
| MRI | 21 (21) |
| Unknown | 2 (2) |
| Number at initial
diagnosis |
| 1–2 | 53 (53) |
| >2 | 47 (47) |
| Location |
|
Supratentorial | 95 (95) |
|
Infratentorial | 4 (4) |
| Both | 1 (1) |
| Symptoms |
| Yes | 71 (71) |
| No | 25 (25) |
| Unknown | 4 (4) |
| Hemorrhage |
| Yes | 22 (22) |
| No | 71 (71) |
| Unknown | 7 (7) |
| Size |
| <20 mm | 48 (48) |
| >20 mm | 51 (51) |
| Midline shift |
| Yes | 18 (18) |
| No | 72 (72) |
| Unknown | 10 (10) |
| Brain edema |
| Yes | 67 (67) |
| No | 25 (25 |
| Unknown | 8 (8) |
Seventy-one percent of patients were diagnosed with
additional extracranial metastases and in 46%, more than one organ
system was affected, including lung in 54 patients (54%), liver in
35 patients (35%), bone in 16 patients (16%) and skin in 42
patients (42%). Sixteen percent of patients had distant lymph node
metastases.
In 71% of patients, CNS symptoms from cerebral
metastasis were reported and included seizures, behavioral changes,
headache and speech disorder; in 25% of patients, cerebral
metastases were asymptomatic and an incidental finding during
melanoma staging investigations.
Treatment characteristics (Table III)
Prior to diagnosis of brain metastasis, 50 patients
(50%) were treated systemically with dacarbazine (6%), interferon α
(23%) or a combination of both (21%). In the entire patient
collective, 45 patients (45%) received WBRT which was delivered in
an opposing-field photon technique with a median single dose of 2.5
(2–5) Gy and a median total dose of 33
(6–54) Gy. Cranial stereotactic radiotherapy
was carried out as a CT-based hypofractionated radiotherapy or
radiosurgery for 1–3 lesions in 52 patients (52%), in one patient,
it was administered for ≤4 cerebral lesions. A custom-made
individual immobilization mask was used for each patient, median
single dose was 5.5 (2–25) Gy and median total dose 25 (12–50)
Gy.
Systemic treatment was given with either
temozolomide or fotemustine or both (1–12 courses per patient,
median 1 course). Median absolute dose of temozolomide was 270
(min. 140 - max. 420) mg/m2 body surface (equivalent to
200 mg/m2 body surface per day) and patients received
this medication orally for 5 consecutive days, followed by an
interruption of 23 days before the start of the next course.
Fotemustine was daily administered intravenously with a dose of 100
mg/m2 body surface (days 1, 8 and 15), followed by a
break of 5 weeks before maintenance therapy was initiated with 100
mg/m2 body surface once weekly every 3 weeks.
Treatment for patients with multiple brain
metastases was delivered as WBRT in 14% of cases, systemic therapy
in 23% and combined WBRT with systemic chemotherapy in 40%.
Twenty-three percent of patients with multiple brain lesions
received best supportive care only.
Overall survival in the entire patient
collective in univariate analysis
By November 2011, 93 of the 100 patients had already
died and in 84% of cases, death was related to malignant melanoma.
Median follow-up in surviving patients was 32 (4–222) months and it
was 3.5 (0–222) months in all patients.
Median OS (after initial diagnosis) in the entire
patient collective was 3.9 months, 1-year survival rate was 21.4%.
Local and systemic therapy, number of brain metastases and
extracranial metastasis were identified as significant predictors
for OS in the entire patient collective.
Patients who received local therapy (either surgery
and/or stereotactic radiotherapy) at any time had a superior OS
(6.9 months, n=46) compared to patients who never received local
therapy (2.6 months, n=54, p<0.001) (Fig. 1A). Use of local therapy in the
primary treatment (n=42) was associated with better OS (7.5 vs. 2.8
months, p<0.001) (Fig. 1B) and
use of systemic therapy resulted in a median OS of 5.1 months
(n=55) compared to 3.1 months without use of systemic treatment
(n=45, p=0.002) (Fig. 1C).
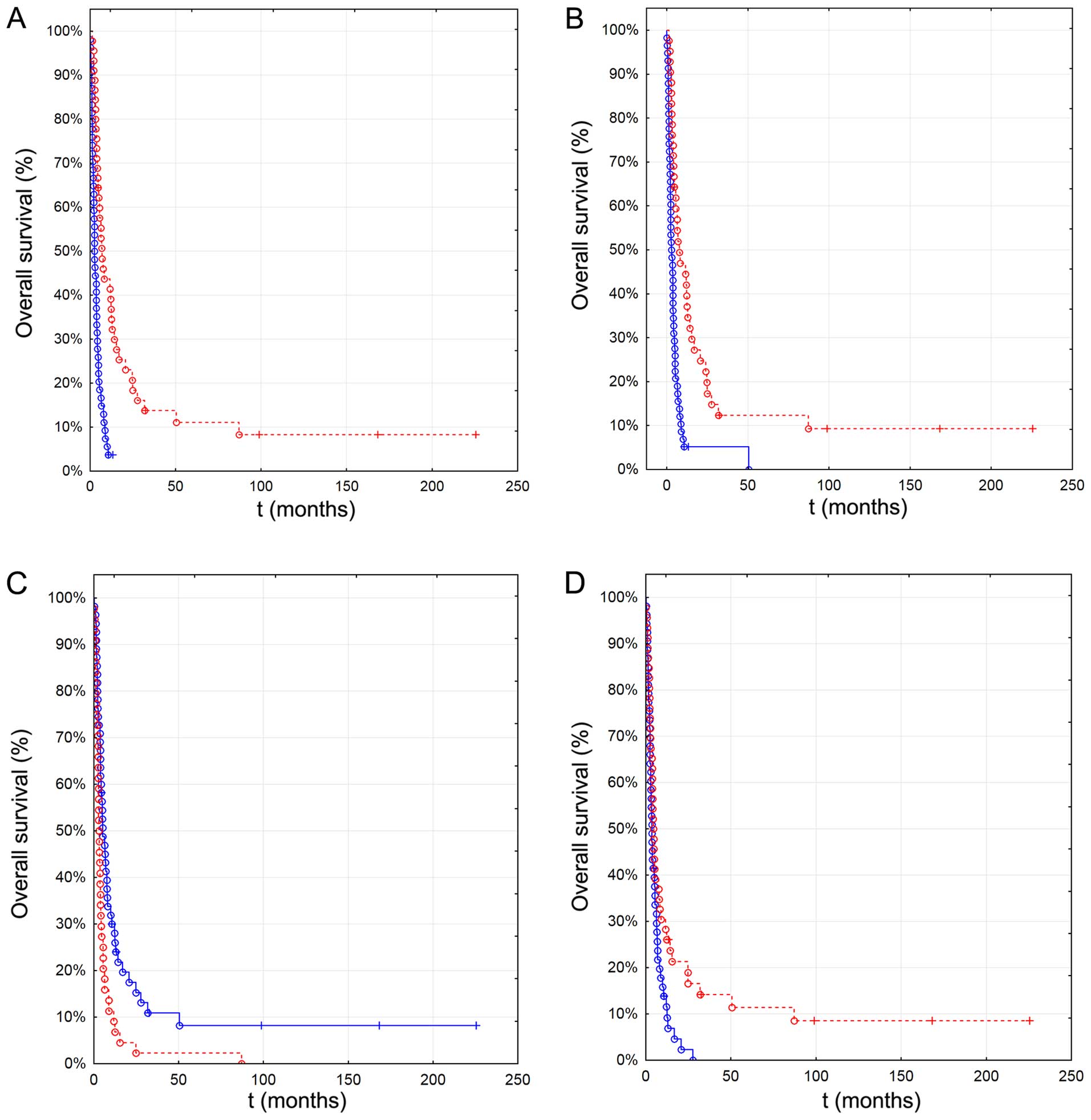 | Figure 1Association of overall survival with
clinical and treatment characteristics in the primary therapy in
the entire patient cohort (n=100). (A) Use of any local therapy
(6.9 months, n=46, blue continuous line) vs. no local therapy (2.6
months, n=54, red dotted line). (B) Use of local therapy in primary
treatment (7.5 months, n=42, blue continous line) vs. no local
therapy in primary treatment (2.8 months, n=58, red dashed line).
(C) Use of any systemic therapy (5.1 months, n=55, blue continuous
line) ever vs. no systemic therapy (3.1 months, n=45, red dashed
line). (D) One to two brain metastasis (6.5 months, n=53, red
dashed line) vs. ≥2 brain metastasis (2.6 months, n=47, blue
continuous line). |
Increasing number of brain metastasis significantly
reduced OS which was 6.5 months in patients with one solitary
lesion (n=40), 6.4 months in patients with 2 metastatic lesions
(n=13), 3.8 months in those with 3 lesions (n=7) and 2.5 months in
patients with >3 brain metastases (n=40, p<0.001). OS was
significantly lower in patients with >3 metastatic brain lesions
(n=47) compared to patients with 1–2 lesions (2.6 vs. 6.5 months,
p=0.029) (Fig. 1D). Patients with
extracranial metastases had a median OS of 3.9 months compared to
4.4 months in patients where metastases were confined to brain
(p=0.022, n=71).
No association was determined between OS and number
of extracranial metastatic sites (p=0.98), location of extracranial
metastases (p=0.4), lactate dehydrogenase (LDH) levels (p=0.11) and
WBRT in the primary treatment concept (p=0.09) even though patients
who received WBRT (n=45) lived longer than patients without WBRT
(4.8 vs. 3.5 months, p=0.85).
Different treatment modalities yielded different OS
(p<0.001) depending on whether they were used in the primary
treatment or whether they were part of the whole treatment
(including further treatments after disease progression) (Table IV).
 | Table IVMedian OS (months) in patients with
respect to therapy approach as part of primary or whole treatment
concept. |
Table IV
Median OS (months) in patients with
respect to therapy approach as part of primary or whole treatment
concept.
| Therapy
approach | Primary
therapy | Whole therapy |
|---|
| WBRT only | 3.2 | 3.2 |
| Systemic therapy
only | 3.3 | 1.6 |
| Local therapy
only | 6.4 | 4.4 |
| WBRT + systemic
therapy | 4.1 | 4.7 |
| WBRT + local
therapy | 8.2 | 5. 6 |
| Systemic + local
therapy | 12.7 | 14.2 |
| WBRT + systemic +
local therapy | 2 | 8 |
| No therapy | 1.5 | 1.5 |
The subgroup which received local plus systemic
therapy at any time (initial treatment or over the course of the
disease) had the best OS (12.7 and 14.2 months, n=10) (Fig. 2). If triple therapy (WBRT +
systemic + local therapy) was used in the primary treatment, median
OS was 2 months, however, it was 8 months if all three treatment
modalities were part of the whole treatment concept.
 | Figure 2Overall survival according to
treatment modality in the whole therapy in the entire patient
cohort (n=100). WBRT only (median overall survival 3.2 months,
n=10, blue continuous line), systemic therapy only (1.6 months,
n=9, red dashed line), local therapy only (4.4 months, n=16, green
dashed line), WBRT + systemic therapy (4.7 months, n=19, pink
dashed line), WBRT + local therapy (5.6 months, n=3, black dashed
line), systemic + local therapy (14.2 months, n=15, grey dashed
line), triple therapy (WBRT, systemic and local therapy, 8 months,
n=12, brown dotted line) and no treatment (1.5 months, n=16, olive
continuous line). |
Overall survival in the entire patient
collective in multivariate analysis
The following factors which were significantly
associated with OS in the univariate analysis have been included in
multivariate evaluation: number of brain metastases, use of local
therapy in the primary treatment and presence of extracranial
metastases before diagnosis of brain metastasis.
All tested variables remained independent predictors
for OS and the hazard ratio for patients with >2 brain
metastases was 2.2 compared to patients with 1–2 cerebral
metastases (p=0.0005). Patients who did not receive local therapy
in their primary treatment had an increased risk of death by a
factor 1.8 as opposed to patients with local therapy (p=0.035) and
patients with metastases confined to the brain were less likely to
die (HR=0.6, p=0.035) compared to patients with additional
extracranial metastasis.
OS in patients with multiple cranial
metastases
The subgroup of patients with multiple brain
metastases (n=35) contains all patients who were initially
diagnosed with multiple brain metastases or who developed multiple
brain metastases during their disease course. OS in patients only
receiving systemic treatment was 1.8 months and it was 1.5 months
in patients under best supportive care.
The whole treatment (including sequential modalities
after disease progression) significantly influenced OS in this
subgroup (p=0.007): the best OS in patients with multiple brain
metastases could be achieved if WBRT was combined with systemic
treatment (median OS 3.8 months) in the primary treatment compared
to WBRT alone (3.6 months). However, the survival difference
between these two groups was rather small and the findings did not
reach statistical significance (p=0.058). Patients treated with
systemic therapy only or no therapy at all had a similar median OS
of 1.5 months (Fig. 3).
 | Figure 3Overall survival in patients with
multiple brain metastasis (n=35) according to treatment modality in
overall therapy. WBRT only (median 3.6 months, n=5, blue continuous
line), systemic therapy (1.5 months, n=8, red dashed line), WBRT +
systemic therapy (3.8 months, n=14, green dashed libe) and no
therapy (1.5 months, n=8, pink dashed line). |
Tumor control in relation to different
treatment modalities
In the entire patient cohort, intracranial tumor
control could be evaluated in 58 patients. The intracranial disease
course was significantly associated with OS (p=0.0037) and
intracranial tumor control (until last follow-up) was documented in
28 patients. In 42 patients, no follow-up data on tumor control was
available. Median OS in patients with controlled tumors was 10
months compared to 5 months in patients with intracranial tumor
progression (n=30, p=0.004).
For the subgroup of patients with multiple brain
metastases, no significant association between the two most
commonly performed primary therapies (combined WBRT with systemic
therapy and systemic therapy only) and intracranial tumor control
(p=0.23) could be determined.
When 72 brain metastases from 37 patients were
evaluated for LC per lesion, 50% remained locally controlled and
50% were progressive after a median time interval of 3.6 months. In
this group, the primary treatment modality was significantly
associated with LC which was best after a combination of local
therapy plus systemic therapy in the primary treatment (p=0.011)
(Table V).
 | Table VAssociation of different treatment
modalities and local tumor control per lesion (n=37 patients, n=72
lesions). |
Table V
Association of different treatment
modalities and local tumor control per lesion (n=37 patients, n=72
lesions).
| Therapy
approach | Metastases n
(%) | Median LC
(months) | p-value
(log-rank) |
|---|
| Primary therapy
(n=72) |
| WBRT only | 8 (11) | 3 | 0.011 |
| Systemic therapy
only | 14 (20) | 2 | |
| Local therapy
only | 31 (44) | 2.7 | |
| WBRT + systemic
therapy | 3 (4) | 2 | |
| Local therapy +
WBRT | 6 (9) | 2 | |
| Systemic + local
therapy | 8 (11) | 11.1 | |
| Surgery as primary
therapy (n=72) |
| Yes | 33 (46) | 3 | 0.021 |
| No | 39 (54) | 2.3 | |
| Local therapy in
primary treatment (n=72) |
| Surgery | 27 (38) | 2.8 | 0.042 |
| Stereotactic
radiotherapy | 14 (19) | 2.5 | |
| Surgery +
stereotactic radiotherapy | 6 (9) | 13.2 | |
| No local
therapy | 25 (35) | 2 | |
Metastases which were treated primarily surgically
(n=33) showed a median LC of 2.96 months compared to 2.33 months in
metastases where surgery was not used in the primary treatment
(p=0.021) (Table V).
Use of local therapy approaches in the primary
therapy was also associated with LC (Fig. 4): 6-month LC was 75% when surgery
was combined with stereotactic radiotherapy (median LC 13.2 months,
Table V), it was 53% in the
stereotactic radiotherapy only group and 43% in the surgery only
group (p=0.042).
 | Figure 4Local control in single brain
metastasis (n=35) according to local treatment modality in the
primary therapy (n=72). Surgery only (median 2.8 months, n=27, blue
continuous line), stereotactic radiotherapy only (2.5 months, n=14,
red dashed line), stereotactic radiotherapy + surgery (13.2 months,
n=6, green dashed line) and no therapy (2 months, n=25, pink dashed
line). |
Discussion
The aim of this study was to identify prognostic
factors for survival in stage IV melanoma patients with brain
metastasis. Similar to prior studies, this study is retrospective
(37–39) which underlines the need for
confirmation of our findings in prospective randomized trials.
Predictors for OS in patients with malignant
melanoma metastatic to the brain have been published previously and
include LDH levels, age, Karnofsky index, number of brain
metastasis, leptomeningeal spread, presence of extracerebral
metastases, melanoma ulceration, histology and neurologic symptoms
(10,13,18,25,37,39–42).
The present analysis however, additionally focused
on the subgroup of patients with multiple brain metastases and
evaluated tumor control with respect to different treatment
modalities.
Male gender in our study was more frequent than
female gender (65 vs. 35%) which is similar to the gender
distribution in other reports (10,17,37,39,43).
Unlike the study of Hofmann et al, we did not find a
significant influence of gender on survival (44). Median age at the time of initial
diagnosis of malignant melanoma was 57 years in our cohort which is
slightly higher compared to other studies (10,42)
which may be attributed to the relatively small patient number
(n=100) and long investigation period (1992–2011) in our study.
Fife et al reported a median age at the time of diagnosis of
brain metastasis of 49 and 57 years with a median time from initial
diagnosis of melanoma until occurrence of cerebral metastasis of
2.5 and 3.7 years (10). In our
study, patients were older when brain metastasis was confirmed (62
years), however, median time to development of cerebral metastasis
(2.5 years in our patient group) was comparable with current
literature (1.9–2.7 years) (17,18,37,39).
The impact of age on OS in melanoma patients with
brain metastasis has been reported by several studies (10,18,39,40)
but could not be replicated in our study.
Nodular melanoma was most frequently diagnosed in
our patient cohort (42%) and extracranial metastases were present
in the majority of patients (71%). In the literature, extracranial
metastatic involvement is reported in 65–83% (10,37,39,43,45)
of patients and in 37–51% of cases, multiple organ systems are
affected by extracerebral metastasis (10,37).
In 22% of the patients in our study, lymph nodes
were positive for metastasis upon initial diagnosis of melanoma and
61% of patients developed metastatic spread to lymphatic nodes
during their disease course which stands in line with current
literature, reporting lymph node metastasis in 24–54% (17,39).
Our own results registered multiple site involvement
in 65% of melanoma patients and in 71% of the patients, brain
metastases were symptomatic which is in accordance with the
findings of Raizer et al (39) and Mornex et al (45) who reported neurological symptoms
from brain metastasis in 66–85% of patients.
Compared to other relevant reports which included
17–67% patients with multiple brain metastases (10,37,39,43,45),
the proportion of patients with multiple brain metastases in this
study was 35%.
Median survival in our study was 3.9 months which is
shorter compared to other relevant studies in this field (10,17,18,37,39,41).
Moreover, the majority of our patients displayed extracranial
metastasis and was diagnosed with nodular type melanoma which was
associated with a considerably poor prognosis. In our collective,
seven long-term survivors could be indentified with a median
survival of 32 months.
Number of brain metastasis, presence of extracranial
metastasis and extracranial disease progression but not LDH levels
were significantly associated with survival. Two large
retrospective studies (37,40)
reported LDH levels to be related to survival.
Fifty-three percent of patients in our study had 1–2
brain metastases and lived 6.5 months compared to patients with
>2 cerebral metastases who had a median survival of 2.5 months
which is accordance with current literature (10,37,39,40):
Liew et al (46) reported a
superior survival of patients with 1–3 brain metastases compared to
>4 metastatic brain lesions after stereotactic radiotherapy and
Staudt et al (40) showed a
median survival of 8 months in patients with a solitary brain
lesion as opposed to 3 months in patients with multiple brain
metastases. In the cohort of Eigentler et al (37) patients with multiple brain
metastases had a significantly inferior survival compared to those
with one single brain metastasis and Raizer et al (39) reported extracranial disease and
number of brain metastasis to significantly reduce OS (8 months
with single vs. 3 months with multiple brain metastases).
Interestingly, we could replicate the finding of Raizer et
al (39) that >3 cranial
metastases critically reduced OS.
A significant impact of neurologic symptoms on OS,
which was reported by Bottoni et al (17) and Raizer et al (39), could not be demonstrated by our
study (p=0.52).
Our findings furthermore indicate that location of
metastasis did not impact OS, contrasting the results of Wronski
and Arbit (43) who reported the
infratentorial location to be associated with a significantly
reduced OS in a series of surgically treated stage IV melanoma
patients.
Untreated patients with brain metastasis from
malignant melanoma have a poor prognosis with an expected median
survival of only 1–3 months (47,48)
which accentuates palliation, quality of life and tumor control as
the main treatment focus in stage IV malignant melanoma (49,50).
Surgical treatment is indicated if pathological
confirmation of a cerebral mass is needed and quick symptom relief
from a single dominant lesion is necessary, especially when
obstructive symptoms (i.e., hydrocephalus) or mass effects (i.e.,
midline shift, bleeding) are present (22) and has been shown to improve local
control and survival (10,37,51).
Despite its applicability to differently sized lesions, the
surgical approach is limited to accessible intracerebral
lesions.
Radiosurgery may be suitable for patients with
limited size and number of cerebral lesions (particularly those
<3 cm in size and with mild edema and no mass effect) but in
principle is feasible for brain lesions irrespective of their
location (7,11–13,22,52–54)
with a low complication rate, mortality and morbidity (50,52).
Evidence supports the equivalence of stereotactic radiotherapy and
surgery in the treatment of solitary metastatic cerebral lesions
with a reported 1-year local control rate of 82% after stereotactic
radiotherapy (55).
The rationale for WBRT in the adjuvant setting
(i.e., after local therapy or in inoperable patients who are also
no candidates for stereotactic radiotherapy) is to treat
microscopic disease in order to improve tumor control and possibly
survival (13,56,57).
Compared to untreated patients, WBRT improves survival and may
mitigate neurologic symptoms (30,52,58)
but so far, a significant survival benefit from WBRT could not be
demonstrated (10,22,59,60).
For decades, most cytotoxic drugs which were
available for treatment of metastatic melanoma failed to
significantly improve survival. Little progress could be achieved
when the cytokines interleukin and interferon became available in
the late 1990s while later, cytotoxic drugs such as fotemustine and
temozolomide which pass the blood-brain barrier were routinely used
in malignant melanoma metastatic to the brain (61,62),
yielding response rates of only 25–30% (fotemustine) and 5–17%
(temozolomide) (23,45,63,64).
Combination of radiation with chemotherapy also did not lead to a
significant improvement in survival with response rates of 7.6% and
a median time to progression of only 7 weeks (15–18,22).
Additional use of steroids showed symptom relief and proved to be
superior to best supportive care (22,37).
However, with the approval of new systemic agents in
2011, including immunomodulators such as ipilimumab, a monoclonal
antibody targeting CTLA-4 ligand, considerable progress in the
treatment of advanced melanoma could be achieved (65,66).
Using vermurafinib, a BRAF-inhibitor, in patients with BRAF-mutated
melanoma for instance, resulted in significantly improved overall
and progression-free survival (67–69).
Also other members of recently (2013) approved drugs such as
Dabrafenib and Trametinib showed promising results in phase III
trials so that the portfolio of systemic targeted drugs which can
be used as standard therapy for metastatic melanoma has been
expanded considerably (70).
In a large cohort of 686 patients with brain
metastasis from malignant melanoma, Fife et al (10) reported a superior survival if
surgery was followed by radiotherapy (24%, 8.9 months) or surgery
was given alone (7%, 8.7 months) compared to radiotherapy alone
(36%, 3.4 months). Wronski and Arbit (43) found that consecutive WBRT after
surgical resection of brain metastasis did not improve OS and
recurrence rates. In our study, OS in patients treated with WBRT
alone was 3.2 months which is comparable with the study of Fife
et al (10). Median
survival of patients who received local therapy (either surgery or
stereotactic radiotherapy) followed by WBRT was 8.2 months and the
best OS could be achieved if local therapy was combined with
systemic therapy in the primary treatment (12.7 months).
In the study of Raizer et al, surgery (9.3
vs. 3.9 months), systemic therapy (7.9 vs. 4.1 months) and
stereotactic radio-therapy (10 vs. 4.3 months) but not WBRT
significantly improved survival (39). These results are supported by our
own findings, indicating a superior survival in patients who
received systemic (5.1 vs. 3.1 months, p<0.002) and local
therapy (either stereotactic radiotherapy or surgery, 6.9 vs. 2.6
months, p<0.001). The finding that WBRT non-significantly
prolongs OS could also be replicated (4.8 vs. 3.5 months, p=0.85)
by our study.
Raizer et al reported the best OS if surgery
was combined with stereotactic radiotherapy (13.2 months), followed
by the triple combination of surgery, stereotactic radiotherapy and
WBRT (10.2 months) (39). In our
study, the triple therapy (WBRT, local and systemic therapy)
yielded a median OS of 2 months (primary therapy) and 8 months
(whole treatment) and the best OS could be achieved with the
combination of systemic and local therapy (12.7 months as primary
therapy, 14.2 months as part of the whole treatment concept).
Notably, the study of Raizer et al (39) included 355 patients and systemic
therapy was not evaluated in the combination therapy.
Surgery or stereotactic radiotherapy alone was
reported with a median OS of 8.2 and 9.9 months in the study of
Raizer et al (39). Here,
we found that use local therapy (surgery or stereotactic
radiotherapy) was associated with a median survival of 6.4 (primary
therapy) and 4.4 months (whole therapy). As evidenced by the
current literature, local therapy (stereotactic radiotherapy or
surgery) and systemic therapy remain independent predictors for OS
in the treatment of single brain metastasis (37,39).
For patients with multiple brain metastases, we
found that the combination of WBRT and systemic treatment resulted
in a marginally superior OS compared to WBRT alone (+0.2 months)
which stands in line with current literature (71,72).
We demonstrated that the intracranial disease course
is significantly associated with OS (p=0.0037) and that patients
with intracranial tumor control achieved a better OS (10 months)
compared to patients with uncontrolled intra-cerebral situation (5
months) which supports current studies showing a correlation
between intracranial tumor control and prolonged survival (37,40,49).
If surgery was followed by stereotactic radiotherapy, intracranial
tumor control in our study was superior which supports current
literature, reporting local control rates between 84–94% after
combined surgery and stereotactic radiotherapy (73–75).
In our study, use of local therapy in the primary
treatment significantly increased LC per lesion (p=0.042) which was
best in patients who were treated with a combination of local and
systemic therapy.
When interpreting the results of this study, some
limitations need to be discussed. Our study was of retrospective
design with all limitations inherent to such studies.
Since patients from 1992 up to 2011 were included in
the study, recent developments (i.e., after 2011) in the treatment
of stage IV malignant melanoma, particularly the use of new
systemic agents such as BRAF and MEK targeted drugs or CTLA4 and
PD1 immune checkpoint modulators which were approved by the FDA in
2011 could not be incorporated in this study. Thus, the significant
therapeutic advances achieved by the standard use of these drugs
(76) is not mirrored in this
study which limits the ability of our results to impact on current
treatment or management strategies.
Furthermore, a heterogeneous patient collective
where treatment modalities varied was analyzed and information
regarding causes of death was not routinely available. Thus, no
causal relation between therapies and survival could be determined
by our study.
Also, data on Karnofsky performance score (KPS) and
S100B value were insufficient so that both GPA and RPA scores
(37,40) could not be acquired and
multivariate analyses could not be adjusted for KPS. Therefore, the
impact of the aforementioned parameters on OS could not be
evaluated in our study which limits concrete conclusions of the
effect on OS that each variable may have. It is conceivable that
use of local therapy may be a surrogate for KPS in the dataset
presented in this study.
Finally, analyses regarding the treatments given
could have generated selection effects since only patients who
lived long enough could receive more than one treatment modality.
With respect to the different treatment arms, selection bias cannot
be excluded since only patients with good performance status and
little comorbidity were candidates for surgery for instance.
In conclusion, number of brain metastasis (p=0.004),
presence of extracranial metastases (p=0.035) and use of local
therapy in the primary treatment (p=0.035) are independent
predictors for survival in patients with brain metastases from
malignant melanoma.
For patients with single brain metastasis, a
survival benefit could be demonstrated for local therapy approaches
and systemic treatment but not for WBRT.
Intracranial tumor control (per patient) is
prognostic in malignant melanoma metastatic to the brain. LC (per
lesion) and OS can be most considerably improved by combining local
with systemic therapy. Surgical metastasectomy followed by
stereotactic radiotherapy can increase LC. Patients with multiple
brain metastases benefit from slightly improved OS after a
combination of systemic therapy with WBRT.
Acknowledgements
We would like to thank our colleagues from the
Department of Radiation Oncology and the Department of Dermatology
for their contribution to this study and their continuous
support.
References
|
1
|
Galicich JH: Metastatic brain tumors.
Neurosurgery. 1. 2nd edition. Wilkins RH and Rengachary SS:
McGraw-Hill; New York, NY: pp. 807–821. 1996
|
|
2
|
Nussbaum ES, Djalilian HR, Cho KH and Hall
WA: Brain metastases. Histology, multiplicity, surgery, and
survival. Cancer. 78:1781–1788. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Patchell RA: Metastatic brain tumors.
Neurol Clin. 13:915–925. 1995.PubMed/NCBI
|
|
4
|
Johnson JD and Young B: Demographics of
brain metastasis. Neurosurg Clin North Am. 7:337–344. 1996.
|
|
5
|
Presant CA and Bartolucci AA: Prognostic
factors in metastatic malignant melanoma: The Southeastern Cancer
Study Group Experience. Cancer. 49:2192–2196. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Balch CM and Houghton AN: Diagnosis of
metastatic melanoma at distant sites. Cutaneous Melanoma. 1. 1st
edition. Balch CM, Houghton AN, Milon GW, Sober AJ and Soong SJ:
Lippincott; Philadelphia, PA: pp. 439–467. 1992
|
|
7
|
Sampson JH, Friedman AH and Seigler HF:
Demographics, treatment, and prognosis of 76 patients with
‘primary’ intracerebral melanoma. J Neurosurg. 82:A3571995.
|
|
8
|
Moon D, Maafs E, Peterson-Schaefer K, et
al: A review of 567 cases of brain metastases from malignant
melanoma. Melanoma Res. 3:401993. View Article : Google Scholar
|
|
9
|
Bhatia S, Tykodi SS and Thompson JA:
Treatment of metastatic melanoma: An overview. Oncology (Williston
Park). 23:488–496. 2009.
|
|
10
|
Fife KM, Colman MH, Stevens GN, Firth IC,
Moon D, Shannon KF, Harman R, Petersen-Schaefer K, Zacest AC,
Besser M, et al: Determinants of outcome in melanoma patients with
cerebral metastases. J Clin Oncol. 22:1293–1300. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Buchsbaum JC, Suh JH, Lee SY, Chidel MA,
Greskovich JF and Barnett GH: Survival by radiation therapy
oncology group recursive partitioning analysis class and treatment
modality in patients with brain metastases from malignant melanoma:
A retrospective study. Cancer. 94:2265–2272. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saha S, Meyer M, Krementz ET, Hoda S,
Carter RD, Muchmore J and Sutherland C: Prognostic evaluation of
intracranial metastasis in malignant melanoma. Ann Surg Oncol.
1:38–44. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sampson JH, Carter JH Jr, Friedman AH and
Seigler HF: Demographics, prognosis, and therapy in 702 patients
with brain metastases from malignant melanoma. J Neurosurg.
88:11–20. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
de la Monte SM, Moore GW and Hutchins GM:
Patterned distribution of metastases from malignant melanoma in
humans. Cancer Res. 43:3427–3433. 1983.PubMed/NCBI
|
|
15
|
Patel JK, Didolkar MS, Pickren JW and
Moore RH: Metastatic pattern of malignant melanoma. A study of 216
autopsy cases. Am J Surg. 135:807–810. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Amer MH, Al-Sarraf M, Baker LH and
Vaitkevicius VK: Malignant melanoma and central nervous system
metastases: Incidence, diagnosis, treatment and survival. Cancer.
42:660–668. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bottoni U, Clerico R, Paolino G, Ambrifi
M, Corsetti P and Calvieri S: Predictors and survival in patients
with melanoma brain metastases. Med Oncol. 30:4662013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zakrzewski J, Geraghty LN, Rose AE,
Christos PJ, Mazumdar M, Polsky D, Shapiro R, Berman R, Darvishian
F, Hernando E, et al: Clinical variables and primary tumor
characteristics predictive of the development of melanoma brain
metastases and post-brain metastases survival. Cancer.
117:1711–1720. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jakob JA, Bassett RL Jr, Ng CS, Curry JL,
Joseph RW, Alvarado GC, Rohlfs ML, Richard J, Gershenwald JE, Kim
KB, et al: NRAS mutation status is an independent prognostic factor
in metastatic melanoma. Cancer. 118:4014–4023. 2012. View Article : Google Scholar :
|
|
20
|
McLoughlin JM, Zager JS, Sondak VK and
Berk LB: Treatment options for limited or symptomatic metastatic
melanoma. Cancer Control. 15:239–247. 2008.PubMed/NCBI
|
|
21
|
Carella RJ, Gelber R, Hendrickson F, Berry
HC and Cooper JS: Value of radiation therapy in the management of
patients with cerebral metastases from malignant melanoma:
Radiation Therapy Oncology Group Brain Metastases Study I and II.
Cancer. 45:679–683. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hong A, Fogarty G and Izard MA: The role
of radiation therapy in the management of metastatic melanoma in
the brain. Int J Surg Oncol. 2012:2947352012.PubMed/NCBI
|
|
23
|
Jacquillat C, Khayat D, Banzet P, Weil M,
Fumoleau P, Avril MF, Namer M, Bonneterre J, Kerbrat P, Bonerandi
JJ, et al: Final report of the French multicenter phase II study of
the nitrosourea fotemustine in 153 evaluable patients with
disseminated malignant melanoma including patients with cerebral
metastases. Cancer. 66:1873–1878. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jacquillat C, Khayat D, Banzet P, Weil M,
Avril MF, Fumoleau P, Namer M, Bonneterre J, Kerbrat P, Bonerandi
JJ, et al: Chemotherapy by fotemustine in cerebral metastases of
disseminated malignant melanoma. Cancer Chemother Pharmacol.
25:263–266. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mori Y, Kondziolka D, Flickinger JC,
Kirkwood JM, Agarwala S and Lunsford LD: Stereotactic radiosurgery
for cerebral metastatic melanoma: Factors affecting local disease
control and survival. Int J Radiat Oncol Biol Phys. 42:581–589.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Overett TK and Shiu MH: Surgical treatment
of distant metastatic melanoma. Indications and results. Cancer.
56:1222–1230. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zacest AC, Besser M, Stevens G, Thompson
JF, McCarthy WH and Culjak G: Surgical management of cerebral
metastases from melanoma: Outcome in 147 patients treated at a
single institution over two decades. J Neurosurg. 96:552–558. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Houghton AN: Treatment for advanced
melanoma. Cutaneous Melanoma. 1. 1st edition. Balch CM, Houghton
AN, Milon GW, Sober AJ and Soong SJ: Lippincott; Philadelphia, PA:
pp. 468–497. 1992
|
|
29
|
Ellerhorst J, Strom E, Nardone E and
McCutcheon I: Whole brain irradiation for patients with metastatic
melanoma: A review of 87 cases. Int J Radiat Oncol Biol Phys.
49:93–97. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Morris SL, Low SH, A’Hern RP, Eisen TG,
Gore ME, Nutting CM and Harrington KJ: A prognostic index that
predicts outcome following palliative whole brain radiotherapy for
patients with metastatic malignant melanoma. Br J Cancer.
91:829–833. 2004.PubMed/NCBI
|
|
31
|
Gupta G, Robertson AG and MacKie RM:
Cerebral metastases of cutaneous melanoma. Br J Cancer. 76:256–259.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vlock DR, Kirkwood JM, Leutzinger C, Kapp
DS and Fischer JJ: High-dose fraction radiation therapy for
intracranial metastases of malignant melanoma: A comparison with
low-dose fraction therapy. Cancer. 49:2289–2294. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stridsklev IC, Hagen S and Klepp O:
Radiation therapy for brain metastases from malignant melanoma.
Acta Radiol Oncol. 23:231–235. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Madajewicz S, Karakousis C, West CR,
Caracandas J and Avellanosa AM: Malignant melanoma brain
metastases. Review of Roswell Park Memorial Institute experience.
Cancer. 53:2550–2552. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Douglas JG and Margolin K: The treatment
of brain metastases from malignant melanoma. Semin Oncol.
29:518–524. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
McWilliams RR, Brown PD, Buckner JC, Link
MJ and Markovic SN: Treatment of brain metastases from melanoma.
Mayo Clin Proc. 78:1529–1536. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Eigentler TK, Figl A, Krex D, Mohr P,
Mauch C, Rass K, Bostroem A, Heese O, Koelbl O, Garbe C, et al:
Dermatologic Cooperative Oncology Group and the National
Interdisciplinary Working Group on Melanoma: Number of metastases,
serum lactate dehydrogenase level, and type of treatment are
prognostic factors in patients with brain metastases of malignant
melanoma. Cancer. 117:1697–1703. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Grob JJ, Regis J, Laurans R, Delaunay M,
Wolkenstein P, Paul K, Souteyrand P, Koeppel MC, Murraciole X,
Perragut JC, et al: Radiosurgery without whole brain radiotherapy
in melanoma brain metastases. Club de Cancérologie Cutanée. Eur J
Cancer. 34:1187–1192. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Raizer JJ, Hwu WJ, Panageas KS, Wilton A,
Baldwin DE, Bailey E, von Althann C, Lamb LA, Alvarado G, Bilsky
MH, et al: Brain and leptomeningeal metastases from cutaneous
melanoma: Survival outcomes based on clinical features. Neurooncol.
10:199–207. 2008.
|
|
40
|
Staudt M, Lasithiotakis K, Leiter U, Meier
F, Eigentler T, Bamberg M, Tatagiba M, Brossart P and Garbe C:
Determinants of survival in patients with brain metastases from
cutaneous melanoma. Br J Cancer. 102:1213–1218. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Davies MA, Liu P, McIntyre S, Kim KB,
Papadopoulos N, Hwu WJ, Hwu P and Bedikian A: Prognostic factors
for survival in melanoma patients with brain metastases. Cancer.
117:1687–1696. 2011. View Article : Google Scholar
|
|
42
|
Eisemann N, Jansen L, Holleczek B,
Waldmann A, Luttmann S, Emrich K, Hauschild A, Brenner H and
Katalinic A; GEKID Survival Working Group. Up-to-date results on
survival of patients with melanoma in Germany. Br J Dermatol.
167:606–612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wroński M and Arbit E: Surgical treatment
of brain metastases from melanoma: A retrospective study of 91
patients. J Neurosurg. 93:9–18. 2000. View Article : Google Scholar
|
|
44
|
Hofmann M, Kiecker F, Wurm R, Schlenger L,
Budach V, Sterry W and Trefzer U: Temozolomide with or without
radiotherapy in melanoma with unresectable brain metastases. J
Neurooncol. 76:59–64. 2006. View Article : Google Scholar
|
|
45
|
Mornex F, Thomas L, Mohr P, Hauschild A,
Delaunay MM, Lesimple T, Tilgen W, Bui BN, Guillot B, Ulrich J, et
al: A prospective randomized multicentre phase III trial of
fotemustine plus whole brain irradiation versus fotemustine alone
in cerebral metastases of malignant melanoma. Melanoma Res.
13:97–103. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liew DN, Kano H, Kondziolka D, Mathieu D,
Niranjan A, Flickinger JC, Kirkwood JM, Tarhini A, Moschos S and
Lunsford LD: Outcome predictors of Gamma Knife surgery for melanoma
brain metastases. Clinical article. J Neurosurg. 114:769–779. 2011.
View Article : Google Scholar
|
|
47
|
Garbe C, Hauschild A, Volkenandt M,
Schadendorf D, Stolz W, Reinhold U, Kortmann RD, Kettelhack C,
Frerich B, Keilholz U, et al: Evidence and interdisciplinary
consensus-based German guidelines: Surgical treatment and
radiotherapy of melanoma. Melanoma Res. 18:61–67. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sloan AE, Nock CJ and Einstein DB:
Diagnosis and treatment of melanoma brain metastasis: A literature
review. Cancer Control. 16:248–255. 2009.PubMed/NCBI
|
|
49
|
Steinbach J, Vordermark D and Gutzmer R:
ZNS-Metastasen - eine interdisziplinäre Herausforderung. Onkologie.
36(Suppl 4): 2–6. 2013.(In German). View Article : Google Scholar
|
|
50
|
Flanigan JC, Jilaveanu LB, Chiang VL and
Kluger HM: Advances in therapy for melanoma brain metastases. Clin
Dermatol. 31:264–281. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Miller D, Zappala V, El Hindy N,
Livingstone E, Schadendorf D, Sure U and Sandalcioglu IE:
Intracerebral metastases of malignant melanoma and their
recurrences - a clinical analysis. Clin Neurol Neurosurg.
115:1721–1728. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hanson PW, Elaimy AL, Lamoreaux WT,
Demakas JJ, Fairbanks RK, Mackay AR, Taylor B, Cooke BS, Thumma SR
and Lee CM: A concise review of the efficacy of stereotactic
radiosurgery in the management of melanoma and renal cell carcinoma
brain metastases. World J Surg Oncol. 10:1762012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Fogarty GB and Hong A: Radiation therapy
for advanced and metastatic melanoma. J Surg Oncol. 109:370–375.
2014. View Article : Google Scholar
|
|
54
|
Shaw E, Scott C, Souhami L, Dinapoli R,
Kline R, Loeffler J and Farnan N: Single dose radiosurgical
treatment of recurrent previously irradiated primary brain tumors
and brain metastases: Final report of RTOG protocol 90-05. Int J
Radiat Oncol Biol Phys. 47:291–298. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Swinson BM and Friedman WA: Linear
accelerator stereotactic radiosurgery for metastatic brain tumors:
17 years of experience at the University of Florida. Neurosurgery.
62:1018–1032. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Eichler AF and Loeffler JS:
Multidisciplinary management of brain metastases. Oncologist.
12:884–898. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kocher M, Soffietti R, Abacioglu U, Villà
S, Fauchon F, Baumert BG, Fariselli L, Tzuk-Shina T, Kortmann RD,
Carrie C, et al: Adjuvant whole-brain radiotherapy versus
observation after radiosurgery or surgical resection of one to
three cerebral metastases: Results of the EORTC 22952–26001 study.
J Clin Oncol. 29:134–141. 2011. View Article : Google Scholar :
|
|
58
|
Fonkem E, Uhlmann EJ, Floyd SR, Mahadevan
A, Kasper E, Eton O and Wong ET: Melanoma brain metastasis:
Overview of current management and emerging targeted therapies.
Expert Rev Neurother. 12:1207–1215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hagen NA, Cirrincione C, Thaler HT and
DeAngelis LM: The role of radiation therapy following resection of
single brain metastasis from melanoma. Neurology. 40:158–160. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Schild SE, Behl D, Markovic SN, Brown PD,
Sande JR, Deming RL, Rowland KM Jr and Bearden JD: Brain metastases
from melanoma: Is there a role for concurrent temozolomide in
addition to whole brain radiation therapy? Am J Clin Oncol.
33:633–636. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Gibney GT, Forsyth PA and Sondak VK:
Melanoma in the brain: Biology and therapeutic options. Melanoma
Res. 22:177–183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lyle M and Long GV: The role of systemic
therapies in the management of melanoma brain metastases. Curr Opin
Oncol. 26:222–229. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Bafaloukos D, Tsoutsos D, Fountzilas G,
Linardou H, Christodoulou C, Kalofonos HP, Briassoulis E,
Panagiotou P, Hatzichristou H and Gogas H: The effect of
temozolomide-based chemotherapy in patients with cerebral
metastases from melanoma. Melanoma Res. 14:289–294. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Schadendorf D, Hauschild A, Ugurel S,
Thoelke A, Egberts F, Kreissig M, Linse R, Trefzer U, Vogt T,
Tilgen W, et al: Dose-intensified bi-weekly temozolomide in
patients with asymptomatic brain metastases from malignant
melanoma: A phase II DeCOG/ADO study. Ann Oncol. 17:1592–1597.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Eggermont AM and Robert C: New drugs in
melanoma: it’s a whole new world. Eur J Cancer. 47:2150–2157. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lipson EJ and Drake CG: Ipilimumab: An
anti-CTLA-4 antibody for metastatic melanoma. Clin Cancer Res.
17:6958–6962. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Spagnolo F and Queirolo P: Upcoming
strategies for the treatment of metastatic melanoma. Arch Dermatol
Res. 304:177–184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ravan MC and Matalke MS: Vemurafinib in
patients with BRAF V600E mutation-positive advanced melanoma. Clin
Ther. 34:1474–1486. 2012. View Article : Google Scholar
|
|
69
|
Keating GM: Vemurafinib: in unresectable
or metastatic melanoma. BioDrugs. 26:325–334. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Palathinkal DM, Sharma TR and Koon HB:
Current systemic therapies for melanoma. Dermatol Surg. 40:948–963.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Margolin K, Atkins B, Thompson A, Ernstoff
S, Weber J, Flaherty L, Clark I, Weiss G, Sosman J, Smith W II, et
al: Temozolomide and whole brain irradiation in melanoma metastatic
to the brain: A phase II trial of the Cytokine Working Group. J
Cancer Res Clin Oncol. 128:214–218. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Atkins MB, Sosman JA, Agarwala S, Logan T,
Clark JI, Ernstoff MS, Lawson D, Dutcher JP, Weiss G, Curti B, et
al: Temozolomide, thalidomide, and whole brain radiation therapy
for patients with brain metastasis from metastatic melanoma: A
phase II Cytokine Working Group study. Cancer. 113:2139–2145. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Hartford AC, Paravati AJ, Spire WJ, Li Z,
Jarvis LA, Fadul CE, Rhodes CH, Erkmen K, Friedman J, Gladstone DJ,
et al: Postoperative stereotactic radiosurgery without whole-brain
radiation therapy for brain metastases: Potential role of
preoperative tumor size. Int J Radiat Oncol Biol Phys. 85:650–655.
2013. View Article : Google Scholar
|
|
74
|
Kelly PJ, Lin YB, Yu AY, Alexander BM,
Hacker F, Marcus KJ and Weiss SE: Stereotactic irradiation of the
postoperative resection cavity for brain metastasis: A frameless
linear accelerator-based case series and review of the technique.
Int J Radiat Oncol Biol Phys. 82:95–101. 2012. View Article : Google Scholar
|
|
75
|
Jagannathan J, Yen CP, Ray DK, Schlesinger
D, Oskouian RJ, Pouratian N, Shaffrey ME, Larner J and Sheehan JP:
Gamma Knife radiosurgery to the surgical cavity following resection
of brain metastases. J Neurosurg. 111:431–438. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Foletto MC and Haas SE: Cutaneous
melanoma: New advances in treatment. An Bras Dermatol. 89:301–310.
2014. View Article : Google Scholar : PubMed/NCBI
|


















