Introduction
Gingival squamous cell carcinoma (GSCC) is a rare
tumor comprising <10% of all head and neck squamous cell
carcinomas (1,2). It may occur in either the mandible or
maxilla (3). This type of cancer
typically resembles common periodontal lesion or inflammatory
lesion and usually results in delayed diagnosis. Multiple
prognostic factors such as tumor size and lymph node metastasis are
associated with GSCC, therefore, tumor-node-metastasis (TNM)
classification is used for staging GSCC. Gingival cancer has high
risk of metastases and consequent death with bone invasion on
high-grade tumors (4). In many
case reports, the deaths associated with GSCC is due to delayed
diagnosis and treatments.
Tannins are polyphenols of plant origin found in
vegetables, fruits, red wine, tea, nuts, beans and coffee. Tannins
are grouped into two major categories as hydrolysable and condensed
tannins. Commercially available tannic acid (TA) includes multiple
gallotannins with galloyl esters. Tea and red wine are rich source
of hydrolysable TA (5). TA shows
anticancer activities and cancer protection activities against a
broad spectrum of cancers, including chemically induced cancers
(6–11).
Evidence suggests that, Janus kinase 2/signal
transducer and activator of transcription 3 (Jak2/STAT3) signaling
pathways are associated with oncogenesis, progression and
metastasis of different cancers. Constitutively active STAT3 is
also observed in various malignant transformations in breast
(12), head and neck (13), skin (14), ovarian (15), brain (16) and prostrate (17). Tannins and TA containing foods are
known to have anticancer activities against breast cancer through
modulating the Jak/STAT pathway (18). We have reported that STAT3
modulates VEGF expression through HIF-1α. Similarly our studies
with MSM show that, inhibition of Jak2/STAT3 pathway can restrict
breast tumor growth and pulmonary metastasis (19). It is also proven that TA has the
ability to inhibit the EGF-receptor (20). STAT3 has the direct transcription
control over many genes including survivin (proliferation), VEGF
(angiogenesis), cyclin D1 (cell cycle), Bcl-XL
(apoptosis). Hence, the inhibition of STAT3 should lead to
induction of apoptosis.
In the present study, we explore the role of TA in
modulating the Jak2/STAT3 pathway. We hypothesize that TA induces
proliferation inhibition and G1 phase inhibition in gingival cancer
cells. In addition, we hypothesized that TA can modulate multiple
molecular targets directly related to the mitochondrial apoptotic
pathway and induces intrinsic apoptosis.
Materials and methods
Antibodies and reagents
Roswell Park Memorial Institute medium-1640
(RPMI-1640), 10% fetal bovine serum (FBS) and trypsin-EDTA were
from Gibco-BRL (Grand Island, NY, USA). Jak2, p-Jak2 (Y1007/1008),
p-STAT3 (Y705) and p-STAT3 (S727) antibodies were from Cell
Signaling (Cell Signaling Technologies, MA, USA). STAT3, Bax,
Bcl-2, Bcl-XL, TATA binding protein (TBP), Caspase-3,
cytochrome c, β-actin antibodies and secondary antibody
(rabbit, goat anti-mouse IgG-horseradish peroxidase) were obtained
from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The enhanced
chemiluminescence plus (ECL Plus) detection kit, RT-PCR Premix
kits, oligo(dT), Bcl-2, Bcl-XL, Bax and 18S primer for
RT-PCR were from Bioneer (Daejeon, Korea). DiOC6 was
from Sigma (St. Louis, MO, USA). Mitochondria isolation kit and
Coomassie (Bradford) protein assay kit were from Thermo Scientific
(Thermo Scientific, MA, USA). Restore™ Western Blot Stripping
Buffer and NE-PER kits were from Pierce (Rockford, IL, USA). RNeasy
mini kit, and the Qiaprep spin miniprep kits were from Qiagen
(Hilden, Germany). The electrophoretic mobility shift assay (EMSA)
kit and oligonucleotide probes (STAT3) were from Panomics (Redwood
City, CA, USA). Vybrant FAM poly-caspases assay kit was from
Molecular probes (Eugene, OR, USA) and CaspGLOW™ fluorescein active
caspase-3 staining kit was from eBioscience (San Diego, CA,
USA).
Cell culture and maintenance
YD-38 cell lines were cultured and maintained in
RPMI-1640 medium containing 10% serum and 1%
penicillin/streptavidine, respectively. Unless otherwise specified,
cells were grown in 10-cm dishes to ~80% confluence before being
placed in serum-free media for 18–24 h. Serum-deprived cells were
treated as specified in the figure legends.
Cell proliferation studies using crystal
violet assay
Cell proliferation was analyzed using the crystal
violet assay. The YD-38 cells were seeded on to 6-well plates and
incubated overnight at ambient condition. After 24-h incubation,
the cells were treated with increasing concentration of TA (20–100
μM) for 24 or 48 h. The cells were washed with PBS and incubated
with crystal violet. Excess amount of crystal violet was washed off
with water and the dye captured by the cells were dissolved using
1% SDS. The final colour formed was measured colorimetrically at
570 nm.
Cell cycle analysis
The DNA content of TA or other chemical combinations
treated and non-treated YD-38 cells were determined by BD Cycletest
Plus DNA reagent kit (BD Biosciences, CA, USA) following the
manufacturer's protocol. Briefly, ~5×105 cells were
induced, or not induced with TA or other chemical combinations for
indicated time. The cells were separated, washed twice with PBS and
permeabilized using trypsin buffer. The RNA interaction with PI was
neutralized by treating the cells with trypsin inhibitor and RNase
buffer. These samples were then stained with propidium iodide for
30 min in the dark and analyzed using FACSCalibur (BD FACSCalibur,
CA, USA).
Measurement of apoptosis
Fluorescein-conjugated Annexin V (Annexin V-FITC)
was used to quantify the percentage of cells undergoing apoptosis.
The necrotic cells were counter stained with propidium iodide (PI).
The cells treated or not were washed twice with cold PBS and
resuspended in the binding buffer at a concentration of
1×106 cells/ml. Five microliters each of Annexin V-FITC
and PI were added to the cell suspension. After incubation at room
temperature in the dark for 15 min, the percentages of apoptotic
cells were analyzed by flow cytometry (BD FACSCalibur). Cells
treated with 10 μM camptothecin served as positive control.
Western blotting
The YD-38 cells were treated with TA for determined
times and lysed on ice with radioimmunoprecipitation assay (RIPA)
lysis buffer, containing 1X BD baculogold protease inhibitor
cocktail (BD Bioscience) and 1X PhosSTOP phosphatase inhibitors
(Roche, NJ, USA). Protein concentrations were detected using the
Bradford method. Equal amounts of protein obtained by total lysis
were separated using SDS-PAGE and blotted onto a nitrocellulose
membrane. Blocking was done with either 5% skim milk or BSA in
TBS-T buffer. The membranes were probed with primary antibodies
followed by specific HRP conjugated secondary antibodies. Antibody
detection was done by using enhanced chemiluminescence (ECL) plus
detection kit.
Semi-quantitative reverse transcription
polymerase chain reaction (RT-PCR)
Total RNA from YD-38 cells were prepared using
RNeasy Mini kit (Quiagen, CA, USA) according to the manufacturer's
instructions. Equal amount of RNA from each sample reverse
transcribed using AccuPower RT-premix kit (Bioneer, Korea) and
oligo(dT) primers. PCR was performed using 2 μl of the reverse
transcription product. The PCR reactions were performed in 25–30
cycles of denaturation 94–95°C, annealing 56–60°C and an extension
of 72°C. The primers used for the amplification are listed in the
Table I. After amplification, the
products were visualized in 1.2% agarose containing ethidium
bromide.
 | Table IRT-PCR primers sequences used for the
amplification of multiple human cDNAs. |
Table I
RT-PCR primers sequences used for the
amplification of multiple human cDNAs.
| Sl no. | Genes | Annealing
temperature (°C) | Product size
(bp) | Sequences
(5′-3′) |
|---|
| 1 | Cyclin D1 | 58 | 135 |
F-gctgcgaagtggaaaccatc
R-cctccttctgcacacatttgaa |
| 2 | CDK-4 | 58 | 541 |
F-ctgagaatggctacctctcgatatg
R-agagtgtaacaaccacgggtgtaag |
| 3 | CDK-6 | 58 | 406 |
F-ccgagtagtgcatcgcgatctaa
R-ctttgcctagttcatcgatatc |
| 4 | 18S | 58 | 490 |
F-cggctaccacatccaaggaa
R-ccggcgtcccctcttaatc |
| 5 |
P16Ink4 | 57 | 138 |
F-agccttcggctgactggctgg
R-ctgcccatcatcatgacctgg |
| 6 |
p21Waf1/Cip1 | 58 | 494 |
F-ttagggcttcctcctggaggagat
R-atgtcagaaccggctggggatgtc |
| 7 |
p27Kip1 | 59 | 428 |
F-cctcttcggcccggtggac
R-tttggggaaccgtctgaaac |
| 8 | Bax | 58 | 155 |
F-cccgagaggtctttttccgag
R-ccagcccatgatggttctgat |
| 9 | Bcl-2 | 60 | 459 |
F-ggtgccacctgtggtccacctg
R-ggtgccacctgtggtccacctg |
| 10 |
Bcl-XL | 60 | 780 |
F-ttggacaatggactggttga
R-gtagagtggatggtcagtg |
Electrophoretic mobility shift assay
(EMSA)
STAT3 DNA binding activity was detected using EMSA
(19). Gingival cancer cells were
grown to ~80% confluence and nuclear protein extracts were prepared
using the Nuclear Extraction kit (Affymetrix, CA, USA). EMSA was
performed with EMSA gel shift kit (Panomics) according to the
manufacturer's protocol. Briefly, the nuclear proteins prepared
were subjected for hybridization with a double-stranded,
biotin-labeled oligonucleotide probe containing the
consensus-binding site for STAT3 (sense strand,
5′-CATGTTATGCATATTCCTGTAAGTG-3′). The protein-DNA complexes were
resolved in a 6% non-denaturing PAGE gel and transferred to Pall
Biodyne B nylon membrane (Pall Life Sciences, NY, USA) and detected
using streptavidin-HRP and a chemiluminescent substrate.
Measurement of mitochondrial membrane
potential (ΔΨm)
Changes of mitochondrial membrane potential (ΔΨm)
were measured by DiOC6 staining method. Briefly,
gingival cancer cell lines treated or not treated with TA were
washed and suspended in 0.1 μM DiOC6 solution. Cells
were then incubated at 37°C for 20 min and washed with pre-warmed
DPBS and analysed using FACSCalibur.
Isolation of mitochondria
Mitochrondria from TA-treated and non-treated cells
were isolated using mitochondria isolation kit (Thermo scientific,
USA) following the manufacturer's protocol. Briefly,
2×106 cells were treated with mitochondria isolation
reagent and incubated on ice. Following incubation, Reagent B and C
were added with mixing and incubation on ice between each addition.
The mixture was centrifuged at 700 × g for 10 min and the
supernatant was collected and re-centrifuged. The supernatant was
collected as the cytosol and the mitochondrial pellet obtained was
washed with Reagent C and used for downstream applications.
Poly-caspase assay
Activation of caspases were studied using Vybrant
FAM poly-caspase assay kit, following the manufacturer's protocol.
Briefly, gingival cancer cells treated or non-treated with TA or
other chemical combinations were suspended at a concentration of
1×106 cells/ml culture media. From this 300 μl was mixed
with 10 μl 30X FLICA and incubated 1 h at 37°C and 5%
CO2. Following this, the cells were washed multiple
times using 1X wash buffer. The cells were then analyzed using
FACSCalibur.
Active caspase-3 analysis
Caspase-3 activation studies using CaspGLOW™
fluorescein active caspase-3 staining kit, following the
manufacturer's protocol. Apoptosis is induced using 60 μM TA. In
order to confirm the role of caspase-3 in TA mediated apoptosis,
the cells were pre-treated with Z-VAD-FMK. Following this, 300 μl
of cell suspension containing 1×106 cells/ml was made
and active caspases-3 stained using FITC-DEVD-FMK. The mixture was
incubated at 37°C with 5% CO2 for 1 h. Then the cells
were washed with 1X wash buffer and subjected for FACS
analysis.
Statistical analysis
All experiments were repeated at least three times
and the results expressed as mean ± SEM. Statistical analysis was
performed with ANOVA and Student's t-test of SAS 9.3
program. One-way analysis of variance (ANOVA) was performed
with Duncan's multiple range test. P<0.05 was considered
statistically significant.
Results
Tannic acid induces proliferation
inhibition in gingival cancer cells
The ability of tannic acid to inhibit the
proliferation of YD-38 cells were studied at concentrations ranging
from 20 to 100 μM. TA inhibited the cell viability of YD-38 cells
with IC50 values ranging from 50 to 70 μM/l for 48-h
treatment (Fig. 1). The effects of
TA on cell viability occurred very slowly. Following 48-h
treatment, 60 μM TA decreased cell viability by 50%. Analysis of
cell viability showed, TA did not induced cell death up to 24 h,
rather, it inhibited the proliferation by inducing cell cycle
arrest.
Tannic acid induces G1/S phase arrest in
gingival cancer cells
Studies conducted to investigate the role of TA on
gingival cancer cell proliferation showed a prominent growth
inhibition (Fig. 1). In order to
uncover the mechanism of growth arrest, gingival cancer cells were
treated with TA at different concentrations (40 and 60 μM).
Following TA treatment, the cells were stained using PI and the
distribution of nucleous analyzed using FACSCalibur. The study
revealed that, in cells treated with 40 μM TA, there is an
accumulation of cells in the G1 phase with a decrease in percentage
of cell population on the G2 phase (Fig. 1B). In case of 60 μM TA treated
cells (61%; ***P<0.001), the percentage accumulation
of cells in the G1 phase was comparatively higher than that of the
40 μM treated cells (51%; **P<0.01), showing a
concentration-dependent G1 phase arrest on the gingival cancer
cells (Fig. 1C). Based on the
ability of this concentration to induce cell cycle arrest, the
concentration was used for the subsequent experiments to elucidate
the signaling events involved in TA mediated G1 arrest.
Tannic acid induces apoptosis in YD-38
cells
We next focused on whether TA has the capacity to
induce apoptosis in gingival cancer cells as it does in breast and
AML cells (21). The study
revealed that, in cells treated with TA, there was an accumulation
of cells in the apoptotic phase (Fig.
1D). In case of 60 μM TA-treated cells (27.30%, Fig. 1E; ***P<0.001), the
percentage of apoptosis was similar to that of the positive
control, camptothecin 10 μM treated cells (27.91%, Fig. 1E; ***P<0.001),
confirming induction of apoptosis in the gingival cancer cells.
Tannic acid inhibits the Jak2/STAT3
pathway
The role of STAT3 in the induction of apoptosis was
reported previously (15). In our
study also, TA inhibited the expression as well as phosphorylation
of STAT3 (Fig. 2A). In the
cytoplasm, STAT3 is phosphorylated by upstream kinases Jak2.
Following phosphorylation it forms homo- or hetero-dimers and
translocate to nucleus there it controls transcriptional functions
of multiple genes. Western blot analysis of whole cell extracts
showed a reduction in tyr705 phosphorylated STAT3 together with
total STAT3. Phosphorylation of STAT3 is primarily relying on Jak2
phosphorylation. Hence the Jak2 (Y1007/Y1008) phosphorylation
levels were checked in these cell lines. As expected, TA regulated
Jak2 phosphorylation (Fig. 2A).
The relative expression analysis showed a concentration-dependent
and significant inhibition on the Jak2, STAT3 expression as well as
their phosphorylation (Fig. 2B;
**P<0.01 and ***P<0.001).
Tannic acid suppresses the
transcriptional functions of STAT3
STAT nuclear translocation and DNA binding
activities are influenced by STAT tyrosine phosphorylation rather
than serine phosphorylation (12,13).
Translocation of initiated STATs to the nucleus follows its binding
to a specific response elements in the target gene promoters, and
transcriptionally activates the genes. As shown in Fig. 2C, there was a decrease in the
nuclear level of pSTAT3 in TA-treated cells, when compared with the
control cells. The transcriptional functions of STAT3 is dependent
on its ability to bind with specific response element in the target
genes. EMSA analysis specific to the STAT3-TF showed a decline in
DNA binding activity upon TA treatment (Fig. 2D).
Tannic acid increases the expression of
p21Waf1/Cip1 and p27Kip
The treatment of gingival cancer cells with TA
induced G1 phase arrest. The inhibition of G1/S phase transition is
primarily dependent on the p21Waf1/Cip1 and
p27Kip levels (22) and
its loss leads to uncontrolled cell proliferation. Our studies
revealed that, treatment with TA intensified the expression of
p21Waf1/Cip1 and p27Kip transcriptionally
(Fig. 3A). The elevation in the
expression followed a dose-depended pattern and after a period of
24 h the increase was significant (Fig. 3B; ***P<0.001).
Moreover, the activation of p21Waf1/Cip1 and
p27Kip showed a statistically significant pattern.
Inhibition of Jak2/STAT3 pathway
suppressed the expression of cyclin D1, cyclin E and CDK-4
Members of cyclins and CDKs are important mediators
of the cell cycle. The translational level expression of cyclin D1,
cyclin E and CDK-4 were concentration-dependently inhibited by TA
(Fig. 3C). The level of CDK-4 was
inhibited at protein level (Fig.
3D; ***P<0.001) but non-significantly inhibited
at transcriptional level (Fig.
3B).
Suppression of STAT3/DNA binding activity
leads to decline of anti-apoptotic gene products
RT-PCR studies were carried out in YD-38 cells
treated with increasing concentrations of TA by random priming of
total RNA. Bcl-2 and Bcl-XL was amplified using gene
specific primers. As TA declined the STAT3 and pSTAT3 levels and
their DNA binding activity, it also downregulated the STAT3 target
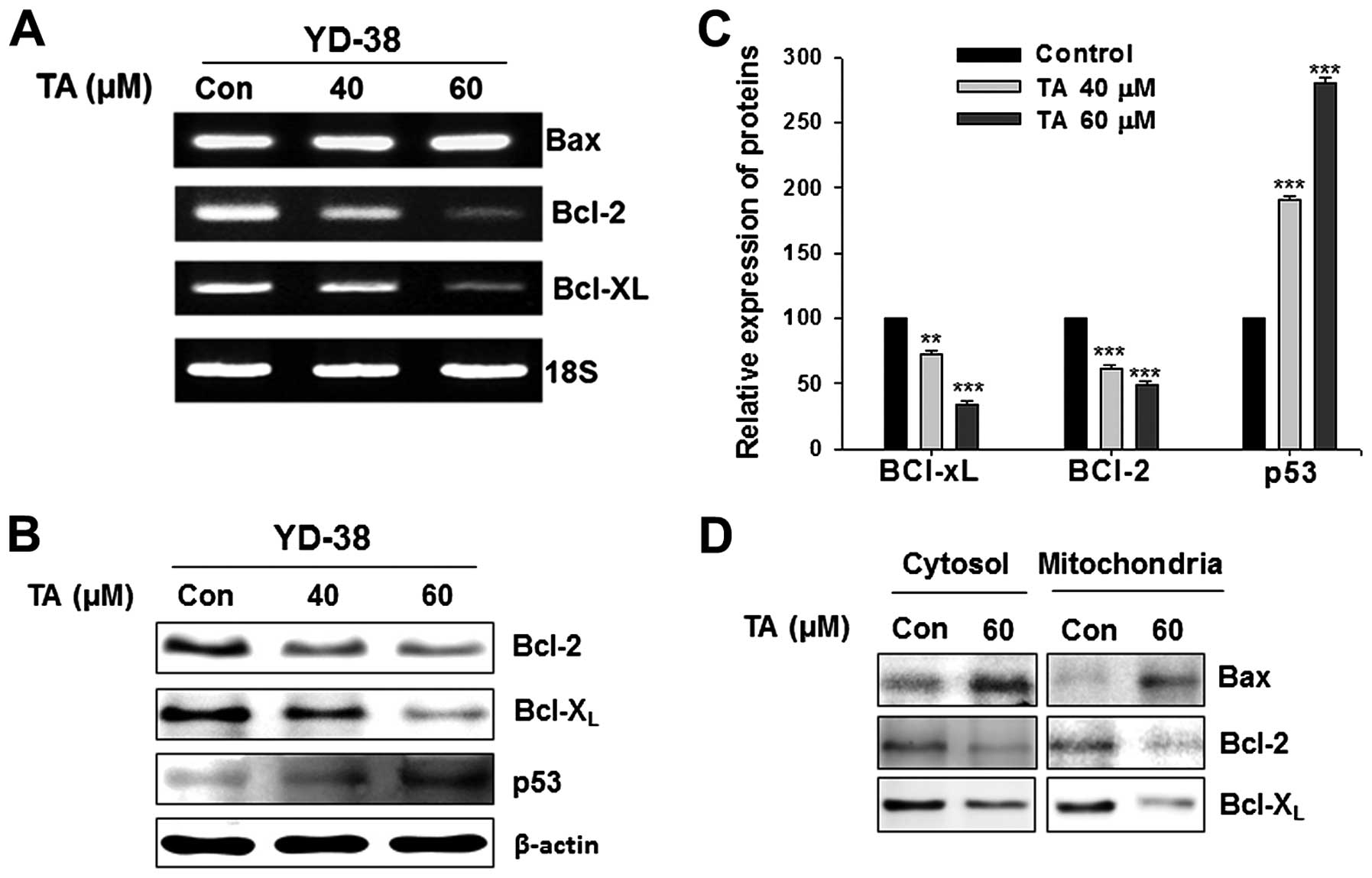
gene products such as Bcl-2, and Bcl-XL (Fig. 4A). By modulating these molecules,
TA can target anti-apoptotic mediators and induce apoptosis.
Tannic acid downregulates the
anti-apoptotic proteins in concentration-dependent manner
Western blotting studies were carried out in YD-38
cells treated with increasing concentrations of TA. The whole cell
lysates were prepared and subjected for the detection of Bcl-2 and
Bcl-XL. TA exposure led to downregulation of STAT3
target gene products, Bcl-2, and Bcl-XL (Fig. 4B). The p53 expression was activated
by the treatment with TA (Fig.
4B). The inhibition of anti-apoptotic genes and activation of
p53 gene were concentration-dependent and statistically significant
(Fig. 4C;
***P<0.001).
Tannic acid inhibits the level of
mitochondrial Bcl-2, Bcl-XL and increases the
mitochondrial localization of Bax
Mitochondria were isolated from the TA-treated and
non-treated gingival cancer cells and the cytosol fraction was
collected. Western blot analysis of the isolated mitochondria
showed inhibition in the level of both Bcl-2 and Bcl-XL
(Fig. 4D). Increase in
mitochondrial localization of Bax was observed with exposure to TA
(Fig. 4D) with increased Bax in
the cytosol. Expression levels of Bax also found increased at
transcriptional level (Fig. 4A).
Similarly, the mitochondrial localization Bcl-2 and
Bcl-XL decreased. Whole cell lysates showed decreased
mitochondrial pore factors confirming our previous findings of TA
on these factors.
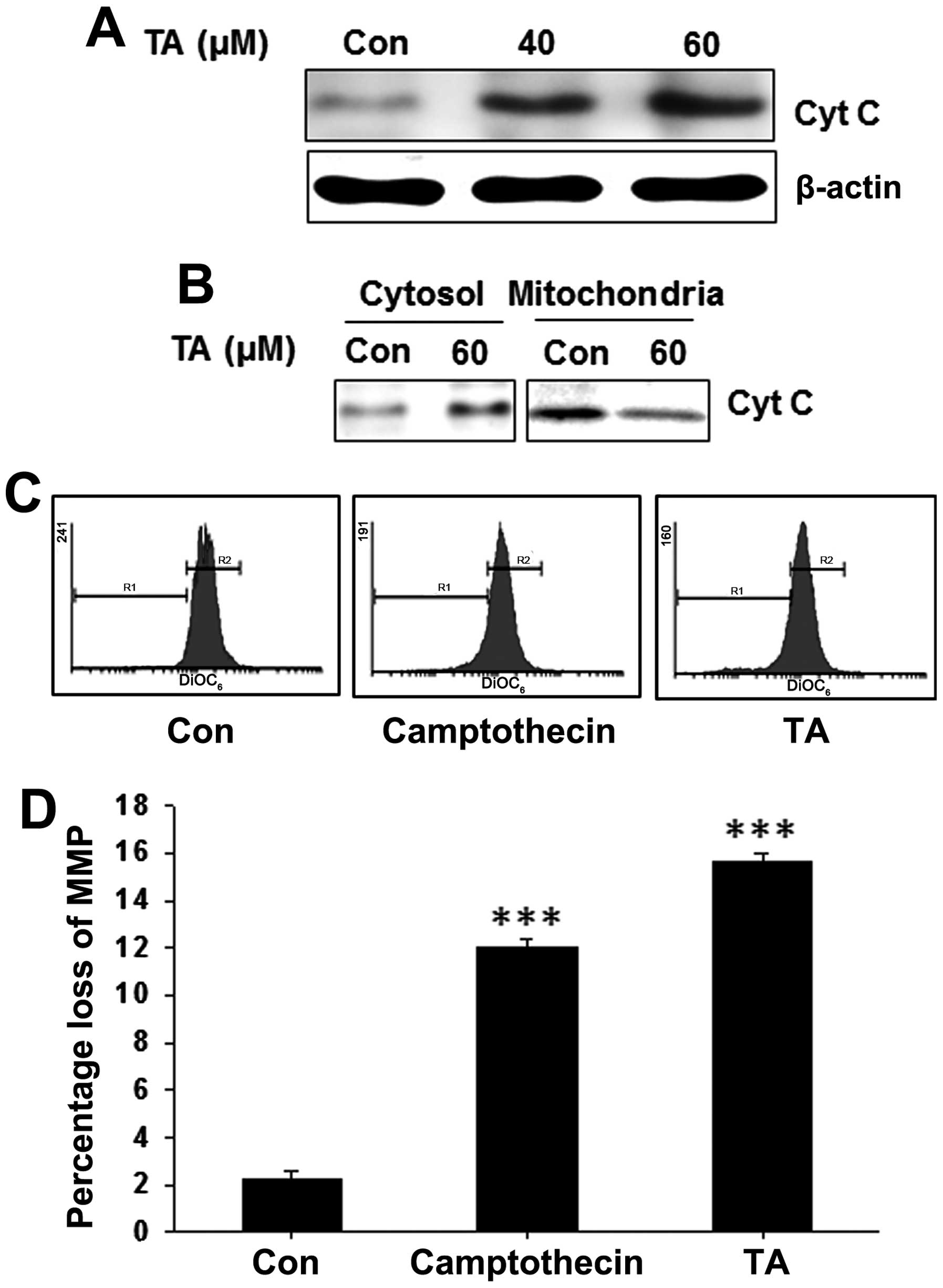
Release of cytochrome c to the cytoplasm
is observed in tannic acid-treated gingival cancer cells
Whole cell lysates were prepared from TA-treated and
non-treated YD-38 cells and subjected for western blot analysis. We
found a concentration-dependent increase on the cytochrome c
level (Fig. 5A). In order to find
the localization of cytochrome c and release of cytochrome
c to the cytosol, mitochondrial and cytosolic fractions of
YD-38 cells were prepared from TA-treated and non-treated cells and
subjected for western blot analysis. In which, we detected a
decrease of cytochrome c in mitochondrial fractions and
increased levels on the cytosolic fractions (Fig. 5B) indicating the release of
cytochrome c to the cytosol and a loss of mitochondrial
membrane potential.
Tannic acid induces loss of mitochondrial
membrane potential (ΔΨm) in gingival cancer cells
The release of cytochrome c from mitochondria
is also usually preceded or accompanied by a reduction in the ΔΨm.
To address whether the TA-induced alteration in pore factors were
associated with the change of ΔΨm, gingival cancer cells were
treated with TA for pre-determined time and stained with
DiOC6 to access ΔΨm (Fig.
5C). Treatment of TA reduced the ΔΨm significantly (Fig. 5D; ***P<0.001),
showing that the mechanism of apoptosis induction by TA was through
the mitochondria-dependent pathway in YD-38 cells.
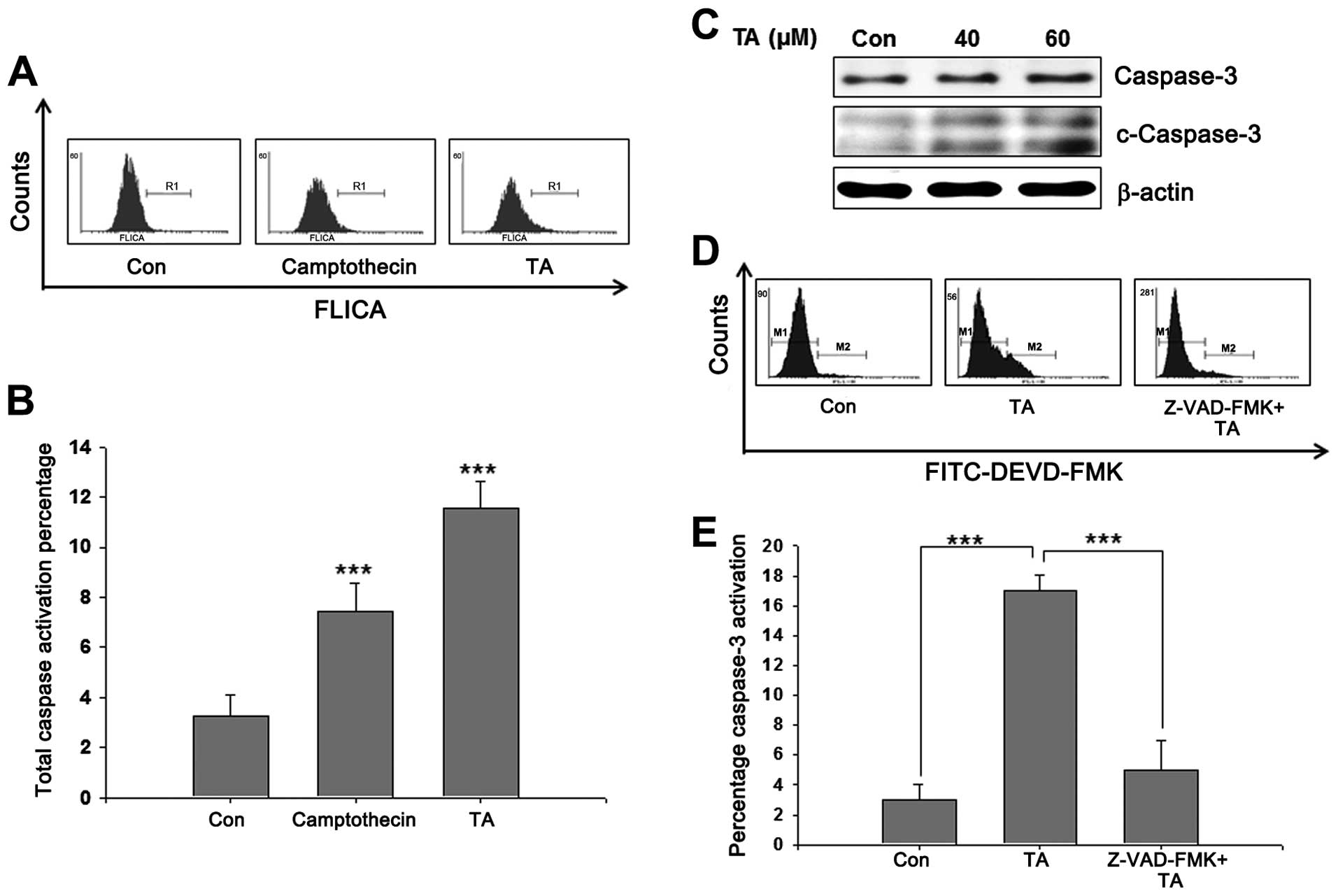
Caspase activation is required for
TA-mediated apoptosis
Mitochondrial apoptotic pathways require active
caspases to ensure apoptosis. Whole caspase activation on TA
challenged cells were analyzed using poly-caspase assay kit, and
showed a prominent increase in caspase activation (Fig. 6A). After observing a significant
increase in whole caspase (Fig.
6B), we analyzed the activation of caspase-3. Western blot
analysis of TA-treated YD-38 cells showed cleaved form of caspase-3
confirming the role of caspases in TA-induced apoptosis (Fig. 6C). In-order to confirm the role of
caspase-3 in TA-induced apoptosis, cells were treated with the
caspase specific inhibitor Z-VAD-FMK prior to TA treatment. The
results showed an increase in active caspase-3 in TA-treated cells
comparing to the non-treated control cells (Fig. 6D). Moreover, inhibition of
caspase-3 prior to TA treatment inhibited apoptosis and caspase-3
activation significantly (Fig.
6E). These data confirmed that TA induces caspase-dependent
apoptosis and activation of caspase-3 is an essential step for
apoptosis induced by TA.
Discussion
Natural compounds are the possible source of
molecules that may have anti-proliferative effects on broad range
of cancers. Multiple natural chemicals are being tested for their
activities on different forms of cancer. Dietary habits and oral
health has direct connection. Foods rich in antioxidants has
multiple therapeutic potential on oral health including, prevention
from inflammation to malignancies through their bio-active,
non-nutrient components (23,24).
Conventional treatment modalities such as chemotherapy, radiation,
surgery and immunotherapy have shown advantages to various extent
in tumor growth retention. However, these modalities can result in
problems like speech impairment, cosmetic issues, and face
deformities.
Cancer cells are usually reported as uncontrolled
cell proliferation occuring as a result of alterations in the
positive and negative regulators of the cell cycle. Similarly
resistance to apoptotic signals cause prolonged lifespan of cancer
cells (25,26). As previously documented,
polyphenols and members of tannin family have the capability to
induce G1 arrest in various cancer cells. Research is being
performed to elucidate the mechanistic aspects of medicinal
properties constituted by TA. TA, a glucoside of gallic acid
polymer, has been shown to possess anti-bacterial, anti-enzymatic,
antitumor and astringent properties. In our study, TA showed
proliferation inhibition capability on YD-38 gingival cancer cells
(Fig. 1A).
Escape from normal apoptotic pathways is a common
phenomenon found in almost all types of cancers. Hence, making the
cells susceptible to apoptosis is a principal approach for
developing drugs against malignancies. Different cytotoxic agents
proved their efficiency in inducing apoptosis and are being used as
chemotherapeutics for the treatment for various human malignancies.
Even though they are effective to an extent, their toxic effect is
associated with side effects. Screening of multiple agents is
taking place to find effective chemotherapeutic agents with the
ability to control cell proliferation without side effects. TA has
been shown to have anticancer properties by inducing apoptosis and
controlling the cancer cell proliferation (21,27).
Previous studies suggest that TA has properties such as the
inhibition of CXCL12 (SDF-1α)/and CXCR4 (28).
In the present study, TA inhibited the
phosphorylation of Jak2 (Fig. 2A).
Jak2 is the major upstream regulator of STAT3 phosphorylation
(29). Inhibition of Jak2
phosphorylation resulted in inhibition of STAT3 phosphorylation.
Generally, STAT3 is phosphorylated on S727 and Y705 residues. Thus,
Y705 is responsible for the nuclear translocation and DNA binding
activities of STAT3 (30). TA
inhibited the phosphorylation of Y705 residues in STAT3. Analysis
of nuclear extracts also confirmed the inhibition of STAT3 nuclear
translocation. Gel shift analysis showed that the DNA binding
activity of STAT3 also inhibited by TA treatment (Fig. 2D). One of the major functions of
STAT3 is to bind to its downstream target genes and
transcriptionally activate them. Transcriptional analysis of STAT3
downstream targets such as cyclin D1, Bcl-2, and Bcl-XL
confirmed the ability of TA in inhibiting transcriptional
activation of STAT3. These targets are directly connected with cell
cycle arrest as well as apoptosis, showing the connection between
inhibition of STAT3 and induction of proliferation regulation.
In nearly all mammalian cells, proliferation is
mainly controlled in G1 phase and it automatically progress through
the remaining phases (31). It is
reported that, G1 arrest is p53-dependent (32). In support of this, our study also
showed an increase in the translational level of p53. Most of the
anticancer agents induced G1 arrest through decreasing the activity
of CDKs and increasing the expression of CKIs (33–35).
In the present study, using TA an inhibition on the positive
regulators of cell cycle, cyclin D1, cyclin E and CDK-4 were shown.
Moreover, it transcriptionally activated the negative regulators of
the cell cycle, p21Waf1/Cip1 and p27Kip1.
Which induced a prominent G1 arrest in TA-treated YD-38 cells
(Fig. 1B).
Apoptosis occurs through different mechanisms, in
the extrinsic pathway; an external signal stimulates the apoptotic
cascade and in the intrinsic pathway, intracellular factors trigger
the apoptotic cascade (36). The
intrinsic pathway is usually under the control of the mitochondria,
and is also known as mitochondrial pathway. The role of Bcl-2 and
Bcl-XL on TA induced apoptosis was confirmed by analysis
of mitochondrial protein levels. In mitochondria, Bcl-2 and
Bcl-XL act as anti-pore factors and inhibit the release
of cytochrome c to the cytosol and inhibit apoptosis
(37,38). Mitochondria isolated from
TA-treated cells showed a reduction on both Bcl-2 and
Bcl-XL pointing to the loss of pore closing factors. It
was previously reported that TA has the capacity to increase the
expression of Bax (39). In our
study we observed that, Bax is highly expressed and localized on
the mitochondria. Changes in the localization of mitochondrial pore
and anti-pore factors lead to the loss of mitochondrial membrane
potential (ΔΨm). Any alteration in ΔΨm leads to the activation of
mitochondrial apoptotic pathway through the release of cytochrome
c to the cytosol (Fig.
5).
Cytochrome c is an activator of zymogenic
caspases. Once the cytochrome c is released to the cytosol,
it activates the pro-caspase to active caspase (40). Fig.
6A shows the activation of poly caspases. Apoptosis is usually
carried out with the activation of the effector caspase-3. Western
blot analysis of TA-treated cells also show the cleaved forms of
caspase-3 (Fig. 6B). Inhibition of
caspase-3 using a specific inhibitor showed a significant recovery
from TA-induced apoptosis (Fig.
6E). This confirmed the caspase-dependent mitochondrial pathway
in the TA mediated apoptosis.
According to the present study, TA induces G1 arrest
and apoptosis in human gingival cancer cells. The mechanistic
aspects of TA mediated apoptosis depend primarily on the inhibition
of the Jak2/STAT3 pathway. Here we report that the Jak2/STAT3
pathway is involved in the cell cycle arrest and the intrinsic
(mitochondrial) apoptotic pathway. We suggest the use of TA and
other drugs targeting STAT3 as a trial drug for inducing G1 arrest
and intrinsic apoptosis.
Acknowledgements
This study was supported by Konkuk University in
2014.
References
|
1
|
Gomez D, Faucher A, Picot V, Siberchicot
F, Renaud-Salis JL, Bussières E and Pinsolle J: Outcome of squamous
cell carcinoma of the gingiva: a follow-up study of 83 cases. J
Craniomaxillofac Surg. 28:331–335. 2000. View Article : Google Scholar
|
|
2
|
Yokoo S, Umeda M, Komatsubara H, Shibuya Y
and Komori T: Evaluation of T-classifications of upper gingival and
hard palate carcinomas - a proposition for new criterion of T4.
Oral Oncol. 38:378–382. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torabinejad M and Rick GM: Squamous cell
carcinoma of the gingiva. J Am Dent Assoc. 100:870–872. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pathak KA, Mathur N, Talole S, Deshpande
MS, Chaturvedi P, Pai PS, Chaukar DA and D'Cruz AK: Squamous cell
carcinoma of the superior gingival-buccal complex. Oral Oncol.
43:774–779. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bian Y, Masuda A, Matsuura T, Ito M,
Okushin K, Engel AG and Ohno K: Tannic acid facilitates expression
of the polypyrimi-dine tract binding protein and alleviates
deleterious inclusion of CHRNA1 exon P3A due to an hnRNP
H-disrupting mutation in congenital myasthenic syndrome. Hum Mol
Genet. 18:1229–1237. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Naus PJ, Henson R, Bleeker G, Wehbe H,
Meng F and Patel T: Tannic acid synergizes the cytotoxicity of
chemotherapeutic drugs in human cholangiocarcinoma by modulating
drug efflux pathways. J Hepatol. 46:222–229. 2007. View Article : Google Scholar
|
|
7
|
Koide T, Kamei H, Hashimoto Y, Kojima T
and Hasegawa M: Tannic acid raises survival rate of mice bearing
syngeneic tumors. Cancer Biother Radiopharm. 14:231–234. 1999.
View Article : Google Scholar
|
|
8
|
Gali-Muhtasib HU, Yamout SZ and Sidani MM:
Tannins protect against skin tumor promotion induced by
ultraviolet-B radiation in hairless mice. Nutr Cancer. 37:73–77.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nepka C, Sivridis E, Antonoglou O,
Kortsaris A, Georgellis A, Taitzoglou I, Hytiroglou P,
Papadimitriou C, Zintzaras I and Kouretas D: Chemopreventive
activity of very low dose dietary tannic acid administration in
hepatoma bearing C3H male mice. Cancer Lett. 141:57–62. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gali HU, Perchellet EM and Perchellet JP:
Inhibition of tumor promoter-induced ornithine decarboxylase
activity by tannic acid and other polyphenols in mouse epidermis in
vivo. Cancer Res. 51:2820–2825. 1991.PubMed/NCBI
|
|
11
|
Tikoo K, Bhatt DK, Gaikwad AB, Sharma V
and Kabra DG: Differential effects of tannic acid on cisplatin
induced nephrotoxicity in rats. FEBS Lett. 581:2027–2035. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Garcia R, Bowman TL, Niu G, Yu H, Minton
S, Muro-Cacho CA, Cox CE, Falcone R, Fairclough R, Parsons S, et
al: Constitutive activation of Stat3 by the Src and JAK tyrosine
kinases participates in growth regulation of human breast carcinoma
cells. Oncogene. 20:2499–2513. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song JI and Grandis JR: STAT signaling in
head and neck cancer. Oncogene. 19:2489–2495. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pedranzini L, Leitch A and Bromberg J:
Stat3 is required for the development of skin cancer. J Clin
Invest. 114:619–622. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Burke WM, Jin X, Lin HJ, Huang M, Liu R,
Reynolds RK and Lin J: Inhibition of constitutively active Stat3
suppresses growth of human ovarian and breast cancer cells.
Oncogene. 20:7925–7934. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schaefer LK, Ren Z, Fuller GN and Schaefer
TS: Constitutive activation of Stat3α in brain tumors: Localization
to tumor endothelial cells and activation by the endothelial
tyrosine kinase receptor (VEGFR-2). Oncogene. 21:2058–2065. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin J, Tang H, Jin X, Jia G and Hsieh JT:
p53 regulates Stat3 phosphorylation and DNA binding activity in
human prostate cancer cells expressing constitutively active Stat3.
Oncogene. 21:3082–3088. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park JH, Darvin P, Lim EJ, Joung YH, Hong
DY, Park EU, Park SH, Choi SK, Moon ES, Cho BW, et al:
Hwanggeumchal sorghum induces cell cycle arrest, and suppresses
tumor growth and metastasis through Jak2/STAT pathways in breast
cancer xenografts. PLoS One. 7:e405312012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lim EJ, Hong DY, Park JH, Joung YH, Darvin
P, Kim SY, Na YM, Hwang TS, Ye SK, Moon ES, et al:
Methylsulfonylmethane suppresses breast cancer growth by
down-regulating STAT3 and STAT5b pathways. PLoS One. 7:e333612012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang EB, Wei L, Zhang K, Chen YZ and Chen
WN: Tannic acid, a potent inhibitor of epidermal growth factor
receptor tyrosine kinase. J Biochem. 139:495–502. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen K-S, Hsiao Y-C, Kuo D-Y, Chou MC, Chu
SC, Hsieh YS and Lin TH: Tannic acid-induced apoptosis and
-enhanced sensitivity to arsenic trioxide in human leukemia HL-60
cells. Leuk Res. 33:297–307. 2009. View Article : Google Scholar
|
|
22
|
Pavletich NP: Mechanisms of
cyclin-dependent kinase regulation: Structures of Cdks, their
cyclin activators, and Cip and INK4 inhibitors. J Mol Biol.
287:821–828. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu RH: Potential synergy of
phytochemicals in cancer prevention: Mechanism of action. J Nutr.
134(Suppl): S3479–S3485. 2004.
|
|
24
|
Venugopal R and Liu RH: Phytochemicals in
diets for breast cancer prevention: The importance of resveratrol
and ursolic acid. Food Sci Hum Wellness. 1:1–13. 2012. View Article : Google Scholar
|
|
25
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cosan D1, Soyocak A, Basaran A, Degirmenci
I and Gunes HV: The effects of resveratrol and tannic acid on
apoptosis in colon adenocarcinoma cell line. Saudi Med J.
30:191–195. 2009.PubMed/NCBI
|
|
28
|
Chen X, Beutler JA, McCloud TG, Loehfelm
A, Yang L, Dong HF, Chertov OY, Salcedo R, Oppenheim JJ and Howard
OM: Tannic acid is an inhibitor of CXCL12 (SDF-1α)/CXCR4 with
antiangiogenic activity. Clin Cancer Res. 9:3115–3123.
2003.PubMed/NCBI
|
|
29
|
Imada K and Leonard WJ: The Jak-STAT
pathway. Mol Immunol. 37:1–11. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bromberg JF, Wrzeszczynska MH, Devgan G,
Zhao Y, Pestell RG, Albanese C and Darnell JE Jr: Stat3 as an
oncogene. Cell. 98:295–303. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pardee AB: G1 events and regulation of
cell proliferation. Science. 246:603–608. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang X, Bai H, Zhang X, Liu J, Cao P, Liao
N, Zhang W, Wang Z and Hai C: Inhibitory effect of oleanolic acid
on hepatocellular carcinoma via ERK-p53-mediated cell cycle arrest
and mitochondrial-dependent apoptosis. Carcinogenesis.
34:1323–1330. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hong C, Kim H-A, Firestone GL and
Bjeldanes LF: 3,3′-Diindolylmethane (DIM) induces a G (1) cell
cycle arrest in human breast cancer cells that is accompanied by
Sp1-mediated activation of p21 (WAF1/CIP1) expression.
Carcinogenesis. 23:1297–1305. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yokota T, Matsuzaki Y, Koyama M, Hitomi T,
Kawanaka M, Enoki-Konishi M, Okuyama Y, Takayasu J, Nishino H,
Nishikawa A, et al: Sesamin, a lignan of sesame, down-regulates
cyclin D1 protein expression in human tumor cells. Cancer Sci.
98:1447–1453. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Garcia HH, Brar GA, Nguyen DHH, Bjeldanes
LF and Firestone GL: Indole-3-carbinol (I3C) inhibits
cyclin-dependent kinase-2 function in human breast cancer cells by
regulating the size distribution, associated cyclin E forms, and
subcellular localization of the CDK2 protein complex. J Biol Chem.
280:8756–8764. 2005. View Article : Google Scholar
|
|
36
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gross A, McDonnell JM and Korsmeyer SJ:
BCL-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen YB, Aon MA, Hsu Y-T, Soane L, Teng X,
McCaffery JM, Cheng WC, Qi B, Li H, Alavian KN, et al:
Bcl-xL regulates mitochondrial energetics by stabilizing
the inner membrane potential. J Cell Biol. 195:263–276. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nam S, Smith DM and Dou QP: Tannic acid
potently inhibits tumor cell proteasome activity, increases p27 and
Bax expression, and induces G1 arrest and apoptosis. Cancer
Epidemiol Biomarkers Prev. 10:1083–1088. 2001.PubMed/NCBI
|
|
40
|
Jiang X and Wang X: Cytochrome c promotes
caspase-9 activation by inducing nucleotide binding to Apaf-1. J
Biol Chem. 275:31199–31203. 2000. View Article : Google Scholar : PubMed/NCBI
|