Introduction
Colorectal cancer (CRC) is the second among women
and third among men of the most commonly diagnosed cancers in the
world (1). A World Health
Organization study from 2012 showed that colorectal cancer exceeds
1,360.500 new cases every year worldwide (1). Furthermore, during the last 5 years,
CRC incidence and mortality rates have increased in all developed
countries. This appears to coincide with a diet change toward more
fat- and carbohydrate-rich foods, and a deficiency in many
nutrients including vitamin D. This so-called urban-industrial or
Western diet is highly based on processed foods (2).
Current colorectal cancer therapy is based on a
combination of cytostatic drugs and surgery where applicable
(3). The chemotherapeutic agent of
choice is a 5-fluorouracil (5-FU), which is used for the treatment
of both the advanced and early stages of colorectal cancer.
Unfortunately, the effectiveness of 5-FU monotherapy of colorectal
cancer is limited to only 10–15% of cases (4). Identification of compounds that can
enhance the response to 5-FU is a key step in the development of
combined chemotherapies. Several studies have shown that
1α,25(OH)2D3 can be administered alone or in
combination with other antitumor agents to enhance the efficacy of
therapy. For example, 1α,25(OH)2D3
downregulates the expression of thymidylate synthase (TS) and
therefore promotes a cytotoxic response to 5-FU (5). Other studies have demonstrated that
1α,25(OH)2D3 enhances cellular sensitivity of
colorectal cancer cells to 5-FU through a calcium-sensing receptor
(5,6).
In humans, vitamin D3 is formed from
7-dehydrocholesterol (cholesta-5,7-dien-3β-ol, 7DHC) in the basal
layer of the epidermis. Initially, the B-ring of 7-DHC undergoes
photolysis upon the exposure of skin to UV-B radiation, leading to
formation of previtamin D3 (7). Pre-vitamin D3 then
isomerizes to vitamin D3, or with further UV-B radiation
to tachysterol3 (T3) and
lumisterol3 (L3). Subsequent activation of
vitamin D3 involves sequential 25- and
1-α-hydroxylations of vitamin D3 (cholecalciferol) which
can occur at both the systemic and local levels. In the liver,
vitamin D3 is hydroxylated by mitochondrial and
microsomal 25-hydroxylases (CYP27A1 and CYP2R1) to
25-hydroxyvitamin D3 (25(OH)D3), also known
as 25-hydroxycholecalciferol or calcifediol) (8). 25(OH)D3 is then
hydroxylated in the kidneys by mitochondrial 1α-hydroxylase
(CYP27B1) (9) producing
1α,25(OH)2D3 (calcitriol), the fully active
form of vitamin D3 (10). A number of tissues and organs such
as intestines and skin can also activate vitamin D3
through these two sequential hydroxylations (11). The activity of 25(OH)D3
and 1α,25(OH)2D3 is limited by the
24-hydroxylase (CYP24A1), which initially transforms them to
24,25(OH)2D3 and
1,24,25(OH)3D3, respectively, prior to their
further oxidation and cleavage of the side chain resultig in
complete inactivation (12). Both
24,25(OH)2D3 and
1,24,25(OH)3D3 possess lower affinity for the
vitamin D receptor (VDR) than 1,25(OH)2D3
(13). In addition, 7DHC, vitamin
D2 and vitamin D3 can be metabolized by the
steroidogenic enzyme, CYP11A1 (14–17).
This enzyme catalyses a series of hydroxylations favoring C20, C22
and C23 but only results in cleavage of the side chain in the case
7DHC (18). CYP11A1, in
combination with the classic vitamin D hydroxylases (CYP27A1,
CYP27B1 and CYP24A1), has recently been shown to generate a series
of vitamin D analogs in cells or tissues incubated ex vivo
with vitamin D precursors (17,19–21),
which are biologically active in cell culture (22) and in vivo (23) models
(19,21,24).
Our previous studies showed that the initial and major product,
20(OH)D3, is non-calcemic (25,26)
with its biologically activity being defined by the nature of
cellular target (21–23,25–28).
Importantly, 20(OH)D3 displays anti-proliferative
properties towards melanoma and other types of cancer (26,31).
Details of the pathways of vitamin D synthesis and metabolism is
presented in Fig. 1.
The active form of vitamin D3,
1α,25(OH)2D3, plays a crucial role in calcium
homeostasis, ensuring proper functioning of bones, muscles and the
nervous system. In the colon it increases the absorption of
calcium, in bones it stimulates release of calcium and phosphate,
while in kidneys together with parathyroid hormone it regulates
calcium reabsorption (32). In
addition, 1α,25(OH)2D3 is involved in
regulating the immune system including reducing the inflammatory
response (33) and influencing the
growth and differentiation of mononuclear cells (34,35).
Several vitamin D analogs are already used for the treatment and/or
prevention of various diseases such as rickets, osteoporosis and
psoriasis (36). The importance of
vitamin D is emphasized by a growing body of evidence that there is
low 25(OH)D3 in the serum of patients with many types of
cancer. Therefore, secosteroids (vitamin D and its derivatives),
have potential applications in the treatment and/or prevention of
several types of cancer, including breast, prostate, colorectal
cancers and melanoma (37–39).
Active metabolites of
1α,25(OH)2D3 are characterized by broad and
diverse biological activities. After entering the cell,
1α,25(OH)2D3 binds to the vitamin D receptor
(VDR) which heterodimerizes with the 9-cis-retinoic acid
receptor (retinoid X receptor, RXR). The VDR-RXR complex acts as a
transcription factor, which recognizes a consensus DNA sequence,
the vitamin D responsive element (VDRE), and regulates the
expression of over 200 genes (13,40).
A wide-range of studies show that
1α,25(OH)2D3 regulates a number of genes
involved in cell proliferation, DNA repair, differentiation,
apoptosis and membrane transport. Among the most important ones are
genes associated with vitamin D3 metabolism, as well as
with calcium and phosphorus homeostasis (41). One of the most well-described
activities of 1α,25(OH)2D3 is the stimulation
of the expression of the CYP24A1 gene (coding
24-hydroxylase) and the inhibition of the expression of the
CYP27A1 (25-hydroxylase) and CYP27B1 (1α-hydroxylase)
genes (42). It is therefore
likely that variations in the expression of genes involved in the
regulation of 1α,25(OH)2D3 activity could
play an important role in determining susceptibility to CRC.
It has been suggested that
1α,25(OH)2D3 can act via an alternative
signaling pathway which involves binding to the endoplasmic
reticulum protein PDIA3 (also known as ERp57) (43). PDIA3 protein belongs to the protein
disulfide isomerases family (PDI) and is a disulfide
oxidoreductase. PDIA3 is involved in the transport and
post-translational modification of glycoproteins, in particular the
heavy chains of the major histocompatibility complex type I (MHC I)
and tyrosinase (44). In addition,
calcium and potassium absorption in the colon is stimulated by
1α,25(OH)2D3 via PDIA3. It is also
interesting that the PDIA3 contains a nuclear localization signal
sequence and a DNA binding domain, and interacts with the
transcription factor, STAT3 (45).
It has also been shown that under specific conditions PDIA3 can
acts as a transcription factor or co-activator.
A number of studies support the potential
preventative and therapeutic effects of vitamin D3, the
precursor to 1α,25(OH)2D3, for anticancer
treatment. Unfortunately, administration of vitamin D3
at its effective therapeutic dose during treatment (>50,000
units/day) is associated with a high probability of hypercalcaemia
occurrence. This can cause such side-effects as nausea, vomiting
and loss of appetite (46,47), or be lethal depending on the
anatomical site of calcium deposition. For this reason many
laboratories have conducted studies on
1α,25(OH)2D3 analogues with little or no
impact on the calcium homeostasis, but still retaining
therapeutically crucial properties (37). In the present study we have
examined the effects of 1α,25(OH)2D3, and
selected analogues with low calcemic activity [calcipotriol,
25(OH)D3, 20(OH)D3], on CRC cell lines (LoVo,
HT29 and HCT116). We also examined the expression of key genes
associated with 1α,25(OH)2D3 activity and
metabolism in CRC cell lines and colon cancer biopsies.
Materials and methods
Cell culture
The CRC cell lines LoVo (colon cancer), HT29
(colorectal adenocarcinoma) and HCT116 (colorectal carcinoma) were
purchased from ATTC (Wesel, Germany). The HT29 cell lines were
cultured in McCoy's medium, LoVo were cultured in Dulbecco's
modified Eagle's medium (DMEM) and F-10 medium (ratio 1:1), while
HCT116 were grown in DMEM only. All media were supplemented with
10% fetal bovine serum (FBS; Sigma) and 1% PSA
(penicillin-streptomycin-amphotericin B solution; Sigma). Cell
cultures were maintained in a 37°C humidified atmosphere of 5%
CO2. Cells were passaged every 2–3 days.
Patients
The study was approved by the local ethics
committee, and informed, written consent regarding the use of
tissue was obtained before surgery or colonoscopy from all CRC
patients and control individuals, respectively (48). The CRC specimens were received from
the Department of General Surgery, Hospital Ministry Internal
Affairs in Gdansk whereas the control samples were collected from
the Department of Hepatology and Gastroenterology of the Medical
University of Gdansk (MUG). Clinical and demographical data were
collected at the time of enrollment. The study included 20 patients
with CRC (11 males and 9 females; mean age 67.2±10.8 years; range,
50–87 years). None of the CRC patients had a second neoplastic
disease or had a history of previous chemo- or radiotherapy. Tumor
CRC samples were collected from: rectum (n=7 cases), sigmoid colon
(n=5), transverse (n=5) and 3 cases from cecum/ascending colon. The
control group comprised 4 healthy individuals (1 males and 3
females; mean age 59±7.9 years; range, 54–65 years) who underwent
colonoscopy as a part of routine surveillance for CRC. None of the
CRC patients or controls suffered from inflammatory bowel disease
or had a family history of CRC. Patients were not on medications at
the time of the investigation.
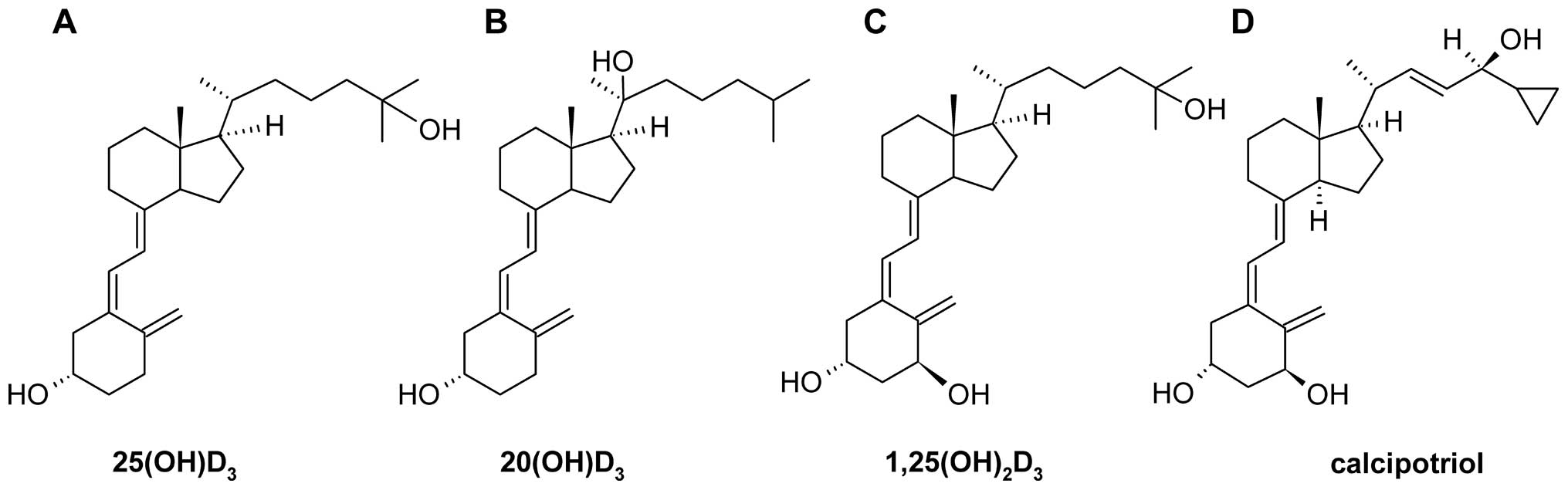
Vitamin D and its analogues
Calcitriol (1α,25(OH)2D3) and
calcifediol (25(OH)D3) were obtained from Sigma (Poznań
Poland). Calcipotriol was obtained from Pharmaceutical Research
Institute (Warsaw, Poland). 20S-Hydroxyvitamin D3
(20(OH)D3;
(3β,5Z,7E)-9,10-secocholesta-5,7,10(19)-trien-3,20-diol) was synthesized and
purified as previously described (15). The structures of these secosteroids
are presented in Fig. 2. They were
dissolved in 99.5% ethanol and stored at −20°C. Their concentration
was calculated using an extinction coefficient of 18,200
M−1cm−1 at 265 nm. For in vitro use,
dilutions were made in the same medium as those for cell culture.
The highest final concentration of ethanol used in the present
study never exceeded 0.2% and had no effect on cell growth.
Proliferation assays
The degree of proliferation of cells following
treatment was measured using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
(MTT) and the sulforhodamine B assay (SRB). Cells were seeded at a
density of ~7×103 (LoVo and HCT116) or 104
(HT29) cells per 100 μl/well in 96-well plates in the appropriate
growth medium, supplemented with 1% PSA and 2% charcoal-treated
FBS, and allowed to attach for 24 h before treatment. The medium
was replaced with fresh medium containing serial dilutions of
1α,25(OH)2D3 or its analogues (0.01 nM - 1
μM) in a volume of 100 μl/well. The plates were incubated at 37°C
for an additional 48 h. For the MTT assay, 20 μl of MTT (5 mg/ml)
were added to each well. After incubation for 4 h, the medium was
removed and 100 μl of the solubilisation solution (1 M acidic
isopropanol) was added to each well. After incubation for 5 min the
absorbance was measured at 570 nm using a microplate reader. For
the SRB assay, after incubation with the vitamin D analogs, 100 μl
of 10% trichloroacetic acid (TCA) was added to each well and plates
were incubated for 1 h at 4°C. Afterwards, the medium was removed
and cells were washed 5 times with deionized water. Following
overnight air-drying, 100 μl of SRB solution [0.4% (w/v) in 1%
acetic acid] was added to each well. After incubation for 15 min
plates were washed 5 times with 1% acetic acid and air-dried. The
protein-bound dye was solubilised with a 10 mM Tris-base solution
(pH 10.5). The absorbance of the dye was recorded at 570 nm with a
microplate reader.
Cell growth in 3D matrigel
The three-dimensional cell growth assay was
performed in a Matrigel Matrix (BD Bioscience, Heidelberg,
Germany). Two drops of cell suspension in Matrigel (~2 mg of
protein/ml, ~1.5×103 cells/40 μl) were added per well to
a 12-well tissue culture plate and plates incubated at 37°C for up
to 30 min to allow the Matrigel to solidify. Wells were then
submerged in growth medium supplemented with the relevant
concentrations of 1α,25(OH)2D3 or it analogs
(final concentrations: 0.5, 0.1 or 0.2 μM). Growth medium was
replaced every third day. Following 10 days of incubation
representative pictures were taken and relative colony size was
determined for 50 random colonies using ImageJ software. Each
experiment was repeated at least four times. Due to the fast growth
rate and the formation of crypt-like structures, only the
inhibition of the formation of ‘megacolonies' was compared.
Sample collection
CRC and control samples were collected as previously
described with some modifications (48). Briefly, CRC and surgical margin
tissue samples from each CRC patient collected during surgery were
placed in sterile vials containing 5 volumes of RNAlater buffer
(Ambion-Life Technologies, Grand Island, NY, USA), incubated for 6
h at 4°C and then stored at −25°C until further analysis. The same
procedure was also applied to control colon biopsies.
cDNA preparation and PCR assays
RNA was extracted using a Total RNA Mini Plus kit
(A&A Biotechnology, Gdynia, Poland) according to the
manufacturer's protocol. Before the RNA isolation, tissue samples
were homogenized in 2 ml vials with the use of ceramic beads
(Blirt-DNA Gdansk, Gdansk, Poland) in the MagNA Lyser apparatus
(Roche Diagnostics Deutschland GmbH, Mannheim, Germany) for 45 sec
at 6,000 rpm. The concentration and purity of isolated RNA was
measured using a NanoDrop® spectrophotometer. A total of
2 μg of RNA were subjected to reverse transcription using a
RevertAid™ First Strand cDNA Synthesis kit (Thermo Fisher
Scientific Inc., Waltham, MA, USA). All the primer sequences
(Table I) were designed by the
authors using Primer BLAST database and were purchased from
Sigma-Aldrich (Munich, Germany). Quantification of the expression
of genes of interest was carried out using the StepOnePlus™
Real-Time PCR System (Life Technologies-Applied Biosystems, Grand
Island, NY, USA) with SYBR® Green as a fluorophore. qPCR
reactions were performed in a total volume of 20 μl using Real-Time
HS 2× PCR Master Mix SYBR® kit (A&A Biotechnology),
2 μl of cDNA diluted 4-fold and 200 nM of each primer pair. The PCR
conditions were: 95°C for 5 min followed by 40 cycles of
denaturation for 15 sec at 95°C, annealing for 20 sec at 59°C,
extension for 15–25 sec at 72°C, and fluorescence reading for 5 sec
at 77°C. Dynamic melting curve analysis was performed for all
reactions. All qPCR reactions were performed in duplicate and data
were collected with a StepOnePlus™ software version 2.2 (Life
Technologies). The expression of the genes was normalized by
comparative ΔΔ-Ct method, using β-actin as a
housekeeping gene, followed by calibration (fold-change) to
normalized expression data of samples from controls (ratio=1).
 | Table IThe PCR primers. |
Table I
The PCR primers.
| GENE | Protein name | Primer sequence
(5′-3′) | Amplicon size
(nt) |
|---|
| ACTB | β-actin |
GCTCGTCGTCGACAACGGCTC | |
| |
CAAACATGATCTGGGTCATCTTCT | 353 |
| VDR | Vitamin D
receptor |
CCAGTTCGTGTGAATGATGG | |
| |
GTCGTCCATGGTGAAGGA | 384 |
| PDIA3 | Protein
disulfideisomerase-associated 3 |
CTCCGACGTGCTAGAACTCA | |
| |
CAGGTGTTAGTGTTGGCAGT | 204 |
| CYP24A1 | 24-hydroxylase |
GCAGCCTAGTGCAGATTT | |
| |
ATTCACCCAGAACTGTTG | 335 |
| CYP24SV | 24-hydroxylase |
TCCTGAAGTTGCAGCTGGAGT | |
| |
GAGCTCATCTATTCTGCCCATA | 215 |
| CYP2R1 | 25-hydroxylase |
AGAGACCCAGAAGTGTTCCAT | |
| |
GTCTTTCAGCACAGATGAGGTA | 259 |
| CYP27B1 | 1α-hydroxylase |
TGTTTGCATTTGCTCAGA | |
| |
CCGGGAGAGCTCATACAG | 227 |
Western blotting
Cells were scraped and lysed in the presence of
ice-cold Laemmli buffer supplemented with protease inhibitor
cocktail. Protein concentrations were determined by the Bradford
assay. An equal amount of protein from each sample (50 μg) was
loaded per lane, proteins were separated by SDS-PAGE and then
transferred onto an Immun-Blot™ PVDF membrane (Bio-Rad
Laboratories, Hercules, CA, USA). The membranes were incubated with
primary antibodies: anti-VDR (polyclonal, 1:1,000; Sigma-Aldrich,
SAB2102673), anti-CYP24A1 (polyclonal, 1:500; Santa Cruz
Biotechnology, sc-66851) or HRP conjugated anti-β-actin antibody
(monoclonal, 1:5,000; Santa Cruz Biotechnology, sc-47778) overnight
at 4°C. After three washes in TBST, secondary mouse anti-rabbit
antibodies conjugated to horseradish peroxidase (1:10,000; Santa
Cruz Biotechnology, sc-2537) were added and following incubation
for 1 h at room temperature. Blots were developed with
SuperSignal® West Pico chemiluminescent substrate
(Thermo Fisher Scientific) according to the manufacturer's
protocol.
Statistical analysis
Data were analyzed with the Student's t-test (for
two groups) or one-way analysis of variance and appropriate post
hoc test (the ANOVA Kruskal-Wallis test for comparison of several
groups), using Statistica (StatSoft Inc., Tulsa, OK, USA) or
GraphPad Prism v6.03 (GraphPad Software, San Diego, CA, USA). For
tissue samples, the Mann-Whitney U test of Kruskal-Wallis ANOVA was
applied since the qPCR results for those specimens did not pass the
omnibus test of D'Agostino and Pearson (49). Such procedures allowed the
comparison of each experimental group with the control. Spearman's
test was applied to check the association of paired data
(correlation test). The data are presented as mean ± SD or median
value with range (min-max). Results were considered statistically
significant at P<0.05 and are marked in the figures as:
****P<0.0001; ***P<0.001;
**P<0.01; *P<0.05.
Results
The effect of
1α,25(OH)2D3 and its analogs on the
proliferation of CRC cell lines
The effects of vitamin D analogues on the inhibition
of the proliferation of CRC cell lines, LoVo, HT29 and HCT116, were
assessed by means of MTT and SRB assays (Fig. 3 and Table I). As shown by the MTT assay, the
inhibitory effect of 1α,25(OH)2D3 treatment
was similar for LoVo and HT29 cell lines (Fig. 3A and C). A significant decrease in
cell growth compared to vehicle-treated cells (P<0.001 for LoVo
and P<0.0001 for HT29 cell line) was seen with 1 μM
1α,25(OH)2D3 (Fig. 3D and F), but not for HCT116 cells
where the inhibition of growth did not reach statistical
significance (Fig. 3E). The
IC50 value of 1,25(OH)2D3 for LoVo
(168 nM) with the MTT assay was slightly higher than that for HT29
cells (57 nM) and HCT116 cells (47 nM) (Table II).
 | Table IIThe inhibition of CRC cell
proliferation by 1,25(OH)2D3 and its
analogs. |
Table II
The inhibition of CRC cell
proliferation by 1,25(OH)2D3 and its
analogs.
| LoVo | HT29 | HCT116 |
|---|
|
|
|
|
|---|
| Compound (nM) | MTT | SRB | MTT | SRB | MTT | SRB |
|---|
|
1α,25(OH)2D3 | 168 | 0.77 | 57 | 196 | 47 | 5.9 |
| Calcipotriol | 57 | 0.71 | ND | 4.6 | 5.3 | 3.7 |
|
25(OH)D3 | 6.6 | 35 | 319 | 49 | 457 | 243 |
The IC50 values measured for
1,25(OH)2D3 by the SRB assay differed to
those measured using the MTT assay, presumably due to the different
biochemical parameters measured by these assays, the activity of
NAD(P)H-dependent cellular oxidoreductase enzymes in the case of
MTT and cellular protein content in the case of SRB. The
proliferation of the LoVo cell line (Fig. 3A) measured with the SRB assay was
significantly (P<0.0001) decreased with 1.0 nM
1,25(OH)2D3 compared to vehicle-treated
control cells, with the calculated IC50 being 0.77 nM
(Table III). A statistically significant decrease in the
proliferation of HCT116 and HT29 cells by
1,25(OH)2D3 was also seen using the SRB assay
(Fig. 3B and C) although
IC50 values were considerably higher than for the LoVo
cells (5.9 and 196 nM, respectively (Table II).
Calcipotriol, a low-calcemic analogue of vitamin D
(39), proved to be the most
potent inhibitor of proliferation of the three analogs tested using
both the MTT and SRB assays on the colorectal cancer cell lines
(Fig. 3M–R and Table II). With the SRB assay,
calcipotriol caused significant inhibition of the proliferation of
the LoVo, HCT116 and HT29 cells, compared to vehicle-treated cells,
at concentrations as low as 1, 10 and 10 nM, respectively (Fig. 3M–O).
The potency of 25(OH)D3 relative to
1,25(OH)2D3 varied for the LoVo and HCT116
cells depending on whether the MTT or SRB assay was used (Fig. 3). It should be noted that colon
cells express CYP27B1 and are capable of transforming
25(OH)D3 to 1,25(OH)2D3 (20). With both the MTT and SRB assays,
25(OH)D3 displayed a lower potency than
1,25(OH)2D3 for inhibiting the proliferation
of HCT116 cells (Table II),
likely reflecting the relatively poor ability of these cells to
activate this secosteroid by 1α-hydroxylation (Table II).
Effect of
1α,25(OH)2D3 and its analogues on CRC cell
lines colony formation in three-dimensional Matrigel
The LoVo and HT29 cell lines were used to test the
effects of 1α,25(OH)2D3 and its two low- or
non-calcemic analogues, calcipotriol and 20(OH)D3,
respectively, on cell growth and colony formation in
three-dimensional Matrigel. Our initial tests with the HCT116 cell
line showed that these cells grew too slowly in Matrigel to perform
comparable experiments so this particular cell line was excluded
from the Matrigel assays. In control experiments (without
treatment), the majority of the LoVo and HT29 cells gave rise to
large colonies with complex structure ‘megacolonies', while the
remaining ~10% of cells formed small colonies with limited growth.
Due to the faster growth rate with the formation of crypt-like
structures, only the inhibition of the formation of ‘megacolonies'
was analyzed.
In agreement with the results of the MTT and SRB
assays, vitamin D analogues significantly diminished cell
proliferation as shown by the decrease in both size and the number
of the colonies formed in a presence of the three analogues tested
(Fig. 4). Over the limited range
of concentrations tested (20–500 nM), the potency of
1,25(OH)2D3, calcipotriol and
20(OH)D3 were reasonably similar with a significant
reduction in colony size seen in both LoVo and HT29 cells for all
three analogs at a concentration of 20 nM. The magnitude of the
inhibition (efficacy) varied between the three analogs.
Calcipotriol caused the greatest inhibition (~57%) at the highest
concentration tested (500 nM), while
1,25(OH)2D3 caused ~53% inhibition and
20(OH)D3 ~28% inhibition at this concentration, for both
cell lines.
 | Figure 4The effect of
1α,25(OH)2D3, calcipotriol and
20(OH)D3 on CRC colony formation in Matrigel. Cells in
Matrigel were incubated with varying concentrations (0.02, 0.1 or
0.5 μM) of (A) 1α,25(OH)2D3, (B) calcipotriol
or (C) 20(OH)D3 for 10 days. Light microscopy of
representative ‘megacolonies' formed in 10 days following the
addition of (D–G) 1α,25(OH)2D3 and (H–K)
calcipotriol on LoVo cells are also shown. Original magnification,
×100. The concentrations of 1α,25(OH)2D3 and
25(OH)D3 used were 0 μM (controls, D and H); 0.02 μM (E
and I); 0.1 μM (F and J) and 0.5 μM (G and K). Significance
compared to the control: *P<0.05,
**P<0.01, ***P<0.001. |
1,25(OH)2D3
modulates the expression of some of the genes involved in vitamin D
metabolism and signalling
The expression levels of several genes encoding
proteins inolved in vitamin D signalling (VDR and PDIA3) and
metabolism (CYP24A1 and its splice variant, CYP24SV; CYP27B1 and
CYP2R1) were compared by real-time PCR (qPCR) (Fig. 5). Basal levels of transcripts of
CYP24A1 and its splice variant, CYP24SV, were strongly elevated in
the HT29 and HCT116 cell lines compared to LoVo cells (Fig. 5A and C).
1,25(OH)2D3 caused a marked and statistically
significant elevation of transcript levels for both CYP24A1 and
CYP24SV, most pronounced in the LoVo cell line (3,100-fold for
CYP24A1 and 1,200-fold for CYP24SV) due to the low basal level of
expression. The stimulation of CYP24A1 expression by
1,25(OH)2D3 was confirmed at the protein
level for all three cell lines (Fig.
3A, bottom panels) although the magnitude of the stimulation
appeared less for the LoVo cells than at the transcript level.
 | Figure 5The effects of
1,25(OH)2D3 on the expression of genes
involved in vitamin D3 signaling and metabolism. CRC
cell lines were incubated with 0.1 μM
1,25(OH)2D3 for 24 h and the expression of
24-hydroxylase (CYP24A1 gene) (A), vitamin D receptor
(VDR gene) (B), splicing variant of 24-hydroxylase
(CYP24SV gene) (C), protein disulfide isomerase
(PDIA3 gene) (D), 25-hydroxylase (CYP2R1 gene) (E)
and 1α-hydroxylase (CYP27B1 gene) (F) was analysed by
real-time PCR (qPCR). Data were normalized relative to β-actin mRNA
and further normalized against the basal expression in LoVo cells.
CYP24A1 (A, lower panel) and vitamin D receptor (B, lower panel)
proteins were measured by western blotting, with β-actin used as a
control. The data are presented are mean ± SEM. Significance level
relative to the LoVo cell basal expression: *P<0.05,
**P<0.01, ***P<0.001. Significance
level relative to the untreated control for each cell line is
indicated as #P<0.05. |
For the three cell lines, expression of CYP2R1
transcripts was significantly downregulated by
1,25(OH)2D3 only in the HT29 cells (6.6-fold,
P<0.05) where the basal level was higher than in the other cells
(Fig. 5E).
1,25(OH)2D3 did not alter the level of
transcripts for CYP27B1 in any of the three cell lines (Fig. 5F). Basal levels of CYP27B1
transcripts were significantly lower in HT29 and HCT116 cells than
in LoVo cells. For PDIA3 transcript, basal levels were
significantly higher in HT29 cells than in LoVo cells (Fig. 5D).
1,25(OH)2D3 significantly decreased the the
expression of PDIA3 in LoVo and HT29 cells, but not in the HCT116
cell line. 1,25(OH)2D3 significantly
increased the expression of the VDR at the mRNA level in only the
LoVo cells. Basal expression was also significantly higher in LoVo
cells than in the HT29 or HCT116 cells (Fig. 5B). Consistent with the results at
the transcript level, marginal increases at best were seen for VDR
expression at the protein level following treatment with
1,25(OH)2D3. The highest expression of VDR
was in the HT29 cells (Fig 3B,
lower panels). The splice variant of the VDR protein (lower band)
was observed most prominently in untreated HT29 cells.
Expression of VDR and CYP24A1 genes in
CRC biopsies
The expression of VDR and CYP24A1 genes in
CRC biopsies and control samples were assessed by qPCR (Fig. 6). Matched tumor-surgical margin
biopsies from 20 CRC patients were analyzed in comparison to
healthy mucosal colon biopsies from 4 individuals who underwent
routine colonoscopy. There was a significant decrease in the
expression of VDR in tumor CRC samples in comparison to the
surgical margin and healthy control specimens (Fig. 6A and C). Analysis of the clinical
data revealed that there was no association between the VDR
expression level and either gender, age, tumor progression
(Fig. 6C), G staging or metastasis
presence.
The highest expression of CYP24A1 was observed in
surgical margin specimens in comparison to both tumor and control
samples (P<0.0001; Fig. 6B). No
correlation between CYP24A1 expression and gender or age was found.
When the clinical data were taken into consideration, we observed
that CYP24A1 expression was associated with tumor location
(proximal to distal colon) (rs=0.48; p<0.02). Despite the
general decrease in CYP24A1 mRNA in tumor specimens compared to the
surgical margin, we found increased expression in higher (Stage I
to III, UICC grading 1 to 3) developed tumors (rs=0.36; P<0.05,
Spearman's test, P=0.001; Kruskal-Wallis ANOVA; Fig. 6D). Finally, CYP24A1 expression in
tumor tissue was correlated with VDR mRNA levels (rs=0.57,
P<0.05; Spearman's test; Fig. 6C
and D).
Discussion
The first aim of the study was to investigate the
effects of 1,25(OH)2D3 and its low-calcemic
analogues (particularly calcipotriol and 20(OH)D3) on
the proliferation of CRC cell lines. Calcipotriol has a 100- to
200-fold lower effect on calcium metabolism than
1,25(OH)2D3 (51), while 20(OH)D3 is
non-calcemic at concentration ≤60 μg/kg (26). Three different CRC cell lines were
chosen, representing colon cancer (LoVo), colorectal adenocarcinoma
(HT29) and colorectal carcinoma (HCT116). The LoVo cell line is
well differentiated, HT29 cells are moderately differentiated,
while the HCT116 cell line is poorly differentiated (52).
Overall, our results show that calcipotriol has
similar or greater potency to 1,25(OH)2D3
using MTT, SRB and Matrigel assays of cell proliferation.
25(OH)D3 was generally found to be less potent than
1,25(OH)2D3 but there were exceptions such as
in the MTT assay on LoVo cells and the SRB assay on HT29 cells. It
should be noted that while it is generally accepted that
25(OH)D3 exerts its action following its conversion to
1,25(OH)2D3 by CYP27B1, which is present in
the colon, recent studies indicate that it may have distinct
actions independent of 1,25(OH)2D3 (53).
The treatment of the LoVo and HT29 cell lines with
1,25(OH)2D3, calcipotriol or
20(OH)D3 in Matrigel caused a statistically significant
inhibition of colony size at a concentration of only 20 nM, with
stronger inhibition at 500 nM. Since matrigel resembles a three
dimensional basement membrane complex into which cells can invade,
proliferate and grow (54), these
results indicate that the low calcemic analogs of vitamin
D3 tested in the present study are worthy of future
in vivo testing.
The SRB assay results indicate that the LoVo cell
line is the most sensitive to the vitamin D analogues examined
(lowest IC50 values), while the HTC116 cell line was the
most resistant. Thus, it might be speculated that moderately
differentiated cells (LoVo and HT29 cell lines) are more sensitive
to vitamin D analogs compared with poorly differentiated HCT116
cells. However, this correlation does not hold for the MTT assay
results.
The dose-dependent, anti-proliferative activity of
1α,25(OH)2D3 on human CRC cells has been
previously reported by other researchers (35,55–59).
For instance, treatment of the colon cancer cell line, Caco-2, with
vitamin D3 analogs resulted in growth inhibition by
20–40% (at a concentration of 0.01 μM) (60). This is in general agreement with
our results with calcipotriol and
1,25(OH)2D3, but we observed that
IC50 values varied between the different cell lines
studied. Thus, the observed differences in sensitivity of CRC to
vitamin D analogues may dependent on the unique properties of the
cancer cells (37,38), including the degree of expression
of VDR and CYP24A1 (49), as well
as on the molecular structure of the vitamin D3
analogue. It has recently been suggested that epigenetic changes
may influence the expression of crucial genes which encode proteins
involved in the metabolism of vitamin D, such as CYP24A1 (61). According to the classical pathway,
vitamin D3 acts through the VDR, as supplementation of
VDR knockout animals with vitamin D3 analogues does not
protect them from tumor development (62). Our results indicate that the CRC
cell lines studied differ in their constitutive level of VDR mRNA
and protein. The LoVo cell line showed the highest relative level
of VDR mRNA whereas the HT29 cell line showed the highest
concentration of protein. Thus, the relatively high level of VDR
mRNA in the LoVo cell line did not correlate with the highest level
of its protein product. In fact, the LoVo cell line showed the
lowest concentration of VDR protein in comparison to HT29 and
HTC116 cells. These inconsistencies can be explained by the recent
report showing that the VDR can be regulated at the
post-translational level via proteosomal degradation in a cell-type
specific manner (63).
Notably, epidemiological studies show that vitamin
D deficiency (64), as well as
specific polymorphisms in the VDR gene (65,66),
are strong prognostic factors for development and severity of CRC.
It is also well established that the VDR is expressed by normal
colon epithelial cells, but its expression is decreased during the
progression of colon cancer (67).
A similar reverse correlation between VDR expression and the
aggressiveness of the tumor was reported for human melanomas
(68,69). Our results reveal that there is a
significant downregulation of VDR expression in patients with CRC
at stages I to III. Another interesting observation is the
comparatively high level of VDR transcript expression in surgical
margin specimens in comparison to both tumor and control samples of
healthy colon mucosa. A correlation between tumor progression and
CYP24A1 expression at the mRNA level was observed in the CRC
biopsies. Our results therefore indicate that changes in expression
of the above genes may provide useful information as a prognostic
predictor for CRC metastasis.
The responsiveness of the CRC cell lines to vitamin
D3 analogues may also be affected by their metabolism
and inactivation. Our in vitro results show that there is
correlation between the expression of VDR, and to a lesser degree
CYP24A1, at the mRNA and protein levels in CRC cell lines treated
with 1,25(OH)2D3. This is in an agreement
with the data of Höbaus et al (61) who showed a very high level of
expression of CYP24A1 in HT29 cells and suggested that this
expression is connected with methyltransferase and some histone
deacetylase inhibitors in a cell line-dependent manner.
Furthermore, the present study shows that basal expression of
CYP24A1 and CYP24SV (isoform lacking mitochondrial translocation
signal (50) are highly
upregulated at the mRNA and protein levels in HT29 and HCT116 cell
lines compared to the LoVo cell line. Increased expression of
CYP24A1 in cancer cells may attenuate the effects of
1α,25(OH)2D3 on growth inhibition and
differentiation. Stimulation by 1α,25(OH)2D3
significantly increased the expression of CYP24A1 in LoVo, HT29 and
HCT116 cell lines. It has been shown before that induction of
CYP24A1 expression by 1α,25(OH)2D3 treatment
is more pronounced in responsive CRCs and melanomas in comparison
to resistant ones (56,70,71).
Furthermore, CRC cell lines (HT29 and HCT116) with the highest
basal expression of CYP24A1 at the transcript level were shown to
be less responsive to vitamin D treatment, which is consistent with
other studies (35). However, this
correlation is not so clear at the protein level. The role of
CYP24A1 in tumor biology may be complex as indicated by
clinicopathological studies on patients with melanoma (72).
Although, catabolism of vitamin D by CYP24A1 is
considered as a major regulatory mechanism controlling
25(OH)D3 and 1,25(OH)2D3
concentrations, expression of other genes involved in vitamin D
metabolism may influence the responsiveness of cancer cells to the
treatment (57). Recently, it was
shown that CYP2R1 and not CYP27A1 is the major vitamin D
25-hydroxylase (73). We have
found that that the treatment of HT29 cells with
1,25(OH)2D3 significantly decreased the
expression of CYP2R1 at the mRNA level but the small decreases in
the other cell lines did not reach statistical significance.
Notably, in the responsive HT29 cells, the basal level of CYP2R1
mRNA was 4 times higher than in the HTC116 or LoVo cells.
1,25(OH)2D3 had no effect on
the expression of 1α-hydroxylase (CYP27B1) at the mRNA level in any
of the three cell lines analysed. It is well established now that
CYP27B1 is expressed at extra-renal sites such as normal colon,
brain, placenta, pancreas, lymph nodes and skin (74). Our data show that basal expression
of CYP27B1 at the mRNA level is higher in LoVo cells compared to
the HT29 and HCT116 cell lines. Higher CYP27B1 expression has
previously been reported in well-to-moderately differentiated
states of colon cancer compared to poorly differentiated colon
carcinomas (75–77). A similar reverse correlation
between CYP27B1 expression and tumor progression and aggressiveness
was found in melanomas and ovarian cancer (78,79).
Increased expression of CYP27B1 in cancer tissues may convey some
chemoprevention to these cancers due to increased conversion of
25(OH)D3 of 1,25(OH)2D3. Notably,
the LoVo cells which show the highest expression of CYP27B1,
display the lowest IC50 of the three cell lines for the
inhibition of proliferation by 25(OH)D3.
The VDR is not the only target for active forms of
vitamin D in the cells. For instance, vitamin D3 may
exert some non-genomic actions mediated by PDIA3 (80). The most resistant cell line,
HCT116, showed the lowest basal level of PDIA3 mRNA and the
treatment with 1,25(OH)3D3 did not affect its
expression. In contrast, treatment of LoVo and HT29 cells with
1,25(OH)2D3 resulted in a significant
decrease of PDIA3 mRNA.
It was shown that products of a novel
CYP11A1-dependent pathway of vitamin D metabolism, such as 20(OH)
D3 (which the present study shows to inhibit CRC
proliferation) and 20,23(OH)2D3, can act as
antagonists or inverse agonists on RORα and RORγ (27). These findings may not only give new
insights into local (skin) or systemic regulation of genes by
vitamin D metabolites, but could also explain the pleiotropic
activity of 20(OH)D3 and
20,23(OH)2D3, without the calcemic effects
(22) typically seen for classical
VDR ligands (65). It should be
noted that 20(OH)D3 has been detected in human serum
(19) and epidermis (21) indicating that it may have a
physiological role as an endogenous regulator.
In summary, CRC is becoming a major problem in
modern societies with an aging population as its occurrence
correlates with a lack of physical activity and unhealthy dietary
habits, as well as with vitamin D deficiency (61,81).
Thus, there is an urgent need for an inexpensive, easily available,
safe and effective prevention and treatment for CRC. The present
study indicates that low calcemic calcipotriol or non-calcemic
20(OH)D3, with proven anti-proliferative activity, may
replace 1α,25(OH)2D3 in the treatment of CRC,
preferably in combination with other cytostatic agents.
Additionally, our results indicate that profiling the expression of
genes involved in vitamin D signaling and metabolism, particularly
VDR and CYP24A1, may provide a powerful tool for the planning of
vitamin D3 analogue-based anticancer therapy.
Acknowledgements
The present study was supported in part by grant
AR052190, 1R01AR056666-01A2 and R21AR066505-01A1 from the NIH/NIAMS
(A.T.S.). Students Scientific Association BIO-MED (J.W., A.B.,
T.A., J.N., A.S., Ł.T., M.A.Z.) was awarded with a grant by the
Polish Genetic Society.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
2
|
López-Gómez M, Malmierca E, de Górgolas M
and Casado E: Cancer in developing countries: The next most
preventable pandemic. The global problem of cancer. Crit Rev Oncol
Hematol. 88:117–122. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wolpin BM and Mayer RJ: Systemic treatment
of colorectal cancer. Gastroenterology. 134:1296–1310. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Johnston PG and Kaye S: Capecitabine: A
novel agent for the treatment of solid tumors. Anticancer Drugs.
12:639–646. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu G, Hu X and Chakrabarty S: Vitamin D
mediates its action in human colon carcinoma cells in a
calcium-sensing receptor-dependent manner: Downregulates malignant
cell behavior and the expression of thymidylate synthase and
survivin and promotes cellular sensitivity to 5-FU. Int J Cancer.
126:631–639. 2010. View Article : Google Scholar
|
|
6
|
Milczarek M, Psurski M, Kutner A and
Wietrzyk J: Vitamin D analogs enhance the anticancer activity of
5-fluorouracil in an in vivo mouse colon cancer model. BMC Cancer.
13:2942013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Holick MF, Frommer JE, McNeill SC,
Richtand NM, Henley JW and Potts JT Jr: Photometabolism of
7-dehydrocholesterol to previtamin D3 in skin. Biochem Biophys Res
Commun. 76:107–114. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Holick MF, DeLuca HF and Avioli LV:
Isolation and identification of 25-hydroxycholecalciferol from
human plasma. Arch Intern Med. 129:56–61. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Holick MF, Schnoes HK, DeLuca HF, Gray RW,
Boyle IT and Suda T: Isolation and identification of
24,25-dihydroxycholecalciferol, a metabolite of vitamin D made in
the kidney. Biochemistry. 11:4251–4255. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Holick MF, Schnoes HK, DeLuca HF, Suda T
and Cousins RJ: Isolation and identification of
1,25-dihydroxycholecalciferol. A metabolite of vitamin D active in
intestine. Biochemistry. 10:2799–2804. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hewison M, Zehnder D, Chakraverty R and
Adams JS: Vitamin D and barrier function: A novel role for
extra-renal 1 alpha-hydroxylase. Mol Cell Endocrinol. 215:31–38.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tieu EW, Tang EKY, Tuckey RC and Adams JS:
Kinetic analysis of human CYP24A1 metabolism of vitamin D via the
C24-oxidation pathway. FEBS J. 281:3280–3296. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Haussler MR, Whitfield GK, Haussler CA,
Hsieh JC, Thompson PD, Selznick SH, Dominguez CE and Jurutka PW:
The nuclear vitamin D receptor: Biological and molecular regulatory
properties revealed. J Bone Miner Res. 13:325–349. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Slominski A, Zjawiony J, Wortsman J, Semak
I, Stewart J, Pisarchik A, Sweatman T, Marcos J, Dunbar C and
Tuckey RC: A novel pathway for sequential transformation of
7-dehydrocholesterol and expression of the P450scc system in
mammalian skin. Eur J Biochem. 271:4178–4188. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Slominski A, Semak I, Zjawiony J, Wortsman
J, Li W, Szczesniewski A and Tuckey RC: The cytochrome P450scc
system opens an alternate pathway of vitamin D3 metabolism. FEBS J.
272:4080–4090. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guryev O, Carvalho RA, Usanov S, Gilep A
and Estabrook RW: A pathway for the metabolism of vitamin D3:
Unique hydroxylated metabolites formed during catalysis with
cytochrome P450scc (CYP11A1). Proc Natl Acad Sci USA.
100:14754–14759. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Slominski A, Semak I, Wortsman J, Zjawiony
J, Li W, Zbytek B and Tuckey RC: An alternative pathway of vitamin
D metabolism. Cytochrome P450scc (CYP11A1)-mediated conversion to
20-hydroxyvitamin D2 and 17,20-dihydroxyvitamin D2. FEBS J.
273:2891–2901. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Slominski AT, Li W, Kim TK, Semak I, Wang
J, Zjawiony JK and Tuckey RC: Novel activities of CYP11A1 and their
potential physiological significance. J Steroid Biochem Mol Biol.
151:25–37. 2015. View Article : Google Scholar
|
|
19
|
Slominski AT, Kim TK, Shehabi HZ, Semak I,
Tang EK, Nguyen MN, Benson HA, Korik E, Janjetovic Z, Chen J, et
al: In vivo evidence for a novel pathway of vitamin D3
metabolism initiated by P450scc and modified by CYP27B1. FASEB J.
26:3901–3915. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Slominski AT, Kim TK, Shehabi HZ, Tang EK,
Benson HA, Semak I, Lin Z, Yates CR, Wang J, Li W, et al: In vivo
production of novel vitamin D2 hydroxy-derivatives by human
placentas, epidermal keratinocytes, Caco-2 colon cells and the
adrenal gland. Mol Cell Endocrinol. 383:181–192. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Slominski AT, Janjetovic Z, Kim TK,
Wasilewski P, Rosas S, Hanna S, Sayre RM, Dowdy JC, Li W and Tuckey
RC: Novel non-calcemic secosteroids that are produced by human
epidermal keratinocytes protect against solar radiation. J Steroid
Biochem Mol Biol. 148:52–63. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Slominski AT, Kim TK, Li W, Yi AK,
Postlethwaite A and Tuckey RC: The role of CYP11A1 in the
production of vitamin D metabolites and their role in the
regulation of epidermal functions. J Steroid Biochem Mol Biol.
144:28–39. 2014. View Article : Google Scholar
|
|
23
|
Slominski A, Janjetovic Z, Tuckey RC,
Nguyen MN, Bhattacharya KG, Wang J, Li W, Jiao Y, Gu W, Brown M, et
al: 20S-hydroxyvitamin D3, noncalcemic product of
CYP11A1 action on vitamin D3, exhibits potent
antifibrogenic activity in vivo. J Clin Endocrinol Metab.
98:E298–E303. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Slominski AT, Kim TK, Chen J, Nguyen MN,
Li W, Yates CR, Sweatman T, Janjetovic Z and Tuckey RC: Cytochrome
P450scc-dependent metabolism of 7-dehydrocholesterol in placenta
and epidermal keratinocytes. Int J Biochem Cell Biol. 44:2003–2018.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Slominski AT, Kim TK, Janjetovic Z, Tuckey
RC, Bieniek R, Yue J, Li W, Chen J, Nguyen MN, Tang EK, et al:
20-Hydroxyvitamin D2 is a noncalcemic analog of vitamin D with
potent antiproliferative and prodifferentiation activities in
normal and malignant cells. Am J Physiol Cell Physiol.
300:C526–C541. 2011. View Article : Google Scholar :
|
|
26
|
Slominski AT, Janjetovic Z, Fuller BE,
Zmijewski MA, Tuckey RC, Nguyen MN, Sweatman T, Li W, Zjawiony J,
Miller D, et al: Products of vitamin D3 or 7-dehydrocholesterol
metabolism by cytochrome P450scc show anti-leukemia effects, having
low or absent calcemic activity. PLoS One. 5:e99072010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Slominski AT, Kim TK, Takeda Y, Janjetovic
Z, Brozyna AA, Skobowiat C, Wang J, Postlethwaite A, Li W, Tuckey
RC, et al: RORα and RORγ are expressed in human skin and serve as
receptors for endogenously produced noncalcemic 20-hydroxy-and
20,23-dihydroxyvitamin D. FASEB J. 28:2775–2789. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zbytek B, Janjetovic Z, Tuckey RC,
Zmijewski MA, Sweatman TW, Jones E, Nguyen MN and Slominski AT:
20-Hydroxyvitamin D3, a product of vitamin D3 hydroxylation by
cytochrome P450scc, stimulates keratinocyte differentiation. J
Invest Dermatol. 128:2271–2280. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Slominski AT, Janjetovic Z, Kim TK, Wright
AC, Grese LN, Riney SJ, Nguyen MN and Tuckey RC: Novel vitamin D
hydroxy-derivatives inhibit melanoma growth and show differential
effects on normal melanocytes. Anticancer Res. 32:3733–3742.
2012.PubMed/NCBI
|
|
30
|
Janjetovic Z, Brozyna AA, Tuckey RC, Kim
TK, Nguyen MN, Jozwicki W, Pfeffer SR, Pfeffer LM and Slominski AT:
High basal NF-κB activity in nonpigmented melanoma cells is
associated with an enhanced sensitivity to vitamin D3 derivatives.
Br J Cancer. 105:1874–1884. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang J, Slominski A, Tuckey RC, Janjetovic
Z, Kulkarni A, Chen J, Postlethwaite AE, Miller D and Li W:
20-hydroxyvitamin D3 inhibits proliferation of cancer
cells with high efficacy while being non-toxic. Anticancer Res.
32:739–746. 2012.PubMed/NCBI
|
|
32
|
Holick MF: Vitamin D and bone health. J
Nutr. 126(Suppl): 1159S–1164S. 1996.PubMed/NCBI
|
|
33
|
Hayes CE, Nashold FE, Spach KM and
Pedersen LB: The immunological functions of the vitamin D endocrine
system. Cell Mol Biol (Noisy-le-grand). 49:277–300. 2003.
|
|
34
|
Pálmer HG, Sánchez-Carbayo M,
Ordóñez-Morán P, Larriba MJ, Cordón-Cardó C and Muñoz A: Genetic
signatures of differentiation induced by 1alpha,25-dihydroxyvitamin
D3 in human colon cancer cells. Cancer Res.
63:7799–7806. 2003.
|
|
35
|
Shabahang M, Buras RR, Davoodi F,
Schumaker LM, Nauta RJ and Evans SR: 1,25-Dihydroxyvitamin
D3 receptor as a marker of human colon carcinoma cell
line differentiation and growth inhibition. Cancer Res.
53:3712–3718. 1993.PubMed/NCBI
|
|
36
|
Leyssens C, Verlinden L and Verstuyf A:
The future of vitamin D analogs. Front Physiol. 5:1222014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hansen CM, Hamberg KJ, Binderup E and
Binderup L: Seocalcitol (EB 1089): A vitamin D analogue of
anti-cancer potential. Background, design, synthesis, pre-clinical
and clinical evaluation. Curr Pharm Des. 6:803–828. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Slominski AT and Carlson JA: Melanoma
resistance: A bright future for academicians and a challenge for
patient advocates. Mayo Clin Proc. 89:429–433. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bikle DD: Vitamin D: An ancient hormone.
Exp Dermatol. 20:7–13. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Brown AJ, Dusso A and Slatopolsky E:
Vitamin D. Am J Physiol. 277:F157–F175. 1999.PubMed/NCBI
|
|
41
|
Ebert R, Schütze N, Adamski J and Jakob F:
Vitamin D signaling is modulated on multiple levels in health and
disease. Mol Cell Endocrinol. 248:149–159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Whitfield GK, Jurutka PW, Haussler CA,
Hsieh JC, Barthel TK, Jacobs ET, Encinas Dominguez C, Thatcher ML
and Haussler MR: Nuclear vitamin D receptor: structure-function,
molecular control of gene transcription, and novel bioactions.
Vitamin D. 2nd edition. Feldman D, Pike JW and Glorieux FH:
Elsevier Academic Press; Oxford: pp. 219–261. 2005, View Article : Google Scholar
|
|
43
|
Khanal R and Nemere I: Membrane receptors
for vitamin D metabolites. Crit Rev Eukaryot Gene Expr. 17:31–47.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bouvier M: Accessory proteins and the
assembly of human class I MHC molecules: A molecular and structural
perspective. Mol Immunol. 39:697–706. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Eufemi M, Coppari S, Altieri F, Grillo C,
Ferraro A and Turano C: ERp57 is present in STAT3-DNA complexes.
Biochem Biophys Res Commun. 323:1306–1312. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ozkan B, Hatun S and Bereket A: Vitamin D
intoxication. Turk J Pediatr. 54:93–98. 2012.PubMed/NCBI
|
|
47
|
Wierzbicka J, Piotrowska A and Żmijewski
MA: The renaissance of vitamin D. Acta Biochim Pol. 61:679–686.
2014.
|
|
48
|
Wierzbicki PM, Adrych K, Kartanowicz D,
Stanislawowski M, Kowalczyk A, Godlewski J, Skwierz-Bogdanska I,
Celinski K, Gach T, Kulig J, et al: Underexpression of LATS1 TSG in
colorectal cancer is associated with promoter hypermethylation.
World J Gastroenterol. 19:4363–4373. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wierzbicki PM, Klacz J, Rybarczyk A,
Slebioda T, Stanislawowski M, Wronska A, Kowalczyk A, Matuszewski M
and Kmiec Z: Identification of a suitable qPCR reference gene in
metastatic clear cell renal cell carcinoma. Tumour Biol.
35:12473–12487. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ren S, Nguyen L, Wu S, Encinas C, Adams JS
and Hewison M: Alternative splicing of vitamin D-24-hydroxylase: A
novel mechanism for the regulation of extrarenal
1,25-dihydroxyvitamin D synthesis. J Biol Chem. 280:20604–20611.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Binderup L: Comparison of calcipotriol
with selected metabolites and analogues of vitamin D3: Effects on
cell growth regulation in vitro and calcium metabolism in vivo.
Pharmacol Toxicol. 72:240–244. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ieta K, Tanaka F, Haraguchi N, Kita Y,
Sakashita H, Mimori K, Matsumoto T, Inoue H, Kuwano H and Mori M:
Biological and genetic characteristics of tumor-initiating cells in
colon cancer. Ann Surg Oncol. 15:638–648. 2008. View Article : Google Scholar
|
|
53
|
Tuohimaa P, Wang JH, Khan S, Kuuslahti M,
Qian K, Manninen T, Auvinen P, Vihinen M and Lou YR: Gene
expression profiles in human and mouse primary cells provide new
insights into the differential actions of vitamin D3 metabolites.
PLoS One. 8:e753382013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Benton G, Arnaoutova I, George J, Kleinman
HK and Koblinski J: Matrigel: From discovery and ECM mimicry to
assays and models for cancer research. Adv Drug Deliv Rev.
79–80:3–18. 2014. View Article : Google Scholar
|
|
55
|
Evans SR, Schwartz AM, Shchepotin EI,
Uskokovic M and Shchepotin IB: Growth inhibitory effects of
1,25-dihydroxyvitamin D3 and its synthetic analogue,
1α,25-dihydroxy-16-ene-23yne-26,27-hexafluoro-19-nor-cholecalcifero
l (Ro 25-6760), on a human colon cancer xenograft. Clin Cancer Res.
4:2869–2876. 1998.PubMed/NCBI
|
|
56
|
Cross HS, Bises G, Lechner D, Manhardt T
and Kállay E: The Vitamin D endocrine system of the gut - its
possible role in colorectal cancer prevention. J Steroid Biochem
Mol Biol. 97:121–128. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Milczarek M, Filip-Psurska B, Swiętnicki
W, Kutner A and Wietrzyk J: Vitamin D analogs combined with
5-fluorouracil in human HT-29 colon cancer treatment. Oncol Rep.
32:491–504. 2014.PubMed/NCBI
|
|
58
|
Milczarek M, Rosinska S, Psurski M,
Maciejewska M, Kutner A and Wietrzyk J: Combined colonic cancer
treatment with vitamin D analogs and irinotecan or oxaliplatin.
Anticancer Res. 33:433–444. 2013.PubMed/NCBI
|
|
59
|
Tanaka Y, Bush KK, Klauck TM and Higgins
PJ: Enhancement of butyrate-induced differentiation of HT-29 human
colon carcinoma cells by 1,25-dihydroxyvitamin D3. Biochem
Pharmacol. 38:3859–3865. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cross HS, Farsoudi KH and Peterlik M:
Growth inhibition of human colon adenocarcinoma-derived Caco-2
cells by 1,25-dihydroxyvitamin D3 and two synthetic analogs:
Relation to in vitro hypercalcemic potential. Naunyn Schmiedebergs
Arch Pharmacol. 347:105–110. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Höbaus J, Fetahu IS, Khorchide M, Manhardt
T and Kallay E: Epigenetic regulation of the 1,25-dihydroxyvitamin
D3 24-hydroxylase (CYP24A1) in colon cancer cells. J
Steroid Biochem Mol Biol. 136:296–299. 2013. View Article : Google Scholar
|
|
62
|
Mordan-McCombs S, Valrance M, Zinser G,
Tenniswood M and Welsh J: Calcium, vitamin D and the vitamin D
receptor: Impact on prostate and breast cancer in preclinical
models. Nutr Rev. 65:S131–S133. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Peleg S and Nguyen CV: The importance of
nuclear import in protection of the vitamin D receptor from
polyubiquitination and proteasome-mediated degradation. J Cell
Biochem. 110:926–934. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zgaga L, Theodoratou E, Farrington SM, Din
FV, Ooi LY, Glodzik D, Johnston S, Tenesa A, Campbell H and Dunlop
MG: Plasma vitamin D concentration influences survival outcome
after a diagnosis of colorectal cancer. J Clin Oncol. 32:2430–2439.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Köstner K, Denzer N, Müller CS, Klein R,
Tilgen W and Reichrath J: The relevance of vitamin D receptor (VDR)
gene polymorphisms for cancer: A review of the literature.
Anticancer Res. 29:3511–3536. 2009.PubMed/NCBI
|
|
66
|
Laczmanska I, Laczmanski L, Bebenek M,
Karpinski P, Czemarmazowicz H, Ramsey D, Milewicz A and Sasiadek
MM: Vitamin D receptor gene polymorphisms in relation to the risk
of colorectal cancer in the Polish population. Tumour Biol.
35:12397–12401. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Vandewalle B, Adenis A, Hornez L,
Revillion F and Lefebvre J: 1,25-dihydroxyvitamin D3
receptors in normal and malignant human colorectal tissues. Cancer
Lett. 86:67–73. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Brożyna AA, Jozwicki W, Janjetovic Z and
Slominski AT: Expression of vitamin D receptor decreases during
progression of pigmented skin lesions. Hum Pathol. 42:618–631.
2011. View Article : Google Scholar
|
|
69
|
Brożyna AA, Jóźwicki W and Slominski AT:
Decreased VDR expression in cutaneous melanomas as marker of tumor
progression: New data and analyses. Anticancer Res. 34:2735–2743.
2014.
|
|
70
|
Reichrath J, Rech M, Moeini M, Meese E,
Tilgen W and Seifert M: In vitro comparison of the vitamin D
endocrine system in 1,25(OH)2D3-responsive
and -resistant melanoma cells. Cancer Biol Ther. 6:48–55. 2007.
View Article : Google Scholar
|
|
71
|
Albertson DG, Ylstra B, Segraves R,
Collins C, Dairkee SH, Kowbel D, Kuo WL, Gray JW and Pinkel D:
Quantitative mapping of amplicon structure by array CGH identifies
CYP24 as a candidate oncogene. Nat Genet. 25:144–146. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Brożyna AA, Jochymski C, Janjetovic Z,
Jóźwicki W, Tuckey RC and Slominski AT: CYP24A1 expression
inversely correlates with melanoma progression: Clinic-pathological
studies. Int J Mol Sci. 15:19000–19017. 2014. View Article : Google Scholar
|
|
73
|
Flanagan JN, Young MV, Persons KS, Wang L,
Mathieu JS, Whitlatch LW, Holick MF and Chen TC: Vitamin D
metabolism in human prostate cells: Implications for prostate
cancer chemoprevention by vitamin D. Anticancer Res. 26:2567–2572.
2006.PubMed/NCBI
|
|
74
|
Zehnder D, Bland R, Williams MC, McNinch
RW, Howie AJ, Stewart PM and Hewison M: Extrarenal expression of
25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol
Metab. 86:888–894. 2001.PubMed/NCBI
|
|
75
|
Cross HS, Bareis P, Hofer H, Bischof MG,
Bajna E, Kriwanek S, Bonner E and Peterlik M: 25-Hydroxyvitamin
D3-1alpha-hydroxylase and vitamin D receptor gene
expression in human colonic mucosa is elevated during early
cancerogenesis. Steroids. 66:287–292. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Bareis P, Bises G, Bischof MG, Cross HS
and Peterlik M: 25-hydroxy-vitamin d metabolism in human colon
cancer cells during tumor progression. Biochem Biophys Res Commun.
285:1012–1017. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Bises G, Kállay E, Weiland T, Wrba F,
Wenzl E, Bonner E, Kriwanek S, Obrist P and Cross HS:
25-hydroxyvitamin D3-1alpha-hydroxylase expression in
normal and malignant human colon. J Histochem Cytochem. 52:985–989.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Brożyna AA, Jóźwicki W, Jochymski C and
Slominski AT: Decreased expression of CYP27B1 correlates with the
increased aggressiveness of ovarian carcinomas. Oncol Rep.
33:599–606. 2015.
|
|
79
|
Brożyna AA, Jóźwicki W, Janjetovic Z and
Slominski AT: Expression of the vitamin D-activating enzyme
1α-hydroxylase (CYP27B1) decreases during melanoma progression. Hum
Pathol. 44:374–387. 2013. View Article : Google Scholar
|
|
80
|
Brown AJ and Slatopolsky E: Vitamin D
analogs: Therapeutic applications and mechanisms for selectivity.
Mol Aspects Med. 29:433–452. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Kim TK, Wang J, Janjetovic Z, Chen J,
Tuckey RC, Nguyen MN, Tang EK, Miller D, Li W and Slominski AT:
Correlation between secosteroid-induced vitamin D receptor activity
in melanoma cells and computer-modeled receptor binding strength.
Mol Cell Endocrinol. 361:143–152. 2012. View Article : Google Scholar : PubMed/NCBI
|