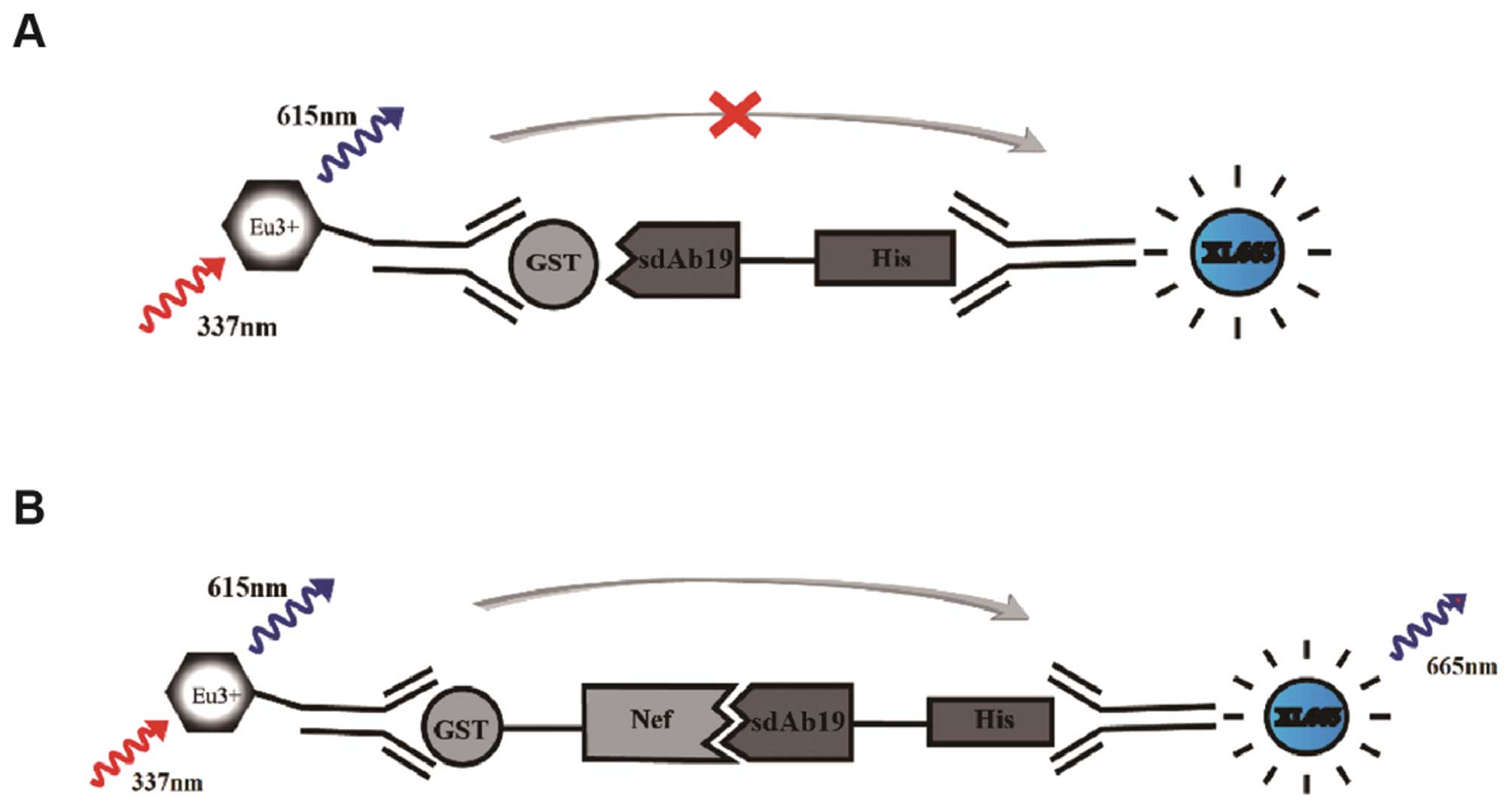
Introduction
Despite the considerable progress made in the field
of anti-HIV drug development, the acquired immune deficiency
syndrome (AIDS) is still a serious health concern across the world
since its discovery more than thirty years ago. Due to its high
mutation rate, HIV is often able to evade the host immune system,
and also the antiviral therapies (1,2).
Therefore, the development of novel drugs and therapeutic methods
against the virus is important. The HIV accessory proteins
represent the alternative targets for anti-HIV drug development. In
particular, the negative factor (Nef), an accessory protein of HIV
which plays a critical role in the physio-pathogenesis of AIDS
could be a good target for the drug research and development. Nef
is expressed early in the viral life cycle and targeted to the
plasma membrane (3,4). It contains an N-myristoylation
cytoplasmic region that is required for its association with
cellular membranes and virtually critical for its complex
biological activities. Nef exists as homodimers in cells, and all
types of Nef are conserved with six structured core domains which
forms one α-helix and five β-layer structures (5). The flexible regions of Nef, such as
its large surfaces accessible for interactions and important
conformational changes, are involved in increasing infectious viral
particles, activating CD4 lymphocytes and preventing infected cells
from apoptosis (6–8).
Recently, antibody therapy has obtained remarkable
results. The single-domain antibodies (sdAb) derived from camelids
is a major breakthrough in the development of antibody therapy
(5,9,10).
The sdAb19, consisting only of a single variable domain which
recognizes the antigenic epitope of the target protein, is
relatively small with a molecular weight of 13 kDa, and can
penetrate in cavities located on the surface of antigens. Previous
studies have demonstrated the sdAb fragments displayed efficient
therapeutic activity in many diseases such as cancer,
neurodegenerative diseases and rheumatoid arthritis (11–13).
The sdAb19 generated by Bouchet et al targeted the HIV Nef
protein with a high affinity and inhibited biologic activities of
Nef both in vitro and in vivo in a
nef-transgenic mouse model, suggesting that inhibition of
Nef function is a potential method to block HIV (9). However, sdAb19 must be ectopically
expressed via an expression vector within the target cell to be
able to exert its neutralizing effect on Nef, while the
extra-cellular administration method turned out to be ineffective.
This might suggest a default of the stability or/and deliverability
of sdAb19. The identification of small molecule compounds capable
of mimicking the neutralizing activity of sdAb19 and displaying
better stability or/and deliverability in vivo would
therefore be the means of circumventing the problem encountered
with sdAb19. In this study, we took advantage of the homogeneous
time-resolved fluorescence (HTRF) technology to carry out a
high-throughput screening of a library of small molecules. HTRF
technology is an ideal platform used for drug discovery in
high-throughput screening, which combines fluorescence resonance
energy transfer technology (FRET) with time-resolved measurement
(TR). The time-resolved characteristic of HTRF technology allows
for the removal of nearly all environmental and compound
interference effects. As a sensitive and reliable method due to its
reduced inter-well variation and fluorescence interference, this
technology has been developed for many antibody-based assays
including GPCR signaling, kinases, cytokines and biomarkers,
bioprocess, as well as the assay for biomolecular interaction
(14–18). We selected the molecules capable of
inhibiting the Nef-sdAb19 interaction by this method, then used the
microscale thermophoresis (MST) technique to identify, among these
candidate molecules, those that fix on Nef. These are the molecules
which probably inhibit the Nef-sdAb19 interaction by competition of
Nef binding with sdAb19, and could possibly mimic the anti-Nef
activity in vivo.
Materials and methods
Antibodies and chemicals
Eu3+ cryptate-conjugated anti-GST
antibody (anti-GST-Eu) and XL665-conjugated anti-6xHis antibody
(anti-6xHis-XL665) were purchased from Perkin-Elmer (France).
Anti-his monoclonal antibody was purchased from Santa Cruz
Biotechnology, Inc. (USA). Tris, sodium dodecyl sulfate (SDS) and
glycine were obtained from Bio-Rad (USA). Protein inhibitor was
purchased from Merk (USA). Isopropyl-1-thio-β-D-galactopyranoside
(IPTG), lysozyme, imidazole and reduced glutathione were purchased
from Applygen (China). Glutathione-Sepharose 4B beads were
purchased from GE (USA), whereas Ni+ beads from Qiagen
N.V. (China). Pharmacologically active compounds (LOPAC) were from
Sigma (USA). Pierce BCA Protein Assay kit was purchased from Thermo
(USA).
Expression and purification of
recombinant proteins
The pGEX-4T-Nef construct encoding GST-Nef and
pcDNA-sdAb19 expressing His-sdAb19 were transformed into
Escherichia coli BL21. The expression of recombinant protein
was induced by the addition of 0.2 mM IPTG for 6–8 h at room
temperature (RT) when transformed bacteria were grown to an OD600
of 0.6–0.8. The cells were collected and lysed by sonication in EBC
buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, pH 7.6)
containing 1 mM phenylmethylsufonyl fluoride (PMSF), 1X complete
mini EDTA-free protein inhibitors and 10 μg/ml lysozyme. Bacterial
extracts were sonicated for 20 min and centrifuged at 12,000 rpm
for 30 min at 4°C to remove cell debris. GST-proteins were purified
using Glutathione Sepharose 4B (GE Healthcare), and His-sdAb19
proteins were purified from supernatant using Ni-NTA Superflow
(Qiagen). Glutathione and imidazole were excluded from the eluted
proteins using 10 kDa-ultrafiltration (Millipore, USA). The
concentration of protein was measured by BCA assay (Beyotime
Biotechnology, China). The purity of recombinant proteins were
determined by SDS-PAGE and coomassie blue straining (CBB).
Digestion by thrombin
Thrombin was added to digest the GST-Nef beads for
16 h at 4°C. The digestion mix was then centrifuged at 12,000 rpm
for 5 min at 4°C to collect the supernatant.
GST pull-down assay
Equal amounts of GST-beads and GST-Nef-beads were
incubated in EBC buffer with 500 μg purified recombinant His-sdAb19
for 6 h at 4°C. The beads were recovered by centrifugation at 1,000
rpm for 2 min at 4°C and washed three times with EBC buffer, and
proteins were eluted into 20 μl of SDS sample buffer by heating to
95°C for 5 min. Proteins were separated using 10% SDS-PAGE gels and
then eletro-blotted to PVDF membrane. Membranes were blocked with
5% non-fat milk for 1 h at RT, and then incubated with anti-His
mouse antibody overnight at 4°C. After washing, the blots were
probed with horseradish peroxidase-conjugated anti-mouse secondary
antibody (ProteinTech Group) for 1 h at RT, and then signals were
visualized using ECL detection system (Beyotime Biotechnology).
Optimization of an HTRF assay for
Nef-sdAb19 interaction
The following experiments were designed to determine
the optimal concentration of HTRF reagents, including GST-Nef,
His-sdAb19, anti-GST-Eu antibody, and anti-6xHis-XL665 antibody. i)
The incubation of GST-Nef having a concentration in a range from 0
to 100 nM with 100 nM His-sdAb19, 5 nM anti-GST-Eu antibody, and
100 nM anti-6xHis-XL665 antibody was to determine the optimal
concentration of GST-Nef through HTRF assay. ii) The incubation of
His-sdAb19 having a concentration in a range from 0 to 100 nM with
100 nM GST-Nef, 5 nM anti-GST-Eu, and 100 nM anti-6xHis-XL665
antibody was to determine the optimal concentration of His-sdAb19
through HTRF assay. iii) Anti-GST-Eu antibody, with the changeable
concentration from 0 to 5 nM, co-incubated with 100 nM
anti-6xHis-XL665 antibody, 100 nM GST-Nef, and 100 nM His-sdAb19
was to determine the optimal concentration of Eu3+-GST
antibody. iv) Anti-6xHis-XL665 antibody, with the increasing
concentrations from 0 to 100 nM, co-incubated with 5 nM anti-GST-Eu
antibody, 100 nM GST-Nef, and 100 nM His-sdAb19 was to determine
the optimal concentration of XL665-His antibody. During these HTRF
assays, GST-Nef was incubated for 4 h at 4°C, and then anti-GST-Eu
and anti-6xHis-XL665 antibodies were added to incubate away from
light for 2 h at RT. The corresponding GST protein was used as
negative control for all the tests. All HTRF reagents were diluted
with Tris-HCl buffer and added to the 384-well plates to a final
volume of 50 μl. Plates were stored in the dark at RT before
reading using Perkin-Elmer VICTORTM X5 (Perkin-Elmer,
USA). The excitation fluorescence of 337 nm was used to excite
Eu3+ and the fluorescence emission at 665 and 615 nm
were detected simultaneously. Signal was expressed in terms of HTRF
ratio ΔF%, as the previous description (19). R=[signal 665/signal 615]*10,000;
ΔR=RNef−RGST; ΔF%=(ΔR/RGST)*100 (Fig. 1).
Nef-sdAb19 interaction inhibition
assay
The library of pharmacologically active compounds
(LOPAC, Sigma-Aldrich, USA) contains 1,280 well-characterized
chemical compounds. The compounds of LOPAC library are stored at a
concentration of 10 mM, dissolved in DMSO in 96-well plates at
−20°C. Before the experiments, we diluted the compounds with the
following procedure. Each compound (5 μl) was mixed with 95 μl DMSO
in other 96-well plates. Each diluted compound (10 μl) was used for
high-throughput screening and added into the 384-well black plate.
Then, 10 μl of 250 nM GST-Nef (a final concentration of 50 nM) was
incubated with the compounds for 2 h at 4°C, following by incubated
with 10 μl of 100 nM His-sdAb19 (a final concentration of 20 nM)
for 4 h at 4°C. Ten microliters of the donor fluorophore
Eu3+ cryptate-conjugated anti-GST antibody (a final
concentration of 2.5 nM) and 10 μl of the acceptor fluorophore
XL665-conjugated anti-6xHis antibody (a final concentration of 100
nM) were added and incubated in dark condition for 2 h at RT. The
GST protein was used as comparison substance. The total volume of
the reaction was 50 μl. There were 3 multiple pores in every dosage
group and negative control group. Plates were read by Perkin-Elmer
VICTOR™ X5 by measurement of fluorescence emission at 665 and 615
nm. The percentage of interaction inhibition was analyzed according
to the following formula (19).
Inhibition (%) = ((F
Nef−FGST)−(Fcom−FGST))/(FNef−FGST)*100;
F=[signal 665/signal 615]*10,000. FNef is the
fluorescence emission ration of GST-Nef group treated with DMSO;
Fcom is the fluorescence emission ration of GST-Nef
group treated with the small molecular compound; FGST is
fluorescence emission ration of GST negative control group treated
with DMSO; F is fluorescence emission ration of 665 nm/615 nm.
Excluding the false-positive
reaction
Microscale thermophoresis (MST) was used to
determine the binding affinities between Nef and the cadidates of
HTRF. a fixed concentration of 200 nM Nef, the candidates which
were titrated with 122.07 to 25,000 nM in PBS buffer. In order to
allow binding, samples were incubated at least 30 min at room
temperature followed by centrifugation for 5 min at 15,000 g to
eliminate potential precipitates. Experimental measurements were
performed in standard or hydrophilic capillaries using a NanoTemper
Monolite™ NT.115 instrument (NanoTemper Technologies GmbH, Germany)
for red fluorescence dye. Thermophoresis signals for each of the 12
capillaries were monitored, which harbor different ratios of
binding partners. The normalized fluorescence at a given time point
was plotted against the concentration of unlabed candidates. The
resulting sigmoidal curves were normalized, and each data point was
determined. Data points were finally fitted by the Hill slope, and
KD value were obtained. Kd Formula (law of mass action): F(c) =
unbound + (bound−unbound)/2 * (FluoConc + c + Kd − Sqrt((FluoConc +
c + Kd)^2 − 4*FluoConc*c) (20).
Results
Expression and purification of GST-Nef
and His-sdAb19
The plasmids pGEX-4T-Nef and pcDNA-sdAb19 were
transformed into E. coli BL21, and the recombinant bacteria
was induced by IPTG in concentration of 0.2 mM for 6–8 h at RT. As
shown in Fig. 2, SDS-PAGE analysis
showed that GST-Nef and His-sdAb19 were expressed in inclusion
bodies in the E. coli cells and their molecular weights were
~54 and 27 kDa (Fig. 2). The two
recombinant proteins were purified with Glutathione-Sepharose 4B
beads and Ni-NTA Superflow, respectively. SDS-PAGE analysis showed
that there was a single band at the expected position, indicating
that the proteins had been purified successfully (Fig. 2).
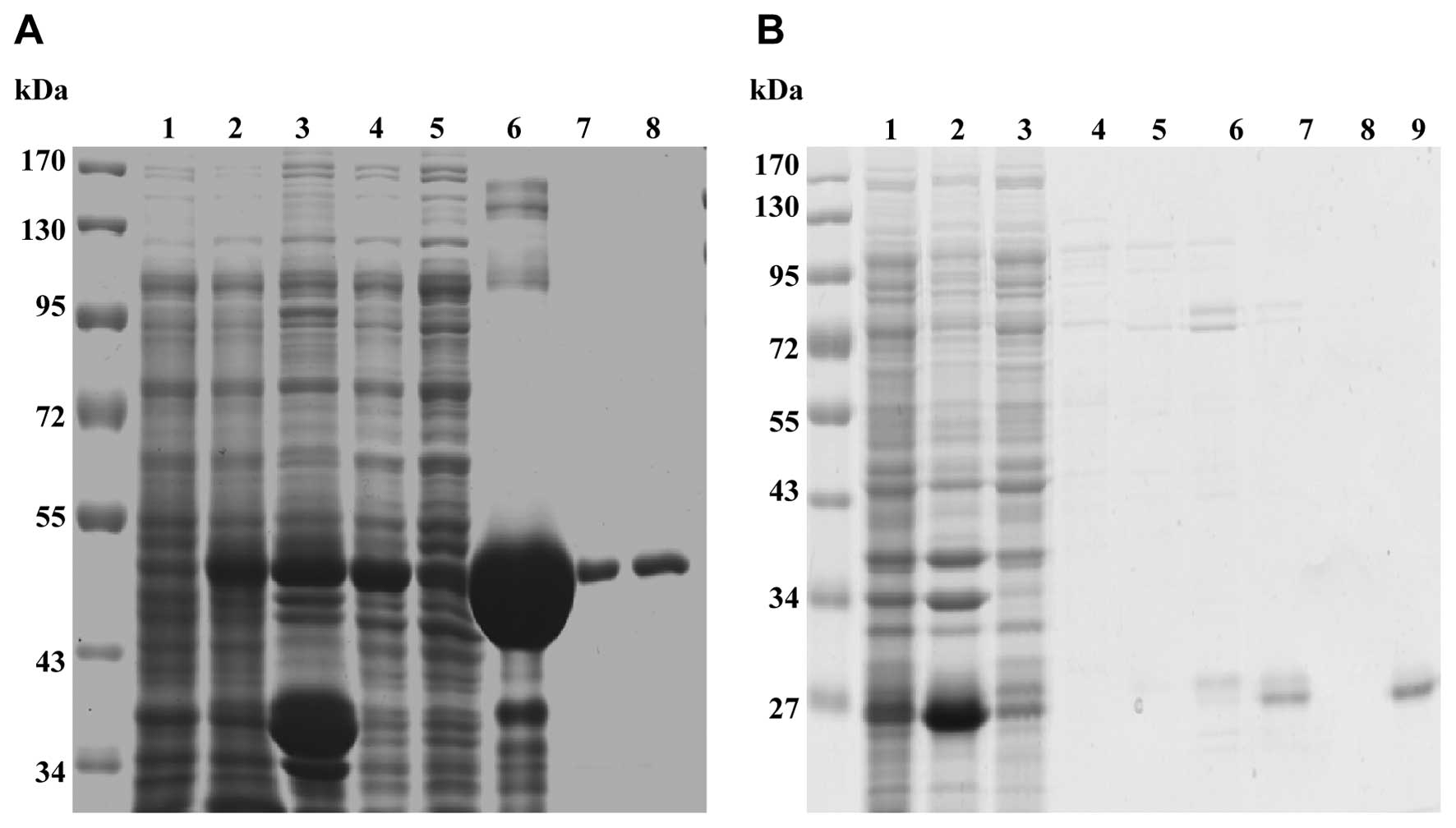 | Figure 2Expression and purification of
recombinant GST-Nef and His-sdAb19. (A) The expression and
purification of recombinant GST-Nef were analyzed by SDS-PAGE. Lane
1, E. coli BL21 transformed with pGEX-Nef without IPTG
induction; lane 2, E. coli BL21 transformed with pGEX-Nef
with IPTG induction; lane 3, the supernatant of cell lysates after
IPTG induction; lane 4, the precipitation of cell lysates; lane 5,
the supernatant of the flow-through; lane 6, GST-Nef recombinant
protein-beads before elution; lane 7, GST-Nef recombinant
protein-beads after elution with 5 mM reduced glutathione; lane 8,
GST-Nef recombinant protein-beads after elution with 5 mM reduced
glutathione. (B) The expression and purification of recombinant
His-sdAb19 were analyzed by SDA-PAGE. Lane 1, E. coli BL21
transformed with pET-sdAb19 without IPTG induction; lane 2, E.
coli BL21 transformed with pET-sdAb19 with IPTG induction; lane
3, the supernatant of cell lysates after IPTG induction; lane 4,
the precipitation of cell lysates; lane 5, the supernatant of the
flow-through; lane 6, His-sdAb19 recombinant protein-beads before
elution; lane 7, His-sdAb19 recombinant protein-beads after elution
with 10 mM imidazole; lane 8, His-sdAb19 recombinant protein-beads
after elution with 50 mM imidazole; lane 9, His-sdAb19 recombinant
protein-beads after elution with 100 mM imidazole. |
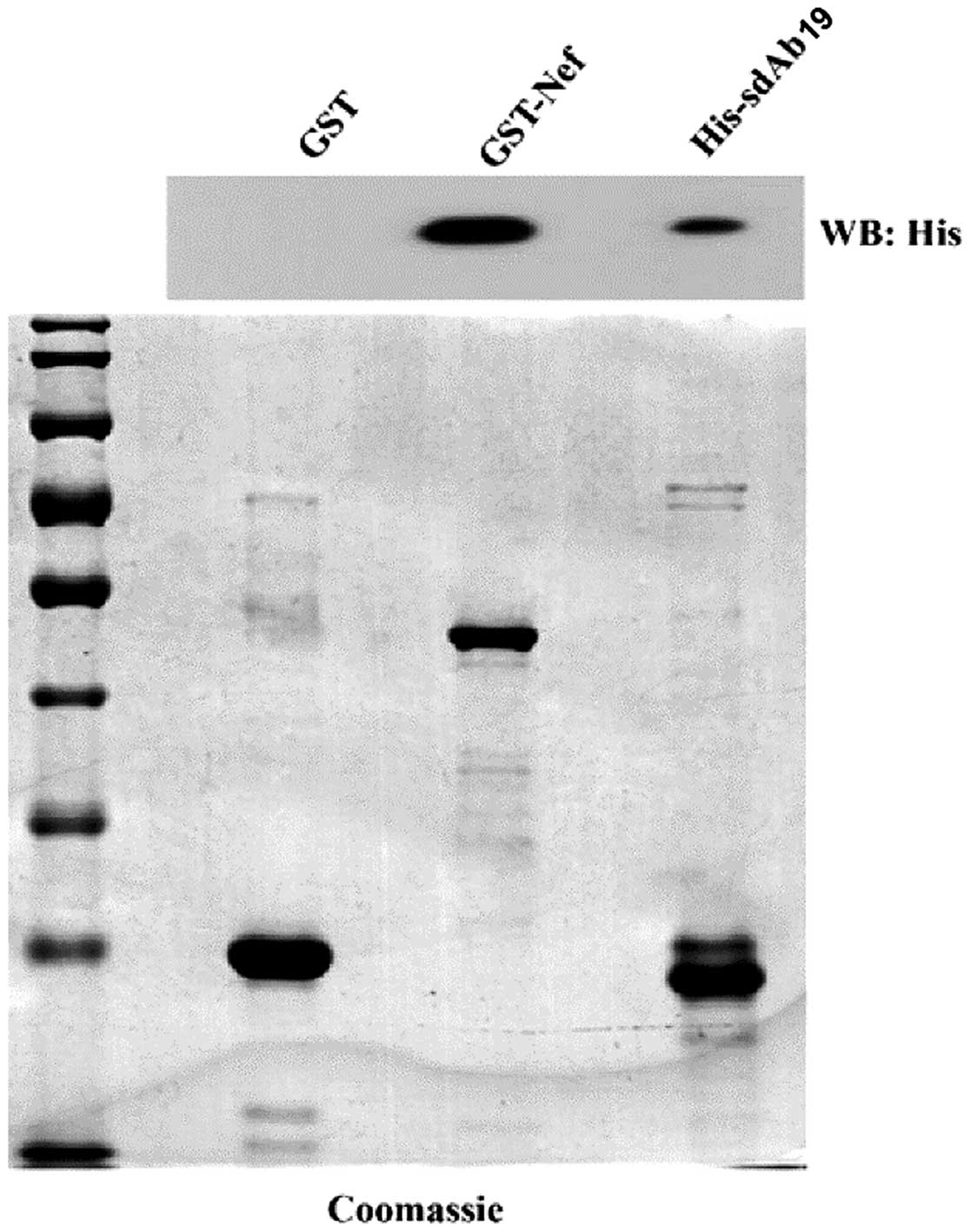
In vitro interaction between GST-Nef and
His-sdAb19
To demonstrate the direct interaction between Nef
and sdAb19 in vitro, we performed an in vitro GST
pull-down experiment using GST-Nef and His-sdAb19 recombinant
proteins. Fig. 3 showed that
His-sdAb19 could be pulled down by the immobilized GST-Nef but not
the immobilized GST, indicating the direct and specific binding of
GST-Nef and His-sdAb19 exists in an acellular context.
Optimization of HTRF assay reagents
To make sure that the interaction of Nef with sdAb19
(presented as HTRF ratio ΔF%) responds sensitively to any
interference coming from the small molecular inhibitors, the used
concentration of four reagents must be optimized, including
GST-Nef, His-sdAb19, anti-GST-Eu antibody, and anti-6xHis-XL665
antibody. As described in detail in Materials and methods, with
excessive His-sdAb19 (100 nM), anti-GST-Eu antibody (5 nM), and
anti-6xHis-XL665 antibody (100 nM), GST-Nef proteins having a
concentration in a range from 0 to 100 nM were added for reactions.
GST proteins were used as negative control. The result showed that
the observed interaction (HTRF ratio ΔF%) increased linearly at the
concentration of GST-Nef <50 nM, while the observed interaction
(HTRF ratio ΔF%) decreased (Fig.
4A) at its concentration >50 nM, suggesting the optimal
concentration of GST-Nef is ~50 nM. Subsequently, the proper
concentration of His-sdAb19 was determined by HTRF assay by the
addition of enough GST-Nef (100 nM), anti-GST-Eu antibody (5 nM),
and anti-6xHis-XL665 antibody (100 nM) with His-sdAb19 (the
concentrations from 0 to 100 nM). The result showed that the
strongest interaction between GST-Nef and His-sdAb19 was observed
when the concentrations of His-sdAb19 reached 40 nM (Fig. 4B). Due to the fact that ΔF% of 20
nM is almost half of 40 nM, and that 100 nM GST-Nef was used in
this reaction twice, than the chosen 50 nM for screening, we
decided on the concentration of 20 nM for the following
high-throughput screening. Whereas, GST at concentration of 100 nM
was the negative control.
 | Figure 4Optimization of HTRF assay reagents.
(A) Optimization of the concentrations of GST-Nef for
high-throughput screening. His-sdAb19 (100 nM), anti-GST-Eu
antibody (5 nM), and anti-6xHis-XL665 antibody (100 nM) were mixed
with different concentrations of GST-Nef from 0 to 100 nM in black
384-wells. GST-Nef and His-sdAb19 were incubated for 4 h at 4°C,
then the anti-GST-Eu and anti-6xHis-XL665 antibodies were added for
2-h incubation at RT. Plates were read using Perkin-Elmer VICTOR
X5. HTRF ratio ΔF% is positively correlated with the Nef-sdAb19
interaction. (B) Optimization of the concentrations of His-sdAb19
for high-throughput screening. GST-Nef (100 nM), anti-GST-Eu
antibody (5 nM), and anti-6xHis-XL665 antibody (100 nM) were mixed
with different concentrations of His-sdAb19 from 0 to 100 nM in the
black 384-wells, followed by a similar procedure of HTRF assay. (C)
Determination of the concentrations of anti-GST-Eu antibody for
high-throughput screening. GST-Nef (100 nM), His-sdAb19 (100 nM),
and anti-6xHis-XL665 antibody (100 nM) were mixed with different
concentrations of anti-GST-Eu antibody from 0 to 5 nM in the black
384-wells, followed by a similar procedure of the HTRF assay. (D)
Determination of the concentrations of anti-6xHis-XL665 antibody
for high-throughput screening. GST-Nef (100 nM), His-sdAb19 (100
nM), and anti-GST-Eu antibody (5 nM) were mixed with different
concentrations of anti-6xHis-XL665 antibody from 0 to 100 nM in the
black 384-wells, followed by a similar procedure of the HTRF
assay. |
Similarly, the suitable concentration of anti-GST-Eu
antibody was detected with enough GST-Nef (100 nM), His-sdAb19 (100
nM), and anti-6xHis-XL665 antibody (100 nM) mixed together with
anti-GST-Eu antibody with the concentrations from 0 to 5 nM for
HTRF detection. The result showed that the maxima of ΔF% appeared
at 2.5 nM anti-GST-Eu antibody, and the strongest interaction
occurred at the anti-6xHis-XL665 antibody concentration >40 nM
(Fig. 4C and D). To guarantee the
effective detection of HTRF signal, the antibodies against GST-Eu
and 6xHis-XL665 need to be excessive. In consideration of these
factors, we chose respectively 2.5 and 100 nM as the proper
concentrations of anti-GST-Eu and anti-6xHis-XL665 antibodies for
the following high-throughput screening.
High-throughput screening of the small
molecules as inhibitors of Nef-sdAb19 interaction
After determination of the proper concentrations of
four reagents for HTRF detection, 50 nM GST-Nef, 20 nM His-sdAb19,
2.5 nM anti-GST-Eu and 100 nM anti-6xHis-XL665 were used for
screening the small molecular inhibitors against Nef-sdAb19
interaction. As described in Materials and methods, the
high-throughput screening by HTRF tests were performed in 384-well
plates in a total 50 μl reaction volume containing the above
reagents with their proper concentration and each small molecular
compound (100 μM) or control DMSO. After incubation, the 384-well
plates were analyzed by Perkin-Elmer VICTOR™ X5. The percentage of
interaction inhibition was calculated with the formula: Inhibition
(%) =
((FNef−FGST)−(Fcom−FGST))/(FNef−FGST)*100.
The results in Fig. 5 showed that
inhibition of most compounds was <50%, the generally
acknowledged standard of effective inhibition in relation to
high-throughput screening experiments. Notably, there were sixteen
candidate compounds showing inhibition (%) >50% (Fig. 5). Their names and classes are shown
in Table I).
 | Table IThe candidates compounds screened
with HTRF and MST assay. |
Table I
The candidates compounds screened
with HTRF and MST assay.
| No. | Name | Class | HTRF | MST |
|---|
| 1 | Trovafloxaxin
mesylate | Immunomodulators
and antibodies | + | + |
| 2 | CL316243 | Adrenoceptor | + | + |
| 3 | Felodipine | Ca2+
channel | + | + |
| 4 | R(+)-IAA-94 | Cl−
channel | + | + |
| 5 | Loratadine | Histamine | + | + |
| 6 |
Isoliquiritigenin | Cyclic
nucleotides | + | + |
| 7 | IC261 |
Phosphorylation | + | + |
| 8 | A3
hydrochloride |
Phosphorylation | + | + |
| 9 | Cystamine | Glutamate | + | − |
| 10 | Artemether | Immunomodulators
and antibiotics | + | − |
| 11 | Colchicine | Cytoskeleton and
ECM | + | − |
| 12 | CNS-1102 | Glutamatc | + | − |
| 13 | Enoximone | Cyclic
nucleotides | + | − |
| 14 | Ketorolac tris
salt | Prostaglandin | + | − |
| 15 | Niclosamide | Antibiotic | + | − |
| 16 | Auranofin |
Phosphorylation | + | − |
Test of Nef binding by MST for
HTRF-positive small molecules
In order to select among the candidate molecules
that bind to Nef, we used microscale thermophoresis to test the
interaction of each of the sixteen candidate molecules obtained and
listed bove. Fig. 6 shows the
SDS-PAGE of the recombinant Nef that we produced and purified for
the MST assays. The red fluorescent dye-labeled Nef was kept
constant at 200 nM, and the sixteen candidate compounds were
titrated from 100 to 25,000 nM. The binding of Nef with the
candidate compounds were monitored by the change in the
thermophoretic property of the fluorescently labeled protein upon
complex formation. The KD was caculated as previously reported
(19). As shown by the MST T-Jump
response in Fig. 7, there were
eight candidate compounds that bind to Nef.
 | Figure 6Affinity purification of Nef.
Coomassie blue staining of the purification of Nef. Lane 1, E.
coli BL21 transformed with pGEX-Nef without IPTG induction;
lane 2, E. coli BL21 transformed with pGEX-Nef with IPTG
induction; lane 3, the precipitation of ultrasound; lane 4, the
supernatant of ultrasound after IPTG induction; lane 5, the
supernatant of ultrasound flowed through the Glutathione Sepharose
4B; lane 6, the filtrate of GST-Nef fusion protein beads digested
by thrombin; lane 7, the digested beads eluted with 3 ml PBS first
time; lane 8, the digested beads eluted with 3 ml PBS second time;
lane 9, the digested beads eluted with 3 ml PBS third time; lane
10, the beads after digested by thrombin and eluted by PBS. |
Discussion
The protein Nef is critical for the pathogenesis of
HIV, and the inhibition of its biological activity represents a
potential way to eradicate HIV. The anti-Nef antibody sdAb19
derived from camelids significantly blocked the growth of HIV
(23). Considering sdAb19
represent an efficient tool to elucidate the molecular functions of
Nef, it is a meaningful strategy to search for new small molecular
inhibitors against Nef-sdAb19 interaction for further inhibiting
the function of Nef. In this study, we utilized HTRF technology to
high-throughput screen the small molecular inhibitors against the
interaction between Nef and sdAb19 in vitro. We obtained
sixteen candidate compounds significantly inhibiting the
interaction between Nef and sdAb19 through screening. Since the
HTRF method that we used in our lab was inappropriate to monitor
the interaction between Nef and small molecules, we used MST to
select the Nef binding molecules, The results showed that only
eight candidate compounds were positive for the binding with Nef in
an extracellular context and represented therefore the candidate
compounds for mimicking the anti-Nef activity of sdAb19 in
vivo. The eight double-positive candidate molecules belong to 4
categories of compounds, including ion channel, phosphorylation,
immunomodulators and antibiotics (Table I).
Ion channels are membrane proteins that are found in
a number of viruses (21). In this
study, felodipine and R(+)-IAA-94 displaying potency in inhibition
of ion channel activity (22) were
selected for their ability of inhibiting Nef-sdAb19 interaction and
binding to Nef. Protein phosphorylation is a reversible
post-translational modification essential for the regulation of
many cellular processes. Phosphorylation can modulate protein
properties including enzymatic activity, stability, subcellular
localization and interaction with binding partners. The importance
of phosphorylation of the replication proteins of negative-strand
RNA viruses has previously been documented (23).
3-[(2,4,6-trimethoxyphenyl)methylidenyl]-indolin-2-one (IC261), a
novel casein kinase-1 delta/epsilon (δ/ɛ) specific inhibitor,
triggers the mitotic checkpoint and induces p53-dependent
postmitotic effects. The effects of IC261 on CK1δ/ɛ might be the
interference of specific phosphorylation of spindle component
proteins (24). Notably, several
recent studies showed that the CK1δ/ɛ is involved in the regulation
of cell survival and cancer progression (25). IC261, as CK1δ/ɛ inhibitor, may
inhibit cancer development through inhibition of microtubule
polymerization (26).
Immunomodulators and antibiotics are bringing great prospects for
clinical trials, since there have been significant advances in
immunotherapeutic field in HIV over the past decade (27). Retinoids, derivatives of vitamin A,
have multiple cellular functions including induction of
differentiation, regulation of apoptosis and inhibition of
proliferation or inflammation (28). Retinoic acid p-hydroxyanilide is a
vitamin A acid analog with antiproliferative activity in cultured
human breast cancer cells (29,30).
Some studies found that all-trans retinoic acid (at RA) might
inhibit HIV-1-induced podocyte proliferation and dedifferentiation
with the cAMP/PKA pathway through Nef-induced MAPK1,2 activation by
retinoic acid receptor α (31,32).
The sdAb19 that derives from camelids, binds with
high affinity (Kd=2×10−9 M) and shows specificity to the
conserved epitope of Nef. The interaction between Nef and sdAb19
was confirmed by GST pull-down in our study. Small organic
molecules could be revealed to be advantageous over antibody- or
protein-based drugs due to their higher stability, bioavailability,
deliverability and lower cost. We developed an HTRF-based screening
assay for the identification of small molecule inhibitors against
Nef-sdAb19 interaction that can be used also for larger scale
screening. We performed HTRF assay with very low protein
concentrations (Nef 50 nM; sdAb19 25 nM) to increase the chances of
finding small-molecule inhibitors. This is a novel trial for
screening the antiviral small molecule drugs using the HTS assays.
Sixteen candidate compounds were screened by HTRF assay, and eight
compounds showed positive against Nef-sdAb19 interaction using
MST.
In conclusion, the research presented in this study
represents a proof of concept of a novel method for the screening
of small molecule compounds, and permited the selection of 8
candidate molecules susceptible to inhibit the biological functions
of HIV Nef protein and the physiopathogenesis of AIDS.
Acknowledgements
We thank Dr Liang Zhou (Quantum Design China &
Nano-Temper Technologies GmbH) for performing the Microscale
Thermophoresis (MST) assay. This study was supported by the
National Program on Key Basic Research Project (973 Program) (grant
no. 2011CB910700), High-Level Talents Project of the Universities
of Guangdong (grant no. [2011]431); National Natural Science
Foundation of China (grant no. 31000628), Fundamental Research
Funds for the Central Universities (grant nos. 21611430, 21610101
and 21609317), Natural Science Foundation of Guangdong Province
(grant no. 9151065004000005).
Abbreviations:
|
HTRF
|
homogeneous time-resolved
fluorescence
|
|
MST
|
microscale thermophoresis
|
|
sdAb
|
single-domain antibodies
|
|
HIV
|
human immunodeficiency virus
|
|
AIDS
|
acquired immune deficiency
syndrome
|
|
FRET
|
fluorescence resonance energy transfer
technology
|
References
|
1
|
Greene WC: The brightening future of HIV
therapeutics. Nat Immunol. 5:867–871. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Foster JL and Garcia JV: HIV-1 Nef: At the
crossroads. Retrovirology. 5:842008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kestler HW III, Ringler DJ, Mori K,
Panicali DL, Sehgal PK, Daniel MD and Desrosiers RC: Importance of
the nef gene for maintenance of high virus loads and for
development of AIDS. Cell. 65:651–662. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fackler OT and Baur AS: Live and let die:
Nef functions beyond HIV replication. Immunity. 16:493–497. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shugars DC, Smith MS, Glueck DH, Nantermet
PV, Seillier-Moiseiwitsch F and Swanstrom R: Analysis of human
immunodeficiency virus type 1 nef gene sequences present in vivo. J
Virol. 67:4639–4650. 1993.PubMed/NCBI
|
|
6
|
Kwak YT, Raney A, Kuo LS, Denial SJ,
Temple BR, Garcia JV and Foster JL: Self-association of the
Lentivirus protein, Nef. Retrovirology. 7:772010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cruz NV, Amorim R, Oliveira FE, Speranza
FA and Costa LJ: Mutations in the nef and vif genes associated with
progression to AIDS in elite controller and slow-progressor
patients. J Med Virol. 85:563–574. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Foster JL, Denial SJ, Temple BR and Garcia
JV: Mechanisms of HIV-1 Nef function and intracellular signaling. J
Neuroimmune Pharmacol. 6:230–246. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bouchet J, Basmaciogullari SE, Chrobak P,
Stolp B, Bouchard N, Fackler OT, Chames P, Jolicoeur P, Benichou S
and Baty D: Inhibition of the Nef regulatory protein of HIV-1 by a
single-domain antibody. Blood. 117:3559–3568. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Muyldermans S: Single domain camel
antibodies: Current status. J Biotechnol. 74:277–302.
2001.PubMed/NCBI
|
|
11
|
Roovers RC, Laeremans T, Huang L, De Taeye
S, Verkleij AJ, Revets H, de Haard HJ and van Bergen en Henegouwen
PM: Efficient inhibition of EGFR signaling and of tumour growth by
antagonistic anti-EFGR Nanobodies. Cancer Immunol Immunother.
56:303–317. 2007. View Article : Google Scholar
|
|
12
|
Gueorguieva D, Li S, Walsh N, Mukerji A,
Tanha J and Pandey S: Identification of single-domain, Bax-specific
intrabodies that confer resistance to mammalian cells against
oxidative-stress-induced apoptosis. FASEB J. 20:2636–2638. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hultberg A, Temperton NJ, Rosseels V,
Koenders M, Gonzalez-Pajuelo M, Schepens B, Ibañez LI,
Vanlandschoot P, Schillemans J, Saunders M, et al: Llama-derived
single domain antibodies to build multivalent, superpotent and
broadened neutralizing anti-viral molecules. PLoS One.
6:e176652011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ohmi N, Wingfield JM, Yazawa H and Inagaki
O: Development of a homogeneous time-resolved fluorescence assay
for high throughput screening to identify Lck inhibitors:
Comparison with scintillation proximity assay and
streptavidin-coated plate assay. J Biomol Screen. 5:463–470. 2000.
View Article : Google Scholar
|
|
15
|
Degorce F: HTRF®: Pioneering
technology for high-throughput screening. Expert Opin Drug Discov.
1:753–764. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jia Y, Quinn CM, Gagnon AI and Talanian R:
Homogeneous time-resolved fluorescence and its applications for
kinase assays in drug discovery. Anal Biochem. 356:273–281. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen X, Longgood JC, Michnoff C, Wei S,
Frantz DE and Bezprozvanny L: High-throughput screen for small
molecule inhibitors of Mint1-PDZ domains. Assay Drug Dev Technol.
5:769–783. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mohamed TM, Zakeri SA, Baudoin F, Wolf M,
Oceandy D, Cartwright EJ, Gul S and Neyses L: Optimisation and
validation of a high throughput screening compatible assay to
identify inhibitors of the plasma membrane calcium ATPase pump - a
novel therapeutic target for contraception and malaria. J Pharm
Pharm Sci. 16:217–230. 2013.
|
|
19
|
Lemay J, Maidou-Peindara P, Bader T,
Ennifar E, Rain JC, Benarous R and Liu LX: HuR interacts with human
immunodeficiency virus type 1 reverse transcriptase, and modulates
reverse transcription in infected cells. Retrovirology. 5:472008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Seidel SA, Dijkman PM, Lea WA, van den
Bogaart G, Jerabek-Willemsen M, Lazic A, Joseph JS, Srinivasan P,
Baaske P, Simeonov A, et al: Microscale thermophoresis quantifies
biomolecular interactions under previously challenging conditions.
Methods. 59:301–315. 2013. View Article : Google Scholar :
|
|
21
|
Fischer WB, Wang YT, Schindler C and Chen
CP: Mechanism of function of viral channel proteins and
implications for drug development. Int Rev Cell Mol Biol.
294:259–321. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Izzedine H, Launay-Vacher V, Deray G and
Hulot JS: Nelfinavir and felodipine: A cytochrome P450 3A4-mediated
drug interaction. Clin Pharmacol Ther. 75:362–363. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Idriss HT: Questions regarding the role of
protein phosphorylation in HIV replication and anti-acquired immune
deficiency syndrome therapy. Saudi Med J. 26:7–10. 2005.PubMed/NCBI
|
|
24
|
Jakubiec A and Jupin I: Regulation of
positive-strand RNA virus replication: The emerging role of
phosphorylation. Virus Res. 129:73–79. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Behrend L, Milne DM, Stöter M, Deppert W,
Campbell LE, Meek DW and Knippschild U: IC261, a specific inhibitor
of the protein kinases casein kinase 1-delta and -epsilon, triggers
the mitotic checkpoint and induces p53-dependent postmitotic
effects. Oncogene. 19:5303–5313. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang WS and Stockwell BR: Inhibition of
casein kinase 1-epsilon induces cancer-cell-selective,
PERIOD2-dependent growth arrest. Genome Biol. 9:R922008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou YX, Karle S, Taguchi H, Planque S,
Nishiyama Y and Paul S: Prospects for immunotherapeutic proteolytic
antibodies. J Immunol Methods. 269:257–268. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Evans TR and Kaye SB: Retinoids: Present
role and future potential. Br J Cancer. 80:1–8. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kaleagasioglu F, Doepner G, Biesalski HK
and Berger MR: Antiproliferative activity of retinoic acid and some
novel retinoid derivatives in breast and colorectal cancer cell
lines of human origin. Arzneimittelforschung. 43:487–490.
1993.PubMed/NCBI
|
|
30
|
Hua S, Kittler R and White KP: Genomic
antagonism between retinoic acid and estrogen signaling in breast
cancer. Cell. 137:1259–1271. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He JC, Lu TC, Fleet M, Sunamoto M, Husain
M, Fang W, Neves S, Chen Y, Shankland S, Iyengar R, et al: Retinoic
acid inhibits HIV-1-induced podocyte proliferation through the cAMP
pathway. J Am Soc Nephrol. 18:93–102. 2007. View Article : Google Scholar
|
|
32
|
Lu TC, Wang Z, Feng X, Chuang P, Fang W,
Chen Y, Neves S, Maayan A, Xiong H, Liu Y, et al: Retinoic acid
utilizes CREB and USF1 in a transcriptional feed-forward loop in
order to stimulate MKP1 expression in human immunodeficiency
virus-infected podocytes. Mol Cell Biol. 28:5785–5794. 2008.
View Article : Google Scholar : PubMed/NCBI
|