Introduction
Ovarian cancer is responsible more than half of the
deaths caused by gynecological malignancies (1). It is a highly metastatic disease that
is rarely diagnosed when it is confined to the ovaries due to a
lack of characteristic symptoms (2). Peritoneal metastasis is the principal
cause of death in patients with advanced or recurrent ovarian tumor
(3–5). Even aggressive peritoneal or
cytoreductive surgery leave large areas of the peritoneal cavity
untreated (6). Although
chemotherapy is a traditional adjuvant choice, experience over the
past few decades has suggested that it does not enhance patient
survival. Therefore, a novel adjuvant therapy is needed
urgently.
Photodynamic therapy (PDT) is a promising
alternative to the conventional therapeutic strategies for the
management of intraperitoneal carcinomas (7). PDT is based on the delivery of a
photosensitizer followed by exposure to light of a specific
wavelength, which converts oxygen into reactive singlet oxygen
(1O2). This causes selective cancer cell
death, because cancer cells uptake more photosensitizers than do
normal cells (8,9). Singlet oxygen eliminates tumor tissue
by causing microvascular acute injury, blocking vessels and also
inducing cancer cell apoptosis (10–12).
Another interrelated mechanism of the antitumor effects of PDT is
the induction of a local inflammatory reaction that leads to the
development of systemic immunity (13).
Hypocrellin B (HB) is a traditional Chinese drug
that is extracted from fungus (Hypocrella bambuase) of a
Chinese herb; it is an excellent natural photosensitizer. In China,
it is commonly used to treat rheumatoid arthritis, and gastric and
skin diseases (14). Several
studies have suggested that it is a promising non-porphyrin
photosensitizer, because it causes high singlet oxygen quantum
yields, has a low tendency for aggregation and rapid metabolism
in vivo (15).
The development of nanotechnology has introduced
more choices for cancer treatment. Nanoparticle delivery systems
allow specific tumor targeting. The leaky vasculature in tumor
tissues promotes the uptake of nanoformulations, and the impaired
and poor lymphatic drainage traps nano-sized particles inside tumor
tissues (16,17) in the so-called ‘enhanced permeation
and retention' (EPR) effect. Therefore, drugs encapsulated in
nanoparticles could accumulate at high levels in tumor tissues for
a longer time. Studies have shown that nanoparticles carrying
anticancer agents prolonged drug retention in tumors, which could
inhibit tumor growth (18–20) and improve the efficiency of cancer
therapy (17). The materials used
for nanoparticle preparation are often biodegradable; therefore,
the drugs they encapsulate can be released gradually and then
degraded slowly. This ensures that the drug concentrations in
target tissues remain high. Recent studies have revealed that
poly(butyl-cyanoacrylate) nanoparticles (PBCA-NPs) are effective
drug carriers (21,22). In the present study, we synthesized
PBCA-NP encapsulated with HB to perform tumor-targeted PDT in
ovarian cancer-bearing Fischer 344 rats.
Materials and methods
Preparation of HB-PBCA-NP
Dextran-70 and Pluronic F-127 (Sigma-Aldrich, St.,
Louis, MO, USA) were dissolved in 5 ml distilled water and the pH
was adjusted to 1.5. The solution was mixed with
α-butyl-cyanoacrylate (BCA; Shunkang Pharmaceutical Co., Ltd.,
Beijing, China) by magnetic stirring at 600 rpm for 4 h at 25°C,
and the pH was then raised to 7.0. The solution was stirred for
another 30 min until polymerization was complete. The filtered
nanoparticles were kept at 4°C for 12 h. Then, 2.5 ml HB (Chinese
Academy of Science, Beijing, China) in ethanol (0.5 mg/ml) was
added to nanoparticle solution and sonicated. The suspension was
then incubated for an additional 12 h at 4°C and vacuum-freeze
dried to obtain HB-PBCA-NP. The size and other features of the
particles were tested using laser dynamic light scatting (DLS;
Malvern Instruments Ltd., Worcestershire, UK) and transmission
electron microscopy (TEM, Tecnai G2 F20 S-TWIN; FEI Co., Hillsboro,
OR, USA).
Cell line and animals
The poorly differentiated Fischer 344 rat-derived
epithelial ovarian cancer line NuTu-19 was maintained in the Basic
Medicine Laboratory of Qilu Hospital. Cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; Sigma) supplemented with
10% heat-inactivated FCS (Gibco-Life Technologies, Grand Island,
NY, USA), and incubated under standardized conditions (37°C, 5%
carbon dioxide and 100% humidity). NuTu-19 cells were harvested
using 0.25% trypsin and 0.02% EDTA, and were washed twice with PBS.
All experiments were performed using exponentially growing
cells.
Animal model
Pathogen-free female Fischer 344 rats (Beijing
Weitong Lihua, Beijing, China), weighing 100–120 g, were housed in
a pathogen-free animal facility and given a standard laboratory
diet and water. A total of 2×106 cells were injected
into the peritoneal cavity of each rat. The temperature of the cage
was kept at 22±2°C, and relative humidity was kept between 45–65%.
The present study was performed in strict accordance with the Guide
for the Care and Use of animals of Qilu Hospital, Shandong
University, China. The protocol was also approved by Ethics
Committee of Animal Experiments of Qilu Hospital of Shandong
University. The experiment was performed in accordance with the
Guide for the Care and Use of Laboratory Animals. The animals were
then observed daily and weighed twice a week. Sodium pentobarbital
was injected intraperitoneally before surgery and all efforts were
made to minimize suffering.
Pharmacokinetic and distribution of HB in
animals
HB-PBCA-NP and HB were freshly prepared prior to use
by dissolving in normal saline and DMSO, respectively.
Tumor-bearing Fischer 344 rats were divided into two groups, which
received 10 mg/kg of either HB-DMSO or HB-PBCA-NP by
intraperitoneal injection. Blood samples were then collected from
the postcava vessel at various time-points (10, 30, 40 min, 1, 2,
4, 6, 12 and 24 h). All blood samples were coagulated for 2 h at
room temperature, and were then centrifuged at 2×103 g
to separate the serum. The rats were sacrificed 1, 2, 4, 6, 12 and
24 h after drug injection, and their tissues (liver, spleen, lung,
kidney, small intestine, uterus and tumor) were harvested. Two
hundred microliters of tetrahydrofuran (THF; Shiyou Biotech Co.,
Ltd., Tianjin, China) was added to the serum and minced tissue
samples, which were then centrifuged again. The supernatants were
analyzed using high performance liquid chromatography (HPLC;
Agilent Technologies, Palo Alto, CA, USA). The concentration of HB
in the different samples was determined at various time-points
according to the areas under the curve. The data were processed
using DAS (drug and statistics for Windows) to obtain
pharmacokinetic parameters.
PDT in vivo
The tumor-bearing rats were divided randomly into
four groups 6 weeks after inoculation. The control groups included
untreated animals (group A) and those that received only
cytoreductive surgery (group B). Animals in group C received
cytoreductive surgery and PDT followed by intraperitoneal injection
with 10 mg/kg HB-DMSO. Group D received surgery and PDT followed by
intraperitoneal injection with 10 mg/kg HB-PBCA-NP. The PDT was
performed when the concentration of drug reached a peak in tumor
tissues. Food was removed from the cages 12 h before the operation,
and rats were anesthetized using 40 mg/kg sodium pentobarbital
(Sigma). An incision ~5 cm in length was made on the abdominal wall
to fully expose the organs in the abdominal cavity. Cytoreductive
surgery was then performed to remove the maximum amount of tumor
lesions. The laser output energy delivered to the surface of the
peritoneal cavity of each rat was 50 J/cm2 with total
energy of 200 mW. During laser irradiation, warm normal saline was
dripped into the peritoneal cavity to reduce the loss of body
fluid. The operations were performed under sterile conditions, and
the animals were kept warm with a heating device until they awoke.
All animals were then followed, and survival data were collected
for further analysis.
Statistical analysis
Data were analyzed using SPSS 13.0 for Windows
(SPSS) software. The significance of differences among groups was
analyzed using two-way ANOVA and Student-Newman-Keuls q-test.
Survival studies were assessed using Kaplan-Meier survival
analysis. P<0.05 was used to indicate statistical
significance.
Results
Features of nanoparticles
The mean size of PBCA-NP was 95 nm in diameter
(range, 30–200 nm). TEM pictures revealed that the nanoparticles
were well separated, and spherical in appearance. The drug
encapsulation efficiency was 92.7% and drug-loading content was 15%
for PBCA-NP; therefore, HB was efficiently encapsulated into the
nanoparticles. The HB-PBCA-NPs were adequate for use in subsequent
experiments.
Fischer 344 rat tumor model
Six weeks after the injection of NuTu-19 cells, all
rats developed ovarian tumors in their abdominal cavity. A large
number of cancer nodules appeared on the surface of the peritoneum,
omentum, diaphragm, bowel, and reproductive organs. The images
presented in Fig. 1A show the
nodules and principal tissues in the abdominal cavity. Malignant
bloody ascites were also detected in the abdominal cavity.
Pathological analysis of tumor tissue sections confirmed the
presence of adenocarcinoma (Fig.
1B).
Pharmacokinetic parameters
Pharmacokinetic parameters were calculated to assess
the controlled drug release tendency of the NP. The half-lives of
HB in the blood delivered using HB-DMSO and HB-PBCA-NP was 9.45 and
12.99 h, respectively. The area under the curve (AUC) of HB-PBCA-NP
was 10.42 μg/ml/h, which was larger than that of HB-DMSO (2.60
μg/ml/h). The time-to-peak was 1 and 4 h for HB-DMSO and
HB-PBCA-NP, respectively. These data confirmed that HB carried by
nanoparticles was cleared more slowly than HB-DMSO.
Distribution of HB in vivo
Serum HB levels increased rapidly in the HB-DMSO
group. The highest concentration was 0.99 mg/l, which was reached
at 1 h after injection. However, very little HB was detected at 12
h after injection. However, HB levels in the serum of the
HB-PBCA-NP group increased much slower. The highest concentration
(0.8954 mg/l) was reached 4 h after injection. More importantly,
the HB concentrations in the serum remained relatively high (0.3187
mg/l) after 12 h compared with the HB-DMSO group (Fig. 2).
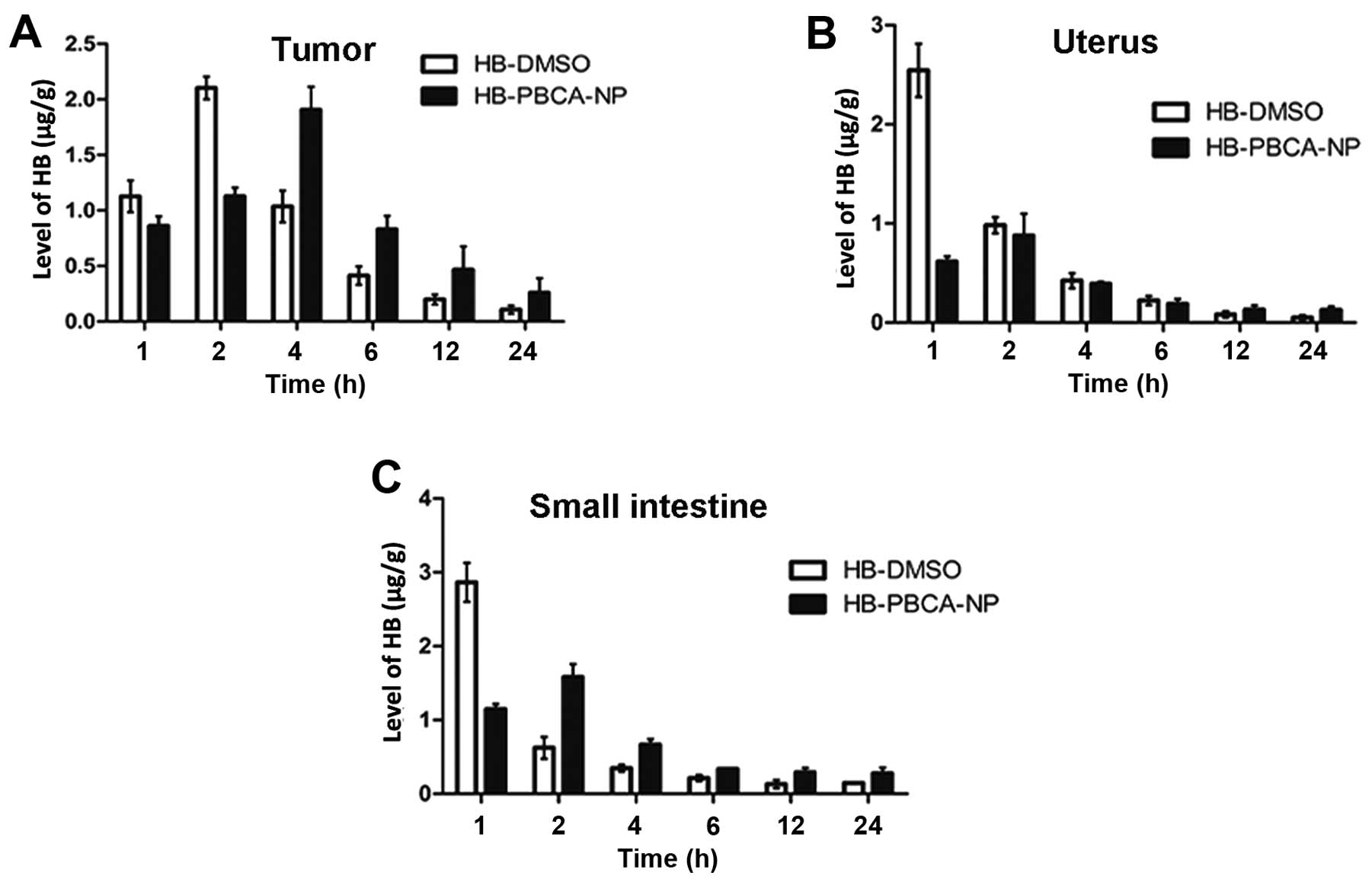
The levels of HB in each tissue at various
time-points after treatment are shown in Figs. 3 and 4. The peak time at which the maximal
concentration of HB was obtained was delayed in the HB-PBCA-NP
group in all tissues compared with the HB-DMSO group. For example,
the peak time in tumor tissue (Fig.
3A) and the liver (Fig. 4A)
was 4 and 2 h in HB-PBCA-NP and HB-DMSO groups, respectively. The
concentrations in the uterus (Fig.
3B), small intestine (Fig.
3C), spleen (Fig. 4B) and lung
(Fig. 4D) peaked at 2 h, which was
later than in the HB-DMSO group (1 h). An additional important
finding was that HB levels in HB-PBCA-NP remained higher than DMSO
group in all tissues even 24 h after drug administration (Figs. 3 and 4). For example, the levels of HB in
tumors in the HB-PBCA-NP group 24 h (0.26±0.13 μg/g) were much
higher than in the HB-DMSO group (0.11±0.04 μg/g). In addition, HB
levels were higher in all tissues in the HB-PBCA-NP compared with
the HB-DMSO group, 4 h after drug administration except for the
uterus and kidney (Fig. 4C).
Fig. 4 demonstrates that the
spleen (Fig. 4B) and liver
(Fig. 4A) of tumor-bearing rats
had the highest concentration of HB, followed by the kidney
(Fig. 4C) and lung (Fig. 4D). The spleen captured a large
number of PBCA-NP (four-times more than the liver). Notably, the
levels of HB in the liver and spleen 4 h after injection in
HB-PBCA-NP group were 16.6±1.67 and 49.62±2.952 μg/g, respectively,
compared with 0.57±0.02 and 4.68±1.17 μg/g in the HB-DMSO group.
The difference of the highest levels of HB in other tissues was not
as great as in the two groups.
Efficacy of PDT
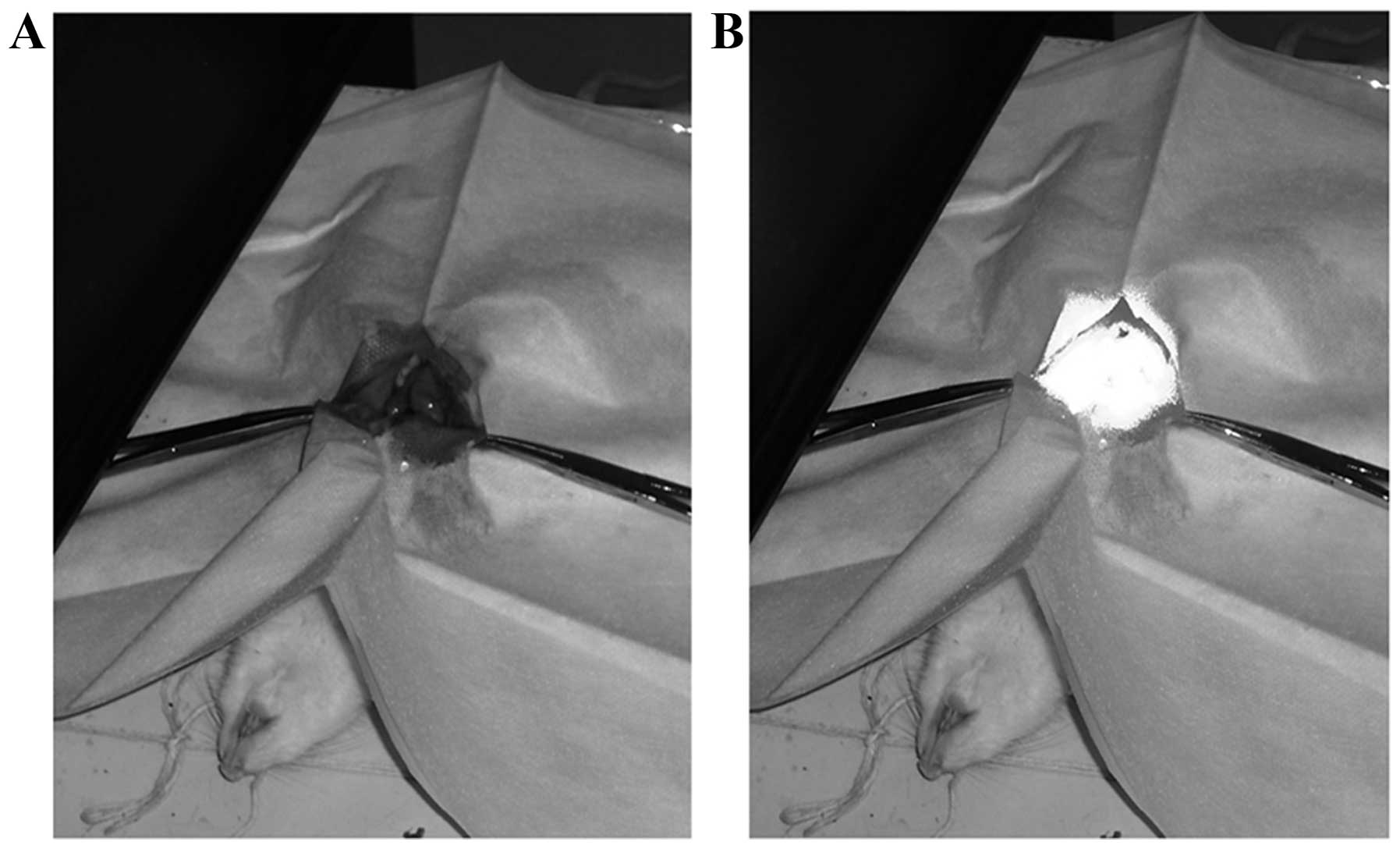
The images of PDT process are shown in Fig. 5. Four rats died within 10 days of
surgery as a result of the invasive operation. These animals were
excluded from the survival analysis. All animals were followed up
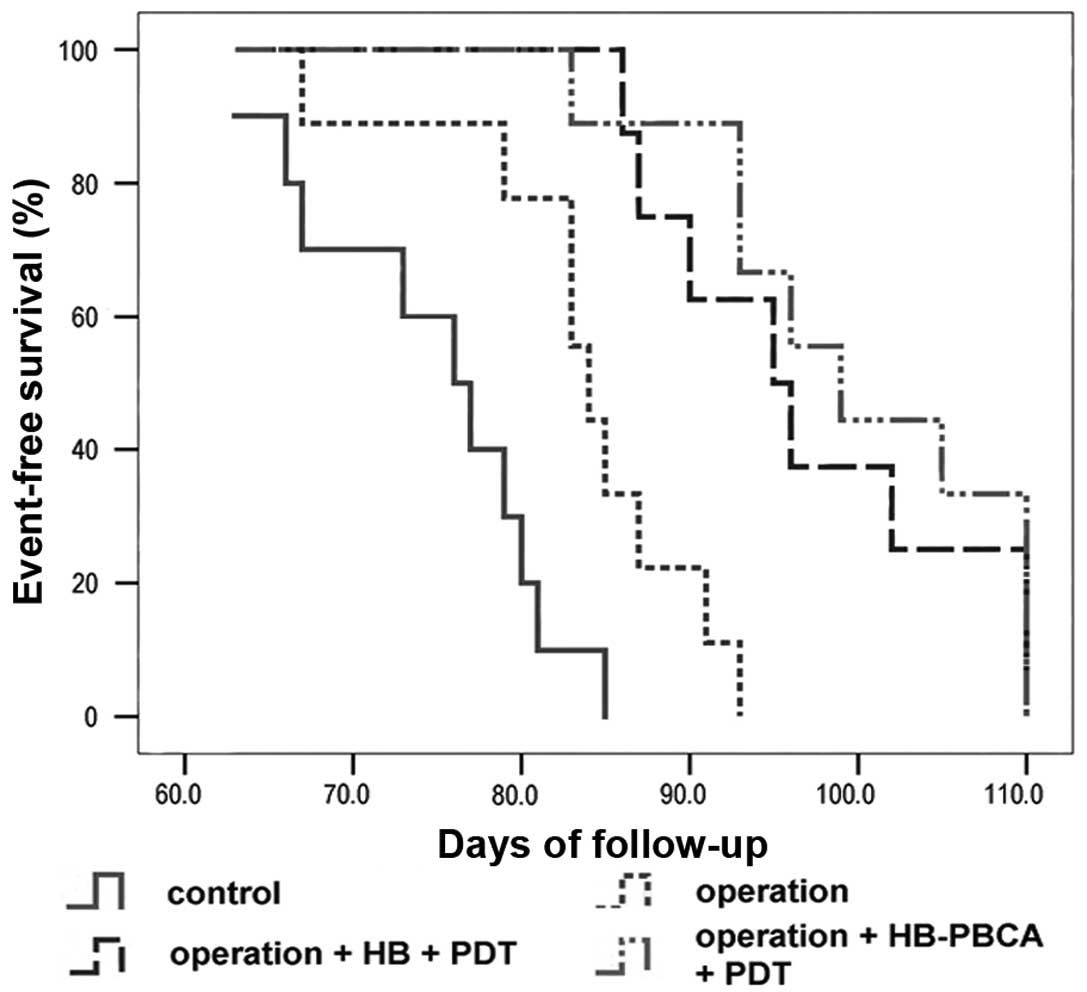
until death. Kaplan-Meier survival curves (Fig. 6) revealed that the median survival
time of groups A and B was 76 (95% CI, 69.8–82.2 days) and 84 days
(95% CI, 81.08–86.92 days), respectively. Rats in both groups
showed progressive cachexy. The median survival time of groups C
and D was 95 (95% CI, 86.68–103.316) and 99 days (95% CI,
90.24–107.74), which were both longer than the control groups.
Surgery alone improved animal survival, whereas surgery combined
with PDT could more effectively extend survival time. These
difference in survival time when each group was compared with the
others individually was significant (P<0.05), except for groups
C and D (P=0.293).
Discussion
Two thirds of patients with ovarian cancer have
already developed peritoneal carcinomatosis at diagnosis (23). The curative rate decreases
substantially, once the disease has metastasized to the pelvic
organs, the abdomen or beyond the peritoneal cavity (2). PDT can destroy cancerous tissue
selectively, sparing normal tissue (24). Therefore, it might be a choice for
the treating the superficial or microscopic lesions that remain
after debulking surgery in ovarian cancer. The feasibility of this
approach was demonstrated in clinical studies (6,25).
Nanoparticle delivery systems allow tumor targeting
and prolonged drug release. Therefore, we hypothesized that
PBCA-based nanoparticle systems carrying the photosensitizer HB
could enhance the efficiency of PDT. HB is an excellent
second-generation natural photosensitizer that is extracted from a
parasitical fungus in China. However, the clinical use of natural
HB is severely restricted by its poor water solubility (15). In the present study, HB was
captured inside PBCA NP to develop a water soluble HB-PBCA-NP
system that could be used directly. Importantly, the HB-PBCA-NP
system significantly delayed the clearance of HB from rat serum.
The peak time was extended from 1 to 4 h by the injection of
HB-PBCA-NP. Accordingly, the half-life of HB in the NP group was
also prolonged from 9.45 to 12.99 h. Twenty-four hours after
injection, HB remained detectable in the serum of the NP group at
relatively high concentrations, which was important for achieving a
minimum effective concentration of HB. The area under the curve of
HB in the NP particle group was also larger than control, meaning a
higher bioavailability of the drug. An ideal drug delivery
formulation should release the drug at a minimum effective
concentration over a longer period of time to achieve maximum
efficiency (17). In the present
study, the HB-PBCA-NP system achieved all these
characteristics.
Similar to the serum metabolism, the peak time of HB
in the NP group was delayed in tissues. The peak time in tumor
tissue was 4 and 2 h in the NP and HB-DMSO groups, respectively.
PDT was administered when HB levels reached their peak. The
concentration in other tissues, particularly in the small intestine
and uterus, was lower. This helped to achieve the optimal
therapeutic effect using a minimum dose of laser. This helped
reduce internal damage, such as perforation of the small intestine.
HB levels remained high in the NP group 24 h after drug
administration, which would theoretically allow PDT to be
repeated.
The HB-PBCA-NP system also altered the
biodistribution of HB. The spleen and liver absorbed more HB than
the other tissues in NP group. In the DMSO group, there was no
significant difference in the HB levels among tissues. The spleen
and liver are both reticuloendothelial system (RES) organs that
capture foreign materials entering the body. In the present study,
HB-PBCA-NPs were delivered intraperitoneally rather than
intravenously; therefore, they were captured in large numbers by
the spleen and liver. To evade the RES, nanoparticles should be
further modified on their surface. To achieve this, we have
synthesized an addition type of NP that is modified by folate on
the surface, making it suitable for intravenous applications
(unpublished data). The maximum concentration of HB in the kidneys
of the HB-PBCA-NP group was lower than in the HB-DMSO group.
Therefore, most PBCA-NP decomposed in the body rather than being
excreted by the kidney.
The Kaplan-Meier survival curves revealed that both
HB-DMSO- and HB-PBCA-NP-based PDT could significantly prolong
animal survival compared with control. This could be attributed to
the killing effect of PDT on the residual nodules that could not be
removed surgically. We demonstrated previously that PDT could
efficiently reduce the volume of subcutaneous tumors in nude mice
using hemoporfin (26). PDT was
performed after surgery without causing excess injury to the
animals. The surgical procedure also allowed an adequate operating
area for the application of PDT. However, there was no statistical
difference in survival time between group C and D. There were many
factors that could have influenced this observation. The
unavoidable capture of NP by the RES might reduce the concentration
of HB in the tumor tissues. Therefore, the nanoparticles need to be
modified further and be made small enough to achieve maximum
targeting to the tumor tissue. Although the levels of HB in the
DMSO group were higher than those in the NP group, this difference
was not significant, and perhaps contributed little to the effect
of PDT in vivo. In addition, photochemical oxygen
consumption might overwhelm the oxygen in the microvasculature
during PDT (27) which might also
influence the outcome of the PDT.
A good animal model is essential for medical
research. Immunodeficiency mice are commonly used for tumor-based
experiments, because they can be used as tumor-bearing animal
models. However, this kind of mouse was not suitable for our
current study because of their susceptibility to microbes. As such,
we needed a model that is strong enough to undergo the surgical and
PDT procedures. We selected the Fischer 344 rat, which has a normal
immune system, to build an ovarian cancer model. The NuTu-19 cell
line is derived from Fischer 344 rat poorly differentiated
papillary serous ovarian adenocarcinoma. Pathological analysis
confirmed that all rats had developed ovarian adenocarcinoma after
the intraperitoneal injection of NuTu-19 cells. The progressive
tumor growth in abdominal cavity resembled the growth of ovarian
cancer in humans; the death that arose from disease complications
was also consistent with human disease (28). Previously, we successfully applied
surgery and PDT on tumor-bearing Fischer 344 rats using an
alternative photosensitizer (hematoporphyrin monomethyl ether) to
prolong the median follow-up time (29). In the present study, the immune
system of Fischer 344 rats was efficient, and only four rats died
after surgery and/or PDT treatment. In addition, the peritoneal
cavity of Fischer 344 rats is large enough. It also allowed the
more convenient collection of blood samples and delivery of laser
light to the abdominal cavity. Therefore, we believe that Fischer
344 rats are particularly well suited for PDT experiments.
In conclusion, although there was no statistically
significant difference between the survival time of the HB-DMSO and
HB-PBCA-NP groups, PBCA-NP remains a promising drug delivery system
for ovarian cancer PDT. PBCA-NP showed potential advantages for
controlled drug release and tumor targeting, which might contribute
to the efficacy of HB-based PDT. PDT combined with surgery could
prolong animal survival time, which might result in a novel choice
for the treatment of ovarian cancer.
Acknowledgements
The present study was supported by grants from the
National Science Foundation of China (no. 81172488) and Outstanding
Young Scientists Foundation of Shandong Province of China
(BS2013YY035).
Abbreviations:
|
PDT
|
photodynamic therapy
|
|
HB
|
hypocrellin B
|
|
PBCA
|
poly(butyl-cyanoacrylate)
|
|
BCA
|
α-butyl-cyanoacrylate
|
|
NP
|
nanoparticles
|
|
TEM
|
transmission electron microscopy
|
|
DLS
|
dynamic light scatting
|
|
HPLC
|
high performance liquid
chromatography
|
|
EPR
|
enhanced permeation and retention
|
|
THF
|
tetrahydrofuran
|
|
AUC
|
area under the curve
|
|
RES
|
reticuloendothelial system
|
References
|
1
|
Feki A, Berardi P, Bellingan G, Major A,
Krause KH, Petignat P, Zehra R, Pervaiz S and Irminger-Finger I:
Dissemination of intraperitoneal ovarian cancer: Discussion of
mechanisms and demonstration of lymphatic spreading in ovarian
cancer model. Crit Rev Oncol Hematol. 72:1–9. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bast RC Jr, Hennessy B and Mills GB: The
biology of ovarian cancer: New opportunities for translation. Nat
Rev Cancer. 9:415–428. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huynh H, Teo CC and Soo KC: Bevacizumab
and rapamycin inhibit tumor growth in peritoneal model of human
ovarian cancer. Mol Cancer Ther. 6:2959–2966. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Armstrong A, Otvos B, Singh S and
Debernardo R: Evaluation of the cost of CA-125 measurement,
physical exam, and imaging in the diagnosis of recurrent ovarian
cancer. Gynecol Oncol. 131:503–507. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Busch TM, Hahn SM, Wileyto EP, Koch CJ,
Fraker DL, Zhang P, Putt M, Gleason K, Shin DB, Emanuele MJ, et al:
Hypoxia and photofrin uptake in the intraperitoneal carcinomatosis
and sarcomatosis of photodynamic therapy patients. Clin Cancer Res.
10:4630–4638. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hendren SK, Hahn SM, Spitz FR, Bauer TW,
Rubin SC, Zhu T, Glatstein E and Fraker DL: Phase II trial of
debulking surgery and photodynamic therapy for disseminated
intraperitoneal tumors. Ann Surg Oncol. 8:65–71. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guyon L, Lesage JC, Betrouni N and Mordon
S: Development of a new illumination procedure for photodynamic
therapy of the abdominal cavity. J Biomed Opt. 17:0380012012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Canter RJ, Mick R, Kesmodel SB, Raz DJ,
Spitz FR, Metz JM, Glatstein EJ, Hahn SM and Fraker DL:
Intraperitoneal photodynamic therapy causes a capillary-leak
syndrome. Ann Surg Oncol. 10:514–524. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hahn SM, Putt ME, Metz J, Shin DB, Rickter
E, Menon C, Smith D, Glatstein E, Fraker DL and Busch TM: Photofrin
uptake in the tumor and normal tissues of patients receiving
intraperitoneal photodynamic therapy. Clin Cancer Res.
12:5464–5470. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Portilho FA, Cavalcanti CE, Miranda-Vilela
AL, Estevanato LL, Longo JP, Almeida Santos MF, Bocca AL, Martins
OP, Simioni AR, Morais PC, et al: Antitumor activity of
photodynamic therapy performed with nanospheres containing
zinc-phthalocyanine. J Nanobiotechnol. 11:412013. View Article : Google Scholar
|
|
11
|
Castano AP, Mroz P and Hamblin MR:
Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer.
6:535–545. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shishkova N, Kuznetsova O and Berezov T:
Photodynamic therapy for gynecological diseases and breast cancer.
Cancer Biol Med. 9:9–17. 2012.PubMed/NCBI
|
|
13
|
Agostinis P, Berg K, Cengel KA, Foster TH,
Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel
D, et al: Photodynamic therapy of cancer: An update. CA Cancer J
Clin. 61:250–281. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma G, Khan SI, Jacob MR, Tekwani BL, Li Z,
Pasco DS, Walker LA and Khan IA: Antimicrobial and antileishmanial
activities of hypocrellins A and B. Antimicrob Agents Chemother.
48:4450–4452. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun Y, Zheng Y, Lei WH, Zhou QX, Hou YJ,
Zhang BW and Wang XS: Oxovanadium(IV) based hypocrellin B complexes
with enhanced photodynamic activity. Dalton Trans. 41:651–657.
2012. View Article : Google Scholar
|
|
16
|
Maeda H: The enhanced permeability and
retention (EPR) effect in tumor vasculature: The key role of
tumor-selective macromolecular drug targeting. Adv Enzyme Regul.
41:189–207. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yallapu MM, Jaggi M and Chauhan SC: Scope
of nanotechnology in ovarian cancer therapeutics. J Ovarian Res.
3:192010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim JH, Kim YS, Park K, Lee S, Nam HY, Min
KH, Jo HG, Park JH, Choi K, Jeong SY, et al: Antitumor efficacy of
cisplatin-loaded glycol chitosan nanoparticles in tumor-bearing
mice. J Control Release. 127:41–49. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Upadhyay KK, Bhatt AN, Mishra AK,
Dwarakanath BS, Jain S, Schatz C, Le Meins JF, Farooque A,
Chandraiah G, Jain AK, et al: The intracellular drug delivery and
anti tumor activity of doxorubicin loaded poly(gamma-benzyl
L-glutamate)-b-hyaluronan polymersomes. Biomaterials. 31:2882–2892.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu Z, Li Y, Li X, Li R, Jia Z, Liu B, Guo
W, Wu W and Jiang X: Paclitaxel-loaded
poly(N-vinylpyrrolidone)-b-poly(ɛ-caprolactone) nanoparticles:
Preparation and antitumor activity in vivo. J Control Release.
142:438–446. 2010. View Article : Google Scholar
|
|
21
|
Yordanov G, Evangelatov A and Skrobanska
R: Epirubicin loaded to pre-polymerized poly(butyl cyanoacrylate)
nanoparticles: Preparation and in vitro evaluation in human lung
adenocarcinoma cells. Colloids Surf B Biointerfaces. 107:115–123.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Duan J, Zhang Y, Han S, Chen Y, Li B, Liao
M, Chen W, Deng X, Zhao J and Huang B: Synthesis and in vitro/in
vivo anti-cancer evaluation of curcumin-loaded chitosan/poly(butyl
cyanoacrylate) nanoparticles. Int J Pharm. 400:211–220. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Muñoz-Casares FC, Rufián S, Arjona-Sánchez
Á, Rubio MJ, Díaz R, Casado Á, Naranjo Á, Díaz-Iglesias CJ, Ortega
R, Muñoz-Villanueva MC, et al: Neoadjuvant intraperitoneal
chemotherapy with paclitaxel for the radical surgical treatment of
peritoneal carcinomatosis in ovarian cancer: A prospective pilot
study. Cancer Chemother Pharmacol. 68:267–274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mroz P, Xia Y, Asanuma D, Konopko A,
Zhiyentayev T, Huang YY, Sharma SK, Dai T, Khan UJ, Wharton T, et
al: Intraperitoneal photodynamic therapy mediated by a fullerene in
a mouse model of abdominal dissemination of colon adenocarcinoma.
Nanomedicine. 7:965–974. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
DeLaney TF, Sindelar WF, Tochner Z, Smith
PD, Friauf WS, Thomas G, Dachowski L, Cole JW, Steinberg SM and
Glatstein E: Phase I study of debulking surgery and photodynamic
therapy for disseminated intraperitoneal tumors. Int J Radiat Oncol
Biol Phys. 25:445–457. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song K, Kong B, Qu X, Li L and Yang Q:
Phototoxicity of Hemoporfin to ovarian cancer. Biochem Biophys Res
Commun. 337:127–132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Seshadri M, Bellnier DA, Vaughan LA,
Spernyak JA, Mazurchuk R, Foster TH and Henderson BW: Light
delivery over extended time periods enhances the effectiveness of
photodynamic therapy. Clin Cancer Res. 14:2796–2805. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rose GS, Tocco LM, Granger GA, DiSaia PJ,
Hamilton TC, Santin AD and Hiserodt JC: Development and
characterization of a clinically useful animal model of epithelial
ovarian cancer in the Fischer 344 rat. Am J Obstet Gynecol.
175:593–599. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Song K, Kong B, Li L, Yang Q, Wei Y and Qu
X: Intraperitoneal photodynamic therapy for an ovarian cancer
ascite model in Fischer 344 rat using hematoporphyrin monomethyl
ether. Cancer Sci. 98:1959–1964. 2007. View Article : Google Scholar : PubMed/NCBI
|