Introduction
Primary brain tumors have differential growth
abilities that can affect the survival rates achieved by the
current anticancer treatments. For example, oligodendroglioma is
characterized by a slow growth rate and a prolonged median survival
of 11.6 years for grade II disease and 3.5 years for grade III
disease (1). Neuroblastoma (NB),
which is most common in children, has a median survival of ~27
months for stage IV disease (2).
The most common brain tumor, glioblastoma multiforme (GBM), is
characterized by aggressive characteristics and rapid growth rate
(3). The median survival of GBM
patients is ~1 year despite the use of treatment strategies such as
chemotherapy, radiotherapy and surgery (4). GBM is derived from astrocytes and has
a poorer prognosis than other brain tumors due to its more rapid
growth rate. We hypothesized that innate characteristics of
astrocytes could be related to the progression of GBM.
In astrocytes, the metabolic flow of lipolysis and
glycolysis is critical for the production of ATP and the
maintenance of neuronal activity. Unlike neurons, astrocytes favor
the production of lactate by aerobic glycolysis even in the absence
of oxygen restriction (called the Warburg effect), while also
efficiently producing ATP by mitochondrial respiration (called the
Pasteur effect) (5). The metabolic
capacity of astrocytes to produce lactate provides critical
metabolic support to neurons (6).
The transport of lactate through astrocytic monocarboxylate
transporter (MCT) 4 has been shown to induce long-term potentiation
and enhance learning and memory in the hippocampus (7). Primary cultured astrocytes isolated
from mouse, release lactate to extracellular space (8) and L-lactate uptake to primary
cultured neurons in embryonic rat (9). Pyruvate, produced from the oxidation
of transported lactate from astrocytes acts as an energy source for
mitochondrial ATP production in neurons. It is proved that pyruvate
dehydrogenase which convert from pyruvate to acetyl coenzyme A has
shown strong immunoreactivity in neuronal cell body of the rat
(10). Thus, the existing evidence
suggests that lactate is involved in increasing growth by acting as
an energy source, and supporting long-term memory formation by
being shuttled between neurons and astrocytes. However, innate
astrocytic metabolism could have a detrimental effect on the brain
in the context of tumor formation, as the glycolytic and lipolytic
fluxes in metabolism could support tumor cell proliferation.
Lactate dehydrogenase (LDH) catalyzes the
interconversion between pyruvate and lactate. LDH-A, which prefer
to catalyze the conversion of pyruvate to lactate, elevates the
glycolytic rate in lactate-producing tissues (11). LDH-B, which prefer to catalyze the
conversion of lactate to pyruvate, allows lactate to be used as an
energy source for oxidative metabolism in aerobic tissues (11). LDH-A expresses primarily in
astrocytes and LDH-B expresses in both astrocytes and neurons from
hippocampus and occipital cortex of human (12). In tumor progression, high-level of
LDH was reported in patients with GBM (13) and it associated with metastasis and
survival in patients with brain tumor treated by radiotherapy
(14). Thus, we hypothesized that
the differential growth rate between GBM and NB are derived from
high expression of inherent LDH. The results of our study could
contribute to the improving therapeutic strategies against GBM by
clarifying the metabolic differences between astrocytes and
neurons.
Materials and methods
Chemicals, reagents and antibodies
Oligomycin (O4876), carbonyl cyanide
m-chlorophenyl hydrazone (CCCP, C2759) and rotenone (R8875)
were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Trihydrochloride and TRIzol were purchased from Invitrogen
(Camarillo, CA, USA). Anti-LDH-A (rabbit polyclonal, NBP1-48336)
and -LDH-B (rabbit monoclonal, EP1566Y) antibodies were purchased
from Novus (Littleton, CO, USA). Anti-GFAP (rabbit polyclonal,
ab7260) and -NeuN (mouse monoclonal, ab104224) antibodies were
purchased from Abcam (Cambridge, MA, USA). The Cell Counting Kit-8
(CCK8) was purchased from Dojindo (Rockville, MD, USA).
Cell culture
Human glioblastoma U87MG cells and
neuroblastoma SH-SY5Y cells
U87MG cells and SH-SY5Y cells were purchased from
the Korean Cell Line Bank (Seoul National University, Seoul, Korea)
and cultured in minimum essential medium (MEM; Welgene, Dalseo-gu,
Daegu, Korea) supplemented with 10% fetal bovine serum (Invitrogen)
and 1% penicillin/streptomycin (Invitrogen) at 37°C in a humidified
atmosphere of 95% air and 5% CO2.
Primary cortical astrocytes
Primary cortical astrocytes were prepared from
postnatal 0–3 days P0–P3 C57BL/6 mice, as previously described
(15). The cerebral cortex was
dissected, freed from adherent meninges, and minced and triturated
into a single-cell suspension. All experimental procedures were
performed in accordance with the institutional guidelines of
Chungnam National University School of Medicine (CNU, Daejeon,
Korea). Cells were grown in Dulbecco's modified Eagle's medium
(DMEM, Invitrogen) supplemented with 25 mM glucose, 10%
heat-inactivated horse serum, 10% heat-inactivated fetal bovine
serum, 2 mM glutamine and 1,000 U/ml penicillin-streptomycin.
Cultures were maintained at 37°C in a humidified 5% CO2
incubator. On the 3rd day of culture, cells were vigorously washed
with repeated pipetting, and the medium was replaced. The next day
(4th day of culture), cells were plated to coverslips
(1×104 per coverslip) coated with 0.1 mg/ml
poly-D-lysine (PDL).
Primary cortical neurons
The cerebral cortex was extracted from embryonic
14–15-day C57BL/6 mice, dispersed by trypsin and DNase I in Hank's
balanced salt solution (HBSS), and then suspended in neurobasal
media (Invitrogen) with 2% B27 supplement, 2 mM L-glutamine and 1%
penicillin-streptomycin. The cells were then filtered, transferred
to 8-well culture dishes coated with polyethyleneimine
(1×105 cells/ml) in medium, and 1 μM astrocyte
inhibitor, cytosine-1-β-D-arabinofuranoside (Sigma), treated after
2 days for purity of primary cultured neurons.
Measurement of cell growth rate
Cells were seeded to 96-well cell culture plates at
2×103 (for U87MG) and 8×103 (for SH-SY5Y)
cells in 0.1 ml growth media. Cell viability was measured after 12,
24, 48 and 72 h using a CCK-8 kit, a sensitive colorimetric assay
for determining the number of viable cells. In this method,
cellular dehydrogenases reduce WST-8 to yield an orange-colored
product (formazan) that is soluble in the tissue culture medium.
The amount of generated formazan dye is directly proportional to
the number of living cells. Absorbance was measured at 450 nm using
a Multiskan Ascent microplate spectrophotometer (Thermo Fisher
Scientific, Waltham, MA, USA).
Measurement of cellular oxygen
consumption rate (OCR) and extracellular acidification rate
(ECAR)
OCR and ECAR were measured using a Seahorse
Bioscience XF24 analyzer (Seahorse Bioscience, North Billerica, MA,
USA). The XF24 biosensor cartridge was activated using 1 ml of XF24
calibration buffer per well at 37°C with no CO2. U87MG
and SH-SY5Y cells were seeded to the XF24 cell culture microplates
at 2×104 and 4×104 cells per well,
respectively, in 0.1 ml growth media. After 1 day, the medium was
replaced with differentiation media, cells were incubated at 37°C
with no CO2 for ≥1 h, and measurements were performed.
The ports of the XF24 biosensor cartridge were filled with 20 μg/ml
oligomycin (an ATPase inhibitor), 50 μM CCCP (an uncoupler) and 20
μM rotenone (a mitochondrial complex I inhibitor) loaded together
into each well, and the XF24 analyzer was operated under the
manufacturer's basal protocol at 37°C.
Real-time polymerase chain reaction (PCR)
analysis
Total RNA was isolated using TRIzol according to the
manufacturer's instructions, and real-time quantitative PCR was
performed using cDNA, SYBR Green PCR Master Mix (iCycleriQ
Real-Time PCR Detection System; Bio-Rad, Hercules, CA, USA), and
the following specific primers: LDH-A F (5′-CTC TGA AGA CTC
TGC ACC CA-3′) and LDH-A R (5′-GCA CCC GCC TAA GAT TCT
TC-3′); PC F (5′-GCC TGG GAA GGT GAT AGA CA-3′) and
PC R (5′-TCC AGT GTC ATG TCC TTG GT-3′); SDH F
(5′-CAA CAC TCT AGC TTG CAC CC-3′) and SDH R (5′-GTA GAG CCC
GTC CAG TTT CT-3′); and PPAR-γ F (5′-TGC TTG TGA AGG ATG CAA
GG-3′) and PPAR-γ R (5′-ATG AGA CAT CCC CAC TGC AA-3′). All
primers were designed by the Primer3 program which is a widely used
for designing PCR primers to amplify fragments that were
appropriately sized (<150 bp) for the Rotor-Gene 6000 real-time
instrument (Qiagen, Valencia, CA, USA). Relative gene expression
was quantified and normalized with respect to the 18s ribosomal RNA
(housekeeping gene as endogenous control) using the Rotor-Gene 6000
real-time rotary analyzer software (Qiagen).
Western blot analysis
Proteins were extracted from U87MG cells, SH-SY5Y
cells, primary cultured astrocytes and neurons with RIPA lysis
buffer [100 mM Tris-HCl (pH 8.5), 200 mM NaCl, 5 mM EDTA and 0.2%
SDS with phosphatase and a protease inhibitor cocktail].
Supernatants were centrifuged at 15,000 rpm and 4°C for 20 min, and
protein levels were measured by the Bradford method. Isolated
proteins (10 μg) were resolved using 10% SDS-PAGE and transferred
onto polyvinylidene fluoride membranes, which were blocked with 5%
BSA in TBST [10 mM Tris-HCl (pH 7.6), 150 mM NaCl, and 0.1%
Tween-20]. The membranes were incubated overnight at 4°C with
primary antibodies against GFAP (1:10,000), NeuN (1:2,000), LDH-A
(1:10,000), LDH-B (1:2,000) and α-tubulin (1:10,000), and then with
a horseradish peroxidase-coupled secondary antibody for 1 h at room
temperature (RT). Finally, the antibody-labeled proteins were
detected using an ECL system (INtRON BioTechnology, Seongnam,
Gyeonggi, Korea).
Immunocytochemistry
U87MG and SH-SY5Y cells were seeded on
poly-L-Lysine-coated coverslips at 2.5×104 cells per
well in 12-well plates. The cells were fixed with 4%
paraformaldehyde at room temperature (RT) for 15 min, permeabilized
with 0.02% BSA in PBS containing 0.25% Triton X-100 for 30 min,
blocked with 5% BSA in PBS at RT for 30 min, and then incubated
overnight at 4°C with primary antibodies against LDH-A (1:200),
LDH-B (1:100), GFAP (1:400), or NeuN (1:300). The cells were then
treated with Alexa Fluor 488-conjugated anti-chicken IgG, Alexa
Fluor 594-conjugated anti-rabbit IgG and Alexa Fluor 647-conjugated
anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA, USA) at RT
for 1 h, and nuclei were stained with trihydrochloride (1:10,000).
After each step, the cells were washed with 0.2% BSA in PBS.
Finally, the cells were mounted on slides with fluorescent mounting
medium (Dako North America, Carpinteria, CA, USA), and
immunofluorescent images were acquired using an Olympus confocal
microscope (Olympus, Center Valley, PA, USA).
Statistical analysis
The results are shown as mean values ± SEM (error
bars) and reflect at least three independent experiments.
Statistical analyses included the 2-tailed unpaired Student's
t-test and one-way analysis of variance (ANOVA), and were performed
using GraphPad Instat software (GraphPad Software, San Diego, CA,
USA). A P-value <0.05 was considered statistically significant,
and such values are indicated in the figures by:
*P<0.05, **P<0.01 and
***P<0.001.
Results
U87MG cells grow faster than SH-SY5Y
cells
To examine the factors capable of affecting the
growth rate of GBM, we first sought to characterize the growth rate
of U87MG cells in a model system. We used the Cell Counting Kit-8
(CCK-8) assay to compare the proliferation ability of U87MG cells,
which represent fast-growing GBM, and SH-SY5Y cells, which
represent slower-growing NB (16)
and were used herein as a control cancer cell line. As expected, we
found that U87MG cells grew more rapidly (>2-fold) than SH-SY5Y
cells over a period of 48 h (Fig.
1).
U87MG cells show high-level of ECAR
production but no difference in OCR
According to the Warburg effect, activation of
aerobic glycolysis in cancer cells affects cell growth by
supporting the production of ATP through the mitochondrial
respiratory chain (17,18). To compare mitochondrial function
between U87MG and SH-SY5Y cells, we used an XF24 analyzer to
evaluate the oxygen consumption rate (OCR) (Fig. 2A) and extracellular acidification
rate (ECAR) (Fig. 2B) (19). Interestingly, there was no
difference in the basal mitochondrial OCR (U87MG cells, 1.798±0.09
nmol and SH-SY5Y cells, 2.025±0.077 nmol) (Fig. 2C). In contrast, ECAR was ~3-fold
higher in U87MG cells compared to SH-SY5Y cells (U87MG cells,
0.407±0.025 nmol and SH-SY5Y cells, 0.125±0.007 nmol) (Fig. 2D). Thus, the metabolic flow seems
to favor aerobic glycolysis for ATP generation in U87MG cells.
Following treatment of cells with carbonyl cyanide m-chlorophenyl
hydrazone (CCCP), which is potent mitochondrial oxidative
phosphorylation uncoupler (20),
U87MG cells were found to have a higher capacity for maximal OCR
(U87MG cells, 3.369±0.109 nmol and SH-SY5Y cells, 2.233±0.036 nmol)
(Fig. 2E). Furthermore, rotenone
treatment increased ECAR compared to the basal level in U87MG cells
(28.294±1.107 versus 21.842±1.446 nmol, respectively) (Fig. 2F). Density of cell in each well is
important because the XF-analyzer measures the oxygen or proton in
a small area in the middle of the well. Consequently, we seeded 95%
of the area in the well with U87MG and SH-SY5Y cells, respectively.
These results indicate potential mitochondrial function revealing
high capacity in the U87MG cells in addition to glycolytic
metabolic capacity. Consistent with this, we observed high-level
expression of the gene encoding succinate dehydrogenase
(SDH), which is involved in the TCA (Fig. 3C).
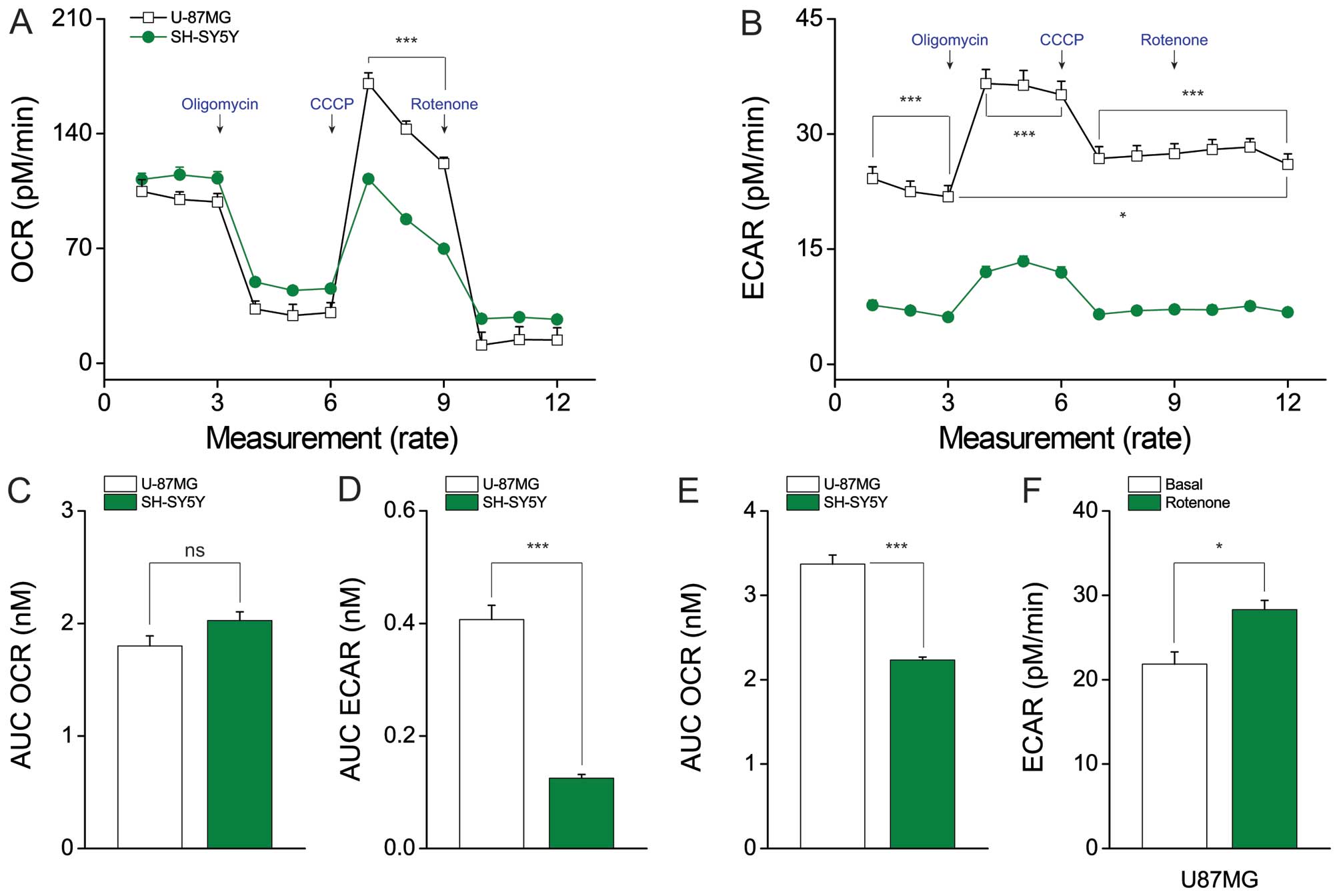 | Figure 2Lactate production differs between
U87MG and SH-SY5Y cells, whereas mitochondrial function does not.
(A) The oxygen consumption rate (OCR), which indicates
mitochondrial function, and (B) extracellular acidification rate
(ECAR), which indicates lactate production, were analyzed with a
Seahorse XF24 analyzer in cells treated with 2 μg/ml oligomycin (an
ATPase inhibitor), 5 μM CCCP (an uncoupler), or 2 μM rotenone (a
mitochondrial complex I inhibitor). The AUC (area under the curve)
of (C) basal OCR and (D) basal ECAR were measured from the first to
third time-points. (E) The AUC of the maximal OCR was measured from
the seventh to ninth time-points. (F) U87MG cells showed increased
ECAR after rotenone treatment, compared with the basal ECAR. Data
represent the means ± SEM (error bars) from three experiments
(U87MG, n=10; and SH-SY5Y, n=10). NS, not significant;
*P<0.05; and ***P<0.001 (by one-way
ANOVA) compared with SH-SY5Y cells (A–E) and basal ECAR compared
with rotenone-treated ECAR condition (F). |
U87MG cells favor aerobic glycolysis at
the transcriptional level
Lactate is a critical substrate for supplying energy
in cancer (via the Warburg effect), and is believed to help
determine low pH during carcinogenesis (21). Furthermore, the activation of
aerobic glycolysis can trigger lactate production, and as a
byproduct, it can also supply nicotinamide adenine dinucleotide
(NAD)+ as a co-substrate for glycolysis which induce
rapid cancer cell growth (22).
Here, we first examined the mRNA expression of LDH-A, which is the
main enzyme responsible for lactate production. Consistent with the
ECAR results, the level of LDH-A was ~3-fold higher in U87MG cells
compared to SH-SY5Y cells (Fig.
3A). The mRNA expression level of pyruvate carboxylase
(PC), which mediates gluconeogenesis (23), was relatively low in U87MG cells,
supporting the use of pyruvate to produce lactate in GBM cells
(Fig. 3B). Thus, the metabolic
flow appears to tend toward aerobic glycolysis, which encourages
fast growth. Moreover, the gene encoding proliferator-activated
receptor-gamma (PPAR-γ), which is involved in the production of
palmitate (a component of the cellular membrane) and supports
cellular division and growth, was induced in U87MG cells (Fig. 3D).
LDH protein expression is high in U87MG
cells
The LDH-A and LDH-B subunits catalyze the forward
and reverse reactions, respectively, between pyruvate and lactate.
To assess the protein expression levels of LDH-A and LDH-B, we
performed immunoblotting using specific antibodies (Fig. 4A). Consistent with the results of
our mRNA expression analysis, the protein expression level of total
LDH was ~1.5-fold higher in U87MG cells compared to SH-SY5Y cells
(Fig. 4B and C). Furthermore,
immunofluorescent staining showed that expressional levels of LDH-A
and B were consistent with our immunoblotting results (Fig. 4D and E). As U87MG and SH-SY5Y cells
are derived from astrocytes and neurons, respectively, we stained
these cells for GFAP (an astrocytic marker) and NeuN (a neuronal
marker) to examine remnant cellular characteristics (Fig. 4D). Interestingly, those cells have
expression of GFAP and NeuN, respectively, and U87MG cells which
contain high expression of LDH seems to derive from original
cellular peculiarity, the astrocyte. The levels of intensity with
LDH-A and LDH-B were over 2.5-fold and 3-fold higher in U87MG cells
compared to SH-SY5Y cells, respectively (Fig. 4F and G). The date were processed by
the corrected total cell fluorescence (CTCF). The value of CTCF
indicates that integrated density without measured fluorescence by
background.
LDH protein expression is high in primary
cultured cortical astrocytes
Astrocytes and neurons have different metabolic
profiles, allowing them to carry out their specific roles in the
brain. Lactate production and glycogen storage tend to be higher in
astrocytes than neurons (24).
Here, we evaluated LDH expression in the astrocytes and neurons of
primary cultures (Fig. 5A). The
markers for astrocytes (GFAP) and neurons (NeuN) were only
expressed by astrocytes and neurons, respectively (Fig. 5B and C). As expected, the
expression levels of LDH-A and LDH-B were higher in astrocytes than
in neurons (Fig. 5D and E). These
results may suggest that the increased lactate production of
astrocytes is maintained in GBM cells, supporting high-level
cellular growth by fulfilling intracellular ATP demands.
Discussion
Cancer cells require a particular environment to
flourish, including the acidic conditions that arise from the
production of lactate via aerobic glycolysis (25). Furthermore, lactate inhibits the
T-cell response and interrupts the immune system in cancer cells
(26). Thus, the proliferation of
cancer cells can be regulated by restricting lactate production
(27). Here, we present evidence
suggesting that the high proliferative capacity of GBM cells is
related to the innate metabolic phenotype of astrocytes, which
tends toward lactate production.
Uncontrolled proliferation of tumor cells is an
obstacle for devising therapeutic strategy in GBM patients with
temozolomide (common drug of GBM). In several patients, GBM cells
have been shown to resist temozolomide treatment through the repair
of DNA damages (28). In an effort
to identify a therapeutic target with prominent effects on the
proliferation of GBM cells, we focused on the metabolic
organization related to GBM. Under conditions of unrestricted
oxygen, normal cells process energy via the Pasteur effect, with
mitochondrial respiration for producing ATP at high efficiency
(29). In cancer cells, however,
the metabolic organization is altered to favor the Warburg effect,
wherein ATP preferentially is produced by glycolysis flux with low
efficiency of mitochondrial oxidative phosphorylation and lactate
is generated, even in the presence of a sufficient oxygen supply
(17). Interestingly, astrocytes
have higher metabolic capacity than neuron and contribute to
metabolic support to neurons through production of lactate,
concomitantly, astrocyte produce ATP in mitochondria for
self-energy needs. Consistent with this, we found that U87MG cells
simultaneously had higher ECAR, higher potential mitochondrial
function, and higher growth ability compared to SH-SY5Y cells.
Moreover, ECAR remained relatively high in U87MG cells following
rotenone treatment, and cultured astrocytes had higher LDH
expression than neurons in our system. Together, these findings
indicate that, similar to astrocytes, U87MG cells favor lactate
production.
A previous transcriptional profiling showed that
other TCA cycle enzymes, including citrate synthase and malate
dehydrogenase, were more highly expressed in astrocytes than in
neurons (30). Here, we noted that
succinate dehydrogenase was also expressed at a relatively higher
level in GBM cells compared to SH-SY5Y cells (Fig. 3C). The production of a co-substrate
capable of activating the mitochondrial respiratory chain from the
TCA cycle (e.g., FADH or NADH) could increase cell division and
proliferation, as seen in adenocarcinoma originating from a primary
unknown metastatic cancer (31).
Moreover, we noted higher PPAR-γ expression in GBM cells,
which could contribute to their rapid proliferation. Consistent
with this, a previous study found that PPAR-γ activation
induced tumor formation and reduced the overall survival time in
pancreatic cancer (32). GBM cells
have high-level of LDH as glycolytic enzyme and SDH
as enzyme for TCA cycle compared to NB cells. Expression of
PPAR-γ was elevated and is capable of contribution to
cellular membrane formation rather than NB cells. Otherwise, NB
cells contain high-level of PC for energy production with
transported lactate from astrocyte compared to GBM cells (Fig. 6).
The polarity of the metabolic organization that
support the rapid growth of GBM cells appears to be related to the
upregulation of both glycolysis- and TCA cycle-related enzymes.
Here, we report that U87MG cells have a metabolic phenotype similar
to that of astrocytes, but mitochondrial respiration does not
appear to be different between U87MG and SH-SY5Y cells. The
determining factor for rapid growth appears to be a difference in
lactate dehydrogenase expression in GBM cells, leading to a higher
ECAR. According to recent reports, inhibition of LDH-A has
anticancer effect by restriction of energy in human lymphoma and
pancreatic xenograft tumor growth (33,34).
Furthermore, inhibition of LDH-A suppresses proliferation in
separated cells from mammary gland tumor (35). Based on these findings, we suggest
that the inherent peculiarity of astrocytic metabolism, wherein the
Pasteur and Warburg effects are both at work, facilitates
glioblastoma. This new understanding of the metabolic
characteristics of brain tumor cells could help guide the future
development of novel targeted drugs.
Acknowledgements
Primary culture of astrocytes and neurons received
help from the laboratory of C. Justin Lee in Korea Institute of
Science and Technology. This study was supported by the National
Research Foundation of Korea (NRF) grant funded by the Ministry of
Science, ICT & Future Planning (MSIP) (2014R1A2A1A11051231),
(2014R1A1A1037655) and by the Ministry of Education
(2014R1A6A1029617) and research fund of Chungnam National
University.
References
|
1
|
Ohgaki H and Kleihues P: Population-based
studies on incidence, survival rates, and genetic alterations in
astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol.
64:479–489. 2005.PubMed/NCBI
|
|
2
|
Campbell AM, Rennie JS, Moos KF and Patton
D: Neuroblastoma presenting as mandibular swelling in a
two-year-old girl - a short case report. Br J Oral Maxillofac Surg.
25:422–426. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Diehn M, Nardini C, Wang DS, McGovern S,
Jayaraman M, Liang Y, Aldape K, Cha S and Kuo MD: Identification of
noninvasive imaging surrogates for brain tumor gene-expression
modules. Proc Natl Acad Sci USA. 105:5213–5218. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Buckner JC: Factors influencing survival
in high-grade gliomas. Semin Oncol. 30(Suppl 19): S10–S14. 2003.
View Article : Google Scholar
|
|
5
|
Bouzier-Sore AK and Pellerin L: Unraveling
the complex metabolic nature of astrocytes. Front Cell Neurosci.
7:1792013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bélanger M, Allaman I and Magistretti PJ:
Brain energy metabolism: Focus on astrocyte-neuron metabolic
cooperation. Cell Metab. 14:724–738. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suzuki A, Stern SA, Bozdagi O, Huntley GW,
Walker RH, Magistretti PJ and Alberini CM: Astrocyte-neuron lactate
transport is required for long-term memory formation. Cell.
144:810–823. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Walz W and Mukerji S: Lactate production
and release in cultured astrocytes. Neurosci Lett. 86:296–300.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dringen R, Wiesinger H and Hamprecht B:
Uptake of L-lactate by cultured rat brain neurons. Neurosci Lett.
163:5–7. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bagley PR, Tucker SP, Nolan C, Lindsay JG,
Davies A, Baldwin SA, Cremer JE and Cunningham VJ: Anatomical
mapping of glucose transporter protein and pyruvate dehydrogenase
in rat brain: An immunogold study. Brain Res. 499:214–224. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cahn RD, Zwilling E, Kaplan NO and Levine
L: Nature and Development of Lactic Dehydrogenases: The two major
types of this enzyme form molecular hybrids which change in makeup
during development. Science. 136:962–969. 1962. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bittar PG, Charnay Y, Pellerin L, Bouras C
and Magistretti PJ: Selective distribution of lactate dehydrogenase
isoenzymes in neurons and astrocytes of human brain. J Cereb Blood
Flow Metab. 16:1079–1089. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Crane CA, Austgen K, Haberthur K, Hofmann
C, Moyes KW, Avanesyan L, Fong L, Campbell MJ, Cooper S, Oakes SA,
et al: Immune evasion mediated by tumor-derived lactate
dehydrogenase induction of NKG2D ligands on myeloid cells in
glioblastoma patients. Proc Natl Acad Sci USA. 111:12823–12828.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nieder C, Marienhagen K, Dalhaug A,
Aandahl G, Haukland E and Pawinski A: Prognostic models predicting
survival of patients with brain metastases: Integration of lactate
dehydrogenase, albumin and extracranial organ involvement. Clin
Oncol (R Coll Radiol). 26:447–452. 2014. View Article : Google Scholar
|
|
15
|
Woo DH, Han KS, Shim JW, Yoon BE, Kim E,
Bae JY, Oh SJ, Hwang EM, Marmorstein AD, Bae YC, et al: TREK-1 and
Best1 channels mediate fast and slow glutamate release in
astrocytes upon GPCR activation. Cell. 151:25–40. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Biedler JL, Helson L and Spengler BA:
Morphology and growth, tumorigenicity, and cytogenetics of human
neuroblastoma cells in continuous culture. Cancer Res.
33:2643–2652. 1973.PubMed/NCBI
|
|
17
|
Warburg O: On respiratory impairment in
cancer cells. Science. 124:269–270. 1956.PubMed/NCBI
|
|
18
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang R, Novick SJ, Mangum JB, Queen K,
Ferrick DA, Rogers GW and Stimmel JB: The acute extracellular flux
(XF) assay to assess compound effects on mitochondrial function. J
Biomol Screen. 20:422–429. 2015. View Article : Google Scholar
|
|
20
|
Park JW, Lee SY, Yang JY, Rho HW, Park BH,
Lim SN, Kim JS and Kim HR: Effect of carbonyl cyanide
m-chlorophenylhydra-zone (CCCP) on the dimerization of lipoprotein
lipase. Biochim Biophys Acta. 1344:132–138. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie J, Wu H, Dai C, Pan Q, Ding Z, Hu D,
Ji B, Luo Y and Hu X: Beyond Warburg effect - dual metabolic nature
of cancer cells. Sci Rep. 4:49272014.
|
|
22
|
Chiarugi A, Dölle C, Felici R and Ziegler
M: The NAD metabolome - a key determinant of cancer cell biology.
Nat Rev Cancer. 12:741–752. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jitrapakdee S, St Maurice M, Rayment I,
Cleland WW, Wallace JC and Attwood PV: Structure, mechanism and
regulation of pyruvate carboxylase. Biochem J. 413:369–387. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brown AM and Ransom BR: Astrocyte glycogen
and brain energy metabolism. Glia. 55:1263–1271. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gatenby RA and Gillies RJ: Why do cancers
have high aerobic glycolysis? Nat Rev Cancer. 4:891–899. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fischer K, Hoffmann P, Voelkl S,
Meidenbauer N, Ammer J, Edinger M, Gottfried E, Schwarz S, Rothe G,
Hoves S, et al: Inhibitory effect of tumor cell-derived lactic acid
on human T cells. Blood. 109:3812–3819. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hirschhaeuser F, Sattler UG and
Mueller-Klieser W: Lactate: A metabolic key player in cancer.
Cancer Res. 71:6921–6925. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Van Meir EG, Hadjipanayis CG, Norden AD,
Shu HK, Wen PY and Olson JJ: Exciting new advances in
neuro-oncology: The avenue to a cure for malignant glioma. CA
Cancer J Clin. 60:166–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stickland LH: The Pasteur effect in normal
yeast and its inhibition by various agents. Biochem J. 64:503–515.
1956. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lovatt D, Sonnewald U, Waagepetersen HS,
Schousboe A, He W, Lin JH, Han X, Takano T, Wang S, Sim FJ, et al:
The transcriptome and metabolic gene signature of protoplasmic
astrocytes in the adult murine cortex. J Neurosci. 27:12255–12266.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim HM, Kim H, Jung WH and Koo JS:
Metabolic phenotypes in primary unknown metastatic carcinoma. J
Transl Med. 12:22014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nakajima A, Tomimoto A, Fujita K, Sugiyama
M, Takahashi H, Ikeda I, Hosono K, Endo H, Yoneda K, Iida H, et al:
Inhibition of peroxisome proliferator-activated receptor gamma
activity suppresses pancreatic cancer cell motility. Cancer Sci.
99:1892–1900. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Omar HA, Berman-Booty L, Kulp SK and Chen
CS: Energy restriction as an antitumor target. Future Oncol.
6:1675–1679. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Le A, Cooper CR, Gouw AM, Dinavahi R,
Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL and Dang
CV: Inhibition of lactate dehydrogenase A induces oxidative stress
and inhibits tumor progression. Proc Natl Acad Sci USA.
107:2037–2042. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fantin VR, St-Pierre J and Leder P:
Attenuation of LDH-A expression uncovers a link between glycolysis,
mitochondrial physiology, and tumor maintenance. Cancer Cell.
9:425–434. 2006. View Article : Google Scholar : PubMed/NCBI
|




















