Introduction
Gastric and colon cancers remain the leading cause
of cancer mortality throughout the world. Overall, neoplasms of the
gastrointestinal (GI) tract account for 25% of all neoplasms and
for 9% of deaths from all causes in the world (1). Therefore, chemoprevention by dietary
constituents is gaining interest as a possible approach to reduce
cancer risk (2). The GI tract
works in a constant link with the external environment and
modifications in the diet may amplify or prevent exposure to
carcinogenetic factors. In this context, identifying dietary
components with antineoplastic activity and investigating their
mechanisms of action are assuming importance in a new strategy of
prevention for blocking or, at least, delaying GI cancer onset
(2,3).
Among the substances naturally found in foods,
evidence suggests that dietary vitamin K (VK) compounds are able to
prevent the growth of many types of cancer cells, as demonstrated
by studies performed in both in vitro and in vivo
models (4–7).
VK is the family name for a series of fat-soluble
compounds, classically associated to bone health and metabolism and
in regulation of blood clotting (8). There are two structurally related
natural forms of vitamin K: the vitamin K1 (VK1), phylloquinone and
the vitamin K2 (VK2), menaquinone. The first is mainly found in
green leafy vegetables, the latter is synthesized by the intestinal
flora. Another compound, the vitamin K3 (VK3), menadione, is not
considered a natural VK, but rather a synthetic analogue acting as
a provitamin (8).
Most of data on the antineoplastic effects of VK
family derives from studies performed on VK2 and VK3 and the
treatment of hepatocellular carcinoma (9–11).
As regards VK1, it has been proven to be effective in glioma and
pancreatic cancer cell lines (12,13).
However, in spite of its features as a nutritional factor, the
potential of VK1 against neoplasms of the GI tract has received
less or no attention so far (14).
In addition, the exact mechanism of action of VK family in
affecting the neoplastic growth is still object of investigation,
even if it has been reported that VK compounds may alter the
expression of some genes involved in cell cycle control and induce
apoptosis in different cancer cell lines (15,16).
A number of molecules, such as the natural polyamines: putrescine,
spermidine and spermine, could be of interest in VK action.
Polyamines are ubiquitous short-chain polycationic alkylamines
actively involved in cell proliferation and differentiation. They
interact with anionic macromolecules including DNA, RNA and
protein, thereby modulating chromatin structure, gene
transcription, translation and DNA stabilization (17). Moreover, it has been demonstrated
that polyamines are also involved in signal transduction and in
cell death pathway (18).
The rise in intracellular polyamine concentration,
mostly occurring through an upregulation of the biosynthetic enzyme
ornithine decarboxylase (ODC), correlates with increased cell
proliferation and tumorigenesis (19). Thus, the mucosal polyamine levels
can be considered markers for neoplastic proliferation (20). As a consequence, not only polyamine
deprivation, but also impairment of polyamine uptake into
neoplastic cells and modulation of the polyamine metabolic pathway,
can represent a way of cancer chemoprevention and chemotherapy
(21). Drugs that affect polyamine
biosynthesis and catabolism have been extensively evaluated in both
in vitro and in vivo studies as possible alternative
tools for the management of cancer (22). Besides, some components of diet,
particularly flavonoids, polyphenols and probiotics, have already
been reported to reduce the hyperproliferative role of polyamines
in GI neoplasms (23–25). On the contrary, the polyamine
pathway as potential target for dietary VK has not been evaluated
and no data are available on possible different susceptibility to
VK1 by gastric or colon neoplastic cell lines.
In order to shed light on how VK1 could affect the
growth of neoplasms originating from different GI tracts, the aim
of the present study was to evaluate and compare the effects of
increasing concentrations of VK1 on the cell proliferation and
apoptosis of a gastric (HGC-27) and a colon (SW480) cancer cell
line. Additionally, to investigate which mechanisms could be
involved in affecting cell growth, the polyamine biosynthesis, as
well as the phospho-ERK 1/2 (P-ERK 1/2) expression, were examined
in the same cell lines. The latter signaling molecule has been
linked to the regulation of cellular growth, apoptosis and
chemoresistance (26).
Materials and methods
Cell culture conditions
HGC-27 cell line (neoplastic cells from
undifferentiated carcinoma of the stomach) and human colon
adenocarcinoma-derived SW480 cell line (low differentiated cells
derived from colonic adenocarcinoma grade III–IV) were obtained
from the Interlab Cell Line Collection (Genoa, Italy). As
previously published (27), cells
were routinely cultured in Dulbecco’s modified Eagle’s medium
(DMEM) and Leibovitz L-15 medium, respectively, supplemented with
10% fetal bovine serum (FBS), 2 mM glutamine, 100 U/ml penicillin,
100 μg/ml streptomycin in a monolayer culture and incubated at 37°C
in a humidified atmosphere containing 5% CO2 in air. At
confluence, the cells were harvested by trypsinization and serially
sub-cultured with a 1:4 split ratio. All cell culture components
were purchased from Sigma-Aldrich (Milan, Italy).
Vitamin K1 treatment
HGC-27 and SW480 cells (25th–30th passage) were
seeded at a density of 2×105 cells/5 ml of supplemented
Leibovitz L-15 and DMEM medium, respectively, in 60-mm tissue
culture dishes (Corning Costar Co., Milan, Italy). After 24 h, to
allow for attachment, the medium was removed and supplemented
culture medium containing increasing concentrations of VK1 (10, 50,
100 and 200 μM) added to cells for 24, 48 and 72 h. Triplicate
cultures were set up for each treatment and for the control, and
each experiment was repeated 4 times. In the set of experiments
investigating the role of ERK 1/2 on the VK1-induced apoptosis, the
cells were treated with 20 μM MEK inhibitor (UO126) 2 h prior to
100- and 200-μM VK1 treatment.
Assessment of cell proliferation
After HGC-27 and SW480 cells had been cultured for
24, 48 and 72 h with increasing concentrations of VK1, the
proliferative response was measured by colorimetric 3-(4,5
di-methylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
test.
To determine cell growth by colorimetric test, MTT
stock solution (5 mg/ml in medium) was added to each dish at a
volume of one tenth the original culture volume and incubated for 2
h at 37°C in humidified CO2. At the end of the
incubation period, the medium was removed, and the blue formazan
crystals were solubilized with acidic isopropanol (0.1N HCl
absolute isopropanol). MTT conversion to formazan by metabolically
viable cells was monitored by spectrophotometer at an optical
density of 570 nm (28).
Apoptosis
The apoptosis was measured by evaluation of Bax and
Bcl-2 mRNA expression [using quantitative PCR (qPCR) method with
SYBR1-Green dye] and protein expression of Bax, Bcl-2, caspase-3,
caspase-9 and P-ERK 1/2 (using western blot analysis).
Cells were washed twice in phosphate-buffered saline
(PBS) and then trypsinized and centrifuged at 280 × g. The cell
pellets were re-suspended in 0.3 ml of pure distilled water and
used for RNA extraction. Total cell RNA was extracted using TRI
Reagent (Molecular Research Center, Inc., Cincinnati, OH, USA),
following the manufacturer’s instructions. Approximately 2 μg total
cell RNA, extracted from both the control and treated cells, was
used for cDNA synthesis. Reverse transcription (RT) was carried out
in 20 μl of the final volume at 41°C for 60 min, using 30 pmol
antisense primers for analyses of Bax, Bcl-2 and β-actin gene
(28). The β-actin gene was
utilized as an internal control and it was chosen as a reference
housekeeping gene. Real-time PCRs were performed in 25 μl of final
volume containing 2 μl of cDNA, Master Mix with SYBR-Green (iQ SYBR
Green Supermix; Bio-Rad Laboratories, Milan, Italy) and sense and
antisense primers for Bax, Bcl-2 and β-actin gene.
Real-time PCRs were carried out in a CFX96 Real-Time
PCR detection system (Bio-Rad Laboratories) using the following
protocol: 45 cycles at 95°C for 3 min, 95°C for 10 sec, 55°C for 30
sec followed by a melting curve step at 65–95°C with a heating rate
of 0.5°C per cycle for 80 cycles. The PCR products were quantified
by external calibration curves, one for each tested gene. Curves
were obtained with serial dilutions of known copy number of
molecules (102–107 molecules). All expression
data were normalized by dividing the target amount by the amount of
β-actin. The specificity of the PCR product was confirmed by gel
electrophoresis.
For western blotting, HGC-27 and SW480 cells were
collected and lysed on ice in RIPA buffer (Pierce Ripa buffer;
Thermo Fisher Scientific, Rockford, IL, USA). After homogenization
and centrifugation at 14,000 rpm for 15 min at 4°C, protein
concentration was measured by a standard Bradford assay (Bio-Rad
Laboratories). Aliquots of 50 μg of total proteins were separated
in 4–12% pre-cast polyacrylamide gels (Invitrogen, Life
Technologies, Carlsbad, CA, USA) and transferred onto a PVDF
membrane (Bio-Rad Laboratories) with the Trans-Blot Turbo (Bio-Rad
Laboratories). Bax, Bcl-2, caspase-3, caspase-9, P-ERK 1/2 and
β-actin protein expressions were evaluated by 1:500 diluted Bax,
Bcl-2 (D55G8), cleaved caspase-3 (Asp175), caspase-3 (3G2), cleaved
caspase-9, caspase-9, phospho-p44/42 mitogen-activated protein
kinase (MAPK) (ERK 1/2) (197G2), p44/42 MAPK (L34F12) and β-actin
antibody, respectively (Cell Signaling Technology, Danvers, MA,
USA). After overnight incubation, the membranes were further
incubated with a horseradish peroxidase-conjugated goat secondary
antibody (Bio-Rad Laboratories). The proteins were detected by
chemiluminescence (ECL; Thermo Fisher Scientific). The
densitometric analysis of each protein-related signal was obtained
by using the Molecular Imager ChemiDoc™ (Bio-Rad Laboratories) and
normalized against β-actin expression.
Polyamine biosynthesis
For the evaluation of polyamine levels after VK1
treatment, each cell culture pellet was homogenized in 700 μl of
0.9% sodium chloride mixed with 10 μl (200 nmol/ml) of the internal
standard 1,10-diamino-decane (1,10-DAD). An aliquot of the
homogenate was used to measure the total protein content. Then, to
precipitate proteins, 50 μl of perchloride acid (PCA) 3M was added
to the homogenate. After 30 min of incubation in ice, the
homogenate was centrifuged for 15 min at 7,000 × g. The supernatant
was filtered (Millex-HV13 pore size 0.45 μm; Millipore, Bedford,
MA, USA) and lyophilized. The residue was dissolved in 300 μl of
HCl (0.1 N). Dansylation and the extraction of dansyl-polyamine
derivatives were performed as previously described (29). After extraction, aliquots of 200 μl
were injected into a high-performance liquid chromatography system
(UltiMate 3000; Dionex Corp., Sunnyvale, CA, USA) equipped with a
reverse-phase column (Sunfire C18, 4.6×100 mm, 3.5 μm particle
size; Waters Corp., Milford, MA, USA). Polyamines were eluted with
a linear gradient ranging from acetonitrile-water (50:50, v:v) to
acetonitrile (100%) for 30 min. The flow was 0.5–1.0 ml/min from 0
to 12 min and then set at a constant rate (1.0 ml/min) until the
30th min. The fluorescent intensity was monitored by a fluorescence
detector (UltiMate 3000 RS; Dionex Corp.) with excitation at 320 nm
and emission at 512 nm. Polyamine levels were expressed as
concentration values in nmol/mg of protein.
ODC activity was measured with a radiometric
technique that estimated the amount of 14CO2
liberated from DL-[1-14C]-ornithine (specific activity,
56.0 mCi/mmol; New England Nuclear, Boston, MA, USA) (30). The cell culture pellet
(2–4×106 cells) was homogenized in 0.6 ml ice-cold
Tris-HCl (15 mM, pH 7.5) containing 2.5 mM dithiothreitol, 40μM
pyridoxal-5-phosphate, and 100 μM ethylene diamine tetra acetate
and then centrifuged at 30,000 × g for 30 min at 4°C. An aliquot of
supernatant (200 μl) was added to a glass test tube containing 0.05
μCi DL-[1-14C]-ornithine and 39 nmol DL-ornithine. After
incubation for 60 min at 37°C, the reaction was stopped by adding
trichloroacetic acid (TCA) to a final concentration of 50%.
14CO2 liberated from
DL-[1-14C]-ornithine was trapped on filter paper
pre-treated with 40 μl NaOH (2N), which was suspended in a center
well above the reaction mixture. Radioactivity on the filter papers
was determined by a liquid scintillation counter (model 1219
Rackbeta; Pharmacia LKB Biotechnology AB, Uppsala, Sweden). ODC
activity was expressed as pmolCO2/h/mg of protein.
Enzymatic activity was found to be linear within the range of
50–600 μg of protein (r2 = 0.99). The intra-assay and inter-assay
variation coefficients (CV%) were 6 and 8%, respectively.
The effects of VK1 treatments on ODC mRNA levels
were evaluated using the above described quantitative PCR method
with SYBR1-Green dye and the appropriate primers.
Statistical analysis
Due to the non-normal distribution of the data,
non-parametric tests were performed. Data were analyzed by
Kruskal-Wallis analysis of variance and Dunn’s multiple comparison
test. All data are expressed as mean ± SEM. Differences were
considered significant at P<0.05. The software package SigmaStat
for Windows, version 3.00 (SPSS, Inc., San Jose, CA, USA) was
used.
Results
Effects of VK1 on cell proliferation
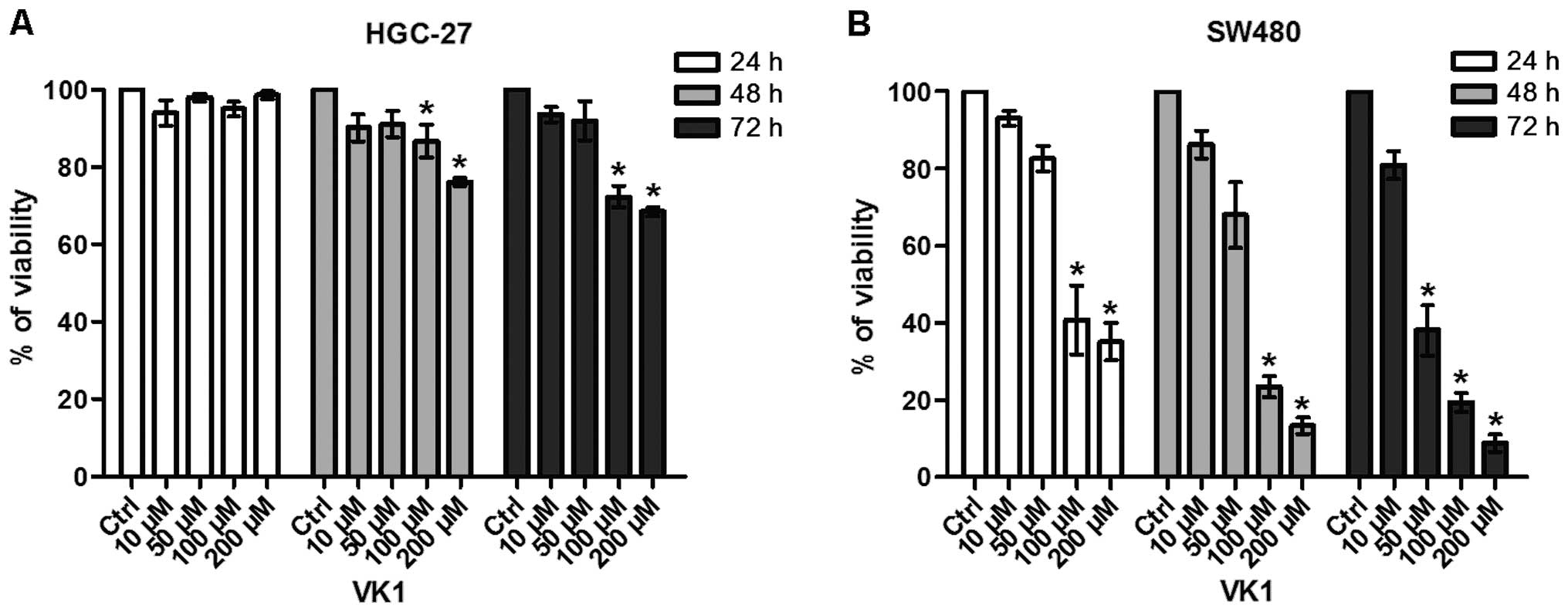
Fig. 1 reports the
effects of exposure of HGC-27 and SW480 cell lines to increasing
concentrations of VK1 (namely 10, 50, 100 and 200 μM) for 24, 48
and 72 h. VK1 produced considerable antiproliferative effects in
both cell lines.
In particular, VK1 administration caused a
significant (P<0.05) reduction in the conversion of the MTT
tetrazolium salt in HGC-27 cells compared to untreated control with
concentrations starting from 100 μM after 48 and 72 h of treatment
(−13 and −28%, respectively) (Fig.
1A).
In SW480 colon cancer cells this effect was more
evident (−59%) and started earlier, after 24 h of 100 μM treatment
(Fig. 1B). After 72 h, VK1
concentrations ≥50 μM were able to cause a significant and marked
reduction (−62%) of cell proliferation (P<0.05).
Effects of VK1 on apoptosis
Fig. 2 shows the
effects of increasing VK1 concentrations on apoptosis of the HGC-27
and SW480 cell lines, evaluated by Bax and Bcl-2 mRNA levels and
expressed as Bax/Bcl-2 ratio.
HGC-27 cell line exhibited a significant (P<0.05)
and dramatic increase in the Bax/Bcl-2 ratio at 48 h and with the
highest concentrations of VK1 (100 and 200 μM) compared to control
cells (+343 and +522%, respectively) (Fig. 2A). Similarly, in SW480 cells a
significant (P<0.05) and even more dramatic increase in the
Bax/Bcl-2 ratio was evident at 48 h and with the highest
concentrations of VK1 (100 and 200 μM) compared to control cells
(+750 and +500%, respectively) (Fig.
2B). The induction of apoptosis continued to be significant
after 72 h of treatment with the concentration of 100 μM (+300%),
while the Bax/Bcl-2 ratio dramatically decreased by 42% with the
highest 200 μM concentration, probably as a consequence of VK1
toxic effect.
The protein levels of Bax, Bcl-2, caspase-3 and
caspase-9 were evaluated by western blot analysis. In HGC-27 cells,
no significant changes in Bax and Bcl-2 proteins or in Bax/Bcl-2
ratio occurred at all the times and concentrations used (data not
shown). On the contrary, an increase in apoptotic proteins was
observed only after 48 h of treatment in SW480 cells (Fig. 3A). More specifically, Bax protein
significantly (P<0.05) increased by ~200% in cells treated with
VK1 concentrations ≥100 μM compared to untreated cells (Fig. 3B). Bcl-2 significantly (P<0.05)
decreased by 50% with VK1 concentrations ≥10 μM compared to control
cells (Fig. 3C). A significant
(P<0.05) 200% increase in the Bax/Bcl-2 ratio was observed with
VK1 concentrations equal to 50 and 100 μM compared to untreated
cells (Fig. 3D). With the highest
200 μM concentration, Bax/Bcl-2 ratio dramatically increased by
400% compared to control cells. Of note, the addition of UO126
blocked the VK1 mediated induction of apoptosis in SW480 colon
cancer cells.
As for the involvement of caspases in VK1-induced
apoptosis, no significant cleavage of caspase-3, an executioner
caspase, or caspase-9, an initiator caspase, was observed in the
two cell lines. This evidence suggests that VK1 may induce
apoptosis in these cells without activation of caspases (data not
shown).
P-ERK 1/2 phosphorylation was evaluated by treating
cells with increasing VK1 concentrations (from 10 to 200 μM) for
24, 48 and 72 h. No variation, at all the tested times was observed
in P-ERK 1/2 in HGC-27 cells (data not shown). On the contrary, a
dose-dependent significant (P<0.05) increase in P-ERK 1/2 was
observed only after 48 h in SW480 cells with VK1 concentrations
starting from 10 μM (+100%) (Fig.
4). When cells were pretreated for 2 h with 20 μM of the MEK
inhibitor UO126 followed by VK1 treatment (100 and 200 μM) for 48
h, no induction of P-ERK 1/2 was observed. Therefore, as for Bax
and Bcl-2 proteins, the addition of UO126 blocked SW480 cells from
VK1 mediated induction of apoptosis, thus suggesting a contribution
of the MAPK pathway in this process.
Effects of VK1 on polyamine
biosynthesis
Table I shows the
polyamine profile in HGC-27 and SW480 cell lines following
administration of increasing VK1 concentrations up to 72 h. VK1
treatment led to a decrease in the single and total polyamine
contents in both cell lines. In detail, a significant (P<0.05)
reduction in the spermidine and total polyamine content was
observed in HGC-27 cells after 48 h (−41 and −14%, respectively)
and 72 h (−45 and −28%, respectively) of exposure to VK1
concentrations ≥100 μM compared to control cells. In SW480 cells,
the effect started after 24 h with VK1 concentrations ≥100 μM that
induced a significant (P<0.05) decrease of all the single and
total polyamine levels compared to control cells. Of note, the
effect persisted after 48 and 72 h of treatment.
 | Table IThe polyamine profile. |
Table I
The polyamine profile.
| HGC-27 | | Control | K1 10 μM | K1 50 μM | K1 100 μM | K1 200 μM |
|---|
| 24 h | Put | 0.17±0.02 | 0.14±0.02 | 0.14±0.03 | 0.11±0.02 | 0.14±0.02 |
| Spd | 10.06±0.17 | 9.46±0.20 | 9.6±0.20 | 9.53±0.09 | 9.60±0.23 |
| Spm | 17.26±0.93 | 17.81±0.54 | 17.03±1.0 | 17.31±0.68 | 17.56±0.51 |
| Total | 27.50±0.79 | 27.42±0.76 | 26.77±1.7 | 26.94±0.79 | 27.39±0.73 |
| 48 h | Put | 0.15±0.02 | 0.16±0.02 | 0.21±0.02 | 0.22±0.04 | 0.17±0.02 |
| Spd | 9.92±0.29 | 8.73±0.39 | 7.6±0.40 |
5.81±0.22a |
5.83±0.44a |
| Spm | 17.77±0.27 | 17.40±0.37 | 17.87±0.14 | 17.90±0.37 | 16.21±0.23 |
| Total | 27.85±0.52 | 26.29±0.45 | 25.65±0.72 |
23.93±0.50a |
22.21±0.54a |
| 72 h | Put | 0.18±0.01 | 0.11±0.02 | 0.16±0.03 | 0.07±0.01 | 0.04±0.01 |
| Spd | 8.94±0.23 | 7.04±0.31 | 6.70±0.20 |
4.93±0.34a |
4.25±0.22a |
| Spm | 19.01±0.28 | 16.77±0.83 | 16.49±0.81 | 15.17±0.31 | 14.90±0.37 |
| Total | 28.13±0.55 | 23.93±0.46 | 23.35±0.64 |
20.17±0.44a |
19.19±0.47a |
| SW480 |
| 24 h | Put | 2.10±0.19 | 1.33±0.09 | 0.98±0.12 |
0.60±0.10a |
0.67±0.14a |
| Spd | 12.77±0.30 | 11.24±0.12 | 9.22±0.30 |
8.01±0.40a |
7.72±0.31a |
| Spm | 12.45±0.30 | 11.62±0.19 | 10.30±0.30 |
8.83±0.16a |
8.75±0.20a |
| Total | 27.32±0.77 | 24.80±0.45 | 20.02±0.78 |
17.44±0.61a |
17.70±0.50a |
| 48 h | Put | 1.50±0.15 | 0.98±0.20 | 0.67±0.10 |
0.26±0.05a |
0.25±0.09a |
| Spd | 9.40±0.28 | 8.20±0.37 | 7.10±0.39 |
5.95±0.35a |
5.20±0.50a |
| Spm | 12.61±0.42 | 12.5±0.60 | 11.70±0.20 |
11.00±0.31a |
11.30±0.23a |
| Total | 23.51±0.50 | 21.62±0.45 | 19.47±0.69 |
17.50±0.50a |
16.75±0.53a |
| 72 h | Put | 1.68±0.11 | 1.45±0.12 | 1.53±0.10 |
0.80±0.12a |
0.81±0.14a |
| Spd | 6.80±0.20 | 6.00±0.24 | 5.82±0.20 |
4.40±0.27a |
4.27±0.20a |
| Spm | 12.10±0.23 | 11.82±0.19 | 11.90±0.36 |
11.1±0.15a |
10.97±0.20a |
| Total | 20.58±0.50 | 19.27±0.48 | 19.25±0.63 |
16.30±0.44a |
16.02±0.50a |
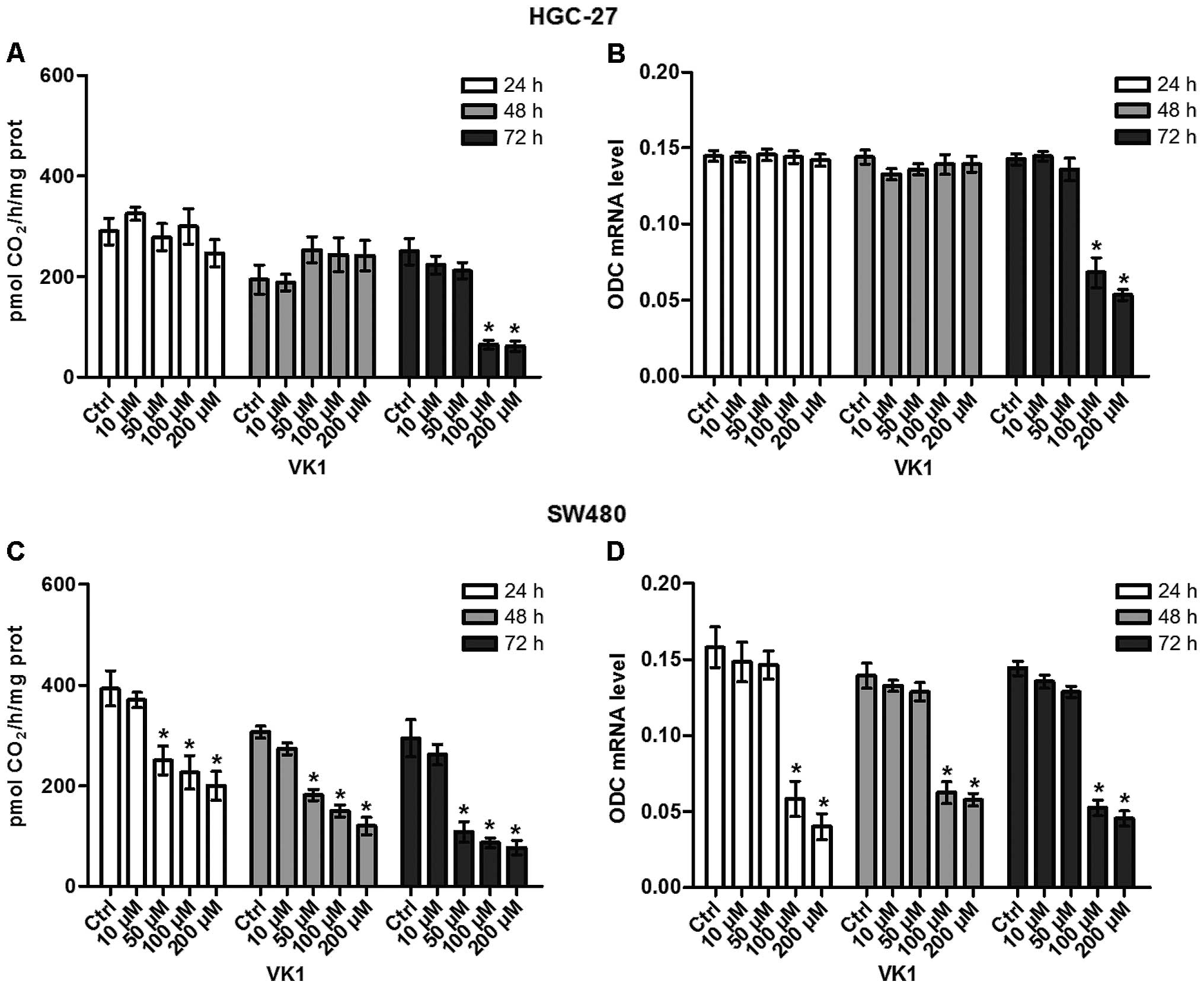
The effects of VK1 administration on ODC activity
and ODC expression in HGC-27 and SW480 cell lines were studied at
increasing concentrations (from 10 to 200 μM) up to 72 h. As shown
in Fig. 5A and B, after 72-h
administration, VK1 concentrations ≥100 μM significantly reduced
(P<0.05) both ODC activity (−74%) and ODC expression mRNA (−50%)
in HGC-27 cells compared to the control ones. Overall, the effect
of reduction by VK1 in SW480 cells was more rapid, since ODC
activity was significantly reduced (P<0.05) by 36% starting from
24 h of treatment with VK1 concentrations ≥50 μM compared to the
untreated cells (Fig. 5C).
Regarding ODC expression, 24-h administration of VK1 concentrations
≥100 μM caused a significant reduction (P<0.05) by 62.5% of ODC
mRNA level compared to untreated cells (Fig. 5D). Notably, the significant effect
persisted up to 72 h.
Discussion
Along with the proven effects on bone metabolism and
blood clotting, compounds belonging to VK family have also been
proposed as antineoplastic factors. However, the majority of data
on this issue derives from studies performed on VK2 and VK3 and the
exact mechanisms of action are still not completely elucidated
(6,31,32).
It has been reported that VK1 can inhibit survival
and induce apoptosis in pancreatic cancer cell lines (33); it is also able to enhance
sorafenib-induced growth inhibition in human hepatocellular
carcinoma and malignant glioma (12,34),
but scarce data are available on its potential against GI neoplasms
(14). Hence, one of the aims of
the present research was to compare the effects of VK1
administration on growth of two different human GI cancer cell
lines, one originating from undifferentiated carcinoma of the
stomach (HGC-27), the other from colon adenocarcinoma (SW480)
paying attention to the involvement of polyamine biosynthesis.
The main finding of the present study is that VK1
administration not only reduced cell proliferation and induced
apoptosis in both HGC-27 and SW480 cells, but also caused a
significant decrease in the polyamine biosynthesis. Even if the
response to increasing concentrations of VK1 shown by the two GI
cancer cell lines was very similar, some peculiar differences must
be underlined.
Firstly, as regards cell proliferation, VK1
administration to HGC-27 cells with concentrations ≥100 μM
significantly reduced the MTT conversion after 48 h. In SW480
cells, the inhibitory effect started rapidly after 24 h with the
same concentration and, thus, demonstrating a more pronounced
susceptibility of colonic cells to VK1 action.
Secondly, in accordance with MTT data, also the
single and total polyamine levels decreased significantly after VK1
treatment in the same time-dependent manner. However, after 48 h of
VK1 administration to HGC-27 cells, a significant reduction
occurred only in the spermidine as well as total polyamine contents
with the highest concentrations (100 and 200 μM). In SW480 cells,
all the single and total polyamines significantly decreased already
after 24 h of treatment with concentrations ≥100 μM.
Polyamines can be considered reliable markers of
neoplastic proliferation, since abnormal hyperproliferative cells,
such as those in neoplastic and pre-neoplastic tissue, exhibit very
high requirements for these substances to sustain cell growth
through elevated DNA, RNA and protein synthesis (18). Furthermore, it is known that
polyamines influence the expression of various genes involved in
cell proliferation, tumor invasion and metastatis (35). There is a vast body of literature
describing the relationship between increased levels of poly-amines
and cancer, most of which suggests an association of induced
polyamine biosynthesis with neoplasms (36). An important role is also played by
ODC, a key-regulator enzyme in the polyamine biosynthesis, that it
is now considered as a true oncogene (37).
VK1 administration induced a significant decrease in
ODC activity and expression in both the gastric and colon cancer
cells. In HGC-27 cell line the decrease was significant at a late
stage (after 72 h) with the highest concentrations (100 and 200
μM). On the contrary, in SW480 cells, a significant decrease in ODC
activity occurred earlier with 50 μM of VK1 after 24 h of treatment
and this effect persisted up to 72 h. A concomitant and significant
inhibition of ODC gene expression was observed starting from 100 μM
of VK1.
Data from the present study are in agreement with
published studies performed using other nutritional components such
as flavonoids, polyphenols and probiotics that have demonstrated
how these factors are able to reduce the polyamine biosynthesis in
different cancer cells, including the GI ones (38).
Functionally, ODC is in part responsible for the
variations in the single and total polyamine contents. This enzyme
affects mainly spermidine and putrescine levels, that are more
involved in cell proliferation than spermine; the latter is
implicated essentially in cell differentiation and neoplastic
transformation, with different processes engaged in maintaining its
critical levels (39).
Polyamines are also pivotal in the regulation of ion
transport and the stabilization of several cellular components,
such as cell membranes and chromatin structure. Therefore, a
reduction of the levels of these polycations might induce
destabilization of important cell structures, leading to loss of
cell integrity and finally inducing cell death (39,40).
It has been observed that the depletion of polyamines by treatment
with the ODC inhibitor, difluoromethylornithine (DFMO), resulted in
growth inhibition leading to a significant increase in apoptosis by
altering both Bax and Bcl-2 expression (41,42).
Based on the above, the inhibitory effect on
polyamine biosynthesis by VK1 could also be linked to the observed
increase of apoptosis. VK1 concentrations ≥100 μM also induced
apoptosis in both cell lines after 48 h of treatment, as indicated
by the significant increase in the Bax/Bcl-2 ratio of mRNA levels.
However, in HGC-27 no significant changes in Bax and Bcl-2 proteins
occurred, probably due to the fact that VK1 exerted its effects on
the modulation of gene expression without any consequence on the
protein structure in this cell line. In the contrary, the increase
in Bax/Bcl-2 ratio of mRNA levels in SW480 cells was caused by a
significant increase in the pro-apoptotic Bax protein levels and a
significant decrease in the anti-apoptotic Bcl-2 protein
levels.
As regards the possible involvement of the MAPK
pathway in the apoptotic process, treatment with VK1 showed a
significant dose-dependent increase in ERK phosphorylation, with a
peak increase after 48 h in SW480 cells, but not in HGC-27 ones. Of
note, when SW480 cells were simultaneously co-treated with a MEK
inhibitor and VK1, the induction of ERK phosphorylation was
prevented and VK1-mediated induction of apoptosis was blocked,
indicating the participation of the MAPK pathway in this
process.
Although it is accepted that ERK pathway stimulates
cell growth and protects cells from death (43), our findings agree with reports in
literature proposing new interpretations of the ERK
phosphorylation. Zhu et al (44) demonstrated a proapoptotic role of
the ERK pathway in T-cells where the inhibition of ERK
phosphorylation antagonized apoptosis. Similar results were
obtained by Showalter et al (13) in a study performed on pancreatic
cancer cell lines treated with either VK1 or VK2 administered at
inhibitory doses. ERK phosphorylation was also induced by two VK
analogs in different cancer cells and had a strong correlation with
the growth-inhibitory effect (45). The increase in ERK phosphorylation
could be closely linked to the decrease in polyamine contents
observed after VK1 treatment. Previous studies have shown that the
inhibitory effect on polyamine biosynthesis by DFMO treatment
increases ERK phosphorylation and signaling through the MAPK
pathway in human breast cancer cells (46).
Finally, a caspase-dependent induction in apoptosis
induced by VK1 was not observed in the cell lines, since western
blot analysis revealed no active products of caspase-3, an
executioner caspase, and caspase-9, an initiator caspase. Even if
caspases are established players of apoptosis in various models,
increasing evidence demonstrates that apoptosis may be induced
without their activation (47).
In conclusion, to the best of our knowledge, this is
the first study aimed at comparing the effects of the naturally
occurring VK1 on gastric and colorectal cancer cell growth and
exploring its mechanisms of action. Our findings show an inhibition
of cell proliferation and induction of apoptosis involving
polyamine biosynthesis and MAPK pathway. Moreover, this evidence
indicates a different proliferative behavior and a different
response to VK1 by gastric and colon cancer cells, with the latter
showing a more pronounced susceptibility to VK1 action. This cell
line demonstrated growth ability closely linked to the high
capacity of de novo polyamine biosynthesis and polyamine
uptake (48). This evidence
supports the hypothesis that VK1 may have different effects on
different types of cell lines.
Although future studies are needed to better
elucidate the exact mechanisms underlying VK1 anticancer properties
and to check these effects also in in vivo models, our
preliminary data imply potential for novel therapeutic strategies.
VK1 is safe and without known toxicities in adult humans (49), so it could be effective in
prevention and treatment of selected GI neoplasms. In particular,
as hypothesized for other diet components, a combined
chemopreventive and therapeutical intervention using protocols
based on the use of this naturally substance, along with polyamine
inhibitors and/or analogues would enhance VK1 properties. This
could represent a suitable alternative option for improving the
efficacy of chemotherapy and chemoprevention and the management of
patients with cancers of the digestive tract.
References
|
1
|
Lepage C, Hamza S and Faivre J:
Epidemiology and screening of colon cancer. Rev Prat. 60:1062–1067.
2010.(In French).
|
|
2
|
Milner JA, McDonald SS, Anderson DE and
Greenwald P: Molecular targets for nutrients involved with cancer
prevention. Nutr Cancer. 41:1–16. 2001.
|
|
3
|
MacFarlane AJ and Stover PJ: Convergence
of genetic, nutritional and inflammatory factors in
gastrointestinal cancers. Nutr Rev. 65:S157–S166. 2007. View Article : Google Scholar
|
|
4
|
Hitomi M, Yokoyama F, Kita Y, Nonomura T,
Masaki T, Yoshiji H, Inoue H, Kinekawa F, Kurokohchi K, Uchida N,
et al: Antitumor effects of vitamins K1, K2 and K3 on
hepatocellular carcinoma in vitro and in vivo. Int J Oncol.
26:713–720. 2005.PubMed/NCBI
|
|
5
|
Amalia H, Sasaki R, Suzuki Y, Demizu Y,
Bito T, Nishimura H, Okamoto Y, Yoshida K, Miyawaki D, Kawabe T, et
al: Vitamin K2-derived compounds induce growth inhibition in
radioresistant cancer cells. Kobe J Med Sci. 56:E38–E49.
2010.PubMed/NCBI
|
|
6
|
Ogawa M, Nakai S, Deguchi A, Nonomura T,
Masaki T, Uchida N, Yoshiji H and Kuriyama S: Vitamins K2, K3 and
K5 exert antitumor effects on established colorectal cancer in mice
by inducing apoptotic death of tumor cells. Int J Oncol.
31:323–331. 2007.PubMed/NCBI
|
|
7
|
Lamson DW and Plaza SM: The anticancer
effects of vitamin K. Altern Med Rev. 8:303–318. 2003.PubMed/NCBI
|
|
8
|
Shearer MJ and Newman P: Metabolism and
cell biology of vitamin K. Thromb Haemost. 100:530–547.
2008.PubMed/NCBI
|
|
9
|
Yao Y, Li L, Zhang H, Jia R, Liu B, Zhao
X, Zhang L, Qian G, Fan X and Ge S: Enhanced therapeutic efficacy
of vitamin K2 by silencing BCL-2 expression in SMMC-7721
hepatocellular carcinoma cells. Oncol Lett. 4:163–167.
2012.PubMed/NCBI
|
|
10
|
Ozaki I, Zhang H, Mizuta T, Ide Y, Eguchi
Y, Yasutake T, Sakamaki T, Pestell RG and Yamamoto K:
Menatetrenone, a vitamin K2 analogue, inhibits hepatocellular
carcinoma cell growth by suppressing cyclin D1 expression through
inhibition of nuclear factor kappaB activation. Clin Cancer Res.
13:2236–2245. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kanamori T, Shimizu M, Okuno M,
Matsushima-Nishiwaki R, Tsurumi H, Kojima S and Moriwaki H:
Synergistic growth inhibition by acyclic retinoid and vitamin K2 in
human hepatocellular carcinoma cells. Cancer Sci. 98:431–437. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Du W, Zhou JR, Wang DL, Gong K and Zhang
QJ: Vitamin K1 enhances sorafenib-induced growth inhibition and
apoptosis of human malignant glioma cells by blocking the
Raf/MEK/ERK pathway. World J Surg Oncol. 10:602012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Showalter SL, Wang Z, Costantino CL,
Witkiewicz AK, Yeo CJ, Brody JR and Carr BI: Naturally occurring K
vitamins inhibit pancreatic cancer cell survival through a
caspase-dependent pathway. J Gastroenterol Hepatol. 25:738–744.
2010. View Article : Google Scholar
|
|
14
|
Orlando A, Linsalata M, Tutino V, D’Attoma
B, Notarnicola M and Russo F: Vitamin K1 exerts antiproliferative
effects and induces apoptosis in three differently graded human
colon cancer cell lines. BioMed Res Int. Article ID 296721 (In
press).
|
|
15
|
Tokita H, Tsuchida A, Miyazawa K,
Ohyashiki K, Katayanagi S, Sudo H, Enomoto M, Takagi Y and Aoki T:
Vitamin K2-induced antitumor effects via cell-cycle arrest and
apoptosis in gastric cancer cell lines. Int J Mol Med. 17:235–243.
2006.PubMed/NCBI
|
|
16
|
Kawakita H, Tsuchida A, Miyazawa K, Naito
M, Shigoka M, Kyo B, Enomoto M, Wada T, Katsumata K, Ohyashiki K,
et al: Growth inhibitory effects of vitamin K2 on colon cancer cell
lines via different types of cell death including autophagy and
apoptosis. Int J Mol Med. 23:709–716. 2009.PubMed/NCBI
|
|
17
|
Igarashi K and Kashiwagi K: Modulation of
cellular function by polyamines. Int J Biochem Cell Biol. 42:39–51.
2010. View Article : Google Scholar
|
|
18
|
Moschou PN and Roubelakis-Angelakis KA:
Polyamines and programmed cell death. J Exp Bot. 65:1285–1296.
2014. View Article : Google Scholar
|
|
19
|
Nowotarski SL, Woster PM and Casero RA Jr:
Polyamines and cancer: implications for chemotherapy and
chemoprevention. Expert Rev Mol Med. 22;15:e32013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ramani D, De Bandt JP and Cynober L:
Aliphatic polyamines in physiology and diseases. Clin Nutr.
33:14–22. 2014. View Article : Google Scholar
|
|
21
|
Laukaitis CM and Gerner EW: DFMO: Targeted
risk reduction therapy for colorectal neoplasia. Best Pract Res
Clin Gastroenterol. 25:495–506. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Seiler N: Thirty years of
polyamine-related approaches to cancer therapy. Retrospect and
prospect Part 1 Selective enzyme inhibitors. Curr Drug Targets.
4:537–564. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Linsalata M and Russo F: Nutritional
factors and polyamine metabolism in colorectal cancer. Nutrition.
24:382–389. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Linsalata M, Orlando A, Messa C, Refolo MG
and Russo F: Quercetin inhibits human DLD-1 colon cancer cell
growth and polyamine biosynthesis. Anticancer Res. 30:3501–3507.
2010.PubMed/NCBI
|
|
25
|
Linsalata M, Cavallini A, Messa C, Orlando
A, Refolo MG and Russo F: Lactobacillus rhamnosus GG influences
polyamine metabolism in HGC-27 gastric cancer cell line: A strategy
toward nutritional approach to chemoprevention of gastric cancer.
Curr Pharm Des. 16:847–853. 2010. View Article : Google Scholar
|
|
26
|
MacKeigan JP, Collins TS and Ting JP: MEK
inhibition enhances paclitaxel-induced tumor apoptosis. J Biol
Chem. 275:38953–38956. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Orlando A, Linsalata M, Notarnicola M,
Tutino V and Russo F: Lactobacillus GG restoration of the gliadin
induced epithelial barrier disruption: the role of cellular
polyamines. BMC Microbiol. 14:192014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Linsalata M, Notarnicola M, Tutino V,
Bifulco M, Santoro A, Laezza C, Messa C, Orlando A and Caruso MG:
Effects of anandamide on polyamine levels and cell growth in human
colon cancer cells. Anticancer Res. 30:2583–2589. 2010.PubMed/NCBI
|
|
29
|
Linsalata M, Russo F, Notarnicola M,
Berloco P and Di Leo A: Polyamine profile in human gastric mucosa
infected by Helicobacter pylori. Ital J Gastroenterol Hepatol.
30:484–489. 1998.PubMed/NCBI
|
|
30
|
Garewal HS, Sloan D, Sampliner RE and
Fennerty B: Ornithine decarboxylase assay in human colorectal
mucosa. Methodologic issues of importance to quality control. Int J
Cancer. 52:355–358. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kayashima T, Mori M, Yoshida H, Mizushina
Y and Matsubara K: 1,4-Naphthoquinone is a potent inhibitor of
human cancer cell growth and angiogenesis. Cancer Lett. 278:34–40.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Adnan H, Antenos M and Kirby GM: The
effect of menadione on glutathione S-transferase A1 (GSTA1): c-Jun
N-terminal kinase (JNK) complex dissociation in human colonic
adenocarcinoma Caco-2 cells. Toxicol Lett. 214:53–62. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wei G, Wang M and Carr BI: Sorafenib
combined vitamin K induces apoptosis in human pancreatic cancer
cell lines through RAF/MEK/ERK and c-Jun NH2-terminal kinase
pathways. J Cell Physiol. 224:112–119. 2010.PubMed/NCBI
|
|
34
|
Wei G, Wang M, Hyslop T, Wang Z and Carr
BI: Vitamin K enhancement of sorafenib-mediated HCC cell growth
inhibition in vitro and in vivo. Int J Cancer. 127:2949–2958. 2010.
View Article : Google Scholar :
|
|
35
|
Gerner EW and Meyskens FL Jr: Polyamines
and cancer: Old molecules, new understanding. Nat Rev Cancer.
4:781–792. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Casero RA Jr and Marton LJ: Targeting
polyamine metabolism and function in cancer and other
hyperproliferative diseases. Nat Rev Drug Discov. 6:373–390. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pegg AE: Regulation of ornithine
decarboxylase. J Biol Chem. 281:14529–14532. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Linsalata M, Orlando A and Russo F:
Pharmacological and dietary agents for colorectal cancer
chemoprevention: effects on polyamine metabolism (Review). Int J
Oncol. 45:1802–1812. 2014.PubMed/NCBI
|
|
39
|
Deloyer P, Peulen O and Dandrifosse G:
Dietary polyamines and non-neoplastic growth and disease. Eur J
Gastroenterol Hepatol. 13:1027–1032. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Seiler N and Raul F: Polyamines and
apoptosis. J Cell Mol Med. 9:623–642. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Davidson NE, Hahm HA, McCloskey DE, Woster
PM and Casero RA Jr: Clinical aspects of cell death in breast
cancer: The polyamine pathway as a new target for treatment. Endocr
Relat Cancer. 6:69–73. 1999. View Article : Google Scholar
|
|
42
|
Gao JH, Guo LJ, Huang ZY, Rao JN and Tang
CW: Roles of cellular polyamines in mucosal healing in the
gastrointestinal tract. J Physiol Pharmacol. 64:681–693. 2013.
|
|
43
|
Sui X, Kong N, Ye L, Han W, Zhou J, Zhang
Q, He C and Pan H: p38 and JNK MAPK pathways control the balance of
apoptosis and autophagy in response to chemotherapeutic agents.
Cancer Lett. 344:174–179. 2014. View Article : Google Scholar
|
|
44
|
Zhu L, Yu X, Akatsuka Y, Cooper JA and
Anasetti C: Role of mitogen-activated protein kinases in
activation-induced apoptosis of T cells. Immunology. 97:26–35.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Osada S, Osada K and Carr BI: Tumor cell
growth inhibition and extracellular signal-regulated kinase (ERK)
phosphorylation by novel K vitamins. J Mol Biol. 314:765–772. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Manni A, Washington S, Hu X, Griffith JW,
Bruggeman R, Demers LM, Mauger D and Verderame MF: Effects of
polyamine synthesis inhibitors on primary tumor features and
metastatic capacity of human breast cancer cells. Clin Exp
Metastasis. 22:255–263. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Matsumoto K, Okano J, Nagahara T and
Murawaki Y: Apoptosis of liver cancer cells by vitamin K2 and
enhancement by MEK inhibition. Int J Oncol. 29:1501–1508.
2006.PubMed/NCBI
|
|
48
|
Duranton B, Holl V, Schneider Y,
Carnesecchi S, Gossé F, Raul F and Seiler N: Polyamine metabolism
in primary human colon adenocarcinoma cells (SW480) and their lymph
node metastatic derivatives (SW620). Amino Acids. 24:63–72.
2003.PubMed/NCBI
|
|
49
|
Mizuta T, Ozaki I, Eguchi Y, Yasutake T,
Kawazoe S, Fujimoto K and Yamamoto K: The effect of menatetrenone,
a vitamin K2 analog, on disease recurrence and survival in patients
with hepatocellular carcinoma after curative treatment: a pilot
study. Cancer. 106:867–872. 2006. View Article : Google Scholar : PubMed/NCBI
|